Abstract
Excessive consumption of carbonated drinks contributes to the dietary surplus of carbohydrates, and is a main driver of the obesity epidemic in the USA. From a public health standpoint, it is therefore crucial to develop strategies that enable individuals to regulate this calorie-rich, but nutrient-poor food intake. However, conservative medical approaches to this end have met with limited success. Using a pharmacological strategy to eliminate the effervescent aspect of carbonated drinks, we report significant weight loss in a patient with long-standing obesity. Administration of low-dose acetazolamide, a carbonic anhydrase inhibitor, resulted in altered taste of carbonation, and in turn a marked reduction in the patient’s carbonated drink intake and the loss of almost 1 kg of body weight per week. The pharmacological intervention also resulted in appetite suppression, which might synergistically contribute to weight loss. These findings point to the use of low-dose acetazolamide as a novel weight reduction strategy.
INTRODUCTION
Obesity is one of the most significant public health issues in our time. It is often compared to an epidemic in terms of its detrimental effects on health and associated long-term morbidity for a significant proportion of individuals in our society [1–4]. It is thought that various dietary and lifestyle changes that have occurred over the past four decades are responsible for this phenomenon [1, 2]. In particular, the consumption of carbonated, sugar-sweetened drinks has more than doubled during the aforementioned time period, mirroring the rising incidence of obesity in the USA [1].
While dietary restriction of carbonated drinks and consumption of sugar-free carbonated beverages are popular approaches to mitigate this epidemic, their degrees of success are modest and transient [5, 6]. In several studies, these approaches have also had counterintuitive effects on body weight [7]. For instance, a significant dose–response relationship has been described between the daily consumption of artificially sweetened carbonated drinks (i.e. net zero calories) and prospective weight gain [7]. These results point to the possibility that carbonated drinks contribute to weight gain and obesity not only via added dietary sugars and calories.
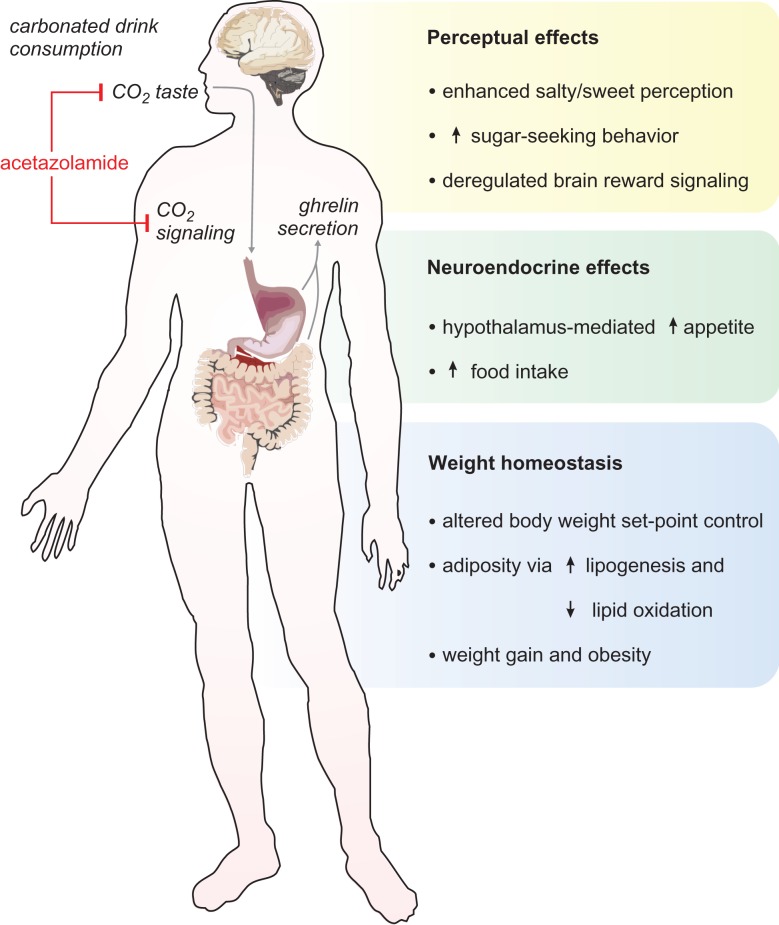
In particular, the carbonation of sweetened drinks is a property that might directly interact with physiological factors underlying body weight homeostasis and adiposity (Fig. 1) [8–10]. These interactions constitute pathways through which carbonation—via carbon dioxide signaling—might promote obesity. Furthermore, the effervescent aspect of carbonated beverages enhances the perception of sweetness and promotes sugar-seeking behavior in consumers [11–13]. Given these findings, we reasoned that affecting the taste and associated signaling of carbonation in beverages might diminish their appeal to consumers, limiting their intake and effects on energy balance and body weight. Acetazolamide is a carbonic anhydrase inhibitor, which has been shown to eliminate the sparkling or ‘fizzy’ taste of carbonated beverages [14, 15]. Here we tested the effect of administration of low-dose acetazolamide as part of a weight loss strategy in an obese patient with an excessive daily consumption of carbonated, sugar-sweetened beverages.
Figure 1:
Effects of the taste of carbonation and CO2 signaling in gustatory perception, appetite and body weight homeostasis. Acetazolamide (red) is a carbonic anhydrase inhibitor that eliminates the taste of carbonation on gustatory receptors at the mouth and, if administered systemically, might inhibit CO2 signaling in the gastrointestinal tract.
CASE REPORT
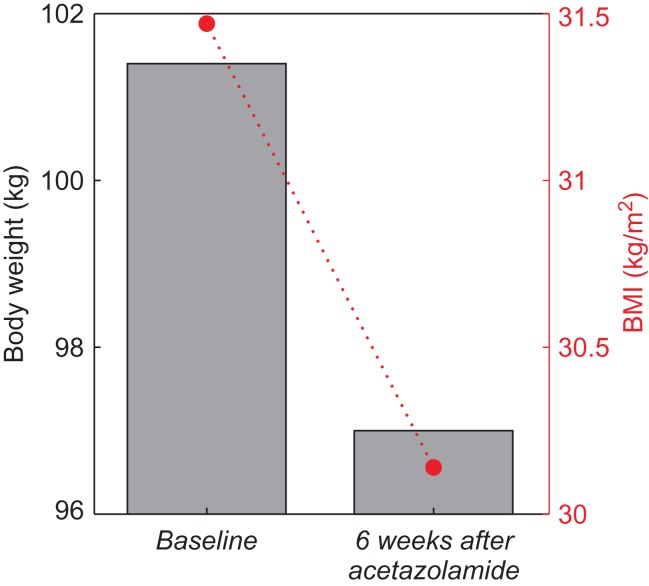
An otherwise healthy 48-year-old Caucasian man with long-standing obesity presented to the clinic with complaints of inability to lose weight, despite regular exercise and attempts at dieting, and with a self-reported addiction to carbonated, sugar-sweetened drinks. He indicated drinking ~6–8 non-diet carbonated beverages daily, often with other snacks (i.e. chips and cookies) while at work. Carbonated drinks represented an estimated 32–42% percentage of his daily caloric intake (average of 2613 kcal/day) to sustain 101.4 kg of body weight and body mass index (BMI) of 31.47 kg/m2 (Table 1 and Fig. 2). The patient reported several unsuccessful attempts at decreasing his carbonated drink consumption. At this initial visit, his physical exam was notable for obesity, and chemistry/metabolic and other laboratory testing were otherwise unremarkable (Table 1). Given the large contribution of carbonated drinks to his daily caloric intake, the patient was given a low dose of acetazolamide (125 mg orally daily, off-label use) in an attempt to discourage the consumption of carbonated drinks throughout the day.
Table 1:
Patient’s weight, BMI and other laboratory analyses at baseline and 6 weeks after initiation of administration of low-dose acetazolamide
| Baseline | Six weeks after acetazolamide | |
|---|---|---|
| Body weight (kg) | 101.4 | 97 |
| BMI (kg/m2) | 31.47 | 30.14 |
| White blood cell count (×103/μL) | 7 | – |
| Hemoglobin (g/dL) | 15.6 | – |
| Hematocrit (%) | 44.5 | – |
| Serum sodium (mmol/L) | 143 | 139 |
| Serum potassium (mmol/L) | 4.6 | 4.7 |
| Serum chloride (mmol/L) | 101 | 98 |
| Serum bicarbonate (mmol/L) | 25 | 22 |
| Blood urea nitrogen (mg/dL) | 12 | 21 |
| Creatinine (mg/dL) | 0.96 | 1.17 |
| Glucose (mg/dL) | 101 | 101 |
| Hemoglobin A1C (%) | 5.3 | – |
| Serum calcium (mg/dL) | 9.4 | 10.1 |
| Aspartate transaminase | 25 | 28 |
| Alanine transaminase | 40 | 35 |
| Alkaline phosphatase | 88 | 90 |
| Total serum bilirubin | 0.3 | 0.4 |
| Total serum protein | 8 | 9 |
| Serum albumin | 4.7 | 5.1 |
Figure 2:
Effect of daily acetazolamide administration on weight and BMI after 6 weeks. Bar plot (gray) represents weight, and dashed line (red) traces BMI as a function of time.
On follow-up at 6 weeks after onset of daily acetazolamide administration, the patient exhibited a 4.4 kg weight loss, with a total body weight of 97 kg and BMI of 30.14 kg/m2 (Fig. 2). The patient reported good adherence to acetazolamide treatment, missing ~1–2 doses/week. Furthermore, the patient’s observed weight loss is in agreement with the estimated weight loss of 4.5–6 kg (at an estimated rate of 0.75–1 kg/week), given the reported daily calorie deficit of 828–1104 kcal due to eliminated intake of carbonated drinks. Upon taking acetazolamide, the patient reported that carbonated drinks had a ‘flat’ taste, making it easier for him to avoid their consumption. However, the craving for carbonated drinks was not affected, with occasional consumption of carbonated drinks (1–2 times/week), in spite of the perceived change in taste. The patient denied altered taste of any other food or drink consumed, and overall good tolerance and satisfaction with acetazolamide at this dose. During the treatment period, the patient continued his usual physical activity with regular exercise 1–3 times/week, and made no other modifications to his diet in an attempt to promote further weight loss. However, the patient reported decreased appetite (a documented side effect of acetazolamide) [16] and reduced consumption of some of the snacks that often accompanied his routine consumption of carbonated drinks. The patient reported that the snacks were not as appealing when taken without the carbonated drinks.
The patient denied the occurrence of adverse events that have been associated with acetazolamide administration, such as flushing, ataxia, confusion, seizures, depression, dizziness, drowsiness, flaccid paralysis, headache, paresthesias, visual or auditory disturbances, anaphylaxis, rashes or other skin lesions, diarrhea, melena, nausea, vomiting, hematuria, polyuria, fatigue, malaise, or fever [16]. On laboratory analyses 6 weeks after initiation of acetazolamide therapy, the patient did not exhibit any alterations in his electrolyte and acid–base status, and had normal serum levels of hepatic enzymes and renal function markers (Table 1) [16].
DISCUSSION
Carbonated drinks are extremely popular throughout the world, and considered the principal contributors of added sugars in the American diet [1–4]. The rise in their consumption has mirrored the increased prevalence of obesity in the USA [1]. While their direct caloric contribution in fueling the obesity epidemic is recognized, the effect of their carbonation is also postulated to be crucial, although the underlying mechanism(s) remain poorly understood [8, 9].
In this line, a dose–response relationship has been reported between the net intake of carbonated drinks and the magnitude of prospective weight gain, irrespective of whether these beverages are sweetened with sugar or artificial substitutes (i.e. with or without the calories) [7]. Moreover, greater consumption of carbonated sugar-sweetened beverages is associated with stronger correlations between an individual’s genetic predisposition (as assessed by the expression profile of 32 BMI-associated loci) and the severity of their obesity [4]. These findings suggest that interactions between genetic and diet/environmental factors may have synergistic effects in individuals with excessive consumption of carbonated beverages, potentiating their weight gain and other metabolic disturbances.
In fact, behavioral and functional magnetic resonance imaging studies have demonstrated that carbonated drink consumers engage distinct brain perceptual pathways, exhibit diminished salty and sweet taste perception, and have significant biases for sweet foods (Fig. 1) [11–13]. Additionally, studies in animal models and human subjects suggest that carbon dioxide stimulates ghrelin secretion by the stomach and/or small intestine, promoting appetite, deregulating brain reward signaling and possibly enhancing the addictive properties of carbonated drinks (Fig. 1) [8–10].
A substantial body of evidence supports the notion that strategies to limit the consumption of carbonated beverages may have a significant impact in weight loss and in reducing obesity at the population level [5, 6]. The widespread availability and marketing of carbonated drinks poses difficulties to implementing these strategies. Although policies to restrict their sale at low cost and/or in excessive portions have been proposed, these regulations have not seen widespread adoption. Moreover, as mentioned above, while in principle the use of artificial sweeteners in carbonated beverages should eliminate their caloric contributions and promote weight loss, epidemiological studies suggest that the consumption of these beverages is instead positively correlated with weight gain and obesity [7]. Although the mechanisms behind this counterintuitive result are not well understood, it is conceivable that carbonation itself might promote appetite and the deregulation of energy balance and body weight homeostasis (Fig. 1). However, medical strategies that target carbon dioxide taste and signaling to limit the consumption of carbonated beverages and promote weight loss have not been adequately explored to date.
In this way, carbonic anhydrase inhibition by low-dose acetazolamide for weight loss, as discussed in this case study, holds potential to expand the therapeutic options available for the management of obesity. This pharmacological strategy has an excellent risk-to-benefit profile, with virtually no side effects or adverse events, as documented in the present case and as inferred from the extensive body of literature on the clinical use of acetazolamide and its safety profile [16]. Furthermore, acetazolamide administration and associated reduction in carbonated drink consumption can set the stage for, or complement, long-term multimodal approaches to obesity that emphasize lifestyle modifications and promote the development of balanced, healthy nutritional habits. In particular, this strategy might help reduce long-term consumption of carbonated drinks by virtue of its effects at multiple levels, such as preventing the sensation or taste of carbonation (effervescence or ‘fizz’) and rendering carbonated drinks less pleasurable (Fig. 1). Moreover, acetazolamide might curtail carbon dioxide’s effect(s) on ghrelin signaling that stimulate appetite, and in turn counter its effects on reinforcement learning and addiction, possibly leading to the extinction of deregulated food-seeking behaviors that are not guided by metabolic needs (Fig. 1).
Although our findings are currently limited to a single case (and recently another patient with early promising results as well), the extent of clinical improvement demonstrated here suggests the potential for future longitudinal studies on this strategy for weight loss. This benefit was observed for an administered dose of 125 mg, which is lower than the more typical doses of 250 mg for acute altitude sickness and doses of 500 mg for other indications, suggesting that efficacy may be achieved at even lower-than-typical doses. It would be interesting to postulate and potentially later explore whether there may be a dose–response effect at the more commonly administered doses of this medication.
As a precaution, it is important to consider dose adjustments for patients with renal dysfunction, as impaired acetazolamide clearance and abnormally elevated serum levels have been associated with acid–base derangements [17, 18]. Of note, low doses of acetazolamide (<250 mg once daily), such as the dose employed here, have not been associated with significant serum pH changes (i.e. 0–0.05 unit change in pH) [19, 20], and our daily regimen allows for a sufficient pharmacological clearance window (half-life: 2–4 h) [16]. In fact, our patient exhibited a normal serum bicarbonate level after 6 weeks of daily acetazolamide treatment (Table 1), suggesting a normal acid–base status. As an alternative mode of administration that circumvents potential systemic side effects, acetazolamide could be given as a solution for topical oral mouth rinses, which have been shown effective in attaining a localized inhibition of carbonic anhydrase at taste receptors and eliminating the taste of carbonation in beverages [14]. This mode of administration could also help elucidate the contribution of the role of carbon dioxide in central perceptual versus gastrointestinal neurohormonal effects on appetite and weight gain (Fig. 1).
Acknowledgements
We are grateful to Richard Hardstone for helpful discussions and comments on the article.
Conflict of Interest statement
The authors report no conflicts of interest.
Funding
This work was supported by the National Institutes of Health [T32GM07308 to W.M.].
Ethical Approval
None required.
Consent
Consent provided by the patient.
Guarantor
Steven Lamm.
REFERENCES
- 1. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 2. Caprio S. Calories from soft drinks—do they matter? N Engl J Med 2012;367:1462–3. [DOI] [PubMed] [Google Scholar]
- 3. Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med 2012;367:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397–1406. [DOI] [PubMed] [Google Scholar]
- 6. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16:1894–1900. [DOI] [PubMed] [Google Scholar]
- 8. Cuomo R, Savarese MF, Sarnelli G, Nicolai E, Aragri A, Cirillo C, et al. The role of a pre-load beverage on gastric volume and food intake: comparison between non-caloric carbonated and non-carbonated beverage. Nutr J 2011;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eweis DS, Abed F, Stiban J. Carbon dioxide in carbonated beverages induces ghrelin release and increased food consumption in male rats: implications on the onset of obesity. Obesity Res Clin Pract 2017;11:534–43. [DOI] [PubMed] [Google Scholar]
- 10. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60. [DOI] [PubMed] [Google Scholar]
- 11. Green E, Murphy C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol Behav 2012;107:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Salle F, Cantone E, Savarese MF, Aragri A, Prinster A, Nicolai E, et al. Effect of carbonation on brain processing of sweet stimuli in humans. Gastroenterology 2013;145:537–539.e533. [DOI] [PubMed] [Google Scholar]
- 13. Sartor F, Donaldson LF, Markland DA, Loveday H, Jackson MJ, Kubis HP. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite 2011;57:237–46. [DOI] [PubMed] [Google Scholar]
- 14. Graber M, Kelleher S. Side effects of acetazolamide: the champagne blues. Am J Med 1988;84:979–80. [DOI] [PubMed] [Google Scholar]
- 15. Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, et al. The taste of carbonation. Science 2009;326:443–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acetazolamide. Lexi-Drugs Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed January 20, 2018.
- 17. Heller I, Halevy J, Cohen S, Theodor E. Significant metabolic acidosis induced by acetazolamide. Not a rare complication. Arch Intern Med 1985;145:1815–7. [PubMed] [Google Scholar]
- 18. Chapron DJ, Gomolin IH, Sweeney KR. Acetazolamide blood concentrations are excessive in the elderly: propensity for acidosis and relationship to renal function. J Clin Pharmacol 1989;29:348–53. [DOI] [PubMed] [Google Scholar]
- 19. Galdston M. Respiratory and renal effects of a carbonic anhydrase inhibitor (diamox) on acid-base balance in normal man and in patients with respiratory acidosis. Am J Med 1955;19:516–32. [DOI] [PubMed] [Google Scholar]
- 20. Brechue WF, Stager JM, Lukaski HC. Body water and electrolyte responses to acetazolamide in humans. J Appl Physiol (1985) 1990;69:1397–1401. [DOI] [PubMed] [Google Scholar]