Abstract
Background
MEF2C (Myocyte-specific enhancer factor 2C) has been associated with neurodevelopmental disorders. This study aimed at delineating the clinical profiles of MEF2C gene mutations.
Methods
In total, 112 Chinese patients with intellectual disability (ID) were recruited, including 44 patients presented with Rett syndrome (RTT) or RTT-like syndrome, and 68 patients with non-syndromic ID. Targeted next-generation sequencing (NGS) was performed. Detailed clinical information was collected.
Results
Five heterozygous MEF2C gene mutations were identified, of which three were novel. The MEF2C mutant rate was 4.5% (5/112) in total, and 6.8% (3/44) in the RTT (−like) cohort. All patients with MEF2C gene mutation presented with cognitive impairment, gross motor delay, speech disorder and autistic features. Four patients had epilepsy, which responded well to antiepileptic drugs. One female was diagnosed with classical RTT, two females with RTT-like syndrome, and two males with non-syndromic ID. Generally, the phenotype of two males with relatively downstream mutations (c.565C > T, p.Arg 189*; c.766C > T, p.Arg 256*) was milder than that of three females with upstream mutations (c.48C > G, p.Asn16Lys; c.334G > T, p.Glu112* and c.403-1G > T).
Conclusions
Our findings expanded the current understanding of the consequences of MEF2C dysfunctions, especially MEF2C point mutations. MEF2C mutations are associated with a broad clinical spectrum, ranged from classical RTT to non-syndromic ID. Through our study, it can be inferred that there is correlation between the phenotype and MEF2C-genotype, the mutation site. Overall, the MEF2C gene mutational analysis should be performed in ID cohort, especially in patients with features overlapped with RTT.
Electronic supplementary material
The online version of this article (10.1186/s12881-018-0699-1) contains supplementary material, which is available to authorized users.
Keywords: MEF2C, Rett (−like) syndrome, Non-syndromic intellectual disability, Genotype-phenotype correlation
Background
MEF2C (Myocyte-specific enhancer factor 2C), a member of MEF2 subfamily, is a transcriptional activator binding specifically to the MEF2 element, which has pivotal role in myogenesis, hematopoiesis, neurogenesis and synaptogenesis [1, 2]. Especially, MEF2C is crucial for normal neuronal development, distribution, and electrical activity in the neocortex [3]. MEF2C haploinsufficiency caused by either large deletions encompassing MEF2C, or intragenic mutations of MEF2C, is associated with neurodevelopmental disorders [4].
In this study, we performed targeted next-generation sequencing (NGS) in Chinese patients with intellectual disability (ID) of unknown causes. MEF2C mutations were identified in five patients, including three females with RTT or RTT-like features, and two males with non-syndromic ID. The phenotypes and genotype of MEF2C mutation in Chinese ID patients were reported here.
Methods
Patients
In total, 112 Chinese patients with ID were enrolled into this study from August 2015 to March 2018, including 68 females and 44 males, aged from 13 months to 12.5 years. Among them, 25 girls were initially diagnosed with RTT (21 with typical RTT, 2 with Han-RTT, 1 with Zappella variant of RTT and 1 with congenital RTT), 16 females and 3 males with RTT-like syndrome. Mutations in MECP2, CDKL5 and FOXG1 have been ruled out in the patients of RTT (−like) cohort. The other 68 patients presented with non-syndromic ID. Metabolic or mitochondrial disorders, central nervous system infection or other known etiologies were excluded.
Detailed clinical information including clinical manifestation, electroencephalogram (EEG), magnetic resonance imaging (MRI), and family history, etc., was collected. Genomic DNA was extracted from peripheral leukocytes of the patients and their parents. This study was approved by the Medical Ethics Committee, Peking University First Hospital. Written informed consent was obtained from the parents of the patients.
Targeted next-generation sequencing
A genetic panel which is related to ID was designed, containing 512 genes (supplemental material, Additional file 1: Table S1) in total. Especially, genes associated with RTT (−like) syndrome were included. Library preparation including end repair, adapter ligation and PCR enrichment were carried out as recommended by the NEBNext Rfast DNA Fragmentation and Library Prep Set for Ion Torrent. Targeted regions were captured with a customized SeqCap panel (Roche). The enriched libraries were sequenced on an Ion Torrent Proton sequencer with 200 bp single end read. Signal processing and base calling were carried out using Torrent Suite 5.04 software. Reads were aligned to the human reference genome build UCSC hg19 using Tmap. Variants were called using the variant caller from Torrent Suite.
Variants were annotated using ANNOVAR (http://annovar.openbioinformatics.org/en/latest/), assessment of the variants were carried out following the ACMG guidence. Common sites with population allele frequency above 5% according to dbSNP 138, 1000 Genome Project, esp6500si and ExAC databases were excluded. Rare loss-of-function variants were selected for follow-up analyses. Selected variants with depth lower that 20X and allelic fraction lower than 30% were manually reviewed with IGV. Functional variants annotated by HGMD, Clinvar or predicted to be deleterious by SIFT and Polyphen2 were regraded as candidates.
Results
Molecular analysis
Causative gene mutations were identified in 14 patients of our RTT (−like) cohort, with detection rate of 31.8% (14/44), incluing 3 MEF2C mutations. In the non-specific ID group, 9 patients (13.2%, 9/68) were identified with pathogenic mutations, including 2 MEF2C mutations. The wild type and mutant allele of MEF2C is approximately at 1:1 (ranging from 39.1 to 47.2%), so mosaicism were not considered.
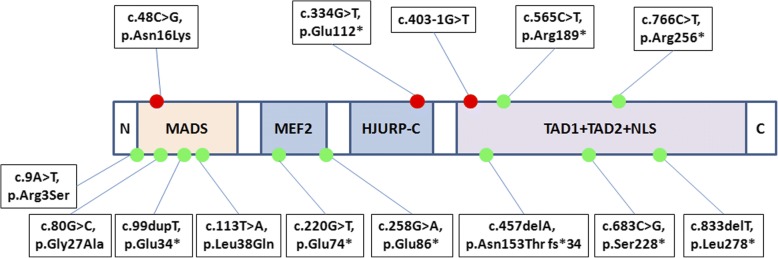
Five heterozygous mutations in MEF2C were identified in three females with RTT (−like) syndrome and two males with non-syndromic ID. The MEF2C mutant rate was 4.5% (5/112) in total, whereas 6.8% (3/44) and 2.9% (2/68) in Rett (−like) cohort and non-syndromic ID group, respectively. These five mutations included three nonsense mutations, one splicing site mutation, and one missense mutation. Three MEF2C mutations (c.48C > G, p.Asn16Lys; c.403-1G > T; c.766C > T, p.Arg256*) arose de novo, and the other two (c.565C > T, p.Arg189*; c.334G > T, p.Glu112*)were of unknown origin, as the parental DNA was not available. Among these mutations, two mutations, c.565C > T, p.Arg189* and c.766C > T, p.Arg256*, were reported pathogenic, the other three mutations, c.48C > G, p.Asn16Lys; c.334G > T, p.Glu112*; and c.403-1G > T, were novel. They were predicted as pathogenic by Mutationtaster, the missense mutations, c.48C > G, p.Asn16Lys, was predicted as pathogenic by PolyPhen-2 and SIFT as well. A schematic representation of the MEF2C protein, including the mutations identified in this study as well as the mutations previously reported, is shown in Fig. 1.
Fig. 1.
The structure of the MEF2C protein and the location of MEF2C mutations. Red dots: Novel mutations found in this study. Green dots: Reported mutations. The upper line: Five MEF2C mutations identified in this study. The lower line: Nine MEF2C mutations that has been described previously. N, N-terminus; MADS, MCM1, agamous, deficiens, serum response factor; MEF2, myocyte enhancer factor 2; TAD, transcriptional activation domain; NLS, nuclear location signal; C, C-terminus
Clinical profiles of patients with MEF2C mutations
Patient 1 is a girl aged 5 years and 9 months, the first child of a healthy nonconsanguineous couple. Her psychomotor milestones were profoundly delayed, with raising head at 8 months, sitting alone at 1 year old, and still unable to walk by herself at 5 years and 9 months. Poor eye contact, hand clapping, hand wringing and bruxism were observed at 1 year old, followed by deterioration of hand skills. Epileptic attack occurred at 20 months old, and she responded to valproate (VPA), oxcarbazepine (OXC), and topiramate (TPM) combined therapy. Seizure free was achieved at 5.5 years old. EEG demonstrated spike-slow waves at right medial and posterior temporal, with generalization. MRI (1 year old) revealed enlargement of frontal subarachnoid space. Above manifestations led to the diagnosis of typical RTT. However, mutational analysis of MECP2, CDKL5 and FOXG1 was negative. Through this study, a de novo missense MEF2C mutation, c.48C > G, p.Asn16Lys, was identified, which was a novel mutation.
Patient 2 is a 2.5 years old girl presented with RTT-like ID. she displayed profound psychomotor retardation, with controlling head at 5 months, sitting alone at 8 months. She remained unable to walk independently and still had no speech at 2.5 years old. She developed stereotypic hand movements and bruxism at 2 years of age. No seizure was reported, but there was epileptiform discharge on EEG at age of 2 years. Brain MRI revealed high T1 and T2 signal at posterior horn of bilateral ventricle. A nonsense mutation, c.565C > T, p.Arg189*, of MEF2C was discovered, a known disease causing mutation (https://www.ncbi.nlm.nih.gov/clinvar/).
Patient 3 is a 23-month-old girl who presented with RTT-like features. Feeding difficulties caused concern at 3 months of age. No epilepsy was observed, though she had medical history of febrile convulsions at 9 months old. There was significant delay in her developmental milestones, without obvious retrogression. She could sit alone at 1 year old, and walk at 23 months of age, with abnormal gaits. Unmeaningful language began at 12 months. She also presented with poor eye contact, stereotypic actions, breathing disturbance, and sleeping abnormalities. She suffered from recurrent respiratory infections frequently after 15 months of age. MRI revealed hypomyelination at 1 year and 10 months of age. A novel nonsense mutation, c.334G > T, p.Glu112*, in MEF2C was identified.
Patient 4 was a boy aged 7 years and 8 months. He achieved the gross motor developmental milestones somewhat delay, with rising head at 1 year old, sitting alone at 1 year and 2 months, and walking at 1.5 years. Lack of speech was another problem, that he still cannot speak a single word so far (7 years and 8 months). He was always immersed in his own world, showed little interest to the others, and lacked eye-contact. Febrile seizures attacked at 1 year, which turned into afebrile seizures at 2 years old. Partial seizures occurred 1~ 2 times per month, lasted few minutes to more than half an hour. The epilepsy was fever-sensitive. VPA was used at 2.5 years of age, and no seizures occurred after 4 years of age. EEG at 2.5 years displayed (multi-) spike and slow waves at right occipital region, with slow rhythm on the background. MRI at 3 years of age was normal. A novel mutation, c.403-1G > T, of MEF2C was identified, which arose de novo.
Patient 5 was a boy of 6 years and 4 months. He presented with developmental delay, with rising head at 7 months, sitting alone at 1 year, and walking at 2 years old. Language delay was a main problem, that he could only speak one to two words, such as “give me”, “I want”. He also displayed some autistic behaviors, including occasionally repetitive hand movements, no eye-contact and no interest to others. Febrile seizures initially occurred at 8 months of age, then attacked 2–3 times per year. Seizure-free was achieved for 4 months with levetiracetam (LEV) and VPA. MRI at 4 years old revealed long T1 and T2 signal around bilateral ventricle, and a septum pellucidum cyst. EEG was normal at 4 years of age. A known pathogenic de novo mutation, c.766C > T, p.Arg256*, in MEF2C was discovered.
The genetic data and clinical information from the five patients with MEF2C mutations were summarized in Table 1.
Table 1.
Clinical information of patients with MEF2C (NM_001193350) mutation
| ID | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Sex | F | F | F | M | M |
| Mutation | c.48C > G, p.Asn16Lys | c.565C > T, p.Arg189* | c.334G > T, p.Glu112* | c.403-1G > T | c.766C > T, p.Arg256* |
| Origin | de novo | mother WT; father unknown | father WT; mother unknown | de novo | de novo |
| Age | 5y 9mo | 2 y, 6 mo | 2y 4mo | 7y 8mo | 6y 4 mo |
| Sz (age of onset) | Y (1y 10mo) | N | Y (9mo) | Y (1y) | Y (8mo) |
| Sz types | PS | – | FS | FS, PS, SE | FS |
| Response to AEDs | Seizure free for 4 months, with OXC, VPA, TPM | – | – | seizures free after 4 y, with VPA | seizures free 6y, with LEV, VPA |
| Motor development | Head control (8 mo), sit (12 mo), cannot walk | Head control (5 mo), sit (8 mo), cannot walk, head circumstance 48.5 cm (2y) | Head control (2 mo), sit (12 mo), walk (23 mo), abnormal gaits, head circumstance 45.3 cm (2y 4mo) | Head control (1y), sit (1y 2 mo), walk (1 y, 6 mo) | Head control (7 mo), sit (1y), walk (2y), head circumstance 52 cm (6y 4mo) |
| Cognitive outcome | Severe ID | Severe ID | Severe ID | Severe ID | Severe ID |
| Language | N | N | Unmeaningful murmur | N | 1 to 2 words |
| Poor eye-contact | Y | Y | Y | Y | Y |
| Repetitive behaviour | Y | Y | Y | Occassionlly | Occassionally |
| Poor hand skills | Y | Y | Y | Normal | Normal |
| Hypotonia | Y | Y | Y | N | N |
| Family history | N | N | N | N | N |
| EEG | Sharp-slow waves at right medial and posterior temporal, followed by generalization (2y, 11mo) | NA | Normal (2y, 4mo) | Sharp-and-slow waves around Pz(4y,1mo) | Normal (4 y) |
| MRI | enlargement of frontal subarachnoid space (1 y) | Long T1 and T2 signal at posterior horn of bilateral ventricle. | Hypomyelination (1y, 10 mo) | Normal (3y) | Long T1 and T2 signal around bilateral ventricle, septum pellucidum cyst (4y) |
| Other symptoms | hypalgesia | _ | Feeding difficulty, sleeping disturbance, irritability, recurrent respiratory infection, bruxism | No interest to others | No interest to others |
F, female; M, male; Y, yes; N, no; NA, not avaliable; ID, intellectual disability; VPA, valproate; LEV, levetriacetam; OXC, oxcarbazepine; TPM, topiramate; y, year; mo, month; AEDs, antiepileptic drugs
Discussion
In this study, five MEF2C mutations were identified in RTT (−like) syndrome or non-syndromic intellectual disability patients, of which three mutations were novel. The MEF2C gene was originally identified as the phenocritical gene in the 5q14.3 microdeletion syndrome [5, 6]. Consecutively, intragenic deletions/insertions and point mutations of MEF2C were discovered in patients with neurodevelopmental disorders, including intellectual disability and epilepsy [7]. As most patients were detected having multi-gene microdeletions involved MEF2C, the phenotype of MEF2C gene mutation is not well recognized [8, 9]. Up to now, only 14 point mutations in MEF2C have been described in patients with neurological disorders [7, 10–13], including mutations discovered in this study, as is shown in Fig. 1. Our findings provide support for delineating the clinical features of patients with MEF2C point mutations, as well as the genotype-phenotype relationship.
MEF2C gene, located at 5q14, encodes myocyte enhancer-binding factor 2C, a transcription factor that is indispensable in early neuroprogenitor development [2, 14]. The human MEF2C protein consists five core structural domains, including MADS (MCM1, agamous, deficiens, serum response factor), myocyte enhancer factor 2 (MEF2), transcriptional activation domain 1 (TAD1), transcriptional activation domain 2 (TAD2), and nuclear localization signal (NLS) [15]. The MADS domain contains 56 amino acids, the MEF2 domain starts from amino acid 57 to 86, and the HJURP-C domain consists of 30 amino acids, while the stereostructure of rest domians remains unknown. The main role of MADS domain and the adjacent MEF2C domain is to mediate dimerization and DNA binding, whereas the rest domains act as the transcriptional activator [15]. The MEF2C mutations are scattered through the whole MEF2C protein, and there’s no hot mutant region (Fig. 1).
Typical clinical manifestations in patients with MEF2C gene mutations encompass global developmental delay, intellectual disability, absent speech, poor eye-contact and various minor brain anomalies on MRI [11]. A subset of patients with MEF2C haploinsufficiency phenotypically resembles RTT, including psychomotor stagnation, stereotypical behavior, poor hand skills, etc., [4, 7, 16] Our findings basically coincided with previous description. All patients of our cohort presented with cognitive impairment, speech disorder, gross motor delay and lack of eye-contact. MRI was abnormal in four out of five patients, with diverse anomalies, including enlargement of frontal subarachnoid space, hypomyelination, abnormal signals at posterior horn of bilateral ventricle or bilateral ventricle. Among them, one female manifestated as classical RTT and other two females displayed RTT–like phenotype, whereas two males presented with non-syndromic ID.
The overwhelming majority of patients who had macro-deletions partially or entirely encompassing the MEF2C gene, cannot walk independently. However, including 3 of our cases (patients 3–5), 7 (7/14, 50%) patients with MEF2C point mutations acquired the walking abilities [12, 17]. It indicates that MEF2C point mutations might cause milder phenotypes than large intragenic deletions or completely deletions. Additionally, the phenotype of patient 4 (c.565C > T, p.Arg 189*) and patient 5 (c.766C > T, p.Arg 256*) was milder than that of patient 1 (c.48C > G, p.Asn16Lys), patient 2 (c.334G > T, p.Glu112*) and patient 3 (c.403-1G > T). As it was shown in Fig. 1, that the later three mutations located at the upstream, whereas the other two mutations located at the downstream of the MEF2C protein, which suggested that the phenotypic severity may be associated with the mutation site.
Stereotypic behaviors, such as head rocking, hand-mouthing, hand clapping and wringing were reported in some patients with MEF2C mutations [7]. In our cohort, repetitive hand movements were obvious in the three females, whereas it was just occassionally observed in two male cases. Meanwhile, the three females only had limited hand skills, while it was basically normal in both male patients. Besides, bruxism, breathing and sleeping disturbance were also presented in the three females of RTT (−like) cohort. The reason accounted for the different clinical feature in male and female patients with MEF2C gene mutation need to be further studied.
More than half patients with MEF2C haploinsufficiency experienced epilepsy [18]. Seizures generally arised during infancy or early childhood, which were commonly triggered by fever. Tonic-clonic seizures, myoclonic seizures were the most common seizure types, partial seizures, infantile spasms, atypical absence and febrile seizures were also described. Most epilepsy patients responded well to anti-epileptic drugs, whereas refractory in a few cases were also reported [18]. In this study, four (4/5, 80%) patients developed epilepsy within one year old. Febrile seizures were occurred in all of them, partial seizures were reported in two patients, and one patient had history of epileptic status. Seizure-free was achieved in all of them by mono- or multi-antiepileptic drugs (AEDs) therapy.
Mild to severe hypotonia was reported in most of the patients [12]. In our cohort, severe hypotonia was observed in three females. MRI showed nonspecific abnormalities in four of our patients, including hypomyelination, enlarged ventricles, abnormal signal on posterior horn of bilateral ventricle, just as that was reported in the literature [5].
Recently, MEF2C mutations were founded in patients with heart disorders, including patent ductus arteriousus (PDA), double outlet right ventricle (DORV), ventricular septal defect (VSD) and dilated cardiomyopathy (DCM), accompanied with or without neurological symptoms [19–21]. All patients of our cohort had no medical history of heart diseases. As DCM is usually adult-onset, a longer term of ultrasonic cardiogram monitoring is necessary for patients with MEF2C mutations. There was no relationship between the genotype and phenotype with or without heart disorders and/or neurological symptoms. The clinical heterogeneity might indicated the partly incomplete penetrance.
Conclusions
Our findings expanded the spectrum of point mutations of MEF2C gene, and also showed some relationship between phenotype and MEF2C-genotype. The different clinical features in male and female patients with MEF2C gene mutation need to be verified in a larger sample. MEF2C gene mutational analysis was recommended in patients with ID, especially in the RTT (−like) cohort without mutations in traditional RTT genes (MECP2, CDKL5, FOXG1) .
Additional file
Table S1. List of 512 candidate genes. (DOCX 20 kb)
Acknowledgements
We would like to thank the patients and their family for their cooperation in this study
Funding
The study was funded by 985 Peking University and Clinical Hospital Cooperation Project (2013-1-06), Clinical Research Special Fund of Wu Jieping Medical Foundation (320.6750.17091), and Technology Innovation Talents Special Fund of Harbin Science and Technology Bureau (2016RAXYJ089).
Availability of data and materials
Raw exome data and associated variant calls will not be shared, as part of our work has not been published.
Abbreviations
- AEDs
antiepileptic drugs
- DCM
dilated cardiomyopathy
- DORV
double outlet right ventricle
- EEG
electroencephalogram
- ID
intellectual disability
- LEV
levetiracetam
- NGS
next-generation sequencing
- OXC
oxcarbazepine
- PDA
patent ductus arteriousus
- TPM
topiramate
- VPA
valproate
- VSD
ventricular septal defect
Authors’ contributions
XHB, XRW, and SJY obtained the funding and designed the research. QPZ, YC and YXW acquired clinical data and samples from the patients. QPZ also made contribution in the analysis of NGS data. YC and YXW also provided help in clinical evaluation of the patients. JPW validated the parental origin of candidate variants by PCR-Sanger sequencing, made routine follow-up of the patients and drew up the manuscript. XRW also made contribution in English editing of the manuscript. All the authors have read and approved the manuscript and ensure that this is the case.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee, Peking University First Hospital. Guardians of all participants have signed the informed consent.
Consent for publication
Consent for publication was obtained from the parents of the patients. All the guardians consented to publish the medical information of the patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiaping Wang, Email: wangjiaping321@126.com.
Qingping Zhang, Email: zhangqp1988@126.com.
Yan Chen, Email: cydoc1991@163.com.
Shujie Yu, Email: yushujie2008520@163.com.
Xiru Wu, Email: wxrwwn@gmail.com.
Xinhua Bao, Email: zwhang@pku.eud.cn.
Yongxin Wen, Email: angel124@163.com.
References
- 1.Potthoff MJ, Olson EN. MEF2C: a central regulator of diverse developmental programs. Development. 2007;134(23):4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 2.Leifer D, Krainc D, Yu YT, McDermott J, Breitbart RE, Heng J, et al. MEF2C, a MADS/MEF2-family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci U S A. 1993;90(4):1546–1550. doi: 10.1073/pnas.90.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, et al. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U S A. 2008;105(27):9397–9402. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zweier M, Rauch A. The MEF2C-related and 5q14.3q15 microdeletion syndrome. Mol Syndromol. 2012;2(3–5):164–170. doi: 10.1159/000337496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotz A, Hellenbroich Y, Sperner J, Linder-Lucht M, Tacke U, Walter C, et al. Microdeletion 5q14.3 and anomalies of brain development. Am J Med Genet A. 2013;161A(9):2124–2133. doi: 10.1002/ajmg.a.36020. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso C, Boys A, Parrini E, Mignon-Ravix C, McMahon JM, Khantane S, et al. Periventricular heterotopia, intellectual disability, and epilepsy associated with 5q14.3-q15 deletion. Neurology. 2009;72(9):784–792. doi: 10.1212/01.wnl.0000336339.08878.2d. [DOI] [PubMed] [Google Scholar]
- 7.Le Meur NH-EM, Jaillard S, Goldenberg A, Joriot S, Amati-Bonneau P, Guichet A, Barth M, Charollais A, Journel H, Auvin S, Boucher C, Kerckaert JP, David V, Manouvrier-Hanu S, Saugier-Veber P, Frébourg T, Dubourg C, Andrieux J, Bonneau D. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe intellectual disability with stereotypic movements, epilepsy and/or cerebral malformations. J Med Genet. 2010;47(1):22–29. doi: 10.1136/jmg.2009.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowakowska BA, Obersztyn E, Szymanska K, Bekiesinska-Figatowska M, Xia Z, Ricks CB, et al. Severe intellectual disability, seizures, and hypotonia due to deletions of MEF2C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(5):1042–1051. doi: 10.1002/ajmg.b.31071. [DOI] [PubMed] [Google Scholar]
- 9.Engels H, Wohlleber E, Zink A, Hoyer J, Ludwig KU, Brockschmidt FF, et al. A novel microdeletion syndrome involving 5q14.3-q15: clinical and molecular cytogenetic characterization of three patients. Eur J Hum Genet. 2009;17(12):1592–1599. doi: 10.1038/ejhg.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zweier M, Gregor A, Zweier C, Engels H, Sticht H, Wohlleber E, et al. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe intellectual disability and diminish MECP2 and CDKL5 expression. Hum Mutat. 2010;31(6):722–733. doi: 10.1002/humu.21253. [DOI] [PubMed] [Google Scholar]
- 11.Bienvenu T, Diebold B, Chelly J, Isidor B. Refining the phenotype associated with MEF2C point mutations. Neurogenetics. 2013;14(1):71–75. doi: 10.1007/s10048-012-0344-7. [DOI] [PubMed] [Google Scholar]
- 12.Vrečar IIJ, Jones EA, Kingston H, Reardon W, Kerr B, Clayton-Smith J, Douzgou S. Further clinical delineation of the MEF2C Haploinsufficiency syndrome: report on new cases and literature review of severe neurodevelopmental disorders presenting with seizures, absent speech, and involuntary movements. J Pediatr Genet. 2017;6(3):129–141. doi: 10.1055/s-0037-1601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava S, Cohen JS, Vernon H, Baranano K, McClellan R, Jamal L, et al. Clinical whole exome sequencing in child neurology practice. Ann Neurol. 2014;76(4):473–483. doi: 10.1002/ana.24251. [DOI] [PubMed] [Google Scholar]
- 14.Cesaretti C, Spaccini L, Righini A, Parazzini C, Conte G, Crosti F, et al. Prenatal detection of 5q14.3 duplication including MEF2C and brain phenotype. Am J Med Genet A. 2016;170A(5):1352–1357. doi: 10.1002/ajmg.a.37594. [DOI] [PubMed] [Google Scholar]
- 15.Dong C, Yang XZ, Zhang CY, Liu YY, Zhou RB, Cheng QD, et al. Myocyte enhancer factor 2C and its directly-interacting proteins: a review. Prog Biophys Mol Biol. 2017;126:22–30. doi: 10.1016/j.pbiomolbio.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Lambert L, Bienvenu T, Allou L, Valduga M, Echenne B, Diebold B, et al. MEF2C mutations are a rare cause of Rett or severe Rett-like encephalopathies. Clin Genet. 2012;82(5):499–501. doi: 10.1111/j.1399-0004.2012.01861.x. [DOI] [PubMed] [Google Scholar]
- 17.Rocha H, Sampaio M, Rocha R, Fernandes S, Leao M. MEF2C haploinsufficiency syndrome: report of a new MEF2C mutation and review. Eur J Med Genet. 2016;59(9):478–482. doi: 10.1016/j.ejmg.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Paciorkowski ARTR, Rosenfeld JA, Hoover JM, Harris CJ, Winter S, Lacassie Y, Bialer M, Lamb AN, Schultz RA, Berry-Kravis E, Porter BE, Falk M, Venkat A, Vanzo RJ, Cohen JS, Fatemi A, Dobyns WB, Shaffer LG, Ballif BC, Marsh ED. MEF2C haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal development pathways. Neurogenetics. 2013;14(2):99–111. doi: 10.1007/s10048-013-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan F, Qiu ZH, Wang XH, Sun YM, Wang J, Li RG, et al. MEF2C loss-of-function mutation associated with familial dilated cardiomyopathy. Clin Chem Lab Med. 2018;56(3):502–511. doi: 10.1515/cclm-2017-0461. [DOI] [PubMed] [Google Scholar]
- 20.Qiao XH, Wang F, Zhang XL, Huang RT, Xue S, Wang J, et al. MEF2C loss-of-function mutation contributes to congenital heart defects. Int J Med Sci. 2017;14(11):1143–1153. doi: 10.7150/ijms.21353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu CX, Wang W, Wang Q, Liu XY, Yang YQ. A novel MEF2C loss-of-function mutation associated with congenital double outlet right ventricle. Pediatr Cardiol. 2018. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of 512 candidate genes. (DOCX 20 kb)
Data Availability Statement
Raw exome data and associated variant calls will not be shared, as part of our work has not been published.