SUMMARY
SETTING:
Hawassa Prison, Southern Region of Ethiopia.
OBJECTIVE:
To determine the burden of pulmonary tuberculosis (TB) using active case finding among prisoners.
DESIGN:
In this cross-sectional study, prisoners were screened for TB using a symptom screen. Those with cough of ⩾2 weeks had spot and morning sputum samples collected for acid-fast bacilli (AFB) smear microscopy and molecular diagnostic testing (Xpert® MTB/RIF).
RESULTS:
Among 2068 prisoners, 372 (18%) had a positive cough screen. The median age of these 372 persons was 23 years, 97% were male and 63% were from urban areas. Among those with a positive symptom screen, 8 (2%) were AFB sputum smear-positive and 31 (8%) were Xpert-positive. The point prevalence of pulmonary TB at the prison was 1748 per 100 000 persons. In multivariate analysis, persons with cough >4 weeks were more likely to have TB (OR 3.34, 95%CI 1.54–7.23).
CONCLUSION:
A high prevalence of TB was detected among inmates at a large Ethiopian prison. Active case finding using a cough symptom screen in combination with Xpert had high utility, and has the potential to interrupt transmission of Mycobacterium tuberculosis in correctional facilities in low- and middle-income, high-burden countries.
Keywords: active TB case finding, prison, Xpert MTB/RIF
RÉSUMÉ
CONTEXTE :
Prison de Hawassa, Sud de l’Ethiopie.
OBJECTIF :
Déterminer le poids de la tuberculose (TB) pulmonaire parmi les détenus en utilisant la recherche active des cas.
SCHÉMA:
Etude transversale. La TB a été recherchée parmi les détenus grâce à un dépistage sur les symptômes ; ceux ayant une toux de ⩾2 semaines ont eu un recueil de crachats spot et matin pour une microscopie de frottis et un test de diagnostic moléculaire (Xpert® MTB/RIF).
RÉSULTATS:
Parmi 2068 prisonniers, 372 (1%) ont déclaré avoir une toux de ⩾2 semaines. L’âge médian de ces 372 personnes a été de 23 ans, 97% ont été des hommes et 63% venaient de zones urbaines. Parmi ceux avec une toux, 8 (2%) ont eu un résultat positif de frottis de crachats à la recherche de bacilles acido-alcoolo-résistants et 31 (8%) ont eu un Xpert positif. La prévalence ponctuelle de la TB pulmonaire dans la prison a été de 1748 sur 100 000 personnes. En analyse multivariée, les personnes ayant une toux de >4 semaines ont été plus susceptibles d’avoir la TB (OR 3,34 ; IC95% 1,54–7,23).
CONCLUSION:
Une prévalence élevée de TB a été détectée parmi les détenus d’une vaste prison éthiopienne. La recherche active des cas basée sur le dépistage de la toux en combinaison avec l’Xpert a été très utile et a le potentiel d’interrompre la transmission de Mycobacterium tuberculosis dans les structures carcérales des pays très touchés avec des revenus faibles et moyens.
RESUMEN
MARCODEREFERENCIA:
La prisión de Hawassa, una región en el sur de Etiopía.
OBJETIVO:
Determinar mediante la búsqueda activa de casos la carga de morbilidad por tuberculosis (TB) pulmonar en las personas encarceladas.
MÉTODO:
Fue este un estudio transversal. Se practicó la detección sintomática de la TB en las personas encarceladas; en los reclusos que referían tos con una duración de ⩾2 semanas, se recogió una muestra inmediata de esputo y una muestra matinal con fines de baciloscopia y diagnóstico molecular (Xpert® MTB/RIF).
RESULTADOS:
En 372 de los 2068 prisioneros la detección por la tos fue positiva (18%). La mediana de la edad de estas personas fue 23 años, el 97% era de sexo masculino y el 63% residía en zonas urbanas. De las personas con una selección positiva por la tos, el resultado de la baciloscopia fue positivo en ocho (2%) y 31 tuvieron una prueba Xpert positiva (8%). La prevalencia puntual de TB pulmonar en la prisión fue 1748 por 100 000 personas. En un análisis multivariante, la probabilidad de sufrir TB fue mayor en las personas con tos de >4 semanas de duración (OR 3,34; IC95% 1,54–7,23).
CONCLUSIÓN:
Se detectó una alta prevalencia de TB en las personas recluidas en una extensa prisión de Etiopía. La búsqueda activa de casos mediante una detección sintomática por la tos, en asociación con la prueba Xpert, fue de gran utilidad y puede interrumpir la transmisión de Mycobacterium tuberculosis en los establecimientos correccionales de los países con ingresos bajos y medianos y alta carga de morbilidad.
THE BURDEN OF TUBERCULOSIS (TB) disease is higher in prisons than in the general population. Prisons are often neglected reservoirs for TB disease, and can be significant amplifiers of disease in both prison and the community.1,2 Transmission of drug-resistant strains, overcrowding, poor living conditions, limited health care, inadequate TB treatment and control strategies and high rates of human immunodeficiency virus (HIV) infection all contribute to the disproportionate burden of TB in prisons.2 The World Health Organization (WHO) estimates TB prevalence in prisons to be 10–100-fold higher than that in the general population.3 The median estimated fraction of TB in the general population attributable to exposure in prisons for TB is 8.5%.4
Close to 3 million cases of active TB worldwide remain undiagnosed by existing health systems each year,5 including many in the prison system, especially in sub-Saharan Africa.6,7 Lack of active surveillance and monitoring programmes and well-equipped laboratory facilities for TB diagnosis contribute to low case finding among prison inmates.8 Further-more, overcrowding of prisons in low- and middle-income countries (LMICs) provides a favourable environment for the transmission of Mycobacterium tuberculosis. In high TB burden countries, prisoners often come from underprivileged communities with higher risk and rates of TB.9
The impact of TB in prisons extends beyond the prison walls into the surrounding communities.10 As failure to control TB in prisons leads to enhanced TB transmission in the community, including drug-resistant disease,11 TB control in prisons is a major public health priority. However, there is limited understanding regarding TB epidemiology in African prisons. Studies carried out in African prisons reported 10–35-fold higher TB prevalence in prisoners than in the general population.12–15 In many high TB burden settings in LMICs, there is no effective TB control programme in place in prisons.
Ethiopia is among the high TB burden countries, with an incidence rate of 192 per 100 000 population.5 There are six federal and 120 regional prisons and detention centres in Ethiopia.16 Most prisoners are incarcerated in an overcrowded and poorly ventilated environment. The health service in prisons is often poorly organised, and lacks skilled manpower and laboratory facilities for TB diagnosis.4 Although prison TB prevention and control efforts are emerging in Ethiopia, these have been limited to a few prisons. Studies in Ethiopian prisons have reported point prevalences of TB ranging from 349 to 1913/100000.2,17–23 However, there are no data on the burden of TB in Hawassa prison, Hawassa, one of the largest prisons in Southern Ethiopia.
We aimed to estimate the burden of TB in Hawassa prison and assess the value of active TB case finding in a prison setting.
METHODS
Study design/setting
A cross-sectional study design was used to screen prison inmates for pulmonary TB (PTB) as described below from 15 June to 13 July 2015; HIV serological testing was offered from 13 January to 10 February 2016 at Hawassa prison (capacity approximately 2500 inmates, average stay 18 months per inmate). The prison has a clinic that provides general health care and performs sputum microscopy.
Study population
All prisoners without known TB were eligible to participate; informed consent was required for enrolment. Enrolment was performed by nurses from the prison clinic and by the prison’s health committee (prison inmates selected by prison authorities to facilitate health work between inmates and the prison clinic). They provided study information to prisoners by visiting their cells, and asked those interested in participating to attend the prison clinic to receive further details about the study. All study participants underwent a cough screen; those with a positive screen (cough ⩾2 weeks) provided informed consent and were interviewed, including for the presence of other symptoms, and were asked to submit two sputum samples, one spot and one morning. Five inmates were already on anti-tuberculosis treatment and were excluded, except for estimating point prevalence. We defined PTB patients as prison inmates whose sputum sample was positive on Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA).
HIV screening was carried out after provision of pre-counselling education by a trained prison nurse. HIV testing was offered and performed for participants diagnosed with active TB, after obtaining consent.
Study variables
A structured questionnaire was used to collect patient demographics, history of previous anti-tuberculosis treatment, history of incarceration, tobacco and Khat use based on self-report by prison inmates. Khat is a locally grown plant that is commonly chewed due to its stimulant effects.
Laboratory
For each participant with a positive cough screen, spot and morning sputum samples were collected in the prison health clinic. Acid-fast bacilli (AFB) smear microscopy was performed with regular light microscopy using the Ziehl-Neelsen (ZN) technique.24 The remaining portions of the samples were transported daily in ice boxes to the regional public health laboratory, which was about 500 m away. The two sputum samples were pooled in a single container and stored at −20°C until transport to Armauer Hansen Research Institute (AHRI) in Addis Ababa.
External quality control was performed for all slides by an independent, experienced laboratory technician at AHRI who was blinded to the AFB microscopy and Xpert results.
HIV screening was performed according to the national testing algorithm. Briefly, blood samples from finger-pricks were tested first with HIV (1+2) Antibody Colloidal Gold (Shanghai Kehua Bioengineering, Shanghai, China); positive samples were confirmed using Stat-Pak (Chembio, Medford, Brookhaven, NY, USA), while discordant results were resolved using HIV-1/2 Unigold Recombinant Assay (Trinity Biotech, Bray, Ireland).
Data management
All data were double-entered into an online REDCap database25 and analysed using STATA v1 (StataCorp, College Station, TX, USA). In univariate analysis, differences in categorical variables were tested using the χ2 test; a two-sample t-test was used for continuous variables. A multivariable logistic regression model was used to evaluate the independent association of potential risk factors with TB diagnosis. Model building and selection were based on the purposeful selection of covariates strategy, as previously described, which itself was based on epidemiological findings in the univariate analysis and biological plausibility.26 P < 0.05 was considered significant.
Ethical consideration
The study protocol was approved by the Addis Ababa University, the AHRI Institutional Review Boards and the Ethiopian National Ethics Review Committees in Addis Ababa. Study permission was also obtained from the Ethiopian Regional Health Bureau, Addis Ababa, and the prison administration. Patients with active TB started treatment in the prison clinic. Newly diagnosed HIV-positive participants were linked to a nearby health institution that provided HIV care.
RESULTS
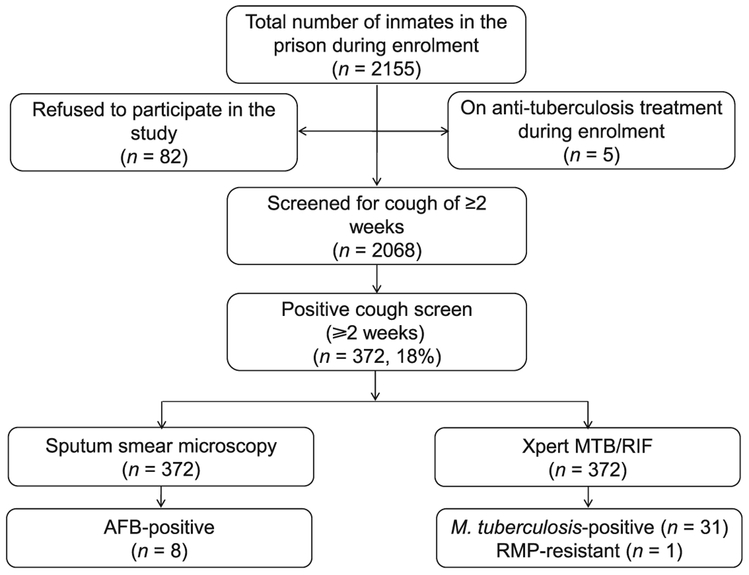
Among 2155 inmates, 2068 (98%) consented to participate and underwent a cough screen; 372 (18%) of these reported a cough of ⩾2 weeks (Figure). Among those with a positive cough screen, the median age was 23 years (interquartile range [IQR] 20–28); 362 (97%) were male and 10 (3%) were female. The majority (n = 329, 88%) had no previous history of incarceration and most were from an urban area (n = 235, 63%) (Table 1). A total of 293 (73%) patients reported fever, 315 (85%) had night sweats and 241 (65%) reported weight loss. The median number of prisoners per cell was 162 (IQR 14–360), for an average cell size of 140 m2, and the median duration of incarceration at the time of screening was 10 months (IQR 0.5–2 years).
Figure.
Study diagram. AFB = acid-fast bacilli; RMP = rifampicin.
Table 1.
Predictors of having pulmonary TB among persons with a positive cough screen
| Characteristic | Total (n = 372) n (%) |
No TB (n = 341) n (%) |
TB (n = 31) n (%) |
Univariate analysis |
|
|---|---|---|---|---|---|
| OR (95%CI) | P value* | ||||
| Male sex | 362 (97) | 331 (97) | 31 (100) | ||
| Age, years, mean | 26 | 26 | 24 | 0.08† | |
| Illiterate | 322 (87) | 294 (86) | 28 (90) | 1.49 (0.43–5.10) | 0.52 |
| Unemployed | 346 (93) | 319 (94) | 27 (87) | 0.46 (0.14–1.44) | 0.18 |
| Not married | 209 (56) | 192 (56) | 17 (55) | 1.06 (0.50–2.22) | 0.87 |
| Duration of cough, weeks | |||||
| 2–4 | 93 (25) | 78 (23) | 15 (48) | 3.16 (1.49–6.68) | 0.003 |
| >4 | 279 (75) | 263 (77) | 16 (52) | ||
| Fever | 273 (73) | 247 (72) | 26 (84) | 1.97 (0.73–5.30) | 0.17 |
| Night sweats | 315 (85) | 289 (85) | 26 (84) | 0.93 (0.35–2.64) | 0.89 |
| Loss of appetite | 235 (63) | 213 (62) | 22 (71) | 1.46 (0.65–3.28) | 0.35 |
| Weight loss | 241 (65) | 219 (64) | 22 (71) | 1.36 (0.60–3.05) | 0.45 |
| Chest pain | 338 (91) | 308 (90) | 30 (97) | 3.21 (0.42–24.3) | 0.13 |
| Shortness of breath | 252 (68) | 228 (67) | 24 (77) | 1.69 (0.71–4.06) | 0.23 |
| Previous imprisonment | 43 (12) | 42 (12) | 1 (3) | 0.23 (0.03–1.78) | 0.16 |
| Previous anti-tuberculosis treatment | 34 (9) | 31 (9) | 3 (10) | 1.07 (0.30–3.72) | 0.91 |
| Tobacco use (smoking cigarettes) at time of incarceration | 110 (30) | 104 (31) | 6 (19) | 0.54 (0.21–1.37) | 0.19 |
| Chewing Khat | 171 (46) | 159 (47) | 12 (38) | 0.72 (0.34–1.53) | 0.39 |
| Period of incarceration, years | |||||
| ⩽1 | 180 (48) | 161 (47) | 19 (61) | ||
| 1–3 | 113 (30) | 106 (31) | 7 (23) | 0.55 (0.22–1.37) | 0.20 |
| >3 | 79 (21) | 74 (22) | 5 (16) | 0.54 (0.19–1.49) | 0.28 |
| Contact with known TB patient in the prison | 90 (24) | 83 (24) | 7 (23) | 0.90 (0.37–218) | 0.82 |
| Presence of people with cough in the cell | 191 (51) | 175 (51) | 16 (52) | 1.01 (0.48–2.11) | 0.97 |
| Number of prisoners per cell | |||||
| ⩽100 | 35 (9) | 32 (9) | 3 (10) | 0.96 (0.27–3.35) | 0.95 |
| >100 | 337 (91) | 309 (91) | 28 (90) | ||
χ2 test unless otherwise stated.
Two-sample t-test.
TB = tuberculosis; OR = odds ratio; CI = confidence interval.
Pulmonary tuberculosis and human immunodeficiency virus infection
Among those with a positive cough screen, 8 (2%) were AFB sputum-positive and 31/372 (8%) were Xpert-positive, and thus had active PTB disease according to our study definition. The AFB sputum microscopy results concurred with the quality control readings at AHRI. All positive smear microscopy samples were Xpert-positive. Taken together with the five PTB cases who were already on anti-tuberculosis treatment during the study period, the overall point prevalence of PTB at the prison was 1789/100 000. Of the 31 confirmed TB cases, three had a previous history of anti-tuberculosis treatment. The median period of incarceration among the TB cases was 8 months; the majority (n = 19, 61.3%) had been incarcerated for 61 year; ⩽8 (90%) were living with >100 inmates per cell. One TB case with a previous history of anti-tuberculosis treatment had rifampicin (RMP) resistance on Xpert and was confirmed as multidrug-resistant on culture and drug susceptibility testing, with resistance to isoniazid, RMP, streptomycin and ethambutol.
Of the 2186 inmates incarcerated during the testing period, 2040 (93%) agreed to HIV testing; 9 (0.4%) were HIV-positive. HIV testing was performed among 16 of the 31 inmates with PTB; none were positive.
Predictors of pulmonary tuberculosis
Duration of cough was predictive of TB in univariate analysis. In multivariate analysis, the presence of a cough of >4 weeks was independently associated with an increased risk of having PTB (odds ratio 3.34, 95% confidence interval 1.54–7.23; Table 2).
Table 2.
Multivariate analysis of predictors of pulmonary tuberculosis among prison inmates with a positive cough screen
| Multivariate analysis |
||
|---|---|---|
| Characteristics | OR (95%CI) | P value |
| Duration of cough, weeks | ||
| 2–4 | 1.00 | |
| >4 | 3.34 (1.54–7.23) | 0.002 |
| Previous incarceration | 0.32 (0.04–2.50) | 0.28 |
| Tobacco use | 0.63 (0.24–1.64) | 0.35 |
| Period of incarceration, years | ||
| ⩽1 | 1.00 | |
| 1–3 | 0.48 (0.19–1.23) | 0.13 |
| >3 | 0.52 (0.18–1.51) | 0.23 |
OR = odds ratio; CI = confidence interval.
DISCUSSION
Using an active TB case finding strategy combining symptom screening and molecular diagnostic testing, we detected 31 previously undiagnosed cases of active PTB in a large Ethiopian prison. Along with the five known cases of TB, we found a TB prevalence of 1789/100 000 in the prison population. This prevalence is over 16-fold higher than the prevalence found in the general Ethiopian population.27 Our results highlight the usefulness of active TB case finding using a cough screen and Xpert testing among high-risk populations, in this case prisoners, in a high TB burden country.
TB prevalence at Hawassa prison was high despite the low HIV prevalence (0.4%) among those incarcerated. None of those persons found to have PTB in our study were HIV-positive. HIV prevalence among prison inmates in our study was lower than that reported from prisons in other areas of Ethiopia, including Gondar (7.6%),18 Tigray (4.4%)21 and in 13 prisons throughout the country (4.4%).20 The lower prevalence of HIV infection in our study might have reflected a lower HIV prevalence in the southern region compared with other parts of Ethiopia.28 Stigma is in general one of the main factors preventing people from seeking health care services in the country; however, in the prison setting the acceptance rate for HIV screening was high, with 93% of inmates agreeing to HIV testing.
Delays in diagnosis and incomplete treatment of TB are major challenges in most prison settings in resource-limited countries. These factors could be related to the limited availability of health care services in prisons and the lack of TB diagnostics in many prison settings.8,11 In many high-burden LMICs, including Ethiopia,16 TB control activities in prisons are not well integrated into national TB control programmes.8 In prison settings, the use of diagnostic tools with high sensitivity and specificity is recommended.29 Our study highlights the usefulness of active TB case finding using a rapid molecular diagnostic test. Before our study, there was no ongoing surveillance for TB in the prison, and the only available diagnostic tool in the prison, AFB smear microscopy, did not detect 75% of TB cases that were identified using Xpert. Our study provides important data to support an active TB case finding strategy that uses a cough symptom screen plus Xpert in prison settings to increase case detection, identify drug-resistant TB and improve TB control activities by allowing separation of those with active PTB from other inmates.
TB prevalence in Ethiopian prisons has been reported to range from 349 to 1913/100 000 prison population.2,17–23 The observed PTB point prevalence in our study (1789/100 000) was higher than that reported in most previous Ethiopian studies,17−23 but is within this range. The difference in TB prevalence in Ethiopian prisons could be due to the methodological differences employed in the studies for screening and diagnosis of cases, differing prevalence of HIV co-infection among those incarcerated in different regions and differences in the burden of the disease in study areas. Studies conducted in sub-Saharan African prisons have also reported a high prevalence of PTB, ranging from 5.1% to 47.7%.13,30,31 The high prevalence of TB in prison settings can impact TB transmission in communities, and prison settings can amplify TB transmission, with former inmates continuing to spread TB to contacts in the community after release from prison.4,10
Prisons can also be an important source of drug-resistant TB,30 and high levels of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB have been reported in prisons worldwide.8 In a study conducted in Zambia,14 resistance to at least one anti-tuberculosis drug was observed in 40 (23.8%) cases, 16 (9.5%) of whom had MDR-TB. Our study identified one case of RMP-resistant TB using Xpert. This case was confirmed to be MDR-TB using culture and drug susceptibility testing. A recent study also reported an MDR-TB rate of 9.5% in Ethiopian prison settings.32 These findings highlight the emergence of MDR-TB in prison settings and further emphasise the need to strengthen TB control activities in Ethiopian prison settings.
Our study was cross-sectional in nature and was therefore not designed to determine the site of infection with M. tuberculosis (prison vs. community) among those found to have active TB disease. The number of persons per cell was high, and the median length of incarceration among those with TB was 10 months; 61% of those found to have TB using Xpert were incarcerated for ⩽1 year. A study from a Gondar prison reported that an incarceration duration of 2–6 months was associated with TB positivity.18 Further studies are needed to evaluate the site of transmission and impact of screening prisoners at the time of incarceration as an additional TB control measure.
Limitations of the study
The present study had three main limitations. First, HIV testing was performed about 6 months after TB screening rather than concurrently. Given the high turnover in prisons, not all of those screened for TB were present when HIV testing was offered, and vice versa. Second, we relied on Xpert for a definitive diagnosis for TB rather than culture, the gold standard. As culture sensitivity is higher than that of Xpert among those who are smear-negative, our findings may have underestimated the prevalence of PTB. However, as culture is not widely available in many high TB burden, resource-limited countries, including Ethiopia, the use of Xpert is more feasible in many settings. Among the three TB cases with a previous history of anti-tuberculosis treatment, culture was performed in only one, the MDR-TB case with RMP resistance on Xpert. Finally, our strategy of screening only symptomatic cases may have led to an underestimation of the prevalence rate, as asymptomatic or subclinical cases may have been missed.
CONCLUSION
We observed that active TB case finding combining a cough screen plus a commercially available molecular diagnostic test (Xpert) had high utility in detecting active PTB disease among inmates in a large prison in Ethiopia. Despite low HIV prevalence among those incarcerated, the overall PTB prevalence exceeded 1.7% of the prison population at Hawassa prison. Cough of >4 weeks was the only risk factor for TB to be identified among those with a positive symptom screen. Active TB case finding using a symptom screen in combination with Xpert could interrupt transmission of M. tuberculosis in correctional facilities in high-burden LMICs.
Acknowledgements
The authors thank Southern Nations, Nationalities, and Peoples’ Region Health Bureau, REACH Ethiopia, the Hawassa prison administration (Hawassa, Ethiopia), clinic staff and study participants, Armauer Hansen Research Institute (AHRI) TB laboratory staff for their invaluable help to the success of the work; and M Yimer (AHRI) for her assistance.
The study was funded in part from the core AHRI budget (NORAD and SIDA grants), the Addis Ababa University (Addis Ababa, Ethiopia), and the National Institutes of Health (NIH) Fogarty International Center Global Infectious Disease (Bethesda, MD, USA) grant D43TW009127.
Footnotes
Prior presentations: data from this study were presented in part at the 47th Union World Conference on Lung Health, 26–29 October 2016, Liverpool, UK.
Conflicts of interest: none declared.
References
- 1.Valway SE, Greifinger RB, Papania M, et al. Multidrug-resistant tuberculosis in the New York State prison system, 1990–1991. J Infect Dis 1994; 170: 151–156. [DOI] [PubMed] [Google Scholar]
- 2.Abebe DS, Bjune G, Ameni G, Biffa D, Abebe F. Prevalence of pulmonary tuberculosis and associated risk factors in Eastern Ethiopian prisons. Int J Tuberc Lung Dis 2011; 15: 668–673. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Tuberculosis control in prison: a manual for program managers. WHO/CDS/TB/2000.281. Geneva, Switzerland: WHO, 2000. [Google Scholar]
- 4.Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS medicine. December 21 2010; 7(12): e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Global tuberculosis report, 2016. WHO/HTM/TB/2016.13. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 6.Drobniewski F. Tuberculosis in prisons—forgotten plague. Lancet 1995; 346: 948–949. [DOI] [PubMed] [Google Scholar]
- 7.Noeske J, Ndi N, Elo GA, Mfondih SM. Tuberculosis incidence in Cameroonian prisons: a 1-year prospective study. S Afr Med J 2014; 104: 209–211. [DOI] [PubMed] [Google Scholar]
- 8.Biadglegne F, Rodloff AC, Sack U. Review of the prevalence and drug resistance of tuberculosis in prisons: a hidden epidemic. Epidemiol Infect 2015; 143: 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher D, Grzemska M, Coninx R, Reyes H. Guidelines for the control of tuberculosis in prisons. Geneva, Switzerland: World Health Organization & International Committee of the Red Cross, 1998. [Google Scholar]
- 10.PLOS Medicine Editors, Barbour V, Clark J, Jones S, Veitch E. The health crisis of tuberculosis in prisons extends beyond the prison walls. PLOS Med 2010; 7: e1000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Grady J, Hoelscher M, Atun R, et al. Tuberculosis in prisons in sub-Saharan Africa—the need for improved health services, surveillance and control. Tuberculosis 2011; 91: 173–178. [DOI] [PubMed] [Google Scholar]
- 12.Noeske J, Kuaban C, Amougou G, Piubello A, Pouillot R. Pulmonary tuberculosis in the Central Prison of Douala, Cameroon. E Afr Med J 2006; 83: 25–30. [DOI] [PubMed] [Google Scholar]
- 13.Nyangulu D, Harries A, Kang’Ombe C, Yadidi A, Chokani K, T C. Tuberculosis in a prison population in Malawi. Lancet 1997; 350: 1284–1287. [DOI] [PubMed] [Google Scholar]
- 14.Habeenzu C, Mitarai S, Lubasi D, et al. Tuberculosis and multidrug resistance in Zambian prisons, 2000–2001. Int J Tuberc Lung Dis 2007; 11: 1216–1220. [PubMed] [Google Scholar]
- 15.Rasolofo-Razanamparany V, Menard D, Ratsitorahina M, Auregan G, Gicquel B, Chanteau S. Transmission of tuberculosis in the prison of Antananarivo (Madagascar). Res Microbiol 2000; 151: 785–795. [DOI] [PubMed] [Google Scholar]
- 16.Federal Democratic Republic Ethiopia Ministry of Health. Annual TBL Bulletin Addis Ababa, Ethiopia: Ministry of Health, 2015. [Google Scholar]
- 17.Bayu B, Mekiso AB, Legesse T. Prevalence of Pulmonary Tuberculosis and Associated Factors among Prisoners in Wolaita Zone, Southern Ethiopia: Cross-sectional Study. Am J Public Health Res 2016; 4: 142–148. [Google Scholar]
- 18.Moges B, Amare B, Asfaw F, et al. Prevalence of smear positive pulmonary tuberculosis among prisoners in North Gondar Zone Prison, northwest Ethiopia. BMC Infect Dis 2012; 12: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuge TG, Ayanto SY. Prevalence of smear positive pulmonary tuberculosis and associated risk factors among prisoners in Hadiya Zone prison, Southern Ethiopia. BMC Res Notes 2016; 9: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali S, Haileamlak A, Wieser A, et al. Prevalence of pulmonary tuberculosis among prison inmates in Ethiopia, a cross-sectional study. PLOS ONE 2015; 10: e0144040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adane K, Spigt M, Ferede S, Asmelash T, Abebe M, Dinant GJ. Half of pulmonary tuberculosis cases were left undiagnosed in prisons of the Tigray Region of Ethiopia: implications for tuberculosis control. PLOS ONE 2016; 11: e0149453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebrecherkos T, Gelaw B, Tessema B. Smear positive pulmonary tuberculosis and HIV co-infection in prison settings of North Gondar Zone, Northwest Ethiopia. BMC Public Health 2016; 16: 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerdo Z, G. M, Worku A, Ameni G. Prevalence of pulmonary tuberculosis and associated risk factors in prisons of Gamo Goffa Zone, south Ethiopia: a cross-sectional study. Am J Health Res 2014; 2: 291–297. [Google Scholar]
- 24.Federal Democratic Republic Ethiopia Ministry of Health. AFB smear microscopy manual. Addis Ababa, Ethiopia: Ethiopian Publication Health Institute, 2014. [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York, NY, USA: Wiley, 2000: pp 91–142. [Google Scholar]
- 27.Kebede AH, Alebachew Z, Tsegaye F, et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010–2011. Int J Tuberc Lung Dis 2014; 18: 635–639. [DOI] [PubMed] [Google Scholar]
- 28.The Ethiopian Public Health Institute. Report on the 2014 round antenatal care based sentinel HIV surveillance in Ethiopia. Addis Ababa, Ethiopia: EPHI, 2015. [Google Scholar]
- 29.Valenca MS, Scaini JL, Abileira FS, Goncalves CV, von Groll A, Silva PE. Prevalence of tuberculosis in prisons: risk factors and molecular epidemiology. Int J Tuberc Lung Dis 2015; 19: 1182–1187. [DOI] [PubMed] [Google Scholar]
- 30.Rutta E, Mutasingwa D, Ngallaba S, Mwansasu A. Tuberculosis in a prison population in Mwanza, Tanzania (1994–1997). Int J Tuberc Lung Dis 2001; 5: 703–706. [PubMed] [Google Scholar]
- 31.Koffi N, Ngom A, Aka-Danguy E, Seka A, Akoto A, Fadiga D. Smear positive pulmonary tuberculosis in a prison setting: experience in the penal camp of Bouake, Ivory Coast. Int J Tuberc Lung Dis 1997; 1: 250–253. [PubMed] [Google Scholar]
- 32.Ali S, Beckert P, Haileamlak A, et al. Drug resistance and population structure of M.tuberculosis isolates from prisons and communities in Ethiopia. BMC Infect Dis 2016; 16: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]