Abstract
Developmental eye defects in X-linked Ocular Albinism type I (OA1) are caused by G-Protein Coupled Receptor 143 (GPR143) mutations. Mutations result in dysfunctional melanosome biogenesis and macromelanosome formation in pigment cells, including melanocytes and retinal pigment epithelium. GPR143, primarily expressed in pigment cells, localizes exclusively to endolysosomal and melanosomal membranes unlike most GPCRs, which localize to the plasma membrane. There is some debate regarding GPR143 function and elucidating the role of this receptor may be instrumental for understanding neurogenesis during eye development and for devising therapies for OA1. Many GPCRs require association with other proteins to function. These GPCR-interacting proteins also facilitate fine-tuning of receptor activity and tissue specificity. We therefore investigated potential GPR143 interaction partners, with a focus on the melanogenic enzyme tyrosinase. GPR143 co-immunoprecipitated with tyrosinase, while confocal microscopy demonstrated colocalization of the proteins. Furthermore, tyrosinase localized to the plasma membrane when co-expressed with a GPR143 trafficking mutant. The physical interaction between the proteins was confirmed using Fluorescence Resonance Energy Transfer. This interaction may be required in order for GPR143 to function as a monitor of melanosome maturation. Identifying tyrosinase as a potential GPR143 binding protein opens new avenues for investigating the mechanisms that regulate pigmentation and neurogenesis.
Keywords: Tyrosinase, albinism, eye pigmentation
INTRODUCTION
Ocular albinism type I (OA1 or Nettleship-Falls OA) is an X-linked disease characterized by ocular features including nystagmus, photophobia, iris translucency, retinal hypopigmentation and significantly reduced visual acuity due to foveal hypoplasia (King et al., 1995). Cutaneous changes are usually mild or absent, however histological analysis revealed enlarged melanosomes, organelles in which melanin is synthesized, in both epidermal melanocytes and retinal pigment epithelium (RPE)(Garner and Jay, 1980). The RPE appears hypopigmented with melanin concentrated in a few macromelanosomes rather than homogenously dispersed in numerous, smaller melanosomes throughout the cell (Cortese et al., 2005). In addition, macromelanosomes are clustered in the cell periphery with fewer peri-nuclear melanosomes compared to wildtype (Palmisano et al., 2008).
The OA1 gene localizes to the X chromosome and encodes the OA1 protein, GPR143 (Bassi et al., 1995). Despite low protein sequence conservation, GPR143 shares structural and functional similarities with several classes of G protein-coupled receptors (GPCRs; Schiaffino et al., 1999), although it remains unclear as to which family it belongs to. The receptor is highly expressed in pigmented cells (Bassi et al., 1995). Unlike most GPCRs, GPR143 is targeted to melanosomes not the plasma membrane. The mechanisms regulating GPR143 trafficking remain unclear, but studies suggest transport through lysosomal/melanosomal sorting pathways similar to other melanosomal proteins (e.g. tyrosinase (TYR) and tyrosinase-related protein 1 (TYRP1)) (Winder et al., 1993). Two sorting signals, an unconventional dileucine motif and a second unique motif, are necessary and sufficient for intracellular localization of GPR143 (Piccirillo et al., 2006). These sorting signals are recognized by non-melanocytic cells as well, since GPR143 is localized to late endosomes and lysosomes following heterologous expression (Schiaffino et al., 1996; 1999; Shen et al., 2001a). Various pathogenic GPR143 mutations are associated with OA1, many leading to either GPR143 retention in the endoplasmic reticulum (ER) due to receptor misfolding or involving domains critical for GPCR function (Addio et al., 2000).
Various roles have been proposed for GPR143 however a precise function remains to be defined. Lack of GPR143 function results in macromelanosomes formed from abnormal growth of single organelles rather than fusion of multiple mature melanosomes (Incerti et al., 2000). Thus GPR143 appears to regulate melanosome maturation (Samaraweera et al., 2001; Shen et al., 2001b) signaling a halt to melanogenesis-induced growth. GPR143 mutations which affect function may compromise downstream signaling, permitting continuous import of melanosomal proteins into melanosomes and sustained melanogenesis resulting in giant organelles. Based on its topological orientation (Schiaffino et al., 1999; Sone and Orlow, 2007), GPR143 ligands should bind in the organelle lumen and transduce information to the cytosol through heterotrimeric-G protein activation. In this way, GPR143 could function as a “sensor” of melanosomal maturation and prevent formation of macro-organelles (Schiaffino and Tacchetti, 2005).
In vivo studies of GPR143 and TYR double mutant mice demonstrated that GPR143 controls both the number of early stage melanosomes as well as organelle size. In the absence of functional TYR, melanosomes do not mature and macromelanosomes do not form, while in mice lacking functional GPR143 and TYR, the number of early stage melanosomes are altered and no macromelanosomes form (Cortese et al., 2005).
GPR143 regulates transcription of several melanosomal genes through modulation of the microphthalmia-associated transcription factor (MITF), thus forming a feedback loop being both a regulator and target of MITF (Falletta et al., 2014; Vetrini et al., 2004). GPR143 may also control intracellular melanosome transport by regulating microtubule-mediated motility. Maturing melanosomes are transported from the perinuclear area to the cell periphery where mature melanosomes are transferred to keratinocytes. Macromelanosome numbers are increased at the periphery of pigment cells that lack GPR143 expression, with a concomitant decrease in perinuclear melanosomes. GPR143 is co-immunoprecipitated with tubulin, but the precise mechanism of regulation is unclear (Palmisano et al., 2008).
In HeLa cells, GPR143 signaling inhibits delivery of Pmel17 from endosomes to lysosomes and promotes accumulation of Pmel17-containing multivesicular bodies (MVBs) (Burgoyne et al., 2014). GPR143 is therefore thought to form the trafficking fork separating lysosome- and early melanosome-bound proteins. Delaying endosome-lysosome fusion of early melanosomes allows for delivery of melanin-synthesizing enzymes and maturation of melanosomes.
The connection between GPR143 and melanosome biogenesis and maturation was suggested by several studies in vitro and in vivo, but the precise mechanism by which the receptor perceives maturation stage is still unresolved (Cortese et al., 2005; Samaraweera et al., 2001; Schiaffino and Tacchetti, 2005; Shen et al., 2001b). It has been proposed that L-DOPA, produced by the first tyrosinase-catalyzed melanogenic reaction, is a GPR143 ligand which allows the receptor to monitor melanogenesis (Lopez et al., 2008).
A key component of GPCR function is mediated by interaction with other proteins including G proteins, GPCR kinases and arrestins. Recent studies identified GPCR interacting proteins that do not fall into these categories, but play important roles in mediating GPCR signaling. These proteins have been shown to act as tethers, facilitate the effect of agonists and regulate tissue-specific GPCR function (Brady and Limbird, 2002; Ritter and Hall, 2009). Identifying the tissue-specific GPCR binding partners maybe be important, particularly in the clinical setting as they may represent druggable targets. Furthermore, identification of GPR143 binding partners will contribute to our understanding of GPR143 function and open new avenues for investigating the mechanisms regulating pigmentation. Thus, the purpose of our study was to investigate potential melanocyte-specific GPR143 binding partners. Given that intermediates produced during melanogenesis are hypothesized to be GPR143 ligands, we focused on the possible interaction between GPR143 and tyrosinase, the only enzyme indispensable for melanin synthesis. Using several approaches, we demonstrated physical interaction between GPR143 and tyrosinase.
RESULTS
Generation and Characterization of Wildtype (wtGPR143) and Double Mutant GPR143 (mtGPR143)
In order to investigate GPR143 in isolation from other melanosomal proteins, the receptor was exogenously expressed in COS7 cells (COS7s). An expression vector encoding a mutant GPR143 protein was also utilized. GPR143 was shown to be localized to late endosomes and lysosomes due to two separate sorting signals (Piccirillo et al., 2006). Mutagenesis of these sorting signals (L223A-L224A and W329A-E330A) generated a double mutant receptor, mtGPR143, which did not sort to the lysosomal/melanosomal pathway but to the plasma membrane. DNA concentrations of both plasmids were tittered to prevent over-expression, particularly since wtGPR143 localizes to the plasma membrane when highly over-expressed exogenously (Innamorati et al., 2006).
WtGPR143 and mtGPR143 proteins were transiently expressed in COS7s and characterized by immunostaining and Western blot analysis. WtGPR143 was intracellularly expressed in vesicles throughout the cell and close to the perinuclear region (Figure 1a, upper panels), while mtGPR143 was more noticeably distributed in vesicles at the cell periphery and colocalized with the plasma membrane protein cadherin (Figure 1a, lower panels).
Figure 1. Characterization of COS7 cells expressing wildtype or mtGPR143.
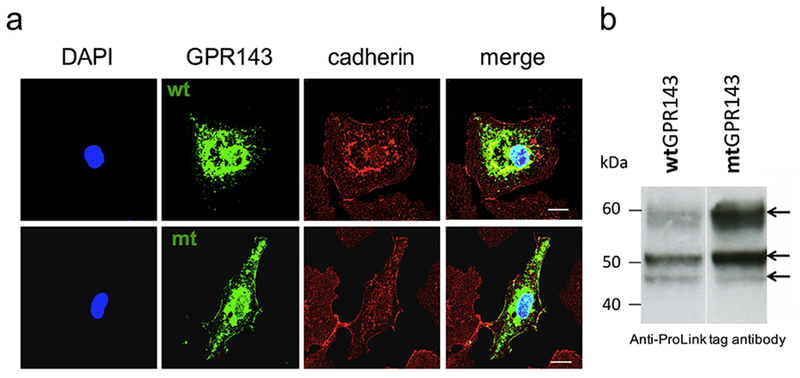
(a) Subcellular localization of wt and mtGPR143 detected by immunofluorescence. Transfected COS7s were fixed and stained with monoclonal anti-ProLink antibody (against PL tagged-GPR143), polyclonal anti-cadherin antibody (plasma membrane marker) and DAPI (nuclei). Scale bar = 20 μm. (b) Immunoblot of protein extracts from transfected COS7s. The anti-ProLink antibody was used to detect GPR143. Each lane was loaded with 30 μg of total amount of protein. Mt, mutant; wt, wildtype.
Western Blot analysis of lysates from transiently transfected COS7s showed the expected glycosylation pattern (Schiaffino, 1996) consisting of a doublet (45 and 48kDa) and a more prominent 60kDa band (Figure 1b). Equal amounts of protein were loaded, however we observed a significant difference in expression, most likely due to variability in the incorporation of expression plasmids following transfection. Furthermore, GPR143 glycosylation was analyzed using Endoglycosidase H (EndoH) and Peptide-N-Glycosidase F (PNGaseF) digestion to characterize oligosaccharide processing in the exogenous system. EndoH did not cause any oligosaccharide cleavage (Figure S1), while PNGaseF caused loss of the 60kDa band. EndoH sensitive moieties have undergone processing in the ER, but not the Golgi, while PNGaseF strips all sugars leaving the protein backbone. Thus the 60kDa band reflects a Golgi-processed, complex oligosaccharide, confirming that exogenously expressed protein is post-translationally modified in a similar fashion to GPR143 in pigment cells.
Co-immunoprecipitation of GPR143 and Tyrosinase
Tyrosinase is a transmembrane enzyme containing C-terminus sorting signals responsible for lysosomal localization in heterologous systems (Simmen et al., 1999). Thus we investigated whether GPR143 and tyrosinase colocalized in COS7s, to determine if they were in fact binding partners, since both proteins sort to the endolysosomal pathway. To assess potential GPR143 and tyrosinase interactions, immunoprecipitation (IP) and Western blot analyses were performed using lysates from transfected cells. It should be noted that it was necessary to utilize different tags to label GPR143 based on the availability of antibodies, which accounts for the difference in the size of the proteins shown in the Western Blots.
COS7s were co-transfected with a ProLink-tagged GPR143 (GPR143-PL) and tyrosinase. GPR143-PL was pulled down using an antibody against the ProLink tag and the IP fractions analyzed by Western Blot Analysis detecting either GPR143 or tyrosinase (Figure 2a, Figure S2). The lysate fractions incubated without antibody were loaded as controls. GPR143 was identified only in transfected COS7s (two 50–60kDa bands), but not untransfected cells. The GPR143 lower band overlaps with the heavy chain anti-ProLink antibody band (~50kDa), which is also visible in untransfected COS7s, however the higher GPR143 band is clearly detectable in both wash and eluate fractions. Tyrosinase was also detected by Western blotting. Antibody chains were not visible since the tyrosinase and ProLink antibodies were generated in different species. Bands corresponding to differential glycosylation tyrosinase patterns (70-84kDa + EGFP 27kDa) were detected in the lysate and eluate fractions of co-transfected COS7s, indicating that the enzyme was interacting with GPR143 and the proteins were pulled down together. Comparable results were obtained targeting tyrosinase using an anti-GFP antibody in co-transfected COS7s (Figure S3). Experiments were repeated co-transfecting mtGPR143 with wildtype tyrosinase. The proteins maintained their ability to interact, since no differences were detected between cell lines expressing either wt or mtGPR143 (Figure 2a. See Figure S2 for entire blot).
Figure 2. Immunoprecipitation (IP) analysis.
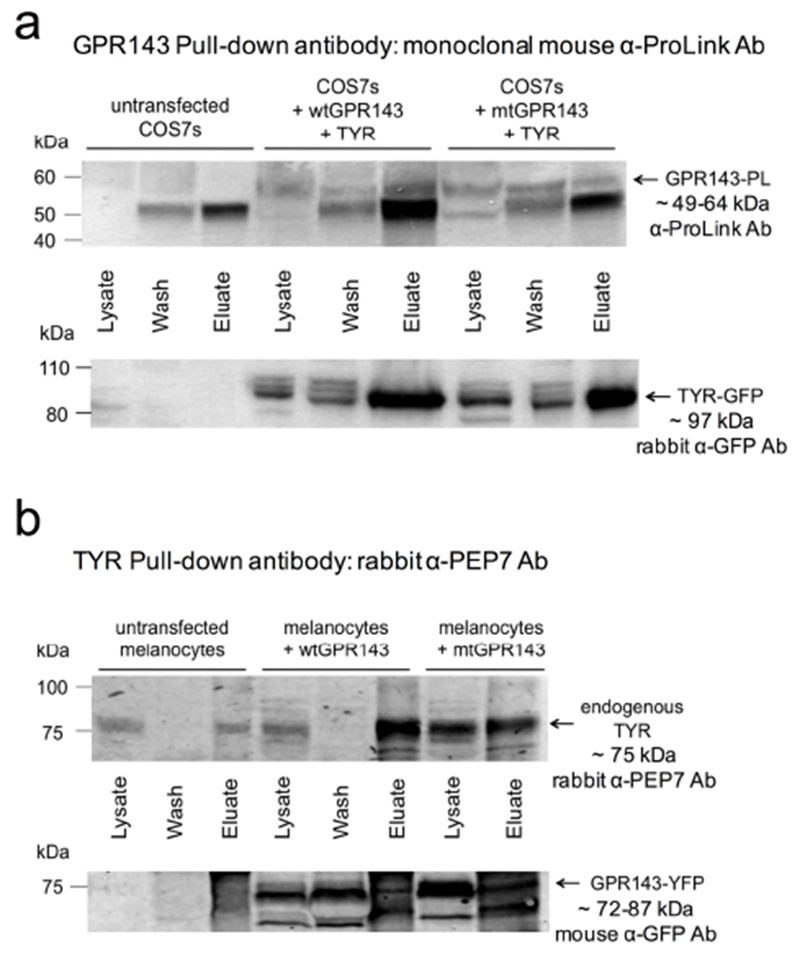
(a) GPR143 was precipitated with anti-ProLink in lysates from COS7s transfected with ProLink tagged wtGPR143 or mtGPR143 and tyrosinase (TYR-EGFP). Untransfected COS7 cell lysates were used as control. The upper blot was hybridized with anti-ProLink to detect tagged-GPR143, and the lower blot with anti-GFP to detect EGFP tagged-TYR. Complete blot is shown in Figure S2. (b) Tyrosinase was precipitated with αPEP7 in melanocytes transfected with wt or mutant GPR143-EYFP. Untransfected melanocytes were used as controls. The upper blot was hybridized with αPEP7 (detects endogenous tyrosinase), and the lower blot with anti-GFP (detects EYFP tagged-GPR143). GAPDH was detected in each fraction and αPEP7 loaded as controls (Figure S4).
The observed findings were validated in melan-a melanocytes established from a wildtype mouse (Bennett et al., 1987). Melanocytes were transfected with human GPR143-EYFP. The human and mouse GPR143 (and tyrosinase) sequences display a high identity and similarity level (Schiaffino and Tacchetti, 2005)). Endogenous mouse tyrosinase was IPed using α-PEP7 (Figure 2b, See Figure S4 for entire blot). A broad band representing tyrosinase was detected above 75kDa. As expected, the band was present in lysates and eluates of all samples, including untransfected melanocytes. A doublet corresponding to GPR143 (45–60kDa + EYFP 27kDa) was observed below 75kDa in the lysates and eluates of transfected melanocytes, confirming interaction between GPR143 and endogenous tyrosinase. α-PEP7 was loaded as a control in order to identify the bands corresponding to the antibody chains. GADPH was used to test IP specificity and as a loading control. The protein amount was similar for the three cell lines (slightly lower for untransfected cells) and the absence of GADPH in the eluates indicated that the precipitation was specific for GPR143 and tyrosinase (Figure S4).
Colocalization of GPR143 and Tyrosinase in COS7 Cells and Melanocytes
To further assess the potential interaction between GPR143 and tyrosinase, we performed colocalization studies using immunofluorescence-confocal microscopy. When wt-GPR143 was co-expressed with tyrosinase in COS7s, the proteins colocalized primarily in the perinuclear region where the ER and Golgi apparatus are located. There was limited colocalization in a few vesicles at the cell periphery (Figure 3a, upper panels). Co-transfection of COS7s with mtGPR143 resulted in prominent colocalization with tyrosinase at the cell surface (Figure 3a, lower panels).
Figure 3. Colocalization of GPR143 and tyrosinase by immunofluorescence in COS7 cells and melanocytes.
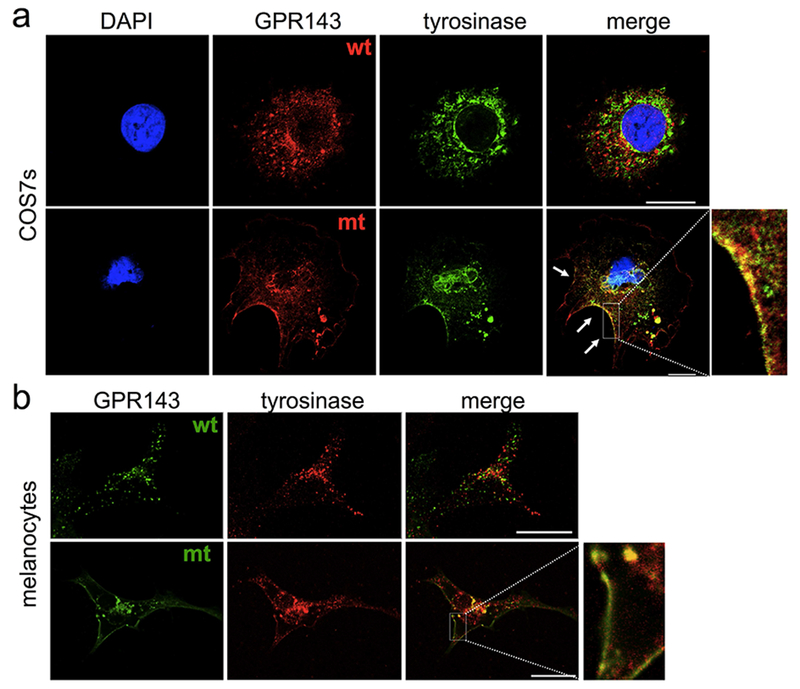
(a) COS7s were co-transfected with GPR143 (wt or mutant) and tyrosinase (TYR-EGFP), fixed and stained with anti-ProLink (against PL tagged-GPR143) and DAPI (nuclei). (b) Melanocytes transfected with GPR143-EYFP (wt or mutant) were fixed and stained with αPEP7 (against endogenous tyrosinase). Scale bar = 20 μm.
Melanocytes were transfected with YFP-tagged human wtGPR143 and colocalization with endogenous tyrosinase investigated. The distribution of the two proteins in melanocytes was similar to that in COS7s, with perinuclear colocalization. In addition, wt-GPR143 was localized in vesicles at the cell periphery which appeared to be distinct, but often close to vesicles containing endogenous tyrosinase (Figure 3b, upper panels). MtGPR143 and endogenous tyrosinase were colocalized at the plasma membrane in melanocytes and vesicles containing tyrosinase were mostly grouped close to the cell surface (Figure 3b, lower panels). These findings indicate that tyrosinase sorting can be disrupted by GPR143 mislocalization.
Fluorescence Resonance Energy Transfer (FRET)
The FRET technique allows assessment of protein-protein interactions. Thus, we established a cell system to study direct GPR143 and tyrosinase interactions. Since previous studies showed that GPR143 is a 7-transmembrane protein (Sone and Orlow, 2007) and tyrosinase is a single membrane-spanning enzyme, both with lumen facing c-termini, we tagged the C-termini of both generating GPR143-EYFP and TYR-ECFP fusion proteins. Thus the fluorescent proteins are located on the same side of the membrane. Constructs were transiently transfected together or individually in COS7s, then analyzed by confocal microscopy.
Sensitized emission was first used to evaluate FRET efficiency in which donor fluorophore excitation (ECFP) leads to acceptor molecule emission (EYFP), if proteins are in close enough proximity for energy transfer to occur (1–10 nm). Images of transfected cells were simultaneously acquired in all three channels (YFP, CFP and FRET, Figure 4). Nikon A1 software was used to calculate correction parameters by means of single transfected COS7s (Figure S5) and FRET efficiency values of each point in a point-to-point manner. Thus a FRET efficiency distribution view was obtained (Figure 4, right panels). The transition from purple to red indicates increase in FRET efficiency from 0 to 100%, which corresponds to intensity of protein interaction. When wtGPR143 was co-expressed with tyrosinase, the FRET signal was observed in several vesicles in the cell periphery, and the perinuclear region (Figure 4). FRET signal was found at the plasma membrane only when tyrosinase was co-expressed with mtGPR143. In this case, FRET efficiency in some plasma membrane regions spanned between 20 and 100% (Figure 4, white arrows).
Figure 4. Fluorescence Resonance Energy Transfer (FRET) of GPR143-EYFP and tyrosinase-CFP in COS7 cells.
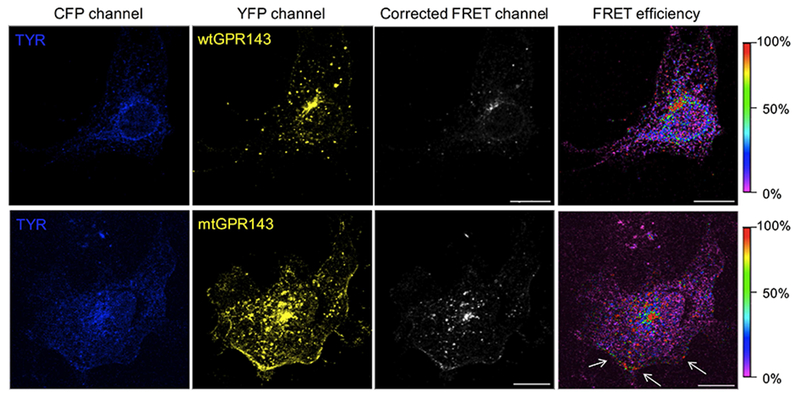
Sensitized emission method was used to detect interaction of GPR143 (YFP channel) and tyrosinase (TYR; CFP channel). FRET signal, corrected by CoA and CoB parameters, and FRET efficiency (color scale on the far right) are shown. White arrows indicate the plasma membrane regions where FRET signal is localized. Controls are shown in Figure S6. Scale bar = 20 μm.
To further validate GPR143 and tyrosinase interaction, we used the acceptor photobleaching method, a quantitative FRET technique which measures donor “de-quenching” in the presence of an acceptor. During FRET, donor fluorescence is channeled to the acceptor, and is thus partially quenched. Photobleaching the acceptor irreversibly eliminates the quenching effect and the level of donor fluorescence increases. Acceptor photobleaching was delimited to specific regions of interest (ROI; Figure S6) which correspond to vesicles in the periphery where wtGPR143 and TYR were present and parts of the plasma membrane where the mtGPR143 and TYR colocalized (control images, Figure S7). Images captured before and after photobleaching display fluorescence in the CFP and YFP channels (Figure S6). ROI fluorescence was used to calculate the ratio of emission intensity after versus before photobleaching and FRET efficiency (Figure 5). The intensity of ECFP emission increased when GPR143-EYFP and TYR-ECFP were expressed together indicating that the pair of fluorophores were involved in a resonance energy transfer before acceptor photobleaching (Figure 5a). The FRET efficacy of the co-transfected COS7s was 21.8% (± 4.5) for the wt-GPR143 with TYR and 16.3% (± 3.4) for the mtGPR143 with TYR (Figure 5b), which was significantly different from the negative controls (single transfected COS7s see Figure S7) and comparable to the positive control (ECFP-EYFP fusion protein: 28.4% (± 4.4)). As a negative control to demonstrate that our observations were not artifacts resulting from over-expression of two transmembrane proteins and were specific to GPR143 rather than a promiscuous interaction with any GPCR, FRET experiments were performed with the GPCR adenosine receptor A2AAR (TYR-CFP + A2AAR-YFP). The fluorescence ratios in the photobleaching experiment confirmed that there is no CFP increase in fluorescence after photobleaching, which indicates that TYR does not interact with the A2AAR. The FRET efficacy calculated for the control experiment is significantly different from the positive control (fusion protein) and from GPR143-TYR samples as well. The second FRET method (sensitized emission) was also performed with the control receptor showing no major colocalization between A2AAR and TYR (Figure S8). In particular, TYR was found to localize intracellularly despite A2AAR was expression at the plasma membrane (Figure S8), excluding any relation between overexpression of TYR or GPR143 with their plasma membrane localization.
Figure 5. Quantification of acceptor photobleaching FRET.
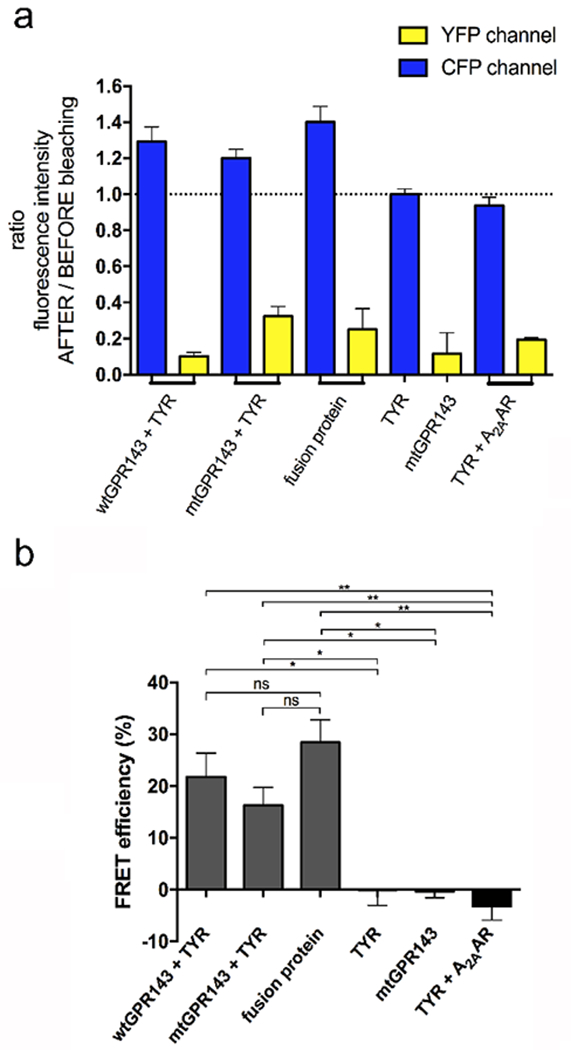
Wildtype or mutant GPR143-EYFP and TYR-ECFP were co-transfected in COS7s. (a) Ratio of emission intensity after:before bleaching. Controls = single transfected COS7s, TYR-ECFP + A2AAR, and ECFP-EYFP fusion. For wtGPR143, photobleaching and relative quantification was performed in intracellular regions. For mtGPR143, portions of plasma membrane were analyzed. (b) FRET efficiency was quantified for co-transfected COS7s. Controls = Single transfected cells, TYR-ECFP + A2AAR, and ECFP-EYFP fusion protein. Data represent means ±SEM of four (co-transfected cells), three (TYR-ECFP + A2AAR) or two control cells. Unpaired Student t-test: * p<0.05, ** p<0.01, ns not significantly different from control. Values refer to limited regions (See Figures S6, S7, S8). A2AAR, adenosine A2A receptor; mt, mutant; TYR, tyrosinase; wt, wildtype.
We therefore demonstrate, by IP and two different FRET methods, that GPR143 and tyrosinase directly interact with each other in several regions of the cell and that this interaction leads to mislocalization of tyrosinase when mtGPR143, that traffics to the plasma membrane, is expressed.
DISCUSSION
GPR143 is an atypical G protein-coupled receptor (GPCR) expressed primarily in pigmented cells with unknown function. Potential GPR143 functions include a sensor of melanosome maturation and a role in delaying MVB-lysosome fusion to facilitate melanosome biogenesis (Burgoyne et al., 2014; Samaraweera et al., 2001). The mechanism underlying GPR143 recognition of melanosome maturation remains unresolved.
Mutations at this locus cause OA1 (King et al., 1995). Most pathogenic mutations cause ER retention of GPR143 while some produce a non-functional receptor (Addio et al., 2000). When GPR143 is not functional, the lysosomal and melanosomal pathways in pigmented cells are not segregated, which may cause enhanced fusion of immature melanosomes and lysosomes (Burgoyne et al., 2014; Giordano et al., 2009). Some of these endosomes containing lysosomal and melanosomal markers, could be targeted by vesicles delivering melanosomal proteins such as tyrosinase and TYRP1. The mutant GPR143 would not be capable of monitoring organelle maturation, resulting in continuous delivery and macromelanosome formation.
The importance of GPCR-interacting proteins is well-established. These proteins facilitate fine-tuning of GPCR activity and contribute to receptor regulation (Brady et al., 2002). More recently, GPCR interacting proteins that do not fit into the three well-known categories; G proteins, GPCR kinases and arrestins; have been identified. Among other roles, these proteins can facilitate tissue specific GPCR activity and mediate the effects of agonists (Bockaert et al., 2004; Ritter and Hall, 2009). GPR143 has been found to associate with several GPCR-interacting proteins. GPR143 activity may require binding to the Gαi3 protein. Introduction of a constitutively active Gαi3 in GPR143 knockout mice corrected the OA1 phenotype (Young et al., 2013). β-arrestin may also regulate late stage melanosome GPR143 signaling. GPCRs participate in biased signaling through arrestin proteins which activate a G protein-independent signaling pathway (Shukla et al., 2014). Once melanosomes has reached an appropriate size, GPR143 may be targeted by β-arrestin , which terminates its basal activity (Innamorati et al., 2006). Thus GPR143 regulation requires canonical GPCR-interacting proteins and may also depend on non-canonical binding partners.
Our investigation focused on tyrosinase, a crucial enzyme required for melanosome maturation, as a GPR143-interacting partner. We hypothesized that since reaction products resulting from tyrosinase-catalyzed metabolism of tyrosine may be GPR143 ligands, tyrosinase is the most likely GPR143-interacting protein.
Tyrosinase activity is not influenced by GPR143 mutations, GPR143 knockout mice express functional tyrosinase which is primarily localized in macromelanosomes (Cortese et al., 2005). However, when tyrosinase activity or trafficking to melanosomes is compromised, macromelanosomes are not formed and melanosome maturation is stalled (Cortese et al., 2005; Paterson et al., 2015). Thus melanosome maturation and GPR143 function at final stages of melanosome maturation require presence of active tyrosinase. Thus, a possible interaction between GPR143 and tyrosinase is feasible.
We generated tagged expression vectors for wtGPR143 and a trafficking double mutant, mtGPR143, lacking two sorting signals necessary for intracellular localization (Piccirillo et al., 2006). GPR143 vectors were transiently expressed in COS7s. Characterization of the receptors demonstrated that wtGPR143 localized to the ER, Golgi apparatus and vesicles which correspond to endosomes and lysosomes (data not shown), in agreement with previous studies (Schiaffino et al., 1999; Shen et al., 2001b). In contrast, mtGPR143 was primarily expressed at the plasma membrane. Investigation of glycosylation patterns revealed that both wt and mutant receptors underwent expected post-translation modification. We next confirmed direct interaction between GPR143 and tyrosinase. Immunoprecipitation studies in COS7s and melanocytes demonstrated that the two proteins are pulled down together from cell lysates using antibodies against either GPR143 or tyrosinase. GPR143 sorting signal mutations did not disrupt this interaction. Physical interaction was validated using two different FRET approaches, which demonstrated close proximity of tyrosinase and wtGPR143 in vesicles and tyrosinase and mtGPR143 at the plasma membrane. FRET efficacy values are in the same order of significance of values shown in other studies involving the ECFP-EYFP pair (Gu et al., 2004; Karpova et al., 2003; Wilson et al., 2002) and can thus be considered accurate and reliable.
Colocalization of wtGPR143 and tyrosinase was confirmed using confocal microscopy. mtGPR143 and tyrosinase colocalized at the plasma membrane and in vesicles close to the cell surface in both COS7s and melanocytes. Tyrosinase is delivered to melanosomes even if GPR143 is mutated, creating melanin-filled macromelanosomes, therefore tyrosinase trafficking is not typically OA1-dependant (Cortese et al., 2005). We therefore hypothesize that removing GPR143 sorting signals results in the trafficking to transport organelles where tyrosinase is normally located. The physical interaction between GPR143 and tyrosinase may disrupt the sorting signals that would allow tyrosinase to be transported to the melanosome and the bound proteins are transported to the cell membrane instead. Following post-translational modification in the Golgi, wtGPR143 is transported to MVBs and early endosomes which form premelanosomes that become enriched with melanosomal proteins (Raposo et al., 2001). The interaction between mtGPR143 and tyrosinase may thus occur as early as the Golgi, since some perinuclear colocalization is observed between wtGPR143 and tyrosinase.
A limitation of this study is that only melanocytes and heterologous expression in non-pigment cells (COS7s) was investigated. It is possible that GPR143 behavior in RPE, where disruption of function is most consequential in terms of pathogenesis, may be different to that in cutaneous melanocytes. For example, Lopez et al (2008) propose that a fraction of GPR143 is localized to the plasma membrane in RPE suggesting cell-specific effects.
GPR143 controls the rate of melanosome biogenesis, particularly the number of early stage organelles (stage I-II). At the final maturation stages (stage III-IV), GPR143 regulates organelle size (Cortese et al., 2005). GPR143 may regulate melanosome biogenesis by controlling bifurcation of melanosomal and lysosomal pathways (Burgoyne et al., 2014). It was hypothesized that GPR143 delays lysosome fusion until melanin-containing melanosomes become resistant to fusion (Giordano et al., 2009; Lopes et al., 2007). At later stages of melanosome maturation, GPR143 might fulfil different functions and may be involved in regulating organelle size either by monitoring tyrosinase levels or controlling delivery of melanin-related proteins (MRP). It is likely that this is the point at which interaction between tyrosinase and GRP143 is most crucial. The physical interaction between the proteins maybe a direct signal and/or the interaction facilitates access to GPR143 ligands.
In summary, we provide evidence of a direct interaction between GPR143 and tyrosinase. Understanding how GPR143 precisely impacts cellular function and melanogenesis may be instrumental in understanding OA1 pathogenesis, regulation of pigmentation and neurogenesis during optic tract development (Jeffery, 1997).
METHODS
Details are provided in the Supplemental Materials and Methods.
Plasmids
The cloning procedure of GPR143 and tyrosinase coding sequences is reported in the Supplemental Methods.
Immunoprecipitation, Glycosylation studies and Western Blot Analysis
The immunoprecipitation was performed with transfected COS7 or melanocyte lysates incubated with antibodies against GPR143 or tyrosinase. The protocol is reported in the Supplemental Methods as are details of the glycosylation analysis.
Fluorescence Resonance Energy Transfer (FRET)
The GPR143 and tyrosinase coding sequences were linked to EYFP and ECFP, respectively, then transfected into COS7s. The detailed protocol of FRET sensitized emission and acceptor photobleaching methods are reported in the Supplemental Methods.
Supplementary Material
ACKNOWLEDGMENTS
E.D.F. was supported by the Bonn International Graduate School in Drug Sciences (BIGS DrugS) and the state of North Rhine-Westphalia (NRW International Research Graduate School BIOTECH-PHARMA). Prof. Müller provided access to laboratory equipment. Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health (NIH), under Award AR41880 (Seth J. Orlow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Martha Vega and Genevieve Torres for technical assistance and Dr. Seth J. Orlow for helpful discussions.
Abbreviations
- ECFP
enhanced cyan fluorescent protein
- EYFP
enhanced yellow fluorescent protein
- FRET
fluorescence resonance energy transfer
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- IP
immunoprecipitation
- MRP
melanin-related protein
- mtGPR143
GPR143 trafficking mutant
- MVB
multivesicular bodies
- PL
ProLink tag
- Pmel17
premelanosome protein
- OA1
Ocular Albinism type I
- ROI
region of interest
- RPE
retinal pigment epithelium
- TYRP1
tyrosinase-related protein 1
- TYR
tyrosinase
- Wt
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors report no conflicts.
REFERENCES
- Addio M, Pizzigoni A, Bassi MT, Baschirotto C, Valetti C, Incerti B et al. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum Mol Genet 2000;9(20):3011–3018. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Incerti B, Easty DJ, Sviderskaya FV and Ballabi A. Cloning of the murine homolog of the Ocular Albinism type 1 (OA1) gene: sequence, genomic structure and expression analysis in pigment cells. Genome Res 1995;1:880–885. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ and Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer 1987;39(3):414–418. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A and Marin P. GPCR interacting proteins (GIP). Pharmacol Ther 2004; 103(3):203–21. [DOI] [PubMed] [Google Scholar]
- Brady AE and Limbird LE. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal 2002;14:297–309. [DOI] [PubMed] [Google Scholar]
- Burgoyne T, Jolly R, Martin-Martin B, Seabra MC, Piccirillo R, Schiaffino MV et al. Expression of OA1 limits the fusion of a subset of MVBs with lysosomes - a mechanism potentially involved in the initial biogenesis of melanosomes. J Cell Sci 2013;12: 5143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese K, Giordano F, Surace EM, Venturi C, Ballabio A, Tacchetti C et al. The ocular albinism type 1 (OA1) gene controls melanosome maturation and size. Invest Ophthalmol Vis Sci 2005;46(12):4358–64. [DOI] [PubMed] [Google Scholar]
- Falletta P, Bagnato P, Bono M, Monticone M, Schiaffino MV, Bennett DC et al. Melanosome-autonomous regulation of size and number: the OA1 receptor sustains PMEL expression. Pigment Cell Melanoma Res 2014;27(4):565–79. [DOI] [PubMed] [Google Scholar]
- Garner A and Jay BS. Macromelanosomes in X-linked ocular albinism. Histopathology 1980;4:243–254. [DOI] [PubMed] [Google Scholar]
- Giordano F, Bonetti C, Surace EM, Marigo V and Raposo G. The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum Mol Genet 2009;18(23):4530–45. [DOI] [PubMed] [Google Scholar]
- Gu Y, Di W, Kelsell D and Zicha D. Quantitative fluorescence resonance energy transfer (FRET) measurement with acceptor photobleaching and spectral unmixing. J Microsc 2004;215:162–173. [DOI] [PubMed] [Google Scholar]
- Incerti B, Cortese K, Pizzigoni A, Surace EM, Varani S, Coppola M et al. Oa1 knock-out: new insights on the pathogenesis of ocular albinism type 1. Hum Mol Genet 2000;9(19):2781–2788. [DOI] [PubMed] [Google Scholar]
- Innamorati G, Piccirillo R, Bagnato P, Palmisano I and Schiaffino MV. The melanosomal/lysosomal protein OA1 has properties of a G protein-coupled receptor. Pigment Cell Research 2006;19(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery G The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci 1997;20(4):165–169. [DOI] [PubMed] [Google Scholar]
- Jiménez M, Tsukamoto K and Hearing V. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem 1991;266(2):1147–56. [PubMed] [Google Scholar]
- Karpova T, Baumann C, He L, Wu X, Grammer A, Lipsky P et al. Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J Microsc 2003;209:56–70. [DOI] [PubMed] [Google Scholar]
- King RA, Hearing VG, Creel DJ and Oetting WS. Albinism. Metabolic and Molecular Bases of Inherited Disease, in: Ed 8 Scriver CR, Beaudet AL, Sly WS and Valle D 1995:4353–4392. McGraw-Hill, New York. [Google Scholar]
- Lopes VS, Wasmeier C, Seabra MC and Futter CE. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol Biol Cell 2007;18:3914–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez VM, Decatur CL, Stamer WD, Lynch RM and McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol 2008;6(9):e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano I, Bagnato P, Palmigiano A, Innamorati G, Rotondo G, Altimare D et al. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum Mol Genet 2008;17(22):3487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo R, Palmisano I, Innamorati G, Bagnato P, Altimare D and Schiaffino MV. An unconventional dileucine-based motif and a novel cytosolic motif are required for the lysosomal and melanosomal targeting of OA1. J Cell Sci 2006;119(10):2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF and Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol 2001; 152(4): 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter SL and Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol 2009;10(12):819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaraweera P, Shen B, Newton JM, Barsh GS and Orlow SJ. The mouse ocular albinism 1 gene product is an endolysosomal protein. Exp Eye Res 2001;72(3):319–29. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Addio M, Alloni A, Baschirotto C, Valetti C, Cortese K et al. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nature Genetics 1999;23:108–112. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Baschirotto C, Pellegrini G, Montalti S, Tacchetti C, De Luca M et al. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc Natl Acad Sci USA 1996;93:9055–9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino MV and Tacchetti C. The ocular albinism type 1 (OA1) protein and the evidence for an intracellular signal transduction system involved in melanosome biogenesis. Pigment Cell Res 2005;18(4): 227–33. [DOI] [PubMed] [Google Scholar]
- Shen B, Rosenberg B and Orlow SJ. Intracellular distribution and late endosomal effects of the Ocular Albinism type 1 gene product: consequences of disease-causing mutations and implications for melanosome biogenesis. Traffic 2001;2:202–211. [DOI] [PubMed] [Google Scholar]
- Shen B, Samaraweera P, Rosenberg B and Orlow SJ. Ocular Albinism type 1: more than meets the eye. Pigment Cell Res 2001;14:243–248. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Singh G and Ghosh E. Emerging structural insights into biased GPCR signaling. Trends Biochem Sci 2014;39(12):594–602. [DOI] [PubMed] [Google Scholar]
- Simmen T, Schmidt A, Hunziker W and Beermann F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J Cell Sci 1999;53:45–53. [DOI] [PubMed] [Google Scholar]
- Sone M and Orlow SJ. The ocular albinism type 1 gene product, OA1, spans intracellular membranes 7 times. Exp Eye Res 2007;85(6):806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrini F, Auricchio A, Du J, Angeletti B, Fisher DE, Ballabio A et al. The Microphthalmia Transcription Factor (Mitf) controls expression of the Ocular Albinism type 1 gene: link between melanin synthesis and melanosome biogenesis. Mol Cell Bio 2004;24(15):6550–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Meredith D and Halestrap AP. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J Biol Chem 2002;277(5):3666–72. [DOI] [PubMed] [Google Scholar]
- Winder AJ, Wittbjer A, Rosengren E and Rorsman H. The mouse brown (b) locus protein has dopachrome tautomerase activity and is located in lysosomes in transfected fibroblasts. J Cell Sci 1993;166:153–166. [DOI] [PubMed] [Google Scholar]
- Young A, Wang Y, Ahmedli NB, Jiang M and Farber DB. A constitutively active Gαi3 protein corrects the abnormal retinal pigment epithelium phenotype of Oa1 −/− mice. PloS One 2013,8(9):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


