Skeletal myopathies due to variants in the alpha-skeletal isoform of actin, ACTA1, are widely reported, however associated cardiomyopathies are exceedingly rare. We present a unique case of a dominant cardiomyopathy without functional skeletal myopathy associated with a novel variant in ACTA1. This case highlights the implications of a dysfunctional skeletal muscle-enriched protein causing a predominant cardiac phenotype.
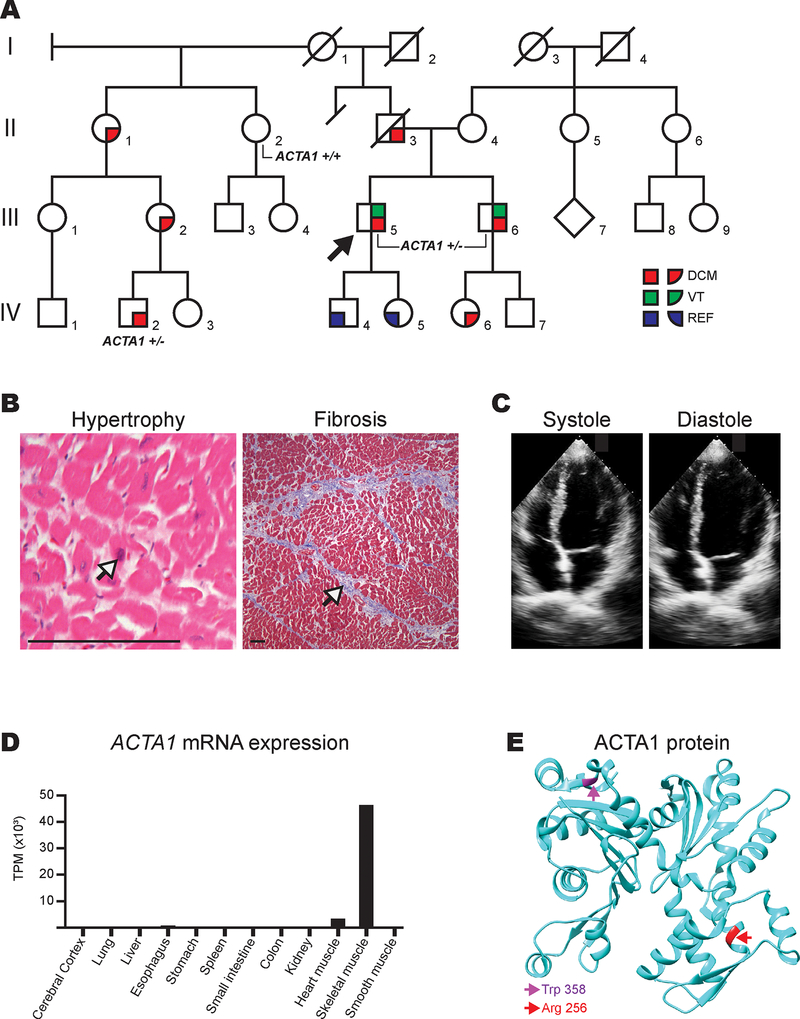
A 36-year-old man (III-5) was admitted after experiencing syncope while playing basketball. Family history was notable for his father’s (II-3) sudden cardiac death at age 56 (Figure A). Imaging revealed a moderately dilated left ventricle with ejection fraction (LVEF) 38%. An electrophysiologic study showed easily inducible polymorphic ventricular tachycardia (VT) to which his syncope was attributed. He was discharged with an implantable cardioverter defibrillator (ICD) and on medical therapy. Over the next five years, he experienced multiple appropriate ICD shocks and progressive left ventricular (LV) dysfunction necessitating continuous inotropic support. He ultimately underwent cardiac transplantation seven years after his initial presentation. Explanted cardiac histology was notable for myocyte hypertrophy and interstitial fibrosis (Figure B).
Figure 1A.
Pedigree of ACTA1 p.Arg256His family. Squares and circles represent males and females, respectively, while the diamond represents unknown gender. Generations are labeled by Roman numerals while individuals are labeled with Arabic numerals. Slashes denote deceased family members. The proband, III-5, is denoted with an arrow. III-5, III-6, and IV-2 were confirmed variant carriers and are thus labeled “ACTA1 +/−”. II-2 was confirmed to not have the variant and is thus labeled “ACTA1 +/+”. Red coloring denotes individuals with dilated cardiomyopathy (DCM). Green denotes individuals who experienced ventricular tachycardia (VT). Blue denotes individuals with reduced ejection fraction (REF). B. Explanted heart histology showing hematoxylin and eosin stain (left) demonstrating well-preserved myocytes with box cart nuclei (denoted by arrow) consistent with cardiac hypertrophy and trichrome stain (right) showing mild interstitial fibrosis (denoted by arrow). Scale bar (bottom left) represents 75 μm. C. Apical 4-chamber views (systole and diastole) of the proband’s brother’s (III-6) initial transthoracic echocardiogram at our institution. Left ventricle was normal in size but calculated LVEF was 34%. D. ACTA1 expression in multiple human tissues using data from the Human Protein Atlas (HPA; www.proteinatlas.org) from the “HPA dataset” located at https://www.proteinatlas.org/ENSG00000143632-ACTA1/tissue. Several non-expressing tissues were excluded for figure simplicity: thyroid gland, parathyroid gland, adrenal gland, appendix, bone marrow, lymph node, tonsil, gallbladder, pancreas, salivary gland, duodenum, rectum, urinary bladder, testis, prostate, epididymis, seminal vesicle, fallopian tube, breast, cervix, endometrium, ovary, adipose tissue, and skin. “TPM” denotes “transcripts per million”. E. ACTA1 protein showing the spatial relationship of p.Arg256His (R256H; colored red and with red arrow) compared to previously reported dilated cardiomyopathy-associated variant, p.Trp358C (W358C; colored magenta and with magenta arrow). Graphic generated using UCSF Chimera package and the Protein Data Bank 1J6Z atomic coordinate file.1
His brother (III-6) was referred for clinical screening soon after the proband’s (III-5) diagnosis. The initial echocardiogram at our institution showed LVEF 34% (Figure C). Medical therapy was initiated, but he was lost to follow-up. He re-presented 15 years later after experiencing symptomatic VT and underwent ICD implantation. Three years later, his LVEF fell to 20%, and ICD interrogation revealed over 1,000 premature ventricular contractions (PVCs) per hour. After antiarrhythmic therapy failure, he underwent successful PVC ablations. A paternal half-first cousin once removed (IV-2) was diagnosed with severe dilated cardiomyopathy at age 15 and soon thereafter underwent left ventricular assist device placement.
Clinical screening of affected individuals’ first-degree relatives was recommended. The proband had two children (IV-4, IV-5) who were found to have mild LV dysfunction in their late teenage years. The proband’s teenage niece (IV-6) had LV dilation with borderline LV function. Individuals III-5, III-6, and their children were physically active without limitations and did not have other symptoms consistent with known inherited cardiomyopathies including intellectual disability, skeletal muscle weakness, lipodystrophy, or atrial arrhythmias.
The proband underwent sequencing of 78 genes associated with cardiomyopathy and arrhythmia through a commercial laboratory (GeneDx, Gaithersburg, Maryland). Individuals IV-2 and IV-4 had sequencing for cardiomyopathy-associated genes through different commercial laboratories (Laboratory for Molecular Medicine, Cambridge, Massachusetts). No pathogenic variants were identified on any test.
Duo whole exome sequencing (WES) of the proband and affected cousin was performed (Personalis, Menlo Park, California) for variant discovery given the suspicion for familial disease and the potential for higher yield with the ability to test distant affected relatives. A sample from the proband’s mother (II-4) was submitted to serve as a proxy for the proband’s deceased affected father’s genotype. Indexed genomic libraries and enrichment were performed with proprietary kits. Sequencing was performed on Illumina sequencers using paired-end reads (Illumina, San Diego, California). Base calling, alignment, variant calling, annotation, and QC reporting were performed using a proprietary pipeline and were manually reviewed.
A heterozygous novel variant in ACTA1, NM_001100.3: c.767G>A, p.Arg256His was detected in the proband, his brother, and his affected cousin (noted amino acid positions follow conventional amino acid numbering, i.e. prior to any protein processing). The variant was not detected in the proband’s paternal half-aunt (II-2), making the proband’s father and three other affected intervening relatives obligate carriers. A copy number gain variant of uncertain significance, 9p22.2p22.1(18,260,501–19,340,501)x3, was identified in individual IV-2 but not in the proband and therefore was not thought to contribute to the cardiac phenotype as it did not track with disease. Upon clinical reevaluation, the screened family members did not exhibit any neuromuscular symptoms1.
ACTA1 is a striated muscle-specific actin with greater than 10-fold greater expression in skeletal muscle than in heart (Figure D).2 There are over 250 known disease-causing variants in ACTA1 that almost exclusively cause skeletal muscle myopathies.3 Only a single ACTA1 variant associated with a dilated cardiomyopathy, p.Trp358Cys, has been previously published, but this individual also suffered from childhood-onset skeletal myopathy.4 This variant is not spatially related to our p.Arg256His variant and may disrupt actin function by a different molecular mechanism (Figure E).
An unpublished p.Arg256Gly variant in the Leiden Open Variation Database also reports an individual with severe dilated cardiomyopathy though with concurrent skeletal myopathy.5 Our p.Arg256His variant is absent from multiple healthy genome datasets, including 1000 Genomes and Exome Aggregation Consortium, and in silico models (including PolyPhen-2 and Sorting Intolerant From Tolerant) predict a deleterious effect on protein function. Furthermore, p.Arg256 is conserved among 93 vertebrate species. These data indicate the importance of preserving p.Arg256 and that p.Arg256His is likely pathogenic and associated with cardiomyopathy though different amino acid substitutions may influence the degree of skeletal muscle involvement.
Here, we first report a novel ACTA1 variant that is associated with a predominant dilated cardiomyopathy without clinical skeletal myopathy. Our work underscores the need to evaluate for the coexistence of inherited skeletal and cardiac myopathies and inspires future investigation into the mechanism by which cardiac muscle can be disproportionately affected by a protein predominantly expressed in skeletal muscle.
Acknowledgments
Sources of Funding: Dr. Reza is supported by the NIH National Human Genome Research Institute Ruth L. Kirschstein Institutional National Research Service T32 Award in Genomic Medicine (T32 HG009495). Dr. Owens is supported by the Winkleman Family Fund in Cardiovascular Innovation.
Footnotes
Disclosures: None
References:
- 1.Pettersen EF, et al. UCSF Chimera - a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. [DOI] [PubMed] [Google Scholar]
- 2.Uhlén M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 3.Sparrow JC, et al. Muscle disease caused by mutations in the skeletal muscle alpha-actin gene (ACTA1). Neuromuscul Disord. 2003;13:519–531. [DOI] [PubMed] [Google Scholar]
- 4.Gatayama R, et al. Nemaline myopathy with dilated cardiomyopathy in childhood. Pediatrics. 2013;131:e1986–e1990. [DOI] [PubMed] [Google Scholar]
- 5.Fokkema IF, et al. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:557–563. [DOI] [PubMed] [Google Scholar]