Abstract
Objective
Test the impact of tailoring CRC screening messages for African Americans (AAs) using novel theoretical variables and to examine moderating effect of communication preferences.
Methods
Participants were randomized to receive two minimally tailored or two enhanced tailored print newsletters addressing CRC. The enhanced intervention was tailored on self-determination theory and other novel psychological constructs. Minimal tailoring only used information available in the patient’s EHR. The primary outcome was CRC screening based on EHR. Participants were AA members aged 50–74 of an integrated health care delivery system not up to date on CRC screening.
Results
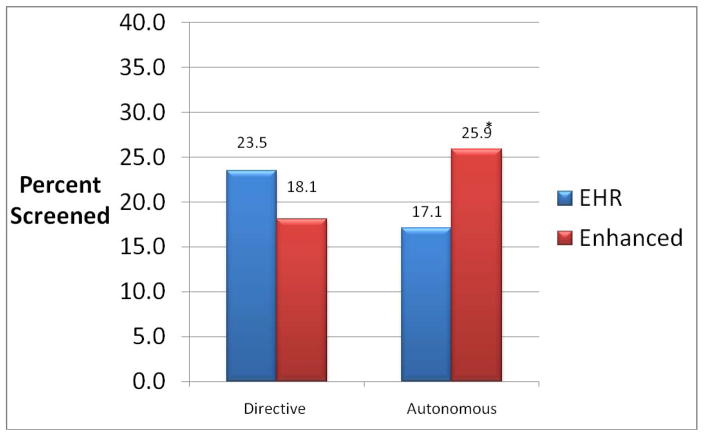
We enrolled 881 participants. CRC screening participation rates at 1-year follow up were 20.5% and 21.5% in the minimally and enhanced tailored groups, respectively. Communication preferences moderated the impact of the intervention. Specifically, among those with an autonomous communication preference, screening rates in the minimally and enhanced tailored groups were 17.1% and 25.9%, respectively, while no intervention effect was evident among those with a directive preference.
Conclusion
Future research is needed to explore the impact of communication preference tailoring for other health behaviors and among other populations.
Practice Implications
Tailored Communications should consider communication style preference to help guide the content and tone of messages.
INTRODUCTION
African Americans (AAs) have higher rates of colorectal cancer (CRC) incidence and mortality, and are more likely to be diagnosed at a later stage of the disease than whites1,2. CRC screening rates among AAs are only slightly lower than whites3–7 and appear to be nearing parity3,8. Like other Americans, however, only around 2 out of 3 AAs age 50 and older are up to date on CRC screening.
Many prior interventions to increase CRC screening have been conducted, with most utilizing clinical reminders and/or educational materials9. Several CRC screening interventions have been tested with AAs but with mixed results10.
Several studies have tested tailored motivational and educational messages to improve CRC screening uptake11–21. Most of these studies have not shown significant effects on screening behavior13–16,17,19,20,22,21, however, most have shown effects on mediators of screening behavior in subsets of the full sample, such as participants with increased CRC risk13–17,21,22. Only two tailored interventions for AAs12,23 have been reported. In one, significant effects were reported for knowledge and attitudes, although screening rates were not reported12 and in the other, results of the tailored intervention were marginally significant for increasing fecal occult blood tests (FOBT) but not for other screening tests23. Rather than simply targeting AAs with interventions tailored only on the group level, there is a need to design and test interventions that account for within-group variation among AAs and other ethnic/racial groups.
Interventions that include decision aid components that help patients clarify their CRC tests preferences and communicate them to their providers have shown promise in predominantly white samples, although no decision aid interventions have been tested exclusively in AAs14,24–26. In summary, the efficacy of tailored interventions and decision aids to increase CRC screening uptake remains equivocal for the general population and understudied among AAs.
One limitation of previous tailored CRC screening interventions is that the theoretical models used although exclusively focused on similar constructs, e.g., stages of change, perceived benefits, barriers, attitudes and knowledge12,16,22,23. More recent tailored programs conducted outside of CRC screening have begun to incorporate other theoretical perspectives, such as Self-Determination Theory as well as personality factors, e.g., decision making style and communication preferences27. More recent tailored programs from our group conducted outside of CRC screening have begun to incorporate other theoretical perspectives, such as Self-Determination Theory as well as personality factors, e.g., decision making style and communication preferences27. This is the approach we employed here.
Prior studies on CRC screening and other health behaviors suggest that AAs may have unique cultural beliefs, preferences, and attributes that offer opportunities for tailoring. These include medical mistrust, perceived racism, gender roles, spirituality28, racial identity28,29, time orientation, and perceived CRC screening norms28–36. The impact of tailoring CRC screening messages on these variables or constructs has not been tested.
Communication preference is one attribute that merits additional exploration. In a prior study of a tailored print dietary intervention designed for AAs, conducted in the same health care delivery system used herein, communication preferences, defined as how much participants preferred patient versus provider-driven communication, moderated the intervention effect27. The extent to which this finding may generalize to other health behaviors such as CRC screening has not been examined.
The aims of the proposed study were twofold;
Test the impact of tailoring CRC screening messages for AAs using constructs based on Self-Determination Theory and personality attributes such as communication preferences compared to minimally tailored messages, based on electronic health record (EHR) information only, and,
Examine potential intervention moderators, particularly communication preferences, i.e., autonomous versus directive communication style.
We hypothesized that individuals preferring autonomous communication would be more responsive to the enhanced intervention which was written in a more autonomy supportive tone and designed to increase autonomous motivation. For those preferring directive communication, messages were written in the same tone for the minimal and enhanced-group participants.
METHODS
Overview
AA members, aged 50–74, from an integrated health care delivery system who were not up to date on CRC screening, were randomized to receive two minimally tailored or two enhanced tailored print newsletters addressing CRC screening. The primary outcome was CRC screening based on electronic health records supplemented by self-reported CRC screening for participants who left the health system during the trial.
Setting and Participants
Eligible participants were AAs aged 50–74 receiving primary care from an integrated health care delivery system serving greater Detroit, Michigan. Health system patients were identified by EHR as study eligible if they: 1) were continuously enrolled in the health system-affiliated health maintenance organization (HMO) for the prior three years; 2) had a visit to a health system-owned primary care clinic in the same time period; and 3) were overdue for CRC screening (i.e., no evidence of colonoscopy screening in the past 10 years, flexible sigmoidoscopy or double contrast barium enema in the past 5 years, and FOBT in the past year). Individuals with a history of CRC, bowel resection, inflammatory bowel disease, or polyps were excluded. A small number of individuals (n=42) were also excluded at the discretion of their primary care physician (PCP).
Recruitment Procedures
Study procedures were reviewed and approved by Institutional Review Boards at each participating institution. Participant recruitment took place between February 2011 and May 2012. Eligible individuals were sent a letter stating they would receive a telephone call from study staff within 2 weeks, a response option card to aid in the upcoming telephone survey, and a $2 bill pre-incentive. Individuals could opt out of study participation by signing and returning the letter. Trained research assistants called eligible individuals who did not opt out to confirm study eligibility and determine interest in participation.
Each individual was called up to 8 times at varying times of the day and days of the week. Those who reported having a colonoscopy appointment scheduled, not living in the state of Michigan or intending to move out of state within the next 12 months, having a family history of familial adenomatous polyposis or hereditary nonpolyposis CRC, personal history of CRC, prior adenomas, or inflammatory bowel disease, or not speaking English were excluded. The baseline survey was administered by trained interviewers using computer-assisted telephone software. Participants were randomized by computer to one of two study arms. In addition to the baseline survey, study participants were also contacted to complete a follow-up survey approximately 12 months following their baseline survey. Those who completed the baseline survey were mailed a $15 gift card and those who completed the follow-up survey were mailed a $20 gift card.
Interventions
Both interventions entailed 1–2 printed newsletters, sent via mail, promoting CRC screening. All participants received their first newsletter around one month after completing their baseline telephone interview. Participants whose EHR data indicated that they had not completed CRC screening at six months post-baseline were mailed a second tailored newsletter at approximately eight months that provided additional messages to get CRC screening. Participants who had completed a CRC screening test by six months post-baseline did not receive a second newsletter.
Both intervention groups received newsletters of equal length and graphic appearance. The first newsletter was eight pages in length and was inserted into the interior pocket of a colorful folder. The folder also included informational inserts, a pen, and a car magnet, which drew upon the overall journey/car theme of the intervention graphics. Participants who received a second newsletter mailing received all of the materials that were included in their first mailing, as well as the second newsletter. Content for the first newsletter focused on addressing barriers to CRC screening, finding personal motivation to get screened such as family, a fictional testimonial describing the experience of getting screened and a second testimonial addressing barriers and motivations to get screened. Content for the second newsletter focused on overcoming procrastination, the importance of screening for health and well-being, confidence for getting screened, addressing persistent barriers, and a fictional testimonial exploring motivational and decision-making style as they pertained to getting screened. Newsletters were written in “plain language” at around a 7th grade reading level.
The minimally tailored intervention used only variables commonly found in an EHR from most health delivery systems. These “EHR variables” included the participant’s name, age, gender, current CRC screening status, and prior CRC screening history and test dates.
The enhanced intervention was tailored on demographic, psychosocial and personality variables measured solely for this study. These variables were collected as part of the baseline survey. Key variables included marital status, family history of CRC, barriers to CRC screening, reasons to get screened, communication preference, time orientation, decisional style, medical mistrust, spirituality, ethnic identity, desire for information about CRC, and procrastination. A key element of the enhanced tailoring was that for those classified with autonomous communication preferences, their newsletters were written in a “pull” message tone, consistent with the principles of Self-Determination Theory and Motivational Interviewing. Participants preferring more directive communications received newsletter content written using a “push” tone. The “pull” vs “push” tailoring permeated the entire newsletters, and was considered the primary tailoring variable. Enhanced intervention participants also received more nuanced tailoring that considered test preferences and physician screening recommendations.
Message content was informed by eight focus groups held in 2009 with 78 African American members of the partnering health care delivery system, stratified by gender, screening status (not screened vs. screened), and test recommendation status.
Measures
The primary outcome was CRC screening as documented in the EHR over the 12 months of the trial. We considered a positive outcome as receipt of any of the following tests; colonoscopy, FOBT, virtual colonoscopy, sigmoidoscopy or barium enema.
During the trial, 141 out of the 881 (16%) baseline cohort members lost their insurance coverage from the sponsoring health system. The rate of lost insurance did not differ significantly between the enhanced and EHR groups, 17% and 15%, respectively. For these 141 participants who did not have CRC screening indicated in their EHR, we used their self-reported screening to supplement EHR data. There were 15 cases (8 in Enhanced and 7 in EHR) where the member lost their insurance, did not have CRC screening in the EHR, and self-reported having CRC screening during the study period, which was assumed to have occurred outside the sponsoring health system.
Demographics assessed on the baseline questionnaire included gender, income (8 categories collapsed into 3 groups (i.e., $40K or less, $40K–$80K, > $80K) and education, measured with 8 categories and then collapsed into 4 (i.e., <high school, high school, some college, and college or greater). Family history was considered positive if the respondent indicated CRC had occurred in any biologic parent, grandparent or sibling.
Communication Preference at baseline was computed from two items (alpha = .71) adapted from a previous study 27, 1) “In general when it comes to my health, I would rather be told what to do”, and 2) “When it comes to my health, I want my doctor to tell me what to do.” Responses were strongly disagree (1) to strongly agree (10). The mean from the two items was dichotomized as Autonomy Preference (mean score < 6) and Directive Preference (mean score >= 6). Using this schema, 43% of the sample was classified as having autonomy preference and 57% as preferring directive communication. This variable was used both as a tailoring variable as well as a moderator in our outcome analyses.
Confidence in screening knowledge was queried at baseline with a single item; ‘How confident are you that you know enough to make a decision about whether or not you want to get checked for colon cancer? “Responses ranged from not at all confident (1) to very confident (10). Motivation to get screened was queried with “How important is it to you to get checked for colon cancer?” Responses ranged from not at all important (1) to extremely important (10), and were analyzed as continuous variables. Both of these items were generated by the study team.
Test Preference was assessed by asking; “Assume that you have decided to get checked for colon cancer and can pick either a colonoscopy or home stool cards. How likely would you be to choose a colonoscopy for your test?”, and “Assuming that you have decided to get checked for colon cancer and can pick either a colonoscopy or home stool cards, how likely would you be to choose home stool cards for your test?” Responses for both items ranged from not at all likely (1) to very likely (10). These items were developed by the study team based on a prior study37.
CRC screening within the health care delivery system where the study was conducted38, like that within many other deliver settings nationwide39, is overwhelmingly conducted by colonoscopy with limited use of other modalities (primarily FOBT).
Process Measures
At 1-year posttest we asked how many newsletters participants recalled receiving, and if they indicated more than zero, how much they read of each (using all, most, some, or none). We also asked, “How much do you feel that the newsletters that you received were written just for you?” Responses were; not at all, somewhat, mostly, completely.
We also queried how much participants agreed with each of the following statements on a scale of 1 to 10, with 1 being strongly disagree and 10 being strongly agree:
“I still have questions about whether or not I should get checked for colon cancer.”
“I still have questions about which colon cancer test is right for me.”
“I trusted the information in the newsletters.”
“The newsletters were culturally offensive.”
“The newsletters fit my cultural background.”
“The newsletters were boring.”
All process items were developed by the study team based on prior studies 40–43.
Analyses
The primary outcome analyses examined CRC screening rates between treatment groups at 1-year follow-up. Chi-square was used for the primary outcome analyses (Aim 1).
Moderator analyses (Aim 2) utilized logistic regression and included interaction terms of selected baseline variables (i.e., possible moderator) by treatment condition, i.e., communication preference, gender, age, CRC test preference, and family history of CRC. If interaction terms were significant, outcomes are presented stratified by the moderating variable.
Sample Size
The study was powered to detect a 10% difference between the minimal and enhanced tailoring groups on CRC screening at 1-year follow-up. Assuming alpha of .05, power of .80, and 20% attrition, we required 880 baseline participants.
RESULTS
A total of 4,266 individuals meeting initial eligibility criteria from the EHR were sent recruitment letters and we were able complete telephone screening for 4,122 of these. From this group, 1,074 were deemed ineligible. The most common reason (n=787) for ineligibility was due to the patient reporting being screened. From the remaining eligible 3,048, we enrolled 881 (29%). We stopped recruitment after meeting our 881 baseline sample target.
A total of 188 of 881 (21.3%) participants had received CRC screening at 1-year follow up based on EHRs, supplemented by self-report for those who left the insurance plan.
Process Measures
An equal percentage of minimal and enhance group members reported receiving at least one of the two project newsletters, 68% and 66%, respectively, p= .72. At follow-up, 49% of the minimally tailored compared to 50% of the enhanced group responded they read most or all of their newsletters, p = .81.
In response to the item, “I still have questions about whether or not I should get checked for colon cancer”, the minimal group had significantly higher scores (F=6.6; p=.01) than the enhanced group, 4.1 and 3.1, respectively, out of a possible 10. In response to, “I still have questions about which colon cancer test is right for me”, the minimal group had significantly higher scores (F=6.4; p=.01) than the enhanced group, 5.0 and 4.0 respectively, out of a possible 10. There were no group differences in perceptions that the newsletters were “boring”, “trustworthy”, “fit my cultural background”, “written for you” and “culturally offensive”. Program satisfaction did not differ between groups, 9.1 and 9.2, in the minimal and enhanced groups, respectively.
Overall Intervention Effects
Follow-up CRC screening rates at 1-year follow up were 21.0% and 21.7% in the minimally and enhanced tailored groups, respectively. This difference was not significant.
Moderation Effects
As hypothesized, baseline communication preference significantly moderated the impact of the intervention on CRC screening rates (interaction beta; −0.85; p = 0.01). Among those with an autonomous preference, CRC screening rates in the minimally and enhanced groups were 17.1% and 25.9%, p < .05, respectively. Among those with a directive preference the screening rates in the minimally and enhanced tailored groups were 23.5% and 18.1%, respectively, and these differences were not significant.
Adjusting for age, gender, and CRC test preference, among those with autonomous preference, the odds of obtaining screening were 1.70 times higher (p=.056;95% CI:0.99–2.8) in the enhanced than the minimally tailoring group, which was borderline significant. Among those with a directive preference, the odds of screening were non significantly lower (OR 0.76; CI: 0.49–1.17) in the enhanced compared to the minimally tailoring group.
Directive preference was significantly (p < .05) more common among those with lower educational status but did not differ by gender or income. Those preferring directive communication were significantly (p < .03) older (mean age 57.7 years) than those preferring autonomous communication (mean age 56.8 years). Finally, those preferring directive versus autonomous communication had significantly (p < .01) higher rated importance for getting CRC screening at baseline, 9.0 and 8.3, respectively, on the 10 point scale.
None of the other proposed moderators, i.e., gender, age, income, education, family history of CRC, or test preference significantly moderated the impact of the intervention.
DISCUSSION
Our tailored intervention designed to increase CRC screening uptake among AAs did not show an overall effect. However, consistent with a prior tailored dietary intervention conducted among AAs in the same health care delivery system, the intervention effects were moderated by communication preference.
Specifically, we found that for participants that reported a preference for autonomous communication, the enhanced tailored intervention was associated with increased CRC screening compared to the minimally tailored group. A key element of the enhanced tailoring was that for those who were classified as preferring autonomous communication, their two newsletters were written in a “pull” versus “push” style, consistent with the principles of Self-Determination Theory and Motivational Interviewing44,45.
The enhanced tailoring did not increase screening among those with a directive communication preference. The newsletters for all those in the minimally tailored group and for those in the enhanced group with a directive preference were written in a “push” tone, consistent with a directive communication preference. Thus, although the enhanced tailored materials included tailoring on other constructs, the lack of intervention effects for those preferring directive communication is not surprising, as at least in terms of tone, these two groups received similar interventions.
In this study, 43% of the sample was classified as having autonomy preference and 57% as preferring directive communication. This is consistent with prior studies which found that AAs may prefer a more directive communication style from their health care practitioners than whites46–48. Specifically, in a prior study conducted partly in the same health system as the current study also with all AAs, we asked “in general, when it comes to my health I would rather an expert just tell me what I should do”. In that study, 57% (242 out of 423) scored >5 on the seven-point disagree–agree scale, suggesting more individuals preferred the directive communication style.
Whether communication preferences impact intervention effectiveness for other social and demographic groups has not been examined. Further, although we have now shown that this attribute moderates the impact of tailored interventions for both dietary change and CRC screening among AAs, whether this finding generalizes to other health behaviors also merits examination.
The overall 1-year uptake of CRC screening in our study was 21.3% across the two study groups. These are similar to the rates we had projected for the minimally tailored group in our power calculations for this trial and are similar to rates reported for intervention group respondents in most tailored intervention studies13,15–17,19,22,23,26,49. Thus, the minimally tailored intervention produced effects in the range of other tailored interventions. It may be that the intervention effects observed in our comparison group represent the ceiling of what can be achieved with this type and dose of intervention.
Over the course of the project 141 (16%) members were dropped from the insurance plan with whom we conducted the study. For these individuals we used self-report to supplement EHR-based results. Although self-reported screening is more susceptible to bias, when we limit the results to only those who remained in the plan and for whom we had EHR evidence the main results and the interaction with communication preference where essentially unchanged, although the odds of obtaining screening were increased slightly to 1.72 (up from 1.70) in the enhanced than the minimally tailoring group and the difference was now significant (p value decreased to .05 from .057).
Only 68% and 66%, of the minimal and enhanced tailoring group members reported receiving at least one of the two project newsletters and only around 50% of either group responded they read most or all of their newsletters. Thus, lack of intervention uptake may at least in part explain the overall null results.
In addition to the fact that the study only included African Americans, which limits generalizability, another limitation is the lack of data regarding the cost-effectiveness of the intervention, something that has been reported elsewhere50.
Research to elucidate the nature of communication style preferences among other populations and how tailoring on this characteristic may impact other health behaviors is encouraged.
Figure 1. Screening Rates by Communication Preference and Intervention Group.
* p = .04
EHR- Minimally tailored intervention based on Electronic Health Record information
Table 1.
Sample Tailored Messages
| Tailoring Variable | Sample Message |
|---|---|
| Communication Preference – Autonomous | [NAME], what are some good things you feel will come from getting checked for colon cancer? Thinking about reasons to get tested may help you take that next step … |
| Communication Preference – Directive | [NAME], experts agree — regular screening is one of the best ways to help prevent colon cancer. If a screening test finds a problem, doctors can usually diagnose and treat you right away. |
| Reason Why Health Is Important – Being There for Others (Communication Preference – Autonomous) | Being healthy might be important to you because you want to be there to take care of your loved ones, like [TAILORED PERSONS: your son and your granddaughters]. |
| Reason Why Health Is Important – God Expects It (Communication Preference – Directive) | You want to be healthy because you feel that God expects you to do your part in taking care of yourself. |
| Reason Why Health Is Important – Role Model for African American Community (Communication Preference – Autonomous) | Being healthy might be important to you because you want to set an example for other African Americans. |
| Reason Why Health Is Important – Role Model for African American Community (Communication Preference – Directive) | Being healthy is important to you because you want to set an example for other African Americans. |
| Decision Making Style – Planned Testimonial Sample | It’s not like I didn’t do my research. I read about why I should do it. I looked up the details of the different tests. When all was said and done, I knew what I needed to do — I just wasn’t getting around to doing it. (Testimonial Text) |
| Decision Making Style – Impulsive Testimonial Sample | I really didn’t give much thought about getting tested. Like everyone around me, other things were taking up my time. Then one day, I was waiting to have my prescription filled at the pharmacy. To pass the time, I started reading a poster about getting checked. (Testimonial Text) |
Table 2.
Baseline Sample Description
| Minimal EHR Tailoring (n=442) | Enhanced Tailoring (n=439) | Total (n=881) | |
|---|---|---|---|
| Age (range) | 57.5 (50–74) | 57.1 (50–74) | 57.3 (50–74) |
| Gender | |||
| Male | 154 (34.9%) | 144 (32.7%) | 298 (33.8%) |
| Female | 285 (65.1%) | 298 (67.3%) | 583 (66.2%) |
| Education | |||
| <HS | 24 (5.5%) | 19 (4.4%) | 43 (5.0%) |
| HS | 120 (27.6%) | 126 (29.1%) | 246 (28.3%) |
| some college | 212 (48.7%) | 196 (45.3%) | 408 (47.0%) |
| college or > | 79 (18.2%) | 92 (21.2%) | 171 (19.7%) |
| Income | |||
| $40K or less | 147 (41.6%) | 160 (44.2%) | 307 (42.9%) |
| $40K–$80K | 138 (39.1%) | 116 (32.0%) | 254 (35.5%) |
| > $80K | 68 (19.3%) | 85 (23.8%) | 154 (21.5%) |
| Positive Family History of CRC | 34 (7.7%) | 32 (7.2%) | 66 (7.5%) |
| Communication Preference | |||
| Pull | 175 (39.9%) | 205 (46.4%) | 380 (43.1%) |
| Push | 264 (60.1%) | 237 (53.6%) | 501 (56.9%) |
Acknowledgments
Supported by National Cancer Institute Grant P50-CA101451
Footnotes
Conflict of interest statement: All authors received funding from National Cancer Institute grant P50-CA101451 but no other financial support for this work.
No author has any financial disclosures beyond support by the NIH grant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ken Resnicow, University of Michigan, School of Public Health, Department of Health Behavior & Health Education, 109 Observatory Street, Room 3867 SPH I, Ann Arbor, MI 48109-2029.
Yan Zhou, University of Michigan, Ann Arbor, MI, USA.
Sarah Hawley, University of Michigan, Ann Arbor, MI, USA.
Masahito Jimbo, University of Michigan, Ann Arbor, MI, USA.
Mack T. Ruffin, University of Michigan, Ann Arbor, MI, USA.
Rachel E. Davis, University of South Carolina, Columbia, SC USA.
Deirdre Shires, Henry Ford Health System, Detroit, MI, USA.
Jennifer Elston Lafata, Virginia Commonwealth University, Richmond, VA.
Literature Cited
- 1.American Cancer Society. Colorectal Cancer Facts & Figures, 2011–2013. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed November 13, 2013];Colorectal Cancer Rates by Race and Ethnicity. 2013 http://www.cdc.gov/cancer/colorectal/statistics/race.htm.
- 3.Joseph DA, King JB, Miller JW, Richardson LC. Prevalence of colorectal cancer screening among adults--Behavioral Risk Factor Surveillance System, United States, 2010. MMWR. Morbidity and mortality weekly report. 2012 Jun 15;61(Suppl):51–56. [PubMed] [Google Scholar]
- 4.Prevention CfDCa. Cancer screening - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41–56. [PubMed] [Google Scholar]
- 5.Prevention CfDCa. Vital signs: Colorectal cancer screening, incidence, and mortality--United States, 2002–2010. MMWR Morb Mortal Wkly Rep. 2011 Jul 8;60(26):884–889. [PubMed] [Google Scholar]
- 6.Prevention CfDCa. Vital signs: colorectal cancer screening among adults aged 50–75 years - United States, 2008. MMWR Morb Mortal Wkly Rep. 2010 Jul 9;59(26):808–812. [PubMed] [Google Scholar]
- 7.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006 Feb;15(2):389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 8.Prevention CfDCa. Vital signs: colorectal cancer screening test use - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013 Nov 8;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 9.Baron RC, Rimer BK, Breslow RA, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008 Jul;35(1 Suppl):S34–55. doi: 10.1016/j.amepre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Naylor K, Ward J, Polite B. Interventions to Improve Care Related to Colorectal Cancer Among Racial and Ethnic Minorities: A Systematic Review. Journal of General Internal Medicine. 2012 Aug 01;27(8):1033–1046. doi: 10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christy SM, Perkins SM, Tong Y, et al. Promoting Colorectal Cancer Screening Discussion: A Randomized Controlled Trial. American Journal of Preventive Medicine. 2013;44(4):325–329. doi: 10.1016/j.amepre.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawl SM, Skinner CS, Perkins SM, et al. Computer-delivered tailored intervention improves colon cancer screening knowledge and health beliefs of African-Americans. Health Education Research. 2012 Oct 1;27(5):868–885. doi: 10.1093/her/cys094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manne SL, Kashy DA, Weinberg DS, Boscarino JA, Bowen DJ, Worhach S. A pilot evaluation of the efficacy of a couple-tailored print intervention on colorectal cancer screening practices among non-adherent couples. Psychology & health. 2013 Sep;28(9):1046–1065. doi: 10.1080/08870446.2013.781601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Dec Making. 2002;22(2):125–139. doi: 10.1177/0272989X0202200210. [DOI] [PubMed] [Google Scholar]
- 15.Costanza ME, Luckmann R, Stoddard AM, et al. Using tailored telephone counseling to accelerate the adoption of colorectal cancer screening. Cancer Detect Prev. 2007;31(3):191–198. doi: 10.1016/j.cdp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Vernon SW, Bartholomew LK, McQueen A, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Ann Behav Med. 2011 Jun;41(3):284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus AC, Mason M, Wolfe P, et al. The efficacy of tailored print materials in promoting colorectal cancer screening: results from a randomized trial involving callers to the National Cancer Institute’s Cancer Information Service. J Health Commun. 2005;10(Suppl 1):83–104. doi: 10.1080/10810730500257754. [DOI] [PubMed] [Google Scholar]
- 18.Albada A, Ausems MG, Bensing JM, van Dulmen S. Tailored information about cancer risk and screening: a systematic review. Patient Educ Couns. 2009 Nov;77(2):155–171. doi: 10.1016/j.pec.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007 Nov 1;110(9):2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 20.Myers RE, Bittner-Fagan H, Daskalakis C, et al. A randomized controlled trial of a tailored navigation and a standard intervention in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013 Jan;22(1):109–117. doi: 10.1158/1055-9965.EPI-12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipkus IM, Skinner CS, Dement J, et al. Increasing colorectal cancer screening among individuals in the carpentry trade: test of risk communication interventions. Prev Med. 2005 May;40(5):489–501. doi: 10.1016/j.ypmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Rawl SM, Champion VL, Scott LL, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns. 2008 May;71(2):215–227. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell MK, James A, Hudson MA, et al. Improving multiple behaviors for colorectal cancer prevention among african american church members. Health Psychol. 2004 Sep;23(5):492–502. doi: 10.1037/0278-6133.23.5.492. [DOI] [PubMed] [Google Scholar]
- 24.Schroy PC, 3rd, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011 Jan-Feb;31(1):93–107. doi: 10.1177/0272989X10369007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruffin MTt, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007 Oct;45(4):267–273. doi: 10.1016/j.ypmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000 Nov 21;133(10):761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 27.Resnicow K, Davis RE, Zhang G, et al. Tailoring a fruit and vegetable intervention on novel motivational constructs: results of a randomized study. Annals of Behavioral Medicine. 2008 Apr;35(2):159–169. doi: 10.1007/s12160-008-9028-9. [DOI] [PubMed] [Google Scholar]
- 28.Harper FW, Nevedal A, Eggly S, Francis C, Schwartz K, Albrecht TL. “It’s up to you and God”: understanding health behavior change in older African American survivors of colorectal cancer. Translational behavioral medicine. 2013 Mar;3(1):94–103. doi: 10.1007/s13142-012-0188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brittain K, Loveland-Cherry C, Northouse L, Caldwell CH, Taylor JY. Sociocultural differences and colorectal cancer screening among African American men and women. Oncol Nurs Forum. 2012 Jan;39(1):100–107. doi: 10.1188/12.ONF.100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz ML, James AS, Pignone MP, et al. Colorectal cancer screening among African American church members: a qualitative and quantitative study of patient-provider communication. BMC Public Health. 2004 Dec 15;4(1):62. doi: 10.1186/1471-2458-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James AS, Daley CM, Greiner KA. Knowledge and Attitudes About Colon Cancer Screening Among African Americans. American Journal of Health Behavior. 2011;35(4):393–401. doi: 10.5993/ajhb.35.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Ge, Burke N, Somkin CP, Pasick R. Considering Culture in Physician— Patient Communication During Colorectal Cancer Screening. Qualitative Health Research. 2009 Jun 1;19(6):778–789. doi: 10.1177/1049732309335269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreuter MW, Skinner CS, Steger-May K, et al. Responses to behaviorally vs culturally tailored cancer communication among African American women. American Journal of Health Behavior. 2004 May-Jun;28(3):195–207. doi: 10.5993/ajhb.28.3.1. [DOI] [PubMed] [Google Scholar]
- 34.Kreuter MW, Sugg-Skinner C, Holt CL, et al. Cultural tailoring for mammography and fruit and vegetable intake among low-income African-American women in urban public health centers. Preventive Medicine. 2005;41(1):53–62. doi: 10.1016/j.ypmed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Holt CL, Clark EM, Kreuter MW, Rubio DM. Spiritual health locus of control and breast cancer beliefs among urban African American women. Health Psychol. 2003 May;22(3):294–299. doi: 10.1037/0278-6133.22.3.294. [DOI] [PubMed] [Google Scholar]
- 36.Christy SM, Mosher CE, Rawl SM. Integrating Men’s Health and Masculinity Theories to Explain Colorectal Cancer Screening Behavior. Am J Mens Health. 2013 Jun 27; doi: 10.1177/1557988313492171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawley ST, McQueen A, Bartholomew LK, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2012 May 15;118(10):2726–2734. doi: 10.1002/cncr.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shires DA, Divine G, Schum M, et al. Colorectal cancer screening use among insured primary care patients. American Journal of Managed Care. 2011;17(7):480–488. [PubMed] [Google Scholar]
- 39.Steele RJ, Kostourou I, McClements P, et al. Effect of repeated invitations on uptake of colorectal cancer screening using faecal occult blood testing: analysis of prevalence and incidence screening. BMJ. 2010;341:c5531. doi: 10.1136/bmj.c5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resnicow K, Davis R, Zhang N, et al. Tailoring a fruit and vegetable intervention on ethnic identity: Results of a randomized study. Health Psychol. 2009 Jul;28(4):394–403. doi: 10.1037/a0015217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resnicow K, Jackson A, Wang T, et al. A motivational interviewing intervention to increase fruit and vegetable intake through Black churches: results of the Eat for Life trial. Am J Public Health. 2001 Oct;91(10):1686–1693. doi: 10.2105/ajph.91.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resnicow K, Jackson A, Braithwaite R, et al. Healthy Body/Healthy Spirit: a church-based nutrition and physical activity intervention. Health Educ Res. 2002 Oct;17(5):562–573. doi: 10.1093/her/17.5.562. [DOI] [PubMed] [Google Scholar]
- 43.Campbell MK, Resnicow K, Carr C, Wang T, Williams A. Process evaluation of an effective church-based diet intervention: Body & Soul. Health Education & Behavior. 2007;34(6):864–880. doi: 10.1177/1090198106292020. [DOI] [PubMed] [Google Scholar]
- 44.Vansteenkiste M, Williams GC, Resnicow K. Toward systematic integration between self-determination theory and motivational interviewing as examples of top-down and bottom-up intervention development: autonomy or volition as a fundamental theoretical principle. Int J Behav Nutr Phys Act. 2012;9:23. doi: 10.1186/1479-5868-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Resnicow K, McMaster F. Motivational Interviewing: moving from why to how with autonomy support. Int J Behav Nutr Phys Act. 2012;9:19. doi: 10.1186/1479-5868-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005 Jun;20(6):531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller S, Khensani N, Beech B. Perceptions of Physical Activity and Motivational Interviewing Among Rural African-American Women with Type 2 Diabetes. Women’s health issues. 2009 Nov 30;:1–7. doi: 10.1016/j.whi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller ST, Beech BM. Rural healthcare providers question the practicality of motivational interviewing and report varied physical activity counseling experience. Patient Education and Counseling. 2009;76(2):279–282. doi: 10.1016/j.pec.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruffin M, Fetters M, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: Results of a randomized controlled trial. Preventive Medicine. 2007;45(4):267–273. doi: 10.1016/j.ypmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Misra S, Lairson DR, Chan W, et al. Cost effectiveness of interventions to promote screening for colorectal cancer: a randomized trial. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2011 May;44(3):101–110. doi: 10.3961/jpmph.2011.44.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]