Abstract
Background:
Globalization of clinical trials fosters inclusion of higher and lower income countries, but the influence of enrolling country income level on heart failure (HF) trial performance is unclear. This study sought to evaluate associations between enrolling country income level, acute HF (AHF) patient profile, protocol completion, and trial endpoints.
Methods and Results:
The ASCEND-HF trial included 7,141 AHF patients from 30 countries. Country income data in gross national income per capita in current US dollars from the year 2007 (i.e., the year trial enrollment began) were abstracted from the World Bank. Patients were grouped by enrolling country income level (i.e., high [>$11,455], upper-middle [$3,706-$11,455], lower-middle [$936-$3,705], and low [<$936]). Income data were available for 29 (97%) countries (N=7,064). There were 3996 (57%), 1518 (21%), and 1550 (22%) patients from high, upper-middle, and lower-middle income countries, respectively. There were no patients from low income countries. Patients from lower-middle income countries tended to be younger with fewer comorbidities and lower utilization of guideline-directed therapies. Rates of adverse events (13.8%) and protocol non-completion (4.9%) during 180-day follow-up were highest among high income countries (all p<0.01). After adjustment for race, geographic region, medications, and clinical characteristics, compared to enrollment from lower-middle income countries, enrollment from higher income countries was associated with increased 30-day mortality or rehospitalization (high income, OR 1.70, 95% CI 1.02–2.85; upper-middle, OR 2.16, 95% CI 1.23–3.81), driven by higher rates of rehospitalization. Mortality was similar at 30 and 180 days. The association between higher country income and the 30-day composite endpoint was similar across geographic regions, with exception of Latin America (p for interaction=0.03).
Conclusions:
In this global AHF trial, patients from higher income countries had lower rates of protocol completion, higher rates of adverse events, and similar mortality rates. After adjustment for race, geographic region, and clinical factors, enrollment from a higher income country was associated with worse clinical outcomes, driven by higher rates of rehospitalization. Variation in enrolling country income level may influence study endpoints and trial performance independent of geographic region.
Keywords: income, country, heart failure, clinical trial
INTRODUCTION
Heart failure (HF) is a global public health problem with an estimated prevalence of 38 million worldwide.1 Clinical outcomes for many of these patients remain poor and similar to many cancers, justifying the continued need for ongoing development of drug and device therapies.2 To successfully execute the large outcome studies necessary to definitively test such treatments, phase III HF trials have experienced a rapid trend towards globalization in recent years.3–5 Although inclusion of multiple global populations fosters inclusive and collaborative research, recent globalization trends may be mostly driven by study execution considerations and the need to efficiently enroll very large patient populations in a reasonable time frame.5–7
Despite uniform selection criteria, existing trial data strongly suggest that patients hospitalized for HF vary markedly by geographic region of enrollment.8–12 The precise mechanism(s) by which region influences patient profile and event rates is unknown, but it is plausible that country income status may be an important mediator of these differences. Consistent with movement towards trial globalization, recent HF studies have consistently included patients from both high income and lower income countries. On one hand, less developed areas may be appealing from a cost and regulatory standpoint and often provide higher enrollment rates than centers from high income countries.13 However, these benefits towards enrollment may be balanced against potential quality control issues.14 To our knowledge, the question of whether country income status influences patient characteristics, downstream patient protocol completion rates, and HF trial endpoints has not been specifically studied. Likewise, the influence of country income status independent of geographic region is unclear. In this context, the global ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) database provides an opportunity to better understand these relationships and rigorously characterize the correlation between enrolling country income status, patient characteristics, protocol completion, and trial endpoints.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
The design and primary results of the ASCEND-HF trial have been published previously.15, 16 Briefly, ASCEND-HF was a prospective, multinational, randomized, placebo-controlled trial studying the effects of nesiritide on dyspnea relief and clinical outcomes among patients hospitalized for acute HF (AHF) with either reduced or preserved ejection fraction. Eligible patients were enrolled within 24 hours of first intravenous HF therapy, had dyspnea at rest or with minimal exertion, ≥1 clinical sign of HF, and ≥1 objective measure of HF. The trial was conducted in accordance with the Declaration of Helsinki and with institutional review board/ ethics committee approval at all sites. All patients provided written informed consent.
Geographic Region and Country Income Level
The ASCEND-HF trial enrolled 7,141 patients at 398 sites across 30 countries in North America, Latin America, Western Europe, Central Europe and Asia-Pacific between May 2007 and August 2010. Per trial protocol, geographic regions were defined as follows: North America – Canada, United States; Latin America – Argentina, Brazil, Chile, Colombia, Mexico; Western Europe – France, Germany, Greece, Israel, Italy, Netherlands, Norway; Sweden; Central Europe – Bulgaria, Lithuania, Poland, Romania, Russia, Ukraine; Asia-Pacific – Australia, China, India, Malaysia, New Zealand, Republic of Korea, Singapore, Taiwan, Thailand.
Country income data in gross national income per capita in current US dollars and classifications from the year 2007 (i.e., the year ASCEND-HF trial enrollment began) were abstracted from the World Bank.17 As defined by the World Bank using data from 2007, countries were assigned to 1 of 4 country income categories as defined by the World Bank: high income (>$11,455), upper-middle income ($3,706-$11,455), lower-middle income ($936-$3,705), and low income (<$936).18 Country income data were available for all countries in ASCEND-HF, with exception of Taiwan which was not listed as a separate country. Thus, the current analysis included data from the 29 remaining countries in the trial (N=7,064). Acknowledging existence of other grading metrics for social and economic development of a country, World Bank income classifications were cross-referenced with classifications per the United Nations Development Programme’s 2007 Health Development Index (HDI).19 Among enrolling countries in ASCEND-HF, application of HDI classification resulted in identical country groupings as compared to the World Bank classification, thus dedicated analysis by HDI status was not performed.
Study Endpoints and Definitions
Pre-specified endpoints for the present study included (i) 180-day all-cause death, (ii) composite 30-day all-cause death or HF hospitalization, (iii) composite 30-day all-cause death or all-cause rehospitalization, and (iv) persistent dyspnea (i.e., no dyspnea improvement) at 6 and 24 hours. Causes of death and hospitalization within 30 days were adjudicated by an independent blinded clinical events committee (University of Glasgow, Glasgow, Scotland, UK). To evaluate trial site performance by country income level, protocol non-completion rate at 180 days was an additional endpoint. Consistent with a prior analysis, protocol non-completion was defined as early withdrawal from the study due to withdrawal of consent, lost to follow-up, or protocol deviation.20 Adverse event rate at 180 days was reported separately. Persistent dyspnea was defined as a binary endpoint using a patient-reported 7-point categorical Likert scale. A response of markedly worse, moderately worse, minimally worse, no change, or minimally better was defined as persistent dyspnea. Definitions for hospitalization for HF and protocol deviation are provided in Supplementary Appendix A.
Statistical Analysis
Baseline characteristics were compared between country income level groups. Continuous variables were reported as median (25th percentile, 75th percentile) and compared using Kruskal-Wallis tests. Categorical variables were presented as frequencies and percentages, and compared using the Pearson Chi-square test or the Fisher’s exact test. Kaplan-Meier event rates for 180-day all-cause death and raw event rates for other endpoints were reported across groups.
Country income level was pre-specified to be examined as both a categorical variable by country income level group and a continuous variable per US $10,000 increase. Unadjusted and adjusted logistic regression models were constructed to evaluate associations between country income level and 30-day endpoints, persistent dyspnea, and protocol non-completion. Unadjusted and adjusted Cox models were used to evaluate associations with 180-day all-cause death. To account for potential clustering of patients within countries, multilevel models were used for the primary analyses. Specifically, models utilized random-intercept multilevel logistic regressions (generalized linear mixed models) and shared frailty Cox regression, each using random effects for country of enrollment. To better discern the influence of geographic region on associations between country income level and study endpoints, a 3-tiered approach was applied to adjusted models. The base model included adjustment for age, sex, and race. The second model included base model variables plus geographic region. The third model (i.e., full model) included variables from the first two models plus 13 additional clinical covariates previously utilized in ASCEND-HF mortality and dyspnea models.20–22 Due to lack of established clinical confounders for protocol non-completion, the third tier of adjustment was not performed for this endpoint. The proportional hazards assumption was tested for the Cox model and linearity assumptions were tested in all models and no violations were found. In addition, interaction testing was pre-specified to examine (i) potential differential associations between country income level and outcomes across geographic regions and (ii) potential differential efficacy of nesiritide by country income level. Multiple imputation was used for missing covariate data; results reflect the combined analysis of 25 imputed datasets. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Two-tailed p<0.05 was considered statistically significant.
RESULTS
Distribution of Country Income Level
Of the 29 countries with available income data, 14 (48%) were high income, 11 were upper-middle income (38%), and 4 (14%) were lower-middle income. These groups comprised 3996, 1518, and 1550 patients, respectively (Figure 1). There were no low income countries. The proportion of countries and patients from each geographic region differed by country income group (Figure 2). For example, North American and Western European countries were exclusively high-income and Latin American countries were exclusively upper-middle income.
Figure 1.
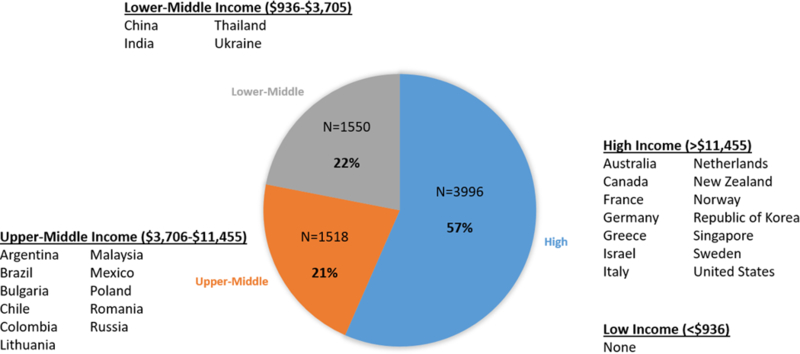
Distribution of enrolling country income level in the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) trial.
Figure 2.
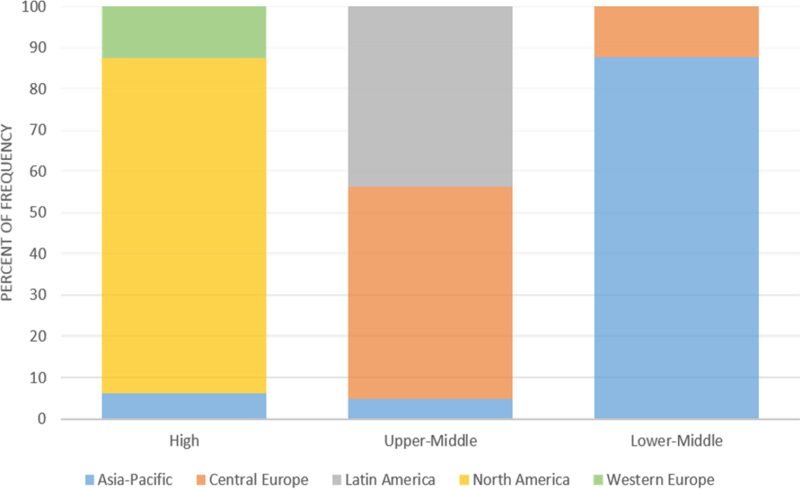
The distribution of patients by geographic region within each country income level category.
Patient Characteristics
Baseline patient characteristics differed by income level category (Table 1). Patients from lower-middle income countries tended to be younger with lower systolic blood pressure and body mass index. These patients were more likely to have reduced ejection fraction (EF) and New York Heart Association class III/IV symptoms prior to decompensation, but less likely to have prior HF hospitalization and many comorbidities such as prior myocardial infarction, coronary revascularization, cerebrovascular disease, atrial fibrillation/flutter, and chronic lung disease. Despite having the highest proportion patients with reduced EF, patients from lower-middle income countries were substantially less likely to receive many guideline-directed medical and device based therapies for HF with reduced EF, including angiotensin-converting enzyme inhibitors/ angiotensin II receptor blockers, beta-blockers, and implantable cardioverter-defibrillators (ICD). For instance, 28% of patients from high income countries had an ICD at baseline compared to <1% among lower-middle income patients.
Table 1.
Baseline Characteristics by Country Income Level Category
| Country Income Level | |||||
|---|---|---|---|---|---|
| Overall (N=7064) |
High >$11,455 (N=3996) |
Upper-Middle $3,706-$11,455 (N=1518) |
Lower-Middle $936-$3,705 (N=1550) |
P value | |
| Age (years) | 67 (56–76) | 69 (57–78) | 69 (59–76) | 61 (52–70) | <0.001 |
| Female | 2412 (34.1) | 1401 (35.1) | 531 (35.0) | 480 (31.0) | 0.012 |
| Race | <0.001 | ||||
| White | 3989 (56.5) | 2632 (65.9) | 1167 (76.9) | 190 (12.3) | |
| Black | 1077 (15.2) | 1043 (26.1) | 34 (2.2) | 0 (0.0) | |
| Asian | 1690 (23.9) | 255 (6.4) | 76 (5.0) | 1359 (87.7) | |
| Other | 307 (4.3) | 65 (1.6) | 241 (15.9) | 1 (0.1) | |
| Region | <0.001 | ||||
| Asia-Pacific | 1685 (23.9) | 249 (6.2) | 75 (4.9) | 1361 (87.8) | |
| Central Europe | 967 (13.7) | 0 (0.0) | 778 (51.3) | 189 (12.2) | |
| Latin America | 665 (9.4) | 0 (0.0) | 665 (43.8) | 0 (0.0) | |
| North America | 3243 (45.9) | 3243 (81.2) | 0 (0.0) | 0 (0.0) | |
| Western Europe | 504 (7.1) | 504 (12.6) | 0 (0.0) | 0 (0.0) | |
| Ejection fraction (%) * | 30 (20–37) | 28 (20–40) | 30 (25–38) | 28 (22–34) | <0.001 |
| Ejection fraction ≥50% * | 648 (9.2) | 530 (13.3) | 87 (5.7) | 31 (2.0) | <0.001 |
| Ischemic HF etiology | 4256 (60.3) | 2411 (60.3) | 866 (57.1) | 979 (63.2) | 0.003 |
| NYHA class † | <0.001 | ||||
| I | 255 (4.4) | 131 (4.3) | 105 (7.8) | 19 (1.3) | |
| II | 1093 (18.8) | 599 (19.8) | 356 (26.3) | 138 (9.6) | |
| III | 2827 (48.6) | 1581 (52.4) | 619 (45.8) | 627 (43.4) | |
| IV | 1642 (28.2) | 709 (23.5) | 273 (20.2) | 660 (45.7) | |
| Vital sign and laboratory findings | |||||
| Systolic blood pressure (mmHg) | 123 (110–140) | 124 (110–140) | 125 (110–140) | 120 (110–135) | <0.001 |
| Diastolic blood pressure (mmHg) | 74 (66–83) | 72 (64–82) | 80 (70–84) | 80 (70–85) | <0.001 |
| Heart rate (bpm) | 82 (72–95) | 80 (70–92) | 82 (72–96) | 88 (78–99) | <0.001 |
| BMI (kg/m2) | 28 (24–33) | 29 (25–35) | 28 (25–32) | 24 (21–27) | <0.001 |
| Hemoglobin (g/dL) ‡ | 12.7 (11.3–14.0) | 12.4 (11.1–13.8) | 13.4 (12.2–14.7) | 12.7 (11.4–14.0) | <0.001 |
| Serum sodium (mmol/L) ‡ | 139 (136–141) | 139 (137–141) | 139 (136–142) | 137 (133–140) | <0.001 |
| BUN (mg/dL) ‡ | 25 (18–39) | 24 (17–36) | 31 (20–47) | 25 (18–37) | <0.001 |
| Creatinine (mg/dL) ‡ | 1.2 (1.0–1.6) | 1.3 (1.0–1.7) | 1.2 (1.0–1.5) | 1.2 (1.0–1.4) | <0.001 |
| NT-proBNP (pg/mL) § | 4492 (2088– 9160) |
4613 (2234– 9202) |
4458 (1978– 8980) |
4453 (2047– 9217) |
0.211 |
| BNP (pg/mL) § | 989 (544–1838) | 1027 (600–1900) | 1570 (737–2984) | 415 (298–553) | <0.001 |
| Signs and symptoms | |||||
| Dyspnea | <0.001 | ||||
| At rest | 4365 (61.8) | 2328 (58.3) | 1044 (68.8) | 993 (64.1) | |
| With minimal activity | 2698 (38.2) | 1667 (41.7) | 474 (31.2) | 557 (35.9) | |
| Orthopnea | 5423 (76.9) | 3189 (79.9) | 1219 (80.3) | 1015 (65.7) | <0.001 |
| Pulmonary Rales | 6126 (86.8) | 3310 (82.9) | 1427 (94.0) | 1389 (89.7) | <0.001 |
| Peripheral edema | 5268 (74.6) | 3163 (79.2) | 1204 (79.3) | 901 (58.1) | <0.001 |
| Jugular venous distention | 3950 (56.0) | 2382 (59.7) | 811 (53.4) | 757 (48.8) | <0.001 |
| Past medical history | |||||
| HF hospitalization within past year | 2755 (39.0) | 1726 (43.2) | 579 (38.2) | 450 (29.1) | <0.001 |
| Previous PCI | 1152 (16.3) | 863 (21.6) | 153 (10.1) | 136 (8.8) | <0.001 |
| Previous CABG | 1305 (18.5) | 1054 (26.4) | 145 (9.6) | 106 (6.8) | <0.001 |
| Previous myocardial infarction | 2475 (35.0) | 1406 (35.2) | 541 (35.6) | 528 (34.1) | 0.628 |
| Cerebrovascular disease/ stroke | 831 (11.8) | 616 (15.4) | 147 (9.7) | 68 (4.4) | <0.001 |
| Hypertension | 5093 (72.1) | 3115 (78.0) | 1151 (75.8) | 827 (53.4) | <0.001 |
| Atrial fibrillation/ flutter | 2650 (37.5) | 1701 (42.6) | 686 (45.2) | 263 (17.0) | <0.001 |
| Diabetes | 3012 (42.6) | 1907 (47.7) | 505 (33.3) | 600 (38.7) | <0.001 |
| Chronic lung disease | 1164 (16.5) | 934 (23.4) | 155 (10.2) | 75 (4.8) | <0.001 |
| Baseline therapies | |||||
| Loop diuretic | 6715 (95.1) | 3894 (97.5) | 1415 (93.2) | 1406 (90.7) | <0.001 |
| Beta-blockers | 4125 (58.4) | 2792 (69.9) | 806 (53.1) | 527 (34.0) | <0.001 |
| ACEI/ ARB | 4292 (60.8) | 2494 (62.4) | 1010 (66.5) | 788 (50.8) | <0.001 |
| MRA | 1981 (28.0) | 930 (23.3) | 580 (38.2) | 471 (30.4) | <0.001 |
| Digoxin | 1879 (26.6) | 913 (22.9) | 422 (27.8) | 544 (35.1) | <0.001 |
| ICD | 1163 (16.5) | 1105 (27.7) | 52 (3.4) | 6 (0.4) | <0.001 |
| CRT | 640 (9.1) | 608 (15.2) | 28 (1.8) | 4 (0.3) | <0.001 |
Data expressed as n (%) or median (25th–75th)
Overall, 5,401 patients had precise ejection fraction measurement data. All 7,064 patients had data for ejection fraction ≥ or <50%.
5,817 patients had data available for New York Heart Association class
There were 6,519, 6,608, 6,522, 6,683 total patients with available hemoglobin, sodium, BUN, and creatinine data, respectively
There were 3,761 and 989 patients with available NT-proBNP and BNP data, respectively
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter-defibriilator; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Protocol Completion and Adverse Events
Rates of protocol non-completion and adverse events at 180 days are displayed in Table 2. Protocol non-completion (4.9%) was most common among high income countries (p<0.001). Adverse event rates increased with higher country income level, with the highest rate (13.8%) among high income countries and lowest rate (3.8%) among lower-middle income countries (p<0.001). After adjustment for demographics and geographic region, categorical analysis with lower-middle income as the referent group suggested >2-fold greater risk of protocol non-completion among high income countries (p=0.033) and similar risk between upper-middle and lower-middle income countries (p=0.782) (Table 3). Increasing continuous country income level was associated with increased likelihood of protocol non-completion at 180 days after adjustment for demographics, but not after adjustment for geographic region.
Table 2.
Protocol Non-completion and Adverse Event Rates at 180 days by Country Income Level Category
| Country Income Level | |||||
|---|---|---|---|---|---|
| Event | Overall (N=7064) |
High >$11,455 (N=3996) |
Upper-Middle $3,706-$11,455 (N=1518) |
Lower-Middle $936-$3,705 (N=1550) |
P value† |
| Protocol non-completion* | 292 (4.1) | 196 (4.9) | 47 (3.1) | 49 (3.2) | <0.001 |
| Withdrawal of consent | 40 (0.6) | 27 (0.7) | 6 (0.4) | 7 (0.5) | 0.368 |
| Lost to follow-up | 122 (1.7) | 60 (1.5) | 30 (2.0) | 32 (2.1) | 0.247 |
| Protocol deviation | 144 (2.0) | 120 (3.0) | 12 (0.8) | 12 (0.8) | <0.001 |
| Adverse event | 744 (10.5) | 553 (13.8) | 132 (8.7) | 59 (3.8) | <0.001 |
Data are expressed as n (%).
Patients may not complete the protocol for multiple reasons and the number of patients with protocol non-completion may not necessarily equal the sum of patients meeting each component.
P values derived from the Pearson chi-square test
Table 3.
Association Between Country Income Level and Protocol Non-completion (referent group = lower-middle income)
| Odds ratio (95% confidence interval) | |||
|---|---|---|---|
| Adjusted Models | |||
| Unadjusted | Base* | Base + Region† | |
| High | 2.26 (0.96–5.34), p=0.063 | 2.69 (1.12–6.46), p=0.026 | 2.82 (1.09–7.31), p=0.033 |
| Upper-middle | 0.82 (0.33–2.04), p=0.676 | 0.97 (0.39–2.43), p=0.949 | 0.86 (0.29–2.51), p=0.782 |
| Per $10,000 increase | 1.19 (1.00–1.41), p=0.050 | 1.22 (1.02–1.45), p=0.026 | 1.06 (0.82–1.37), p=0.650 |
Adjusted for age, sex, and race.
Adjusted for age, sex, race, and geographic region.
Trial Endpoints
Event rates by country income level group are displayed in Table 4. With exception of 30-day all-cause death which was comparable across groups, high income countries and lower-middle income countries had the highest and lowest rates of study endpoints, respectively. Event rates showed a stepwise increase with higher country income group. Adjudicated causes of death through 30 days are presented in Supplementary Table 1.
Table 4.
Event Rates by Country Income Level
| Total N | Events | Event rate, % (95% confidence interval)* |
|
|---|---|---|---|
| 180-day all-cause death | |||
| High, >$11,455 | 3996 | 531 | 13.4 (12.4–14.5) |
| Upper-Middle, $3,706-$11,455 | 1518 | 196 | 13.0 (11.4–14.8) |
| Lower-Middle, $936-$3,705 | 1550 | 164 | 10.7 (9.2–12.3) |
| Total | 7064 | 891 | 12.7 (12.0–13.5) |
| 30-day all-cause death or hospitalization for heart failure | |||
| High, >$11,455 | 3996 | 462 | 11.6 (10.6–12.6) |
| Upper-Middle, $3,706-$11,455 | 1518 | 123 | 8.1 (6.7–9.5) |
| Lower-Middle, $936-$3,705 | 1550 | 90 | 5.8 (4.6–7.0) |
| Total | 7064 | 675 | 9.6 (8.9–10.2) |
| 30-day all-cause death | |||
| High, >$11,455 | 3996 | 139 | 3.5 (2.9–4.0) |
| Upper-Middle, $3,706-$11,455 | 1518 | 70 | 4.6 (3.6–5.7) |
| Lower-Middle, $936-$3,705 | 1550 | 61 | 3.9 (3.0–4.9) |
| Total | 7064 | 270 | 3.8 (3.4–4.3) |
| 30-day hospitalization for heart failure | |||
| High, >$11,455 | 3996 | 331 | 8.3 (7.4–9.1) |
| Upper-Middle, $3,706-$11,455 | 1518 | 55 | 3.6 (2.7–4.6) |
| Lower-Middle, $936-$3,705 | 1550 | 31 | 2.0 (1.3–2.7) |
| Total | 7064 | 417 | 5.9 (5.4–6.5) |
| 30-day all-cause hospitalization | |||
| High, >$11,455 | 3996 | 636 | 15.9 (14.8–17.1) |
| Upper-Middle, $3,706-$11,455 | 1518 | 108 | 7.1 (5.8–8.4) |
| Lower-Middle, $936-$3,705 | 1550 | 65 | 4.2 (3.2–5.2) |
| Total | 7064 | 809 | 11.5 (11.7–12.2) |
Event rate for 180-day all-cause mortality represents a Kaplan-Meier event rate; all other event rates represent raw event rates.
Unadjusted and adjusted outcome analyses for trial endpoints are presented in Table 5. Compared to patients from lower-middle income countries, patients from higher income countries carried ~1.5–2-fold higher risk of the composite of 30-day death or all-cause hospitalization. These results persisted after progressive multivariable adjustment for demographics, geographic region, and the full adjustment model (full model – all p ≤0.042). However, the magnitude of associations changed with stepwise adjustment, where patients from high income countries carried highest risk after base adjustment and upper-middle income patients carried highest risk after adjustment for geographic region and clinical covariates. In continuous variable analysis, every $10,000 increase in country income level was associated with a 10% greater risk of 30-day all-cause death or all-cause hospitalization (p=0.034). The magnitude of this association remained stable after full multivariable adjustment, but was reduced to a statistical trend (p=0.076).
Table 5.
Associations Between Country Income Level and Trial Endpoints (referent group = lower-middle income)
| Hazard ratio or odds ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Adjusted Models | ||||
| Endpoint | Unadjusted | Base* | Base + Region† | Base + Region + Full‡ |
| 180-day all-cause death | ||||
| High | 1.33 (0.87–2.03), p=0.190 | 1.24 (0.74–2.08), p=0.405 | 1.04 (0.54–2.02), p=0.906 | 1.13 (0.61–2.10), p=0.702 |
| Upper-middle | 1.30 (0.85–2.00), p=0.224 | 1.32 (0.79–2.19), p=0.292 | 1.24 (0.64–2.39), p=0.522 | 1.13 (0.61–2.10), p=0.701 |
| Per $10,000 increase | 1.00 (0.92–1.09), p=0.974 | 0.98 (0.89–1.07), p=0.597 | 0.91 (0.78–1.06), p=0.231 | 1.00 (0.87–1.16), p=0.965 |
| 30-day all-cause death or hospitalization for heart failure | ||||
| High | 1.49 (0.85–2.59), p=0.164 | 1.53 (0.84–2.80), p=0.164 | 1.23 (0.74–2.05), p=0.416 | 1.41 (0.74–2.66), p=0.294 |
| Upper-middle | 1.46 (0.84–2.54), p=0.181 | 1.56 (0.86–2.82), p=0.142 | 1.95 (1.09–3.47), p=0.023 | 1.70 (0.87–3.33), p=0.122 |
| Per $10,000 increase | 1.01 (0.91–1.13), p=0.819 | 1.00 (0.89–1.13), p=0.947 | 0.97 (0.82–1.15), p=0.729 | 1.06 (0.90–1.26), p=0.473 |
| 30-day all-cause death or rehospitalization | ||||
| High | 1.98 (1.23–3.19), p=0.005 | 2.11 (1.25–3.58), p=0.005 | 1.54 (1.01–2.33), p=0.043 | 1.70 (1.02–2.85), p=0.042 |
| Upper-middle | 1.48 (0.91–2.39), p=0.112 | 1.61 (0.95–2.73), p=0.076 | 2.32 (1.42–3.79), p=0.001 | 2.16 (1.23–3.81), p=0.008 |
| Per $10,000 increase | 1.10 (1.01–1.21), p=0.034 | 1.10 (1.00–1.22), p=0.044 | 1.07 (0.94–1.23), p=0.307 | 1.13 (0.99–1.29), p=0.076 |
| Persistent dyspnea at 6 hours§ | ||||
| High | 1.04 (0.60–1.80), p=0.897 | 0.97 (0.55–1.72), p=0.929 | 1.16 (0.68–1.99), p=0.589 | 1.08 (0.62–1.89), p=0.790 |
| Upper-middle | 0.98 (0.56–1.72), p=0.957 | 0.93 (0.52–1.64), p=0.794 | 1.18 (0.69–2.00), p=0.552 | 1.13 (0.65–1.96), p=0.668 |
| Per $10,000 increase | 1.00 (0.91–1.10), p=0.986 | 0.99 (0.90–1.09), p=0.852 | 0.96 (0.85–1.09), p=0.527 | 0.99 (0.87–1.12), p=0.868 |
| Persistent dyspnea at 24 hours§ | ||||
| High | 1.37 (0.79–2.39), p=0.260 | 1.22 (0.69–2.15), p=0.490 | 1.57 (0.91–2.68), p=0.103 | 1.56 (0.88–2.76), p=0.126 |
| Upper-middle | 1.02 (0.58–1.79), p=0.937 | 0.92 (0.52–1.62), p=0.771 | 1.39 (0.83–2.34), p=0.215 | 1.39 (0.80–2.41), p=0.244 |
| Per $10,000 increase | 1.06 (0.96–1.17), p=0.238 | 1.05 (0.94–1.16), p=0.391 | 1.04 (0.91–1.19), p=0.549 | 1.07 (0.93–1.22), p=0.366 |
For 180-day all-cause death, data represent hazard ratios; for all other outcomes, data represent odds ratios.
Adjusted for age, sex, and race.
Adjusted for age, sex, race, and geographic region.
Adjusted for age, sex, race, geographic region, body mass index, prior heart failure hospitalization in past 1 year, systolic blood pressure, blood urea nitrogen, serum sodium, smoking status, presence of nocturnal dyspnea, presence of elevated jugular venous pulsation, cerebrovascular disease, history of depression, and background medications (angiotensin-converting enzyme inhibitor/ angiotensin II receptor blocker, beta-blocker, mineralocorticoid receptor antagonist).
Persistent dyspnea was defined as a binary outcome using a self-reported 7-point categorical Likert scale as any of the following responses: as markedly worse, moderately worse, minimally worse, no change, or minimally better.
With regards to other clinical and dyspnea endpoints, there were no statistically significant associations between country income level and outcomes in unadjusted or full adjusted models. Notably, risk of 180-day all-cause death was similar across the spectrum of country income level.
Country Income Level, Geographic Region, and Nesiritide Efficacy
The association between continuous country income level and the composite of 30-day death or all-cause hospitalization differed by geographic region (p for interaction=0.032) (Figure 3). Increasing country income level favored greater risk of the 30-day composite across all geographic regions, with exception of Latin America where increasing country income was strongly and independently associated with lower risk. In contrast, associations between country income level and 180-day all-cause death were consistent across geographic regions.
Figure 3. Risk of trial endpoints by country income level stratified by region of enrollment.
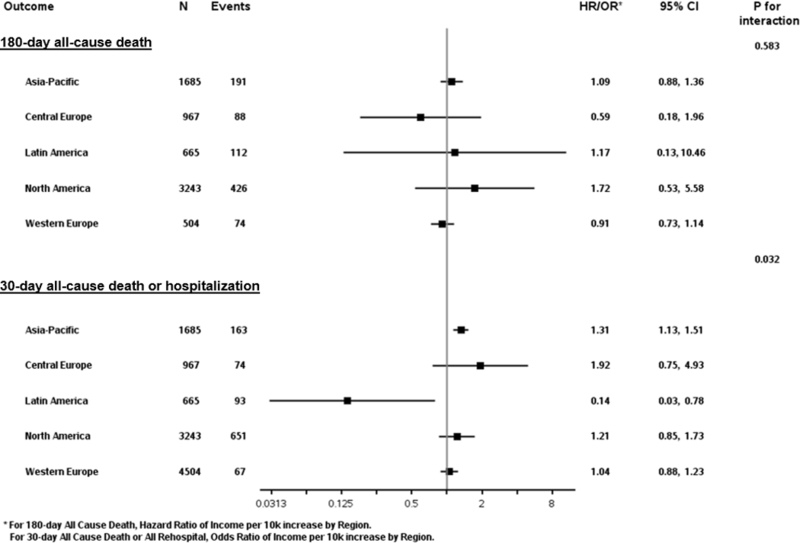
CI, confidence interval; HR, hazard ratio; OR, odds ratio.
The efficacy of nesiritide differed by country income level group (Supplementary Table 2). Specifically, as compared with placebo, nesiritide resulted in statistically significant reductions in 30-day death or HF hospitalization (p for interaction=0.049) and 30-day death or all-cause hospitalization (p for interaction=0.039) among patients in upper-middle income countries. The efficacy of nesiritide on 180-day all-cause death and dyspnea endpoints did not differ by country income level.
DISCUSSION
In this global AHF trial, the majority of patients were enrolled from high income countries, a significant minority of patients were enrolled from middle income countries, and no patients were enrolled from low income countries. Patient profile differed markedly by enrolling country income level with patients from higher income countries tending to have better baseline functional class, higher systolic blood pressure, more comorbidities, and higher uptake of guideline-recommended HF therapies. Adverse events and failure to complete the study protocol were most likely among high income countries. Although there was no association with 180-day all-cause death, patients from higher income countries carried greater risk of 30-day death or hospitalization, and this relationship persisted despite adjustment for race, geographic region, medication use, and other clinical characteristics. Associations between country income level and 30-day outcomes were similar across geographic regions, with exception of Latin America where country income level as associated with lower risk. Likewise, the ability of nesiritide to improve 30-day outcomes varied by country income level, with reductions in clinical endpoints among patients from upper-middle income countries.
To our knowledge, this is the first published analysis of enrolling country income level on AHF trial performance and patient outcomes. Prior studies from AHF clinical trials have centered on patient differences across broadly defined geographic regions without accounting for characteristics of individual countries.9–12 Likewise, although such analyses have consistently found patient profile and event rates to vary widely by global region, they have not investigated specific factors that may mediate such differences. Recognizing that cultural and economic heterogeneity within geographic regions is well established and unaccounted for with simple categorization by region, the present ASCEND-HF analysis offers several strengths that provide granular insight into the role of country income in AHF studies. First, acknowledging that multiple classification schemes for social and economic development exist, World Bank data were cross-referenced with the United Nations Development Programme’s HDI, confirming that country groupings by both criteria were identical.17, 19 Second, the statistical approach included both categorical and continuous variable outcome analysis to explore the impact of the income cutpoints defined by the World Bank. Third, analyses included a diverse set of endpoints, including post-discharge clinical outcomes, patient-reported dyspnea outcomes, and a trial performance outcome defined as protocol non-completion. Simultaneous assessment of these endpoints within a single population was done to broadly capture the patient experience by country income level, while also allowing assessment of potential interplay and tradeoffs between patient profile, event rates, and study execution. Lastly, acknowledging that race, geographic region, and country income status may overlap, a three-tiered stepwise multivariable adjustment strategy was pre-specified to explore the independent association with country income. The robust and independent association between country income category and 30-day death or hospitalization persisted after adjustment for race in the base model, additional adjustment for geographic region in the second model, and further adjustment for clinical factors in the third model. In contrast to existing ASCEND-HF data where 30-day and 180-day mortality were shown to differ by geographic region of enrollment, no independent associations with mortality were seen here.12 Thus, in aggregate, these collective data from ASCEND-HF strongly suggest that geographic region is not a dependable surrogate for country income status. Rather, country income level may vary within geographic regions and may independently predict clinical trial outcomes.
Prior work has speculated on mechanisms by which geographic region may drive patient heterogeneity within global AHF trials.8, 23 Potential domains of variation underlying regional differences include patient factors (e.g., heart failure pathophysiology/genetics, race/ethnicity, comorbidities), trial site factors (e.g., interpretation of trial selection criteria, data collection and accuracy, protocol adherence), healthcare practice factors (e.g., utilization of guideline therapies, physician and patient thresholds for hospital admission/discharge decisions), and healthcare environment factors (e.g., physician/hospital reimbursement incentives, patient access to healthcare, socioeconomics, medico-legal liability climate).8, 11 It is notable that while biologic differences in HF across the world have been reported, differences in patient profile and outcomes may be largely mediated by “non-biologic” inconsistencies in the HF diagnosis, ways in which data are recorded, and ways in which HF care is delivered.24, 25 In the current study, it is notable that associations between country income status and 30-day death or hospitalization were strongly driven by wide variation in rates of rehospitalization. In contrast, risks of 30-day and 180-day mortality were remarkably similar. Collectively, these data generate the hypothesis that preferential access to and use of hospitalization as a care strategy for AHF and other comorbid conditions among higher income countries, rather than disease severity, are predominant drivers of differential rates of hospitalization. Similarly, rates of prior HF hospitalization increased progressively with higher country income group. Although index hospitalization data for presenting HF signs and symptoms are mixed, the combination of higher rates of prior and subsequent hospitalization with similar downstream risk of death could be compatible with increased availability of hospital resources and/or lower thresholds for hospitalization among higher income countries. Alternatively, we acknowledge the possibility of hospitalization under-reporting among lower income countries, but have no objective evidence to support this.
Clinical Trial Implications
Although reflective of the global HF burden and the universal need for improved therapy, the potential adverse consequences of HF trial globalization are becoming increasingly recognized. Rather than a primary intent for inclusive and collaborative research, globalization may be largely driven by relative inability to conduct large trials in high income countries where enrollment is consistently slower and the regulatory environment generally more bureaucratic and expensive.7 Ideally, clinical research should be conducted in populations in proportion to their potential use after therapy approval.5, 6 Unfortunately, although sponsors may find lower income countries appealing from a regulatory and cost perspective, these countries may be paradoxically more likely to find newly approved drugs prohibitively expensive and routine uptake may occur only after years of use in wealthier countries.5, 6
The results of the current study from ASCEND-HF confirm that effects of trial globalization extend beyond geographic location to the economic development of the individual country. The present results highlight country income level as an additional dimension of variation within trials that may influence endpoints. Aside from implications for study power calculations, the potential differential efficacy of nesiritide on 30-day outcomes is consistent with prior examples suggesting the proportion of patients from specific countries may impact final results.26 Thus, in addition to ethical questions regarding inclusion of patients from lower income countries, globalization has the potential to be problematic for regulators, third-party payers, guideline writers, and practicing clinicians who must judge whether results from global trials are generalizable to their respective populations.8 Moreover, although the possibility of underreporting among lower income countries must be acknowledged, the present findings suggesting higher rates of protocol non-completion and adverse events among high income countries may speak to the relative inefficiency of the clinical trial enterprise in wealthier countries. The combination of slower enrollment, higher costs, and lower likelihood of completing the study protocol confirms the complex challenges facing clinical trials in high income countries and the persistent need for major improvements. Although the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) trial suggested quality control issues may be more apparent outside of high income countries, the protocol completion and adverse event data presented here suggest that a unique set of trial performance concerns may apply to high income countries.14, 26 Nonetheless, the collective findings from ASCEND-HF and TOPCAT suggest that attention towards trial conduct may be better directed towards characteristics of the individual country, rather than the broader geographic region. The best approach for anticipating site-specific and country-specific challenges regarding study execution and endpoints is unclear, but a pre-trial registry has been proposed and may carry advantages.27
Limitations
First, although rigorous multivariate modeling with pre-specified covariates was performed, the possibility of residual confounding exists and the retrospective observational nature of this study prohibits definitive determination of cause-effect relationships. Second, although inconsistent with the aims of this hypothesis generating work, adjustment for multiple comparisons may have modified results. Third, the distribution of countries within ASCEND-HF included no low income countries, thus conclusions of the current analysis may not extend beyond high and middle income countries. Fourth, although objective World Bank data were cross-referenced with HDI data for categorizing countries, both may be imperfect surrogates for social and economic development and it is possible that such characteristics could vary by study sites within individual countries (e.g., rural versus urban settings).
CONCLUSIONS
In this global AHF trial, the majority of patients were enrolled from high income countries, a significant minority of patients were from upper-middle and lower-middle income countries, and no patients were from low income countries. Patient characteristics and uptake of guideline-directed therapies varied by enrolling country income level. Patients from higher income countries had lower rates of protocol completion, higher rates of adverse events, and worse 30-day outcomes that persisted after adjustment for race, geographic region, and clinical factors. Mortality rates were similar and differences in clinical outcomes were driven by increased rates of rehospitalization in higher income countries. Variation in enrolling country income level is a challenge to be considered in global HF trials that may influence study endpoints and trial performance independent of geographic region.
Supplementary Material
WHAT IS KNOWN
Prior heart failure clinical trials have shown that patient profile and clinical outcomes vary markedly with the geographic region of patient enrollment.
Consistent with trends towards globalization of clinical trials, recent heart failure trials have included patients from both high income and lower income countries.
WHAT THIS STUDY ADDS
This is the first study to examine the influence of enrolling country income level on heart failure clinical trial endpoints, protocol completion, and adverse events.
Enrolling country income level may impact study endpoints and rates of protocol completion and adverse events, independent of geographic region.
This study suggests that design of future heart failure clinical trials should be mindful of the specific characteristics of individual enrolling countries, in addition to the broader geographic region.
Acknowledgments
Clinical Trial Registration – Clinicaltrials.gov identifier: NCT00475852
SOURCES OF FUNDING
Scios Inc. (Mountain View, CA, USA) provided financial and material support for the ASCEND-HF trial. Database management and statistical analysis was performed by the Duke Clinical Research Institute.
Dr. Greene is supported by National Institutes of Health grant 5T32HL069749–14 and a Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis, and has received research support from Amgen and Novartis. Dr. Hernandez reports consulting fees from AstraZeneca, Bayer, Boston Scientific, Merck, Novartis, Sanofi, and research support from AstraZeneca, GlaxoSmithKline, Luitpold, Merck, and Novartis. Dr. Butler has received research support from the National Institutes of Health, PCORI and the European Union; and serves as a consultant for Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmacautical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, and ZS Pharma. Dr. Ezekowitz reports consulting fees from Pfizer, Abbott Laboratories, and Servier; and research support from Amgen and Johnson & Johnson. Dr. Zannad has received grant funding from Novartis, BG Medicine, and Roche Diagnostics; served on a board for Boston Scientific; and served as a consultant for Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and ResMed. Dr. Metra has received consulting incomes from Bayer, Novartis, and Servier. Dr. Voors has received consultancy fees and/or research grants from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Merck/MSD, Novartis, Servier, and Vifor Pharma. Dr. O’Connor reports consulting for Bayer, Bristol Myers Squib, and Merck. Dr. Mentz receives research support from the National Institutes of Health (U01HL125511–01A1, U10HL110312 and R01AG045551–01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, Luitpold, Medtronic, Merck, Novartis, Otsuka, and ResMed; honoraria from Abbott, AstraZeneca, Bayer, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and ResMed; and has served on an advisory board for Amgen, Luitpold, Merck and Boehringer Ingelheim.
Footnotes
DISCLOSURES
All other authors report no conflicts.
Contributor Information
Stephen J Greene, From the Duke Clinical Research Institute, Durham, NC, USA, University School of Medicine, Durham, NC, USA.
Adrian F Hernandez, From the Duke Clinical Research Institute, Durham, NC, USA, University School of Medicine, Durham, NC, USA
Jie-Lena Sun, From the Duke Clinical Research Institute, Durham, NC, USA.
Javed Butler, Department of Medicine, University of Mississippi Medical Center, Jackson, MS, USA
Paul W Armstrong, Canadian VIGOUR Center, University of Alberta, Edmonton, Alberta, Canada.
Justin A Ezekowitz, Canadian VIGOUR Center, University of Alberta, Edmonton, Alberta, Canada
Faiez Zannad, Centre d’Investigation Clinique Plurithématique 1433, INSERM U1116, Université de Lorraine, CHRU de Nancy, Nancy, France
João P Ferreira, Centre d’Investigation Clinique Plurithématique 1433, INSERM U1116, Université de Lorraine, CHRU de Nancy, Nancy, France
Adrian Coles, From the Duke Clinical Research Institute, Durham, NC, USA.
Marco Metra, Cardiology, University of Brescia, Brescia, Italy.
Adriaan A Voors, University of Groningen, Groningen, Netherlands
Robert M Califf, From the Duke Clinical Research Institute, Durham, NC, USA, Division of Cardiology, Duke University School of Medicine, Durham, NC, USA.
Christopher M O’Connor, Inova Heart and Vascular Institute, Falls Church, VA, USA.
Robert J Mentz, From the Duke Clinical Research Institute, Durham, NC, USA, Division of Cardiology, Duke University School of Medicine, Durham, NC, USA.
REFERENCES
- 1.Braunwald E The war against heart failure: the Lancet lecture. Lancet 2015;385:812–24. [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Braunwald E, Gheorghiade M. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA 2014;312:789–90. [DOI] [PubMed] [Google Scholar]
- 3.Zannad F, Ferreira JP. Globalization of heart failure trials: no turning back on this paradigm. Eur Heart J 2016;37:3175–3177. [DOI] [PubMed] [Google Scholar]
- 4.Kim ES, Carrigan TP, Menon V. International participation in cardiovascular randomized controlled trials sponsored by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2011;58:671–6. [DOI] [PubMed] [Google Scholar]
- 5.Califf RM, Harrington RA. American industry and the U.S. Cardiovascular Clinical Research Enterprise an appropriate analogy? J Am Coll Cardiol 2011;58:677–80. [DOI] [PubMed] [Google Scholar]
- 6.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, Schulman KA. Ethical and scientific implications of the globalization of clinical research. N Engl J Med 2009;360:816–23. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Vaduganathan M, Greene SJ, Mentz RJ, Adams KF Jr., Anker SD, Arnold M, Baschiera F, Cleland JG, Cotter G, Fonarow GC, Giordano C, Metra M, Misselwitz F, Muhlhofer E, Nodari S, Frank Peacock W, Pieske BM, Sabbah HN, Sato N, Shah MR, Stockbridge NL, Teerlink JR, van Veldhuisen DJ, Zalewski A, Zannad F, Butler J. Site selection in global clinical trials in patients hospitalized for heart failure: perceived problems and potential solutions. Heart Fail Rev 2014;19:135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene SJ, Gheorghiade M. Same protocol, different continents, different patients: should we continue to conduct global heart failure trials? Eur J Heart Fail 2015;17:875–8. [DOI] [PubMed] [Google Scholar]
- 9.Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC Jr., Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol 2008;52:1640–8. [DOI] [PubMed] [Google Scholar]
- 10.Mentz RJ, Cotter G, Cleland JG, Stevens SR, Chiswell K, Davison BA, Teerlink JR, Metra M, Voors AA, Grinfeld L, Ruda M, Mareev V, Lotan C, Bloomfield DM, Fiuzat M, Givertz MM, Ponikowski P, Massie BM, O’Connor CM. International differences in clinical characteristics, management, and outcomes in acute heart failure patients: better short-term outcomes in patients enrolled in Eastern Europe and Russia in the PROTECT trial. Eur J Heart Fail 2014;16:614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene SJ, Fonarow GC, Solomon SD, Subacius H, Maggioni AP, Bohm M, Lewis EF, Zannad F, Gheorghiade M. Global variation in clinical profile, management, and post-discharge outcomes among patients hospitalized for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail 2015;17:591–600. [DOI] [PubMed] [Google Scholar]
- 12.Metra M, Mentz RJ, Hernandez AF, Heizer GM, Armstrong PW, Clausell N, Corbalan R, Costanzo MR, Dickstein K, Dunlap ME, Ezekowitz JA, Howlett JG, Komajda M, Krum H, Lombardi C, Fonarow GC, McMurray JJ, Nieminen MS, Swedberg K, Voors AA, Starling RC, Teerlink JR, O’Connor CM. Geographic Differences in Patients in a Global Acute Heart Failure Clinical Trial (from the ASCEND-HF Trial). Am J Cardiol 2016;117:1771–8. [DOI] [PubMed] [Google Scholar]
- 13.Greene SJ, AlKhawam L, Ambrosy AP, Vaduganathan M, Mentz RJ. Hospitalized Heart Failure in the United States: Lessons Learned from Clinical Trial Populations. Heart Fail Clin 2015;11:591–601. [DOI] [PubMed] [Google Scholar]
- 14.de Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA and Rouleau JL. Spironolactone Metabolites in TOPCAT - New Insights into Regional Variation. N Engl J Med 2017;376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr., Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez AF, O’Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K, Califf RM. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF). Am Heart J 2009;157:271–7. [DOI] [PubMed] [Google Scholar]
- 17.GNI, Atlas method (current US$) The World Bank; Available at: https://data.worldbank.org/indicator/NY.GNP.ATLS.CD. Accessed September 24, 2016. [Google Scholar]
- 18.How does the World Bank classify countries? The World Bank; Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/378834-how-does-the-world-bank-classify-countries; Accessed September 24, 2016. [Google Scholar]
- 19.Human Development Report 2009. Overcoming barriers: human mobility and development. United Nations Development Programme Available at: http://hdr.undp.org/sites/default/files/reports/269/hdr_2009_en_complete.pdf; Accessed January 18, 2017. [Google Scholar]
- 20.Greene SJ, Hernandez AF, Sun JL, Metra M, Butler J, Ambrosy AP, Ezekowitz JA, Starling RC, Teerlink JR, Schulte PJ, Voors AA, Armstrong PW, O’Connor CM, Mentz RJ. Influence of Clinical Trial Site Enrollment on Patient Characteristics, Protocol Completion, and End Points: Insights From the ASCEND-HF Trial (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure). Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 21.Khazanie P, Heizer GM, Hasselblad V, Armstrong PW, Califf RM, Ezekowitz J, Dickstein K, Levy WC, McMurray JJ, Metra M, Tang WH, Teerlink JR, Voors AA, O’Connor CM, Hernandez AF, Starling R. Predictors of clinical outcomes in acute decompensated heart failure: Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure outcome models. Am Heart J 2015;170:290–7. [DOI] [PubMed] [Google Scholar]
- 22.Mentz RJ, Hernandez AF, Stebbins A, Ezekowitz JA, Felker GM, Heizer GM, Atar D, Teerlink JR, Califf RM, Massie BM, Hasselblad V, Starling RC, O’Connor CM, Ponikowski P. Predictors of early dyspnoea relief in acute heart failure and the association with 30-day outcomes: findings from ASCEND-HF. Eur J Heart Fail 2013;15:456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitt B, Gheorghiade M. Geographic variation in heart failure trials: time for scepticism? Eur J Heart Fail 2014;16:601–2. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–33. [DOI] [PubMed] [Google Scholar]
- 25.Mentz RJ, Roessig L, Greenberg BH, Sato N, Shinagawa K, Yeo D, Kwok BW, Reyes EB, Krum H, Pieske B, Greene SJ, Ambrosy AP, Kelly JP, Zannad F, Pitt B, Lam CS. Heart Failure Clinical Trials in East and Southeast Asia: Understanding the Importance and Defining the Next Steps. J Am Coll Cardiol HF 2016;4:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 27.Greene SJ, Shah AN, Butler J, Ambrosy AP, Anker SD, Chioncel O, Collins SP, Dinh W, Dunnmon PM, Fonarow GC, Lam CS, Mentz RJ, Pieske B, Roessig L, Rosano GM, Sato N, Vaduganathan M, Gheorghiade M. Designing effective drug and device development programs for hospitalized heart failure: a proposal for pretrial registries. Am Heart J 2014;168:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


