Abstract
Cystic fibrosis is caused by gene mutations that result in an abnormal Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein on the surface of cells. CFTR modulators are a novel class of drugs that directly target the molecular defect. CFTR modulators include potentiators that result in improved activity of the channel; correctors that help the protein traffic to the cell surface properly; and readthrough agents that restore full-length CFTR by suppression of premature termination codons, among other novel classes more recently established. While some of these drugs, CFTR potentiators in particular, have provided remarkable improvements for CF patients, others have yet to achieve profoundly improved outcomes, and many CF patients are not yet impacted by CFTR modulators due to lack of knowledge regarding susceptibility of their mutations to treatment. One limitation to expanding these types of therapies to the maximum number of patients with CF is the lack of rigorously validated clinical biomarkers that can determine efficacy on an individual basis, as well as few pre-clinical tools that can predict whether an individual with a rare combination of mutant alleles will respond to a particular CFTR modulator regimen. In this review, we discuss the various groups of CFTR modulators and their status in clinical development, as well as address the current literature on biomarkers, pre-clinical cellbased tools, and the role of pharmacometrics in creating therapeutic strategies to improve the lives of all patients with cystic fibrosis, regardless of their specific mutation.
Keywords: biomarkers, clinical trials, cystic fibrosis (CF), pharmacology
1 |. INTRODUCTION
The advent of CFTR modulator therapy for the treatment of cystic fibrosis has been among the seminal advances in medicine, providing a key example of precision therapeutics based on a strong foundation of molecular understanding and targeted therapy for the underlying disease. Nevertheless, presently highly efficacious CFTR modulator therapy is available for only a minority of CF individuals, and while patients homozygous for F508del have viable disease modifying CFTR modulator therapy, drug intolerance and more modest efficacy have limited widespread utilization.
In this review, we will summarize recent progress toward accomplishing the goal of highly effective CFTR modulator therapy for all, and discuss key scientific and development hurdles in which the CF community must focus to achieve the aspiration of mutation-specific therapy for as many as CF patients as possible. Reviews of therapies that repair mRNA expression, edit the genome, or insert CFTR by gene therapy are emerging rapidly and are reviewed elsewhere.1,2
2 |. CFTR POTENTIATORS
The archetype CFTR potentiator ivacaftor has demonstrated pronounced clinical benefit in patients with G551D CFTR and other closely related gating mutations, and represents the model for the potential for highly efficacious CFTR modulator therapy. Ivacaftor treatment caused a 10% improvement in FEV1% predicted (absolute change), and was associated with a 55% reduction in exacerbations and a 2.7 kg improvement in body weight.3 CFTR functional improvement was pronounced, as indicated by achieving a mean sweat chloride of 47.8 mmol/L, representing a change of −48.1 mmol/L. Ivacaftor restores between 30% and 50% of CFTR activity, based on in vitro findings with primary HBE cells.4 These clinical and in vitro data, recapitulated in independent post-approval observational studies, stand as an important benchmark for other programs in preclinical development.5,6 Indeed long-term follow up studies in this population have shown a persistent improvement in exacerbation frequency, and consequently the rate of pulmonary decline, which were reduced by nearly 50%, from −1.72% in the control group to −0.91% per year.7
Subsequently, ivacaftor has been shown to improve mucus clearance and GI pH, reduce Pseudomonas isolation, induce bronchodilation, and even reduce structural lung disease as observed by CT scan.5 A number of these improvements were unanticipated, and have revealed the long-term promise for CFTR modulation. For example, improvement in the degree of bacterial load and alter host defense strongly suggest the potential to impact features that will markedly impact future prognosis, and even potentially reverse microbial colonization and structural lung injury, in effect “turning back the clock” onthedisease.8–12 The cumulative results indicate that highly efficacious CFTR modulator therapy has far ranging beneficial effects, beyond immediate effects on readily accessible clinical outcome measures.
CFTR potentiator therapy has successfully expanded to other mutations with gating abnormalities.13 Results in eight related gating mutations were remarkably similar, with the exception of the effect of mutation G970R on sweat chloride, which has recently been proposed to also exhibit aberrant splicing, explaining the apparent discrepancy.14 As in patients with G551D, patients had a −49.2 mmol/L improvement in sweat chloride and a mean change in FEV1 of 10.7 percentage points. ThesefindingsareoverallsurprisinglysimilartopriorstudieswithG551D CF patients and providing early evidence that common outcomes can be expected based on the degree of CFTR modulation achieved.14
R117H is a more complex mutation, since it has both gating abnormalities and conductance defects, and is impacted by its association with the 5T intron variant that reduces surface expression.15 Because it is also associated with residual function, this represented a new treatment group for CFTR potentiator therapy. For patients with R117H CFTR, ivacaftor was shown to be beneficial, although the magnitude of effect is less (mean improvement 2.1 percentage points), and was generally restricted to patients with established airway disease, as opposed to young children with less evidence of disease, and a more frequent association with the mild 7T variant.16 In the GOAL post approval observational study, the beneficial effect of ivacaftor in the R117H population was evident on FEV1 and weight, indicating in appropriately selected patients, beneficial effect on lung function is observed (#190).17
The next challenges for CFTR potentiators will be further expanding the population proven to benefit. It is apparent from case reports that mutations with residual function may benefit from augmented channel gating with ivacaftor therapy, as shown for P67L.18 Splice mutations that do not induce canonical formation of aberrant transcripts (ie, non-cannonical) retain some residual wild type CFRT expression and responded to ivacaftor in a dose-redose shortterm study (#196).19 Recently, in a study evaluating ivacaftor alone and in combination with the corrector tezacaftor (VX-661) in patients with at least one residual function CFTR mutation and an F508del allele was efficacious, even when used as ivacaftor alone (improving FEV1% by 4.7% over 8 weeks), suggesting ivacaftor may be a viable option for the large majority of patients with a residual function allele, even in the absence of F508del. Based on these data, and strong in vitro to in vivo correlations with CFTR missense mutation expressing cell lines, the FDA recently extended the approval of ivacaftor to include both a series of 23 residual function mutations, and selected non-canonical splice mutations that exhibit residual CFTR function. Precision-based approaches on an individual level are also likely to be important as additional populations are considered (Section 7).
Other pharmaceutical approaches are under investigation to evaluate other CFTR potentiators that may be advantageous. One approach is to identify CFTR potentiators that do not destabilize CFTR localized to the cell surface, a finding particularly apparent for ivacaftor when combined with corrector therapy.20–22 Some potentiators in development have been reported to avoid this phenomenon, although the degree of decrement posed by this in vitro observation remains largely uncertain. Minor improvements in the PK of ivacaftor have also been shown by using deuterated derivatives of the same structure, and may be employed to conceive of once daily regimens. It may also be possible to augment CFTR activity when used with potentiators by activating cAMP mediated signaling, such as that induced by longacting beta adrenergic agonists (LABA) or phosphodiesterase (PDE) inhibitors such as roflumilast.23
3 |. CFTR CORRECTORS
CFTR correctors restore trafficking of mutant CFTR, enhancing cell surface expression and are principally directed to the common F508del CFTR mutation. When combined with potentiators, correctors have been shown to sufficiently rescue CFTR in human bronchial epithelial cells (HBE) to be in the range of that expected to provide clinical benefit (20–30% of CFTR activity).24,25 Although they exhibit bioactivity, neither ivacaftor nor lumacaftor are efficacious as single agent therapy for patients homozygous for F508del CFTR.26,27 However, combination therapy has been shown to be modestly beneficial, and led to the approval of lumacaftor/ivacaftor combination therapy.28,29 Employing a complex clinical trial design, Phase 2 testing established high dose ivacaftor was sufficient to overcome indication of its metabolism by lumacaftor,29 and two Phase 3 clinical trials that together were the largest CF clinical trials conducted to date in the CF population established long term efficacy and safety.28 Nevertheless, improved outcomes were overall modest, improving lung function by approximately 3% predicted FEV1, and reducing CF pulmonary exacerbations by 33–40%. Unfortunately, lumacaftor was associated with an increased incidence of respiratory adverse events characterized by wheezing, chest tightness, and dyspnea. Although frequently transient,30 this side effect profile that affected 5–10% of the treated population in the clinical study, and greater numbers of patients in post approval reports,31 particularly among those with low lung function at baseline,32,33 providing an important limitation on the ultimate utility of this therapy. Improved CFTR activity was subsequently estimated in the older CF population in the post-approval PROSPECT study; interim findings indicated sweat chloride improvements of ~18 mEq/L, compatible with expectations posed by in vitro studies and the degree of efficacy seen in the clinic. In an open label study, pediatric CF patients age 6–11 experienced a similar benefit in sweat chloride, and improved airways obstruction as measured by multi-breath washout, a lung clearance index.34 Additional studies seek to reduce the age further, leading to earlier initiation of treatment if supported by CYP pharmacology, which evolves rapidly during the childhood period. Despite this relatively small affect acutely on lung function, improved rate of lung function decline was clear,35 and approached that observed by ivacaftor therapy among patients with the G551D population,7 indicating the beneficial effect of small changes in CFTR activity can be pronounced when experienced over the long term. Unfortunately, lumacaftor-ivacaftor response was not sufficient to achieve clinical benefit in patients with a single F508del allele and a second mutation unresponsive to ivacaftor or lumacaftor, although a decrease in sweat chloride (11 mEq/L) was observed, leaving an important area of unmet medical need.36
Recently, tezacaftor (formerly VX-661) a second corrector derived from the original discovery of VX-809, entered clinical testing in CF patients.37,38 Following a successful Phase 2 dose ranging study that established the most efficacious dose of tezacaftor and that ivacaftor augmented its beneficial effect,37 a large Phase 3 program was initiated that included patient homozygous and heterozygous for F508del CFTR.In patients homozygous for F508del CFTR, combination therapy improved FEV1%by4%predictedascomparedtoplacebo,andwasassociatedwith improved body weight and reduced CF pulmonary exacerbations. CF patients heterozygous for F508del CFTR but with a residual function mutation expected to respond to ivacaftor alone, tezacaftor/ivacaftor was shown to be even more beneficial, with an FEV1 improvement of 6.8% compared to placebo. Moreover, although ivacaftor was beneficial, combination therapy was superior to ivacaftor alone.38 The latter study will support a submission for tezacaftor/ivacaftor approval in a new CF population, comprising approximately 9% of the CF population. As observed with lumacaftor, combination tezacaftor/ivacaftor was not efficacious in F508del heterozygous patients with an unresponsive second allele, and is a major focus for next generation therapies. For these reasons, in addition to natural inefficiency associated with single agent corrector therapy, combined corrector-potentiator therapy is the current approach being advanced for patients heterozygous for F508del CFTR alleles and to maximize efficacy for F508del homozygotes. Given tezacaftor was not associated with off-target respiratory adverse events, as observed with lumacaftor, and has much more stable pharmacokinetic profile, it is expected that tezacaftor will provide an important backbone of multi-agent corrector therapy to be tested in the future.39
As CFTR modulator therapies are further advanced for the F508del CF population, particularly those with only one allele, there are several approaches being pursued to augment efficacy. In the first approach, improved correctors are being advanced to clinical testing by several pharmaceutical companies, including Galapagos/Abbvie and Flately Discovery Therapy in addition to Vertex. Several others are attempting to identify and advance these 2nd generation correctors, and may be informed by an improved understanding of the domain assembly and inter-domain stability necessary to achieve stable F508del CFTR cell surface expression. Several of these strategies rely on the concept that multiple steps of CFTR processing must be targeted to efficiently restore CFTR cell surface expression and stability. In a second approach, add on corrector therapy that augments the activity of approved correctors may be beneficial. Cavanostat (formerly N91115; Nivalis) was one such approach that showed success in an initial study, but was not confirmed in a larger effort40; several other academic labs are pursuing similar approaches. Vertex has also advanced four different second generation corrector agents to clinical testing that are designed to augment the effect of tezacaftor/ivacaftor therapy. Promising data was recently reported in a press release (http://investors.vrtx.com/releasedetail. cfm?ReleaseID=1033559). Regardless of their potential utility as single agents, which is still under investigation, inhibition of ENaC may augment the efficacy of CFTR modulators, perhaps by enhancing the electrochemical gradient for fluid secretion. This is presently being tested in combination with CFTR modulators prospectively, and several other similar trials are on the horizon.
Although correction of F508del CFTR has been the focus, lumacaftor, and other correctors have also been shown to be active on some but not all alleles that exhibit dysfunctional processing. Of note, some of these Class 2 mutations, such as P67L or A455E, only exhibit partially reduced surface CFTR expression, and can exhibit residual activity even in their native state41,42; these properties are one of the confusing aspect of CFTR mutation analysis, since Class 2 alleles have frequently been considered severe by nature. Protein misfolding is also frequently accompanied by other channel deficits, such as deficient gating or accelerated recycling, further complicating matters. Further research to broaden the use of approved correctors is needed, and further the import of individualized testing, described further below.
4 |. CFTR READTHROUGH AGENTS
Inducing translational readthrough of CFTR is an approach with considerable scientific promise, but that has not yet resulted in a drug with sufficient efficacy for approval. Agents that target the ribosome or other aspects of the translational machinery can restore full-length mRNA protein thus, restoring CFTR activity. Aminoglycosides, a class of antibiotics that includes gentamicin, induces readthrough by lowering the fidelity of translation termination process by binding to the eukaryotic ribosomal decoding sites, leading to translational misreading.43,44 Unfortunately, the concentration at which aminoglycosides are active for readthrough limits their use due to enal/ototoxicity with long-term use.45–47 To overcome this problem, novel aminoglycosides derivatives have been synthesized using structure based approach to reduce toxicity and enhance readthrough efficiency.48–50 The NB series (NB84, NB124 Eloxx Pharmaceuticals; Herzliya, Israel) are designed to provide 2.5-fold greater readthrough activity than gentamicin (7% of wild-type levels) and have strong safety profile.50 Current efforts are still going on to bring these forward as potential therapeutic agents.
The need for more potent and safe readthrough compounds led to the development of PTC124 (ataluren), a non-aminoglycoside readthrough agent using nonsense containing reporters.51–54 While successful in some initial clinical studies, its off-target effect on reporter stabilization and formation of multi-substrate adduct inhibitor resulted in questions regarding its overall efficacy.55,56 A multi-center, multi-national phase 3 clinical trial failed to demonstrate its efficacy as a readthrough agent in CF nonsense genotypes, and a follow up study in patients without tobramycin use, which was shown to interfere with its efficacy, did not confirm prior subset analyses.57,58 Based on this, new molecules are urgently needed.
Additional challenges to readthrough have also been noted, and will need to be overcome to achieve levels of CFTR function necessary to restore health, especially given mRNA transcript readthrough rates are relatively modest due to nonsense mediated decay. Even highly efficacious aminoglycosides read through only 4.7% of mRNA transcripts, which could be a potential barrier to achieving efficacy, and the presence of a near-congate but nonidentical amino-acid at the stop-codon position could influence downstream CFTR processing and activity, limiting restoration of function. Considering these challenges to readthrough, multi-agent treatment for PTC mutants may be required, both to enhance readthough efficacy and augment activity of the readthrough product. Recently reported in vitro studies demonstrated that the readthrough efficiency can be substantially increased when administered in combination with CFTR correctors and potentiators.50,59,60 To broaden options, additional screens are in progress in several laboratories including our ongoing collaboration with Southern Research, and may yield novel chemical matter. Escin, an herbal compound is currently being explored, and arose out of a firstgeneration screen.61 The incorporation of CFTR specific HRP assay in capacity to measure trafficking- competent readthrough products.62 Currently, efforts are being made to synthesize multiple scaffolds of hit agents to enhance the efficacy and potency, both alone and in combination with NMD inhibitors, correctors, and potentiators.
5 |. ACTIVATING EXPRESSION AND RELATED PATHWAYS
Other novel approaches are being advanced to augment restoration of CFTR function by CFTR modulator therapy. These approaches could be crucial to achieving maximal efficacy on F508del homozygous patients, particularly individuals that are natively resistant to CFTR correctors, since the efficiency of endogenous CFTR folding can vary widely, and probably acts as an important covariate to CFTR corrector efficacy. Moreover, agents of this sort may be necessary to achieve efficacy in F508del heterozygous patients with recalcitrant alleles not amenable to pharmacologic rescue, such as major insertions, deletions, or splice mutations resistant to antisense oligonucleotide AO therapy.
One approach to augment the activity of CFTR correctors is to increase CFTR expression, increasing the pool of CFTR available for modulator therapy. This has been successfully demonstrated in vitro, and can occur both through molecules that are specific to CFTR or globally enhance gene expression.63 Proteostasis is developing an agent PTI-428 termed a ‘CFTR amplifier, and is thought to be specific to CFTR at the level of mRNA transcription, whereas histone deacetylase (HDAC) inhibitors, as represented by the agent Vorinostat (suberoylanilide hydroxamic acid, SAHA), represents more broadbased effects.64 In interpreting in vitro data care must be taken not to interpret alterations in artificial promoters, a common tool in CFTR cell culture systems that may or may not be relevant to endogenous expression in humans. Whether these agents augment CFTR activity and improve clinical outcomes will be tested in the future, and could add an important tool to the CFTR modulator armamentarium.
A second approach to potentially augment CFTR F508del correction is to restore normal autophagy, which has been shown to be disrupted in response to F508del misprocessing.65,66 Invoking this mechanism, by restoring autophagy pathways by cysteamine or other anti-oxidant like agents, CFTR correction can be restored.67 Intriguing but complicated data that includes in vivo and ex vivo cell analysis has demonstrated modest improvements in sweat chloride and CFTR-mediated halide efflux, when cysteamine is combined with epigallocatechin gallate (EGCG), which is thought to further stabilize CFTR protein. Whether other agents can achieve this effect in a more pharmacologically palatable formulation than cysteamine remains a key question toward moving this concept forward.
A third approach includes stabilizing CFTR once localized to the cell surface. Potential pathways include interrupting CFTR ubiquitination that targets surface localized CFTR for premature degradation,63,68 enhancing the avidity of the PDZ binding motif which stabilizes CFTR.69,70 The reduced membrane half-life of F508del CFTR is particularly vexing, since this may not be completely addressed by CFTR correctors, particular in the setting of CFTR potentiators or activators. Like the other approaches highlighted in this section, increasing the surface CFTR residence time could be beneficial for several different alleles beyond F508del, augmenting the effect of other CFTR modulators.
6 |. CLINICAL BIOMARKERS OF CFTR ACTIVITY
Although biomarkers or CFTR activity are now well established for predicting efficacy on a group-wise basis, they have limited utility in predicting efficacy of CFTR modulators on an individual basis.71–76 The sweat gland, in particular, is the source of several attractive biomarkers for precision medicine. The sweat gland has a distinct CF defect: impaired absorption of salt in the sweat duct, and impaired β adrenergic secretion in the sweat coil.77 The sweat gland epithelium is not affected by sequelae of the defect such as inflammation, tissue destruction, or remodeling seen in other epithelial tissues, such as the airway, and theoretically represents a clear measure of CFTR dysfunction unencumbered by other complications affecting the test (such as mucus obstruction and inflammation). Although sweat chloride has been problematic for predicting response to CFTR modulators on an individual basis,71–73,76 it did predict age-normalized pulmonary severity via the KNorRMA score (#215),17 correlated with in vitro airway epithelial monolayer cultures (#266),17 and as an individual predictor of outcomes.78,79 The inconsistent ability of sweat chloride to predict clinical response for a single patient, and yet predict group-level efficacy, remains a conundrum, and indicates the need for further research. Importantly, the sweat gland is not the primary organ affected in CF, and variation between tissues may reflect organspecific thresholds for drug efficacy that may exist. One approach that could be helpful is evaluating the long-term response of patients as sweat chloride improves with addition of various CFTR modulators. As natural variance in response occurs across genotypes, individual patient response, and the modulator used, this has the potential to elucidate differences in long-term outcome, and could address limitations in individual studies that are limited because of a single population or drug under study, particularly over the short-term.
While some of the variability in sweat chloride may be attributed to variations in test performance, pharmacometric approaches to further develop sweat chloride as a biomarker for precision medicine have not been employed, and may improve utility of the test. As an example, in one study, a single missed dose was thought to contribute to the lack of individual correlation of SC to FEV1,73 suggesting the possibility of a relationship to blood levels of ivacaftor. Studies to correlate blood levels of CFTR modulators with clinical biomarkers could be informative in this regard. Given the multiple covariates that may affect FEV1 at any single time point, it may also be that other clinical outcome measures are better suited to detect a long-term relationship with CFTR activity; for example rate of lung function decline, exacerbation frequency, imaging abnormalities, or nutritional status are all potential outcomes to be considered, and may better reflect long term health status that individual changes in FEV1 over the short term, which can vary due to the state of the airway or baseline level of airway obstruction.
Other potential biomarkers utilizing the unique qualities of the sweat gland include the β adrenergic sweat rate/evaporimetry and the sweat bubble assay, which measure β adrenergic sweat secretion. Both these assays are reported to have sensitivity to distinguish severe and mild CF phenotypes, as well as heterozygotes and non-CF subjects.17,80–85 Although these tests are more labor-intensive and are presently available at only a few centers, with repeated testing, response has been observed in individuals with higher levels of CFTR activity obtained by ivacaftor treatment of R117H, although not with ivacaftor treatment of G551D patients.83–85 This may be due to the low level of CFTR function restored by sweat secretion, placing ivacaftor treated G551D patients below the “floor” of sweat secretion, and points to an important concept that different organs may be impacted by CFTR modulators differently, and have widely different dose-dependency.
Several other CFTR biomarkers have potential to serve as an individual monitoring tool of CFTR activity, but are less well suited for large scale application. Nasal potential difference (NPD) has also been established as a clinical biomarker in multiple studies,57,75,86–90 but the expertise involved limit its utility for wide-spread utilization for monitoring. Other assays, such as CFTR Western blot, mRNA expression, flow cytometry to quantify cell surface expression of either CFTR in monocytes (#60)17 or alterations in non-CFTR membrane proteins in peripheral blood leukocytes (#298)17 all have potential utility, particularly in the clinical trial setting, but like NPD, need improvements for widespread application on a population basis or to determine individual response.
7 |. CELL BASED ASSAYS OF CFTR ACTIVITY
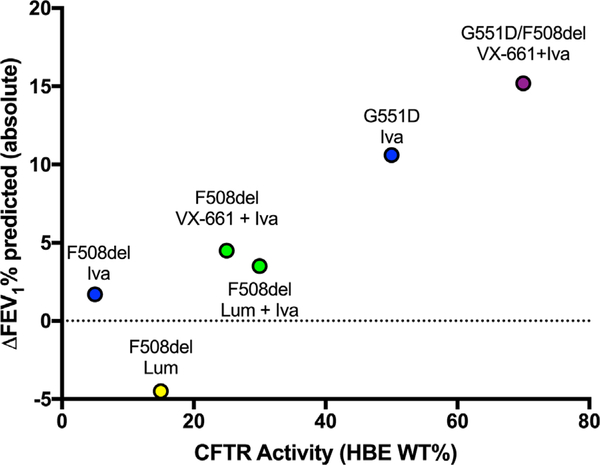
Cell-based assays of CFTR activity have been used for decades for basic studies of CF as well as the development of CFTR modulators. Airway cell monolayers, grown on elevated support membranes at an air-liquid interface usually from primary HBE, have been a mainstay of CF research and are the current gold-standard pre-clinical tool.91 HBE cells provide multiple readouts for CFTR activity (including ion transport, airway surface liquid height, and mucociliary transport rates92–97). Emeging evidence suggests a strong relationship between pulmonary outcome and efficacy of CFTR modulationin vitro on a group-wise basis (Figure 1).Nevertheless, despite these strengths, and improved ability to procure and expand small numbers of cells, large cell numbers are ultimately required and typically come from lung explanted material, limiting their utility for specimen from rare genotypes. Bronchial brushing is an alternate method to obtain lower airway cells,98–100 but requires anesthesia and invasive bronchoscopy; samples epithelia from large central airways, instead of the small airways; and may not result in sufficient numbers for testing a broad variety of agents.
FIGURE 1.
Relationship between CFTR activity measured in vitro with spirometry improvement assessed in clinical trials. Lum = lumacaftor; Iva = ivacaftor; VX-661 = tezacaftor; F508del = homozygous for F508del CFTR unless otherwise noted. Derived from N Engl J Med. 2011;365:1663–72;365:1663–72; N Engl J Med. 2015;373:220–31; Chest 2012; PNAS 2009; PNAS 2011; Lancet Respir Med 2 2014; 527–538; Donaldson et al., NACFC 2015; Fidler et al., NACFC 2015
New research is needed to determine whether HBE cells may be useful as a predictive tool for individual patients, which could enable testing of rare genotypes or complex therapeutic regimens, establishing a patient “theratype.” Particularly as combination treatments are implemented, we need to understand potential discrepancies between in vitro ion transport findings20,21,101 and clinical data,28,35,102,103 as results from our own laboratory suggest that ion transport alone may not predict FEV1 benefit. Comprehensive studies that include downstream effects of CFTR restoration, such as ASL depth, mucus transport, or changes in viscosity, may be more indicative of lung function improvement,94 but require prospective study to confirm. Several other novel methods have the potential to contribute to this area and are of significant interest to the research community.
7.1 |. Intestinal biomarkers
The intestinal epithelium exhibits a distinct CF phenotype, where CFTR is the primary chloride channel in the apical membrane.104–106 Intestinal epithelium is easily obtained using a simple procedure (rectal biopsy), although uptake in adult populations is more limited. Intestinal current measurements are established as a clinical and pre-clinical tool,74,89,107,108 since the biopsy may be mounted on a support and short-circuit current measured across an intact, fully differentiated epithelial layer without compromise from prolonged cell culturing techniques. This test is discriminatory when testing conditions are adjusted appropriately to detect small changes in CFTR activity and correlates with clinical measures in small populations.109 ICM has also been proposed to detect modulator effects quickly (within weeks), but may be limited by drug absorption.110 However, few laboratories are capable of performing the highly-specialized protocols and only a small number of samples can be obtained at a single session, limiting utility.
7.2 |. Intestinal organoids
Three-dimensional models of epithelia have several distinct advantages in that they require minimal sampling, perpetuate long periods inculture, may be restored from cells preserved and shipped long distances, and result in well-differentiated epithelial cultures with simple outcome measures based on fluid secretion. The intestinal organoid is a key recent development, and has been shown to distinguish CFTR activity in a variety of mutations.111–118 This model primarily depends on the forskolin induced swelling (FIS) of organoids, which is a proxy for fluid transport, to evaluate downstream effects of CFTR activity. Measurements are made using live cell microscopy of multiple organoids per subject, providing a sensitive evaluation of CFTR activity and the potential for high-througput.111–114,116–118 The responses of many different CFTR mutations have been characterized, including the response relationship to genotype, disease severity, and CFTR modulator response.17,119 The model has also been used as a drug screen for rare mutations,17 in combination with click chemistry to track CFTR restoration by modulators (S08.4)119; and to evaluate circulating levels of modulators in plasma as a pharmacometric study (#178).119 The same study showed intestinal organoid responses are associated with clinical outcome in a study of ivacaftor treatment of the S1251N gating mutation, in which in vitro response was well correlated with clinical results. The extensive characterization of this model suggests a promising approach to personalized testing of modulators, both as a preclinical predictive tool, as well as an outcome biomarker. An important next step is a larger multi-center validation to evaluate the predictive ability of this model and to extend the technology to additional centers, establishing generalizability. If successful, this could be an important tool for demonstrating efficacy, particularly in rare genotypes in which prospective study are not feasible.
7.3 |. Airway organoids
An ideal pre-clinical cell-based model would be derived from the airway, the tissue of critical importance to the long-term outcome of CF patients, and would have a simple outcome measure(s) that correlate with CFTR activity, its downstream impact, and clinical efficacy. Research in several laboratories is focused on deriving such a model, derived from either upper or lower airway cells. Nasal epithelial cells represent a particularly attractive source for airway epithelia, as they can be obtained repeatedly from subjects with minimally invasive sampling. Several investigators have demonstrated the utility of nasal epithelial monolayers for the study of CF,120–123 and provided comparisons between upper and lower airway epithelial cells, including their ion transport characteristics,119,124 inflammatory state,125 and gene expression.126 Despite these advantages, controversy remains in their application as a surrogate for the lung, as CFTR and other expression profiles may be different from the lower airway, sustenance in culture is more limited, and propensity to differentiate fully is more problematic than lung-derived cells; some of the discrepancies may be mitigated by improved culturing techniques, and there is a concerted effort to optimize culturing technique. To combat the small number of cells obtained from nasal epithelial biopsy, conditional reprogramming92,127–129 or induced pluripotent stem cells129–133 represent attractive approaches. The development of three-dimensional organoid models similar to intestinal organoids may also improve ability to discern useful information from small sample sizes. Such a model could also have a simple outcome measure (ie, forskolin-induced changes in size FIS (#183),17 (#6, #155),119 and potentially reveal downstream factors, such as ciliary beat frequency (#94),17 and viscoelastic properties of mucus (#149).119 A related technology includes the use of primary airway cells in microfluidics-based models of epithelial and endothelial co-culture, more failthfully recapitulating the complex multi-cellular composition of the airway.134,135 These airway models show great promise, with ongoing research to optimize culture conditions, imaging assays, and analysis methods to assess the value of these models as predictive tools. Ultimately, as with other methods, clinical validation in a group and individual basis and prospective clinical validation will be required to fully realize their use as a predictive tool to select and optimize therapy.
8 |. PHARMACOMETRICS
With variable responses seen among F508del homozygotes, and the impossibility of randomized clinical trials for those with rare mutations, predictive biomarkers must be rigorously validated and correlated with clinical outcomes of interest. In order to adequately model biomarkers, the pharmacokinetic/pharmacodynamics of each modulator and modulator combinations must be well understood, particularly as regards the biomarker under study. The PK/PD of all modulators form a critical part of the basic characterization and safety profiles of all modulators under study,103,119,136–138 but individual pharmacometrics have not been evaluated. Recent studies have shown that weight and age may impact efficacy among GOAL patients (S. Heltshe, personal communication), a finding that may reflect the importance of individual pharmacometric analyses. Clinical biomarkers such as sweat glandderived assays and the nasal potential difference assay may be acutely affected by the PK/PD of a given modulator or combination, as shown by existing studies where precise timing of the assay (#27517), or missed doses,73 may contribute to improved correlation with clinical measures or a lack thereof, respectively. Both ivacaftor and lumacaftor are either substrates or inducers of CYP450 enzymes (orboth), and CFpatients are frequently taking other drugs that are highly likely to interact with modulators, placing them at risk for drug toxicity or decreased efficacy.39 Despite development of mass spectrophotometric techniques for determining levels of modulators and their metabolites in body fluids, no recommendations for therapeutic monitoring have so far been made.21,139 Other investigators have recognized the importance of metabolism of these drugs, and the potential impact on biomarker development, and have incorporated some pharmacometric analyses in organoid studies.115 To date, no studies have compared pharmacometric parameters of CFTR modulators in vitro and in vivo, and this may be an important area of research in the future.
9 |. SUMMARY
The positive clinical outcomes of CFTR modulators has brought a new dawn to the lives of many CF patients, particularly those with the most responsive mutations. Lessons drawn from emerging clinical experience and an improved understanding of CFTR molecular biology has opened new opportunities for more sophisticated precision treatments that are most effective when used in combination, combatting multiple deficits in CFTR expression, trafficking, and function. When combined with novel precise biomarkers that will enable individualization of treatment regimens, even for those with ultra-rare mutations, we have the potential to attain our goal of achieving highly efficacious therapy for all CF patients.
REFERENCES
- 1.Alton EW, Boyd AC, Davies JC, et al. Genetic medicines for CF: hypeversus reality. Pediatr Pulmonol. 2016;51:S5–S17. [DOI] [PubMed] [Google Scholar]
- 2.Lee TW, Southern KW, Perry LA, Penny-Dimri JC, Aslam AA. Topicalcystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease. Cochrane Database Syst Rev. 2016;6:CD005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator inpatients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airwayepithelial cell function in vitro by a CFTR potentiator, VX–770. Proc Natl Acad Sci USA. 2009;106:18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of theCFTR potentiator ivacaftor in G551D-Mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawicki GS, Dasenbrook E, Fink AK, Schechter MS. Rate of uptake ofivacaftor use after U.S. food and drug administration approval among patients enrolled in the U.S. cystic fibrosis foundation patient registry. Ann Am Thorac Soc. 2015;12:1146–1152. [DOI] [PubMed] [Google Scholar]
- 7.Sawicki GS, McKone EF, Pasta DJ, et al. Sustained Benefit fromivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015;192: 836–842. [DOI] [PubMed] [Google Scholar]
- 8.Pohl K, Hayes E, Keenan J, et al. A neutrophil intrinsic impairmentaffecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bratcher PE, Rowe SM, Reeves G, et al. Alterations in bloodleukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J Cyst Fibros. 2015;15:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisert KB, Heltshe SL, Pope C, et al. Restoring CFTR function reducesairway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit CareMed.2017;195:1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes D, Jr, Long FR, McCoy KS, Sheikh SI. Improvement inbronchiectasis on CT imaging in a pediatric patient with cystic fibrosis on ivacaftor therapy. Respiration. 2014;88:345. [DOI] [PubMed] [Google Scholar]
- 12.Strang A, Fischer AJ, Chidekel A. Pseudomonas eradication andclinical effectivness of Ivacaftor in four Hispanic patients with S549N. Pediatr Pulmonol. 2017;52:E37–E39. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Burton B, Huang CJ, et al. Ivacaftor potentiation of multipleCFTR channels with gating mutations. J Cyst Fibros. 2012;11: 237–245. [DOI] [PubMed] [Google Scholar]
- 14.De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftorin patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–680. [DOI] [PubMed] [Google Scholar]
- 15.Sheppard DN, Rich DP, Ostedgaard LS, Gregory RJ, Smith AE, Welsh MJ. Mutations in CFTR associated with mild-disease-form Clchannels with altered pore properties. Nature. 1993;362:160–164. [DOI] [PubMed] [Google Scholar]
- 16.Moss RB, Flume PA, Elborn JS, et al. Efficacy and safety of ivacaftor inpatients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The 29(th) Annual North American Cystic Fibrosis Conference. Pediatr Pulmonol. 2015;50:S1–S76. [DOI] [PubMed] [Google Scholar]
- 18.Yousef S, Solomon GM, Brody A, Rowe SM, Colin AA. Improvedclinical and radiographic outcomes after treatment with ivacaftor in a young adult with cystic fibrosis with the P67L CFTR mutation. Chest. 2015;147:e79–e82. [DOI] [PubMed] [Google Scholar]
- 19.Abstracts of the The 28th Annual North American Cystic FibrosisConference Georgia World Congress Center, October 9–11, 2014, Atlanta, Georgia. Pediatr Pulmonol. 2014;49:S1–475. [PubMed] [Google Scholar]
- 20.Veit G, Avramescu RG, Perdomo D, et al. Some gating potentiators, including VX–770, diminish DeltaF508-CFTR functional expression . Sci Transl Med. 2014;6:246ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cholon DM, Quinney NL, Fulcher ML, et al. Potentiator ivacaftorabrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Sci Transl Med. 2014;6:246ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birket SE, Chu KK, Houser GH, et al. Combination therapy with cysticfibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol. 2016;ajplung00395:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert JA, Raju SV, Tang LP, et al. CFTR activation by roflumilastcontributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol. 2013;50:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of theF508del-CFTRproteinprocessingdefectinvitrobytheinvestigational drug VX–809. Proc Natl Acad Sci USA. 2011;108:18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ,Verkman AS. Small-molecule correctors of defective DeltaF508CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study ofVX–809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2011;67:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flume PA, Liou TG, Borowitz DS, et al. Ivacaftor in subjects withcystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest. 2012;142:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-Ivacaftor inpatients with cystic fibrosis homozygous for phe508del CFTR. N Engl J Med. 2015;373:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle MP, Bell SC, Konstan MW, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2:527–538. [DOI] [PubMed] [Google Scholar]
- 30.Marigowda G, Liu F, Waltz D . Effect of bronchodilators in healthyindividuals receiving lumacaftor/ivacaftor combination therapy. J Cyst Fibros. 2017;16:246–249. [DOI] [PubMed] [Google Scholar]
- 31.Jennings MT, Dezube R, Paranjape S, et al. An observational study ofoutcomes and tolerances in patients with cystic fibrosis initiated on Lumacaftor/Ivacaftor. Ann Am Thorac Soc. 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Hubert D, Chiron R, Camara B, et al. Real-life initiation of lumacaftor/ivacaftor combination in adults with cystic fibrosis homozygous for the Phe508del CFTR mutation and severe lung disease. J Cyst Fibros. 2017;16:388–391. [DOI] [PubMed] [Google Scholar]
- 33.Popowicz N, Wood J, Tai A, Morey S, Mulrennan S. Immediateeffects of lumacaftor/ivacaftor administration on lung function in patients with severe cystic fibrosis lung disease. J Cyst Fibros. 2017;16:392–394. [DOI] [PubMed] [Google Scholar]
- 34.Milla CE, Ratjen F, Marigowda G, Liu F, Waltz D, Rosenfeld M,*VXPBIG. Lumacaftor/Ivacaftor in patients aged 6–11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2017;195:912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstan MW, McKone EF, Moss RB, et al. Assessment of safety andefficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5:107–118. [DOI] [PubMed] [Google Scholar]
- 36.Rowe SM, McColley SA, Rietschel E, et al. Lumacaftor/Ivacaftortreatment of patients with cystic fibrosis heterozygous for F508delCFTR. Ann Am Thorac Soc. 2017;14:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donaldson SH, Pilewski JM, Griese M, et al. Tezacaftor/Ivacaftor insubjects with cystic fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am J Respir Crit Care Med. 2017. doi.org/10.1164/rccm.201704-0717OC [Epub ahead of print]. [Google Scholar]
- 38.Two Phase 3 Studies of the Tezacaftor/Ivacaftor Combination Treatment Met Primary Endpoints with Statistically Significant Improvements in Lung Function (FEV1) in People with Cystic Fibrosis. https://investors.vrtx.com/https://investors.vrtx.com/:VertexPharmaceuticalsIncorporated; 2017.
- 39.Jordan CL, Noah TL, Henry MM. Therapeutic challenges posed bycritical drug-drug interactions in cystic fibrosis. Pediatr Pulmonol. 2016;51:S61–S70. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson SH, Solomon GM, Zeitlin PL, et al. Pharmacokinetics andsafety of cavosonstat (N 91115) in healthy and cystic fibrosis adults homozygous for F508DEL-CFTR. J Cyst Fibros. 2017;16:371–379. [DOI] [PubMed] [Google Scholar]
- 41.Van Goor F, Yu H, Burton B, Hoffman BJ. Effect of ivacaftor on CFTRforms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 2014;13:29–36. [DOI] [PubMed] [Google Scholar]
- 42.Sabusap CM, Wang W, McNicholas CM, et al. Analysis of cysticfibrosis-associated P 67L CFTR illustrates barriers to personalized therapeutics for orphan diseases. JCI Insight. 2016;1:e86581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keeling KM, Xue X, Gunn G, Bedwell DM. Therapeutics based onstop codon readthrough. Annu Rev Genomics Hum Genet. 2014;15: 371–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quon BS, Rowe SM. New and emerging targeted therapies for cysticfibrosis. BMJ. 2016;352:i859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheunemann AE, Graham WD, Vendeix FA, Agris PF. Binding ofaminoglycoside antibiotics to helix 69 of 23S rRNA. Nucleic Acids Res. 2010;38:3094–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salas-Marco J, Bedwell DM. Discrimination between defects inelongation fidelity and termination efficiency provides mechanistic insights into translational readthrough. J Mol Biol. 2005;348: 801–815. [DOI] [PubMed] [Google Scholar]
- 47.Kramer EB, Vallabhaneni H, Mayer LM, Farabaugh PJ.A comprehensive analysis of translational missense errors in the yeast Saccharomyces cerevisiae. RNA. 2010;16:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nudelman I, Rebibo-Sabbah A, Cherniavsky M, et al. Development ofnovel aminoglycoside (NB54) with reduced toxicity and enhanced suppression of disease-causing premature stop mutations. J Med Chem. 2009;52:2836–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe SM, Sloane P, Tang LP, et al. Suppression of CFTR prematuretermination codons and rescue of CFTR protein and function by the synthetic aminoglycoside NB54. J Mol Med (Berl). 2011;89: 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue X, Mutyam V, Tang L, et al. Synthetic aminoglycosides efficientlysuppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor. Am J Respir Cell Mol Biol. 2014;50:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du M, Liu X, Welch EM, Hirawat S, Peltz SW, Bedwell DM. PTC124 isan orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci USA. 2008;105:2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorderscaused by nonsense mutations. Nature. 2007;447:87–91. [DOI] [PubMed] [Google Scholar]
- 53.Shoseyov D, Cohen-Cymberknoh M, Wilschanski M. Atalurenfor the treatment of cystic fibrosis. Expert Rev Respir Med. 2016;1–5. [DOI] [PubMed] [Google Scholar]
- 54.Sermet-Gaudelus I, Boeck KD, Casimir GJ, et al. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am J Respir Crit Care Med. 2010;182:1262–1272. [DOI] [PubMed] [Google Scholar]
- 55.Auld DS, Thorne N, Maguire WF, Inglese J. Mechanism of PTC124 activity in cell-based luciferase assays of nonsense codon suppression. Proc Natl Acad Sci USA. 2009;106:3585–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McElroy SP, Nomura T, Torrie LS, et al. A lack of prematuretermination codon read-through efficacy of PTC124 (Ataluren) in a diverse array of reporter assays. PLoS Biol. 2013;11:e1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerem E, Konstan MW, De Boeck K, et al. Ataluren for the treatmentof nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinig AM, Mirzaei S, Berlau DJ. Advances in the treatment ofduchenne muscular dystrophy: new and emerging pharmacotherapies. Pharmacotherapy. 2017;37:492–499. [DOI] [PubMed] [Google Scholar]
- 59.Liang F, Shang H, Jordan NJ, et al. High-throughput screening forreadthrough modulators of CFTR PTC mutations. SLAS Technol. 2017;22:315–324. [DOI] [PubMed] [Google Scholar]
- 60.Xue X, Mutyam V, Thakerar A, et al. Identification of the amino acidsinserted during suppression of CFTR nonsense mutations and determination of their functional consequences. Hum Mol Genet. 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutyam V, Du M, Xue X, et al. Discovery of clinically approvedagents that promote suppression of CFTR nonsense mutations. Am J Respir Crit Care Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang F, Shang H, Jordan NJ, et al. High-Throughput screening forreadthrough modulators of CFTR PTC mutations. SLAS Technol. 2017;22:315–324. [DOI] [PubMed] [Google Scholar]
- 63.Chung WJ, Goeckeler-Fried JL, Havasi V, et al. Increasing theendoplasmic reticulum pool of the F508del allele of the cystic fibrosis transmembrane conductance regulator leads to greater folding correction by small molecule therapeutics. PLoS ONE. 2016;11: e0163615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutt DM, Herman D, Rodrigues AP, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nature Chem Biol. 2010;6:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vu CB, Bridges RJ, Pena-Rasgado C, et al. Fatty acid cysteamineconjugates as novel and potent autophagy activators that enhance the correction of misfolded F508del-Cystic fibrosis transmembrane conductance regulator (CFTR). J Med Chem. 2017;60: 458–473. [DOI] [PubMed] [Google Scholar]
- 66.Luciani A, Villella VR, Esposito S, et al. Targeting autophagy as a novelstrategy for facilitating the therapeutic action of potentiators on DeltaF508 cystic fibrosis transmembrane conductance regulator. Autophagy. 2012;8:1657–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Stefano D, Villella VR, Esposito S, et al. Restoration of CFTRfunction in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy. 2014;10:2053–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu L, Rab A, Tang L, et al. DeltaF508 CFTR surface stability isregulated by DAB2 and CHIP-mediated ubiquitination in postendocytic compartments. PLoS ONE. 2015;10:e0123131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian Z, Xu X, Amacher JF, Madden DR, Cormet-Boyaka E, Pei D. Intracellular delivery of peptidyl ligands by reversible cyclization: discovery of a PDZ domain inhibitor that rescues CFTR Activity. Angew Chem Int Ed Engl. 2015;54:5874–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amacher JF, Zhao R, Spaller MR, Madden DR. Chemically modifiedpeptide scaffolds target the CFTR-associated ligand PDZ domain. PLoS ONE. 2014;9:e103650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA.Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143:14–18. [DOI] [PubMed] [Google Scholar]
- 72.Fidler MC, Beusmans J, Panorchan P, Van Goor F. Correlation ofsweat chloride and percent predicted FEV1 in cystic fibrosis patients treated with ivacaftor. J Cyst Fibros. 2017;16:41–44. [DOI] [PubMed] [Google Scholar]
- 73.Barry PJ, Jones AM, Webb AK, Horsley AR. Sweat chloride is not auseful marker of clinical response to Ivacaftor. Thorax. 2014;69: 586–587. [DOI] [PubMed] [Google Scholar]
- 74.De Boeck K, Kent L, Davies J, et al. CFTR biomarkers: time forpromotion to surrogate end-point. Eur Respir J. 2013;41:203–216. [DOI] [PubMed] [Google Scholar]
- 75.Rowe SM, Liu B, Hill A, et al. Optimizing nasal potential differenceanalysis for CFTR modulator development: assessment of ivacaftor in CF subjects with the G551D-CFTR mutation. PLoS ONE. 2013;8: e66955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Accurso FJ, Van Goor F, Zha J, et al. Sweat chloride as a biomarker ofCFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quinton PM. Cystic fibrosis: lessons from the sweat gland. Physiology (Bethesda). 2007;22:212–225. [DOI] [PubMed] [Google Scholar]
- 78.McGarry ME, Nielson DW . Normalization of sweat chloride concentration and clinical improvement with ivacaftor in a patient with cystic fibrosis with mutation S549N. Chest. 2013;144: 1376–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seliger VI, Rodman D, Van Goor F, Schmelz A, Mueller P. Thepredictive potential of the sweat chloride test in cystic fibrosis patients with the G551D mutation. J Cyst Fibros. 2013;12:706–713. [DOI] [PubMed] [Google Scholar]
- 80.Gonska T, Ip W, Turner D, et al. Sweat gland bioelectrics differ incystic fibrosis: a new concept for potential diagnosis and assessment of CFTR function in cystic fibrosis. Thorax. 2009;64:932–938. [DOI] [PubMed] [Google Scholar]
- 81.Quinton P, Molyneux L, Ip W, et al. Beta-adrenergic sweat secretionas a diagnostic test for cystic fibrosis. Am J Respir Crit Care Med. 2012;186:732–739. [DOI] [PubMed] [Google Scholar]
- 82.Shamsuddin AK, Reddy MM, Quinton PM. Iontophoretic betaadrenergic stimulation of human sweat glands: possible assay for cystic fibrosis transmembrane conductance regulator activity in vivo. Exp Physiol. 2008;93: 969–981. [DOI] [PubMed] [Google Scholar]
- 83.Char JE, Wolfe MH, Cho HJ, et al. A little CFTR goes a long way: CFTR-dependent sweat secretion from G551D and R117H-5T cystic fibrosis subjects taking ivacaftor. PLoS ONE. 2014;9:e88564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Farahmand M, Dunn C, et al. Evaporimeter and bubbleImaging measures of sweat gland secretion rates. PLoS ONE. 2016;11:e0165254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wine JJ, Char JE, Chen J, et al. In vivo readout of CFTR function: ratiometric measurement of CFTR-dependent secretion by individual, identifiable human sweat glands. PLoS ONE. 2013;8:e77114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sad IR, Higa LY, Leal T. et al. Repeatability and diagnostic value ofnasal potential difference in a genetically admixed population. J Clin Med Res. 2016;8:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jaron R, Yaakov Y, Rivlin J, et al. Nasal potential difference in nonclassic cystic fibrosis-long term follow up. Pediatr Pulmonol. 2008;43:545–549. [DOI] [PubMed] [Google Scholar]
- 88.Wilschanski M, Famini H, Strauss-Liviatan N, et al. Nasal potentialdifference measurements in patients with atypical cystic fibrosis. Eur Respir J. 2001;17:1208–1215. [DOI] [PubMed] [Google Scholar]
- 89.Wilschanski M, Yaakov Y, Omari I, et al. Comparison of nasalpotential difference and intestinal current measurements as surrogate markers for CFTR function. J Pediatr Gastroenterol Nutr. 2016;63:e92–e97. [DOI] [PubMed] [Google Scholar]
- 90.Yaakov Y, Kerem E, Yahav Y, et al. Reproducibility of nasal potentialdifference measurements in cystic fibrosis. Chest. 2007;132: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 91.Awatade NT, Uliyakina I, Farinha CM, et al. Measurements offunctional responses in human primary lung cells as a basis for personalized therapy for cystic fibrosis. EBioMedicine. 2015;2: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gentzsch M, Boyles SE, Cheluvaraju C, et al. Pharmacological rescueof conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2017;56:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Astrand AB, Hemmerling M, Root J, et al. Linking increased airwayhydration, ciliary beating, and mucociliary clearance through ENaC inhibition. Am J Physiol Lung Cell Mol Physiol. 2015;308:L22–L32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Birket SE, Chu KK, Houser GH, et al. Combination therapy with cysticfibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol. 2016;310:L928–L939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Button B, Cai LH, Ehre C, et al. A periciliary brush promotes the lunghealth by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oldenburg AL, Chhetri RK, Hill DB, Button B. Monitoring airwaymucus flow and ciliary activity with optical coherence tomography. Biomed Opt Express. 2012;3:1978–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Birket SE, Chu KK, Liu L, et al. A functional anatomic defect of thecystic fibrosis airway. Am J Respir Crit Care Med. 2014;190:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doherty GM, Christie SN, Skibinski G, et al. Non-bronchoscopicsampling and culture of bronchial epithelial cells in children. Clin Exp Allergy. 2003;33:1221–1225. [DOI] [PubMed] [Google Scholar]
- 99.Garratt LW, Sutanto EN, Foo CJ, et al. Determinants of culturesuccess in an airway epithelium sampling program of young children with cystic fibrosis. Exp Lung Res. 2014;40:447–459. [DOI] [PubMed] [Google Scholar]
- 100.Blume C, Davies DE. In vitro and ex vivo models of human asthma. Eur J Pharm Biopharm. 2013;84:394–400. [DOI] [PubMed] [Google Scholar]
- 101.Liu X, Dawson DC. Cystic fibrosis transmembrane conductanceregulator (CFTR) potentiators protect G551D but not DeltaF508 CFTR from thermal instability. Biochemistry. 2014;53:5613–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyle MP, Bell SC, Konstan MW, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase randomised controlled trial. Lancet Respir Med. 2014;2:527–538. [DOI] [PubMed] [Google Scholar]
- 103.Elborn JS, Ramsey BW, Boyle MP, et al. Efficacy and safety oflumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. Lancet Respir Med. 2016;4:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cellspecific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G82–G98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lambert JA, Raju SV, Tang LP, et al. Cystic fibrosis transmembraneconductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol. 2014;50:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Venkatasubramanian J, Ao M, Rao MC. Ion transport in the smallintestine. Curr Opin Gastroenterol. 2010;26:123–128. [DOI] [PubMed] [Google Scholar]
- 107.Derichs N, Mekus F, Bronsveld I, et al. Cystic fibrosis transmembraneconductance regulator (CFTR)-mediated residual chloride secretion does not protect against early chronic Pseudomonas aeruginosa infection in F508del homozygous cystic fibrosis patients. Pediatr Res. 2004;55:69–75. [DOI] [PubMed] [Google Scholar]
- 108.Raju SV, Jackson PL, Courville CA, et al. Cigarette smoke inducessystemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188: 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.J Beekman JM, Sermet-Gaudelus I, de Boeck K, et al. CFTR functionalmeasurements in human models for diagnosis, prognosis and personalized therapy: report on the pre-conference meeting to the 11th ECFS Basic Science Conference, Malta, 26–29 March 2014. J Cyst Fibros. 2014;13:363–372. [DOI] [PubMed] [Google Scholar]
- 110.Graeber SY, Hug MJ, Sommerburg O, et al. Intestinal currentmeasurements detect activation of mutant CFTR in patients with cystic fibrosis with the G551D mutation treated with ivacaftor. Am J Respir Crit Care Med. 2015;192:1252–1255. [DOI] [PubMed] [Google Scholar]
- 111.Beekman JM. Individualized medicine using intestinal responses toCFTR potentiators and correctors. Pediatr Pulmonol. 2016;51: S23–S34. [DOI] [PubMed] [Google Scholar]
- 112.Dekkers JF, Gogorza Gondra RA, Kruisselbrink E, et al. Optimalcorrection of distinct CFTR folding mutants in rectal cystic fibrosis organoids. Eur Respir J. 2016;48:451–458. [DOI] [PubMed] [Google Scholar]
- 113.Dekkers JF, van der Ent CK, Beekman JM. Novel opportunities forCFTR-targeting drug development using organoids. Rare Dis. 2013;1: e27112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dekkers JF, Wiegerinck CL, de Jonge HR, et al. A functional CFTRassay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. [DOI] [PubMed] [Google Scholar]
- 115.J Dekkers R, Vijftigschild LA, Vonk AM, et al. A bioassay usingintestinal organoids to measure CFTR modulators in human plasma. J Cyst Fibros. 2015;14:178–181. [DOI] [PubMed] [Google Scholar]
- 116.Noordhoek J, Gulmans V, van der Ent K, Beekman JM. Intestinalorganoids and personalized medicine in cystic fibrosis: a successful patient-oriented research collaboration. Curr Opin Pulm Med. 2016;22:610–616. [DOI] [PubMed] [Google Scholar]
- 117.Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR byCRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. [DOI] [PubMed] [Google Scholar]
- 118.Zomer-van Ommen DD, Vijftigschild LA, Kruisselbrink E, et al. Limitedpremature termination codon suppression by read-through agents in cystic fibrosis intestinal organoids. J Cyst Fibros. 2016;15:158–162. [DOI] [PubMed] [Google Scholar]
- 119.The 30th Annual North American Cystic Fibrosis Conference,Orange County Convention Center, Orlando, Florida, October 2729, 2016. Pediatr Pulmonol. 2016;51:S1–S507. [Google Scholar]
- 120.de Courcey F, Zholos AV, Atherton-Watson H, et al. Development ofprimaryhumannasalepithelialcellculturesforthestudyofcysticfibrosis pathophysiology. Am J Physiol Cell Physiol. 2012;303:C1173–C1179. [DOI] [PubMed] [Google Scholar]
- 121.Dean N, Ranganath NK, Jones B, et al. Porcine nasal epithelialcultures for studies of cystic fibrosis sinusitis. Int Forum Allergy Rhinol. 2014;4:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grubb BR, Rogers TD, Diggs PC, Boucher RC, Ostrowski LE. Cultureof murine nasal epithelia: model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2006;290:L270–L277. [DOI] [PubMed] [Google Scholar]
- 123.J Mosler K, Coraux C, Fragaki K, et al. Feasibility of nasal epithelialbrushing for the study of airway epithelial functions in CF infants. Cyst Fibros. 2008;7:44–53. [DOI] [PubMed] [Google Scholar]
- 124.Hollenhorst MI, Richter K, Fronius M. Ion transport by pulmonaryepithelia. J Biomed Biotechnol. 2011;2011:174306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ,Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.J Poole A, Urbanek C, Eng C, et al. Dissecting childhood asthma withnasal transcriptomics distinguishes subphenotypes of disease. Allergy Clin Immunol. 2014;133:670–678 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells inducethe conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palechor-Ceron N, Suprynowicz FA, Upadhyay G, et al. Radiationinduces diffusible feeder cell factor(s) that cooperate with ROCK inhibitor to conditionally reprogram and immortalize epithelial cells. Am J Pathol. 2013;183:1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suprynowicz FA, Upadhyay G, Krawczyk E, et al. Conditionallyreprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA. 2012;109:20035–20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu X, Mimms R, Banigan M, et al. Development of glandular modelsfrom human nasal progenitor cells. Am J Respir Cell Mol Biol. 2015;52: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Firth AL, Dargitz CT, Qualls SJ, et al. Generation of multiciliated cellsin functional airway epithelia from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2014;111:E1723–E1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Firth AL, Menon T, Parker GS, et al. Functional gene correction forcystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep. 2015;12:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mou H, Zhao R, Sherwood R, et al. Generation of multipotent lungand airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Benam KH, Villenave R, Lucchesi C, et al. Small airway-on-a-chipenables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13:151–157. [DOI] [PubMed] [Google Scholar]
- 135.Sellgren KL, Butala EJ, Gilmour BP, Randell SH, Grego S. A biomimetic multicellular model of the airways using primary human cells. Lab Chip. 2014;14:3349–3358. [DOI] [PubMed] [Google Scholar]
- 136.Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study ofVX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Davies JC, Cunningham S, Harris WT, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Milla CE, Ratjen F, Marigowda G, et al. Lumacaftor/Ivacaftorin patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2017;195: 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schneider EK, Reyes-Ortega F, Wilson JW, et al. Development ofHPLC and LC-MS/MS methods for the analysis of ivacaftor, its major metabolites and lumacaftor in plasma and sputum of cystic fibrosis patients treated with ORKAMBI or KALYDECO. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1038:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]