Abstract
Bacterial cultivation techniques are classic, basic, and common processes used to characterize the physiological activity of bacteria in their environment. Owing to recent advances in bacterial cultivation techniques, the physiological activity of bacteria can be elucidated at the single‐cell culture level. Here, we report a novel method to monitor the real‐time activity of bacterial growth at the single‐cell level inside giant unilamellar vesicles (GUVs). This method consists of two steps: 1) encapsulation of single bacteria in 1–33 pL scale GUVs and 2) immobilization of the GUVs on a planar lipid bilayer membrane on a glass surface. We directly observed single E. coli cells actively growing to a great number of cells inside GUVs. GUVs also protected the bacteria from external antibiotic compounds during prolonged cultivation for more than 24 h. This approach can be applied widely in the fields of biochemistry, biotechnology, microbiology, and synthetic biology.
Keywords: artificial cell-based incubators, bacterial cultivation, bioreactors, giant unilamellar vesicles, single-cell cultures
Bacteria are important and essential for human activities, such as healthcare, food production, depletion of energy source, and environmental issues.1 The number of bacteria on Earth is estimated to be on the order of 1030 cells.2 Bacterial culture techniques are undoubtedly required to understand their physiological functions and metabolic pathways or to make their metabolic products and chemical compounds.3 Numerous culture techniques have been developed over the past 100 years. Classically, Petri dishes, flasks, and bioreactors are popular tools to culture bacteria, but they have a risk of contamination between cultures because of the difficulty in isolating a single bacterial cell from a huge population.4 To utilize unknown bacteria, technological advances are critically needed to efficiently culture bacteria from a large bacterial population at the single‐cell level in a small, confined space.4,5 Owing to recent technological advances, microfluidic techniques, which use microchips,6 gel droplets,7 and water‐in‐oil (W/O) droplets,8 bacteria can be cultivated at the single‐cell level in a restricted space. Moreover, the cultivation of bacteria in a small, confined space has been adapted for applications in analyzing bacterial function, such as observation of single bacterial behavior,6a,6b,8a phenotypic variability,6c and antibiotic resistance.7a,8b To address the fundamental challenge in understanding or utilizing bacteria, further technical development of cultivation techniques at the single‐cell level is indispensable, and poses significant challenges.
Giant unilamellar vesicles (GUVs) resemble a biological cell membrane that protects an intracellular component from an external environment. Recently, GUVs have been used as a robust microreactor system, especially in the construction of artificial cell‐like systems, which encapsulate biological components and biochemical reaction networks.9 Among numerous GUV synthesis methods, the droplet transfer method, also known as the inverted emulsion method, is very easy and is widely used.10 The droplet transfer method is a very simple mechanism for GUV generation. Lipid‐stabilized W/O droplets (inner leaflet of the membrane) cross the lipid‐stabilized oil–water interface (outer leaflet of the membrane) and the transferred W/O droplets then form unilamellar membrane vesicles. The advantage of GUVs made by droplet transfer is the high efficiency of encapsulation of biomolecules, microspheres, and cells.9c,11 Moreover, GUVs made by droplet transfer have high membrane unilamellarity, which is over 90 % of total GUVs.12 As the membrane of GUVs is unilamellar, it is possible to change the aqueous conditions of the internal and external solutions of GUVs through natural permeability9d,13 or reconstruction of a membrane pore9c,14 or transporter.15 Therefore, GUVs have a great unrealized potential as a bacterial culture system.
Herein, we demonstrate bacterial growth events at the single‐cell level inside single GUVs. We built a robust culture system in which GUVs are immobilized on a planar lipid bilayer membrane at a glass surface. Stabilization of GUVs was assessed by direct observation for over 24 h under culture conditions, and we observed bacterial growth inside GUVs against external antibiotic stimuli. Furthermore, growth events at the single‐cell level were directly monitored in real time and the degree of bacterial growth was analyzed. This system can offer a novel tool to allow users to culture bacteria for bacterial studies.
Figure 1 illustrates the main experimental procedure to produce GUVs containing bacteria and our observation system of bacterial culture within GUVs. To generate bacteria‐containing GUVs, we used the droplet‐shooting and size‐filtration (DSSF) method,10e which is based on the droplet transfer method (Figure 1 a). In this study, 1× lysogeny broth (LB) medium with glucose (200 mm) was used as an outer solution of the GUVs and 1× LB medium with sucrose (200 mm) was used as an inner solution of the GUVs. Water droplets containing bacteria are released from the glass capillary by centrifugal force, and W/O microdroplets containing bacteria are formed in an oil phase involving lipids. W/O microdroplets are transferred through the oil–water interface, and then wrapped in the outer layer of the GUV membrane. The difference in molecular weight (density) between sucrose and glucose also assists the interfacial passage of the W/O microdroplets. We obtained GUVs with sizes ranging from approximately 5 to 40 μm. In our experiment, GUVs were prepared with 1 mm 1‐palmitoyl‐2‐oleoyl‐sn‐glycero‐3‐phosphocholine (POPC) including 0.2 mol % of 1,2‐distearoyl‐sn‐glycero‐3‐phosphoethanolamine‐N‐[biotinyl(polyethyleneglycol)‐2000] (biotin‐PEG‐DSPE) for GUV immobilization on a neutravidin‐coated planar lipid bilayer membrane surface. For direct observation of bacterial cultures inside GUVs, we constructed a modified culture system based on the microreactor system developed by Danelon and co‐workers9d (Figure 1 b). Bacteria‐containing GUVs were immobilized on a neutravidin‐coated planar lipid bilayer membrane surface. To make a planar lipid bilayer membrane, small unilamellar vesicles (SUVs) were created by the extruder method,16 using the same lipid composition (100:0.2 molar ratio of POPC/biotin–PEG–DSPE) of GUVs and were spread on clean cover glass. Next, we added neutravidin to immobilize bacteria‐containing GUVs through biotin–PEG–DSPE binding. Finally, the solution containing GUVs was poured into a handmade chamber, in which the temperature was maintained at 37 °C by using a thermoplate (Figure 1 b).
Figure 1.
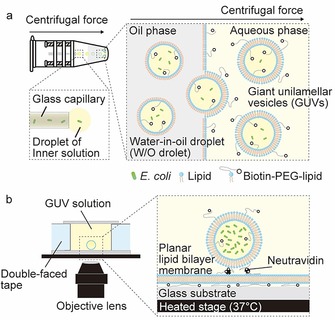
The synthesis of GUVs containing bacteria and the bacterial culture system within GUVs. a) Encapsulation of bacteria inside GUVs by using the droplet‐transfer method. Water droplets containing bacteria are released from the glass capillary by centrifugal force, and W/O microdroplets containing bacteria are formed in an oil phase containing lipids. W/O microdroplets are transferred through an oil–water interface and then wrapped in the outer layer of the GUV membrane by centrifugal force. b) The GUV solution is poured into a handmade chamber. GUVs are immobilized on the surface of a planar‐lipid bilayer through biotin–neutravidin binding and are incubated at 37 °C.
First, we examined the stability of GUVs immobilized on glass substrates coated with a planar lipid bilayer membrane under culture conditions at 37 °C. All GUVs were imaged with phase‐contrast (dark contrast) microscopy. Contrast of all GUVs were caused by differences in the refractive index between internal solution (200 mm sucrose and 1× LB medium) and external solution (200 mm glucose and 1× LB medium) of GUVs (Figure 2 a). If the refractive index of internal solution is higher than that of external solution, inside of the GUVs are darker than external area. A white halo around the edges is an artifact that appears in the phase‐contrast images. Approximately 80 % of GUVs were observed within 2 h, and it was surprising that over 70 % of all GUVs were stably observed and maintained a remarkably consistent sphere even after 24 h. The stability of GUVs was not correlated with GUV size. In damaged GUVs, it was rarely observed that the membrane remained, whereas the inner and outer solutions likely mixed (white arrow in Figure 2 a). This type of damaged GUV is considered to be partially defective, likely because of a small pore in the membrane. When a non‐treated commercial glass substrate was used, GUVs ruptured within 2 h at 37 °C (Figure S1 a). Presumably, GUVs were directly absorbed onto the glass substrate through density differences between the internal and external solutions. In addition, we tested the stability of GUVs immobilized on a glass substrate coated with BSA. Approximately 80 % of all GUVs were observed within 2 h, at the same ratio as in our proposed immobilization technique (Figure S1 b–d). However, some GUVs were deformed, and only 20 % of the GUVs remained after 24 h (Figure S1 b–d). Nonspecific binding between the BSA and GUV membrane may cause deformation and rupture of GUVs. In contrast, in our proposed system, electrostatic repulsion between the GUVs and planar membrane prevented GUVs from directly adhering to the glass substrate. These results suggest that our immobilization technique is superior to stably observe GUVs for a long time under culture conditions.
Figure 2.
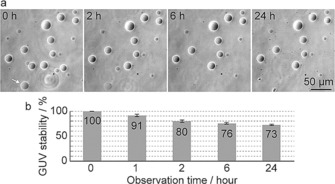
Stabilization analysis of GUVs under bacterial culture conditions (37 °C incubation). a) Phase‐contrast images of GUVs after the indicated incubation time at 37 °C. The white arrow shows a GUV, in which the inner and outer GUV solutions were mixed, likely because of a membrane defect. b) The percentage of intact GUVs remaining at the indicated incubation time.
To investigate the growth of encapsulated bacteria inside GUVs, we used Escherichia coli K12 as a bacterial model. GUVs were stained with 1 μm of rhodamine‐B 1,2‐dihexadecanoyl‐sn‐glycero‐3‐phosphoethanolamine (rhodamine‐DHPE) and E. coli cells were stained with 5 μm SYTO9, which is a membrane‐permeable green fluorescent nucleic acid stain. The presence of E. coli inside GUVs was determined by using confocal laser‐scanning (CLS) microscopy. To encapsulate single or several cells in GUVs, precultured bacterial solution was diluted with 1× LB medium to OD600=0.01–0.015. Figure 3 a shows a fluorescence image of a GUV containing two E. coli cells. As expected, several cells could be successfully encapsulated into GUVs, but GUVs containing more than ten cells were infrequently observed (Figure S2). Further, to confirm whether all E. coli cells are preserved within GUVs, we recorded three‐dimensional (3D) images. More than ten E. coli cells were encapsulated into a GUV (Figure 3 b and see Movie 1 in the Supporting Information), and all cells were observed to move inside the GUV (Figure 3 c and Movie 2). These images and videos demonstrate that this method successfully encapsulates E. coli in GUVs.
Figure 3.
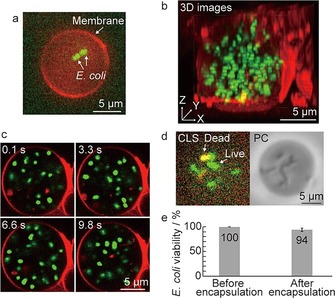
Encapsulation of E. coli into GUVs. a) CLS microscopic image in the equatorial plane of a GUV containing SYTO9‐stained E. coli cells. Visualization of GUV membrane by rhodamine–DHPE staining. b) 3D reconstruction of a GUV containing SYTO9‐stained E. coli cells. c) Snapshots of the random motion of the bacteria inside a GUV. d) Visualization of live and dead E. coli cells inside a GUV using SYTO9 and PI staining by CLS microscopy (left) and phase‐contrast (PC) image of the same GUV (right). SYTO9 (shown in green) stains both live and dead E. coli cells and PI (shown in red) stains only dead E. coli cells. Yellow represents overlap between the two colors, indicating dead cells. e) Quantification of E. coli cell viability before (n=223) and after (n=404) encapsulation in GUVs.
Next, we quantified the viability of E. coli encapsulated inside GUVs by staining the cells with 5 μm SYTO9 and propidium iodide (PI). SYTO9 is a nuclear counterstain that identifies both live and dead cells, whereas PI can only penetrate compromised membranes to visualize dead cells.17 Thus, the colocalization of SYTO9 and PI allows for the unambiguous determination of dead bacteria inside GUVs (Figures 3 d and S3). From our experiments, we determined that 94 % of E. coli cells were alive compared to the number of cells before encapsulation (Figure 3 e), a finding that is consistent with a previous report.11c These results indicate that our approach encapsulates live E. coli in GUVs.
We also performed direct (time‐lapse) observation of bacterial growth at the single‐cell level inside GUVs (Figure 4 a). Initially, E. coli inside GUVs grew from two to four cells during the first 20 min, and then divided to eight cells in the next 50 min (Figure 4 b and Movie 3 in the Supporting Information). Subsequently, the cells repeated the elongation and division processes, with encapsulated E. coli growing to an enormous number of cells over 2–3 h. Furthermore, we successfully observed E. coli growth inside GUVs for about 48 h. As the interdivision time of E. coli is approximately 20–30 min,18 our findings showed that E. coli grows normally and stably inside GUVs.
Figure 4.
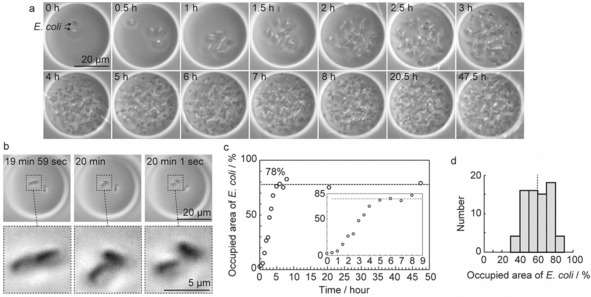
The growth of E. coli inside GUVs in the external presence of ampicillin (1 μg mL−1). a) Time‐lapse images of E. coli growth inside a GUV. Cell numbers increased by repeated cycles of elongation and division. b) Real‐time observation of E. coli division during the first 20 min. c) Time course of E. coli growth inside a GUV. The E. coli growth rate was analyzed as the ratio of the area occupied by E. coli to the GUV area from the original image. d) Histogram of E. coli growth rate after incubation for 5 h, with a mean of 60 % indicated as a vertical dotted line and a standard deviation of 13 % (n=73).
To determine whether the GUV membrane was permeable to substances, we monitored bacterial growth inside GUVs following the addition of an external stimulus, which for this test was the antibiotic ampicillin (1 μg mL−1) outside GUVs. We confirmed that 1 μg mL−1 ampicillin did not affect GUV stability (Figure S4) and that this concentration of ampicillin was sufficient to inhibit growth of E. coli (Figure S5). We found that ampicillin treatment had no effect on the growth of E. coli inside GUVs (Figure 4 a), but E. coli outside GUVs, and thus exposed to ampicillin, only elongated (Figure S6). Furthermore, we observed that E. coli within a defective GUV could not divide in the external presence of ampicillin (Figure S7 a). However, in the absence of ampicillin, we found that the growth of E. coli inside the defective GUVs was similar to that found in intact GUVs (Figures 4 a and S7 b). During these observations, there was no leakage of E. coli from GUVs. Importantly, as shown in the imaging results, a GUV provides a stable and robust chamber, in which bacteria can live and grow for an extended period of time, and it also probably acts as a barrier, with our ampicillin‐based results demonstrating its effectiveness in protecting bacteria from exposure to an external antibiotic stress. However, the barrier effect of the POPC membrane may change by varying the experimental conditions such as lipids and ampicillin concentration. Moreover, our findings from defective GUVs suggest the possibility of controlling the growth of bacteria inside a GUV by external stimuli through altering its structure.
On a final note, to quantify the growth rate of E. coli, we independently analyzed an area inside a GUV occupied by E. coli from the obtained images. A binarized image was made by using ImageJ and the cell‐occupied area inside the GUV was examined (Figure S8). As shown in Figure 4 c, the occupied area reached a saturation level 78 % of the area of the equatorial plane of GUVs within approximately 5 h. The average cell‐occupied area after 5 h was 60±13 % (mean ± SD; n=73; Figures 4 d and S9). The growth rate of E. coli was clearly different among vesicles. We consider four possibilities that account for this observed variation in growth rate: 1) the number of encapsulated cells was not uniform (Figures 3 a, 3 b, and S2), 2) the size of GUVs was not uniform (relating to the amount of nutrients), 3) E. coli died during culture and growth stopped, and 4) variability of growth events between single E. coli.6c Of importance will be to improve control over the number of encapsulated cells and/or uniform size of GUVs. Currently, the reason for this variation is not entirely clear and is a fruitful subject for future study.
In conclusion, we established a new method for bacterial culture from a single cell by using GUVs. We successfully constructed a direct observation system of GUVs by immobilizing GUVs on the surface of a planar lipid bilayer membrane, which dramatically improved stability under culture conditions at 37 °C. This observation system can be applied not only to bacterial culture, but also to other research areas that require long‐term observation, such as protein synthesis,9c,9d gene circuit,19 and metabolic engineering.20 Moreover, our results clearly demonstrate that we succeeded in encapsulating bacteria inside GUVs at the single‐cell level. As our system also observes bacterial growth at the single‐cell level, the risk of contamination between cultures is inherently avoided. In addition, to clarify the role of GUVs as a stable chamber for bacterial culture, we proved using an externally administrated antibiotic that GUVs protect internal bacteria from external stimuli. Further, we showed that the growth state of bacteria can be controlled by the presence of an antibiotic inside defective GUVs possessing a small pore in the membrane. Although these defective GUVs show a vulnerability of our approach, it is expected to be improved by using an artificial cytoskeleton that stabilizes GUVs.21 Moreover, GUVs have recently been used as a suitable material for artificial cell‐like systems, incorporating biological components such as DNA, RNA, and proteins.9c,11,14,15 Artificial cell‐like systems offer a unique possibility to control the condition of the internal solution by changing the substrate or pH through a transmembrane protein.14 Further, controlling the internal condition may affect the growth rate of bacterial cultures; however, our approach does not yet enable control over the condition of the internal solution, but nevertheless, the possibility remains. Our method has potential applicability from artificial cell‐like systems research to the development of bacterial culture systems for a wide range of fields, including microbiology, synthetic biology, and the food industry, and this report is the first step toward culturing living cells inside artificial cell‐like systems.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Supplementary
Supplementary
Supplementary
Acknowledgements
This study was supported by a Leading Initiative for Excellent Young Researchers (LEADER) (16812285) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, a Grant‐in‐Aid for Young Scientist Research (18K18157, 16K21034) from Japan Society for the Promotion of Science (JSPS) to M.M., and Grant‐in‐Aid from MEXT to K.K. (17H06417, 17H06413)
M. Morita, K. Katoh, N. Noda, ChemistryOpen 2018, 7, 845.
Contributor Information
Dr. Masamune Morita, Email: morita.m9@aist.go.jp.
Dr. Naohiro Noda, Email: noda-naohiro@aist.go.jp.
References
- 1. Gilbert J. A., Neufeld J. D., PLoS Biol. 2014, 12, e1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitman W. B., Coleman D. C., Wiebe W. J., Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zengler K., Toledo G., Rappe M., Elkins J., Mathur E. J., Short J. M., Keller M., Proc. Natl. Acad. Sci. USA 2002, 99, 15681–15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaminski T. S., Scheler O., Garstecki P., Lab Chip 2016, 16, 2168–2187. [DOI] [PubMed] [Google Scholar]
- 5. Kou S., Cheng D., Sun F., Hsing I.-M., Lab Chip 2016, 16, 432–446. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Inoue I., Wakamoto Y., Moriguchi H., Okano K., Yasuda K., Lab Chip 2001, 1, 50–55; [DOI] [PubMed] [Google Scholar]
- 6b. Wang P., Robert L., Pelletier J., Dang W. L., Taddei F., Wright A., Jun S., Curr. Biol. 2010, 20, 1099–1103; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c. Hashimoto M., Nozoe T., Nakaoka H., Okura R., Akiyoshi S., Kaneko K., Kussell E., Wakamoto Y., Proc. Natl. Acad. Sci. USA 2016, 113, 3251–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.
- 7a. Eun Y., Utada A. S., Copeland M. F., Takeuchi S., Weibel D. B., ACS Chem. Biol. 2011, 6, 260–266; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b. Connell J. L., Ritschdorff E. T., Whiteley M., Shear J. B., Proc. Natl. Acad. Sci. USA 2013, 110, 18380–18385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.
- 8a. Boedicker J. Q., Vincent M. E., Ismagilov R. F., Angew. Chem. Int. Ed. 2009, 48, 5908–5911; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 6022–6025; [Google Scholar]
- 8b. Jakiela S., Kaminski T. S., Cybulski O., Weibel D. B., Garstecki P., Angew. Chem. Int. Ed. 2013, 52, 8908–8911; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 9076–9079; [Google Scholar]
- 8c. Shim J. U., Olguin L. F., Whyte G., Scott D., Babtie A., Abell C., Huck W. T. S., Hollfelder F., J. Am. Chem. Soc. 2009, 131, 15251–15256; [DOI] [PubMed] [Google Scholar]
- 8d. Schmitz C. H. J., Rowat A. C., Köster S., Weitz D. A., Lab Chip 2009, 9, 44–49; [DOI] [PubMed] [Google Scholar]
- 8e. Frenz L., Blank K., Brouzes E., Griffiths A. D., Lab Chip 2009, 9, 1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.
- 9a. Szostak J. W., Bartel D. P., Luisi P. L., Nature 2001, 409, 387–390; [DOI] [PubMed] [Google Scholar]
- 9b. Nomura S. M., Tsumoto K., Hamada T., Akiyoshi K., Nakatani Y., K, Yoshikawa , ChemBioChem 2003, 4, 1172–1175; [DOI] [PubMed] [Google Scholar]
- 9c. Noireaux V., Libchaber A., Proc. Natl. Acad. Sci. USA 2004, 101, 17669–17674; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9d. Nourian Z., Roelofsen W., Danelon C., Angew. Chem. Int. Ed. 2012, 51, 3114–3118; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 3168–3172. [Google Scholar]
- 10.
- 10a. Pautot S., Frisken B. J., Weitz D. A., Langmuir 2003, 19, 2870–2879; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Pautot S., Frisken B. J., Weitz D. A., Proc. Natl. Acad. Sci. USA 2003, 100, 10718–10721; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10c. Matosevic S., Paegel B. M., J. Am. Chem. Soc. 2011, 133, 2798–2800; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10d. Abkarian M., Loiseau E., Massiera G., Soft Matter 2011, 7, 4610–4614; [Google Scholar]
- 10e. Morita M., Onoe H., Yanagisawa M., Ito H., Ichikawa M., Fujiwara K., Saito H., Takinoue M., ChemBioChem 2015, 16, 2029–2035. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Tan Y.-C., Hettiarachchi K., Siu M., Pan Y.-R., Lee A. P., J. Am. Chem. Soc. 2006, 128, 5656–5658; [DOI] [PubMed] [Google Scholar]
- 11b. Natsume Y., Toyota T., Chem. Lett. 2013, 42, 295–297; [Google Scholar]
- 11c. Chowdhuri S., Cole C. M., Devaraj N. K., ChemBioChem 2016, 17, 886–889. [DOI] [PubMed] [Google Scholar]
- 12. Chiba M., Miyazaki M., Ishiwata S., Biophys. J. 2014, 107, 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.
- 13a. Li S., Hu P., Malmstadt N., Anal. Chem. 2010, 82, 7766–7771; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13b. Li S., Hu P. C., Malmstadt N., Biophys. J. 2011, 101, 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.
- 14a. Tamba Y., Yamazaki M., Biochemistry 2005, 44, 15823–15833; [DOI] [PubMed] [Google Scholar]
- 14b. Mishra A., Lai G. H., Schmidt N. W., Sun V. Z., Rodriguez A. R., Tong R., Tang L., Cheng J., Deming T. J., Kamei D. T., Wong G. C., Proc. Natl. Acad. Sci. USA 2011, 108, 16883–16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.
- 15a. Dezi M., Di Cicco A., Bassereau P., Levy D., Proc. Natl. Acad. Sci. USA 2013, 110, 7276–7281; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. Valkenier H., Mora N. L., Kros A., Davis A. P., Angew. Chem. Int. Ed. 2015, 54, 2137–2141; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 2165–2169. [Google Scholar]
- 16. Hope M. J., Bally M. B., Webb G., Cullis P. R., Biochim. Biophys. Acta. 1985, 812, 55–65. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. J., Immunol. Methods 1991, 139, 271–279; [DOI] [PubMed] [Google Scholar]
- 17b. Stiefel P., Schmidt-Emrich S., Maniura-Weber K., Ren Q., BMC Microbiol. 2015, 15, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.
- 18a. Taheri-Araghi S., Bradde S., Sauls J. T., Hill N. S., Levin P. A., Paulsson J., Vergassola M., Jun S., Curr. Biol. 2015, 25, 385–391; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18b. Reshes G., Vanounou S., Fishov I., Feingold M., Biophys. J. 2008, 94, 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a. Weitz M., Mückl A., Kapsner K., Berg R., Meyer A., Simmel F. C., J. Am. Chem. Soc. 2014, 136, 72–75; [DOI] [PubMed] [Google Scholar]
- 19b. Montagne K., Plasson R., Sakai Y., Fujii T., Rondelez Y., Mol. Syst. Biol. 2011, 7, 466; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19c. Weitz M., Kim J., Kapsner K., Winfree E., Franco E., Simmel F. C., Nat. Chem. 2014, 6, 295–302. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Trentacoste E. M., Shrestha R. P., Smith S. R., Glé C., Hartmanna A. C., Hildebrand M., Gerwick W. H., Proc. Natl. Acad. Sci. USA 2013, 110, 19748–19753; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20b. Wang B. L., Ghaderi A., Zhou H., Agresti J., Weitz D. A., Fink G. R., Stephanopoulos G., Nat. Biotechnol. 2014, 32, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurokawa C., Fujiwara K., Morita M., Kawamata I., Kawagishi Y., Sakai A., Murayama Y., Nomura S. M., Murata S., Takinoue M., Yanagisawa M., Proc. Natl. Acad. Sci. USA 2017, 114, 7228–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Supplementary
Supplementary
Supplementary


