Abstract
Background:
Skull base chordomas are locally invasive tumors which able to extend in different directions with skull base invasion. Although they are histologically benign, they have invasive nature makes total resection virtually impossible to achieve in most cases and this lead to residual tumors after surgery. To decrease postoperative surgical resection morbidity of these tumors, gamma knife radiosurgery (GKRS) was performed as alternative management for these residual chordomas to evaluate its safety and efficacy.
Materials and Methods:
A retrospective study was made on eight residual skull base chordomas treated with GKRS between 2011 and 2015. The mean patient age was 49 years (range 30–73 years). Four patients harboring chordoma were male, and four patients were females with 1:1 ratio. All patients had undergone one prior surgery. Patients were treated with peripheral dose ranged between 12–15 gray (Gy) (mean 13.75 Gy) usually at 35% to 50% isodose curve (mean 38.8%). The maximum dose to the adjacent brain stem area ranged between 10 and 12 Gy. All patients were followed up from 8 to 39 months (mean 18 months).
Results:
The tumor control rate was 50% and 25% after 18 and 36 months, respectively, but we found that their wasdeclined in the tumor control rate with long follow-up time. Four tumors were stable in their size just for 18 months, and then there two of these tumors were progressed in their size, the other four patients showed progression in their tumors in their 1st year of treatment without sign of central tumor necrosis.
Conclusion:
Skull base chordoma patients complained from symptoms due to tumor mass effect which were not prospected to respond to GKRS alone as the aim of this type of treatment was the local tumor control, the tumor control rate declined with long follow-up time and this correlated with radioresistant nature of skull base chordoma. We advise a gross total resection to decrease the tumor volume, and this making gamma knife a reasonable treatment modality.
Keywords: Computed tomography, gamma knife radiosurgery, gray, International Medical Center, magnetic resonance imaging
Introduction
Chordomas are considered as slowly growing, locally aggressive, malignant tumors derived from embryonic remnants of the notochord. Nearly 35% of these neoplasms arise from the skull base, and these constitute 0.1% to 0.2% of primary intracranial tumors. The male/female ratio in chordomas is 1.5:1, with a mean age of patients of 46 years.[1]
Chordomas are located in the posterior fossa with not uncommon extension into the sphenoid sinus, parapharyngeal space, nasopharynx, suprasellar cistern, and middle cranial fossa in 79% of cases. In 65% of cases, we have cavernous sinus involvement, encasement of the internal carotid or basilar artery occurs in 36%. By the time of surgery, one-third of the tumors can exhibit dural erosion.[2]
Macroscopically chordoma is a lobulated, soft gelatinous mass with boney destruction. Calcification is common, it may show areas of hemorrhage and necrosis and usually grossly appears demarcated. We have three subtypes of chordomas identified pathologically.[3]
The most common variant shows no evidence of cartilaginous or additional mesenchymal components. It is formed of cells arranged in cords with eosinophilic cytoplasm lying in a mucinous background. Cytoplasmic vacuolation is common properties. Lobules of tumor cells usually separated by fibrovascular septa. Mitotic figures, nuclear pleomorphism, areas of necrosis, hemorrhage, and calcification may be present without significance in prognostic outcome.[4]
Subtotal tumor resection with preservation of functionally ponents in a highly variable ratio. The cartilaginous component of the tumor is either histologically benign or malignant. Dedifferentiation of these tumors is rare. They contain the areas of conventional chordoma as well as malignant mesenchymal components, which result in poor prognosis and most patients are dead of disease within 6–12 months. Metastases can develop, with the most common sites being the lungs, bones, lymph nodes, liver, and skin.[5]
We can diagnose this case by the clinical presentations which depend on the site of origin and direction of growth. The most typical symptoms are diplopia (49%), headache (24%), and ataxia (4%). Sixth nerve palsy is encountered in 57% of cases, lower cranial nerves in 36%, sensory fifth in 27%, third nerve in 22%, and optic neuropathy in 12%. The median time from symptom onset to diagnosis has been reported as 10 months.[1]
Radiographic assessment of these tumors reveals a midline soft-tissue mass associated with osteolytic bone destruction and calcifications. The typical computed tomography of chordoma showing a well-defined extra-axial mass, with both hyperdense and hypodense areas associated with bone destruction, foci of calcification, and invasions of adjacent neural structures in varying degrees.[6]
On T1-weighted magnetic resonance imaging (MRI), chordomas have low-to-intermediate signal intensity and typically hyperintense signal on T2-weighted images with heterogeneous enhancement after gadolinium is seen.[7]
The surgical goal gross is total resection of the tumor. Even in the best of circumstances, however, this can be accomplished in only 67% of patients due to critical neurovascular structures involvement.[2]
Local recurrences are the rule and most of these appear within 3 years of treatment with the mean interval to the first recurrence being 12.5 months Due to the fact that the invasive nature of the tumor. Despite various salvage treatments, stabilization of disease at the time of recurrence is uncommon.[5]
For improvement of local control and survival in the incomplete tumor resection cases a postoperative radiotherapy can be used.[1,8]
Materials and Methods
Eight residual skull base chordomas treated with gamma knife radiosurgery (GKRS) between 2011 and 2015 in International Medical Center in Cairo. The mean patient age was 49 years (range 30–73 years). Four patients harboring chordoma were males and four patients were females with 1:1 ratio. All patients had undergone one prior surgery. Patients were treated with peripheral dose ranged between 12 and 15 gray (Gy) (mean 13.75 Gy) usually at 35% to 50% isodose curve (mean 38.8%). The maximum dose to the adjacent brainstem area ranged between 10 and 12 Gy.
Results
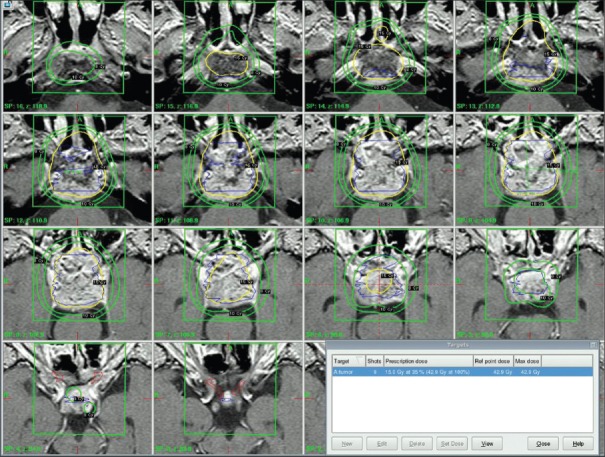
The Elekta Leksell gamma knife was used for the treatment. We did target localization using MRI performed with T1 axial and coronal-weighted sequence at 2 mm slice thickness with and without contrast, to eliminate tumor edema T1-fat saturation sequence and also T2 axial sequence was used. Treatment planning was performed with Elekta Leksell Gamma Plan [Figures 1–3].
Figure 1.
Axial, coronal, saggital cuts of chordoma case targeting
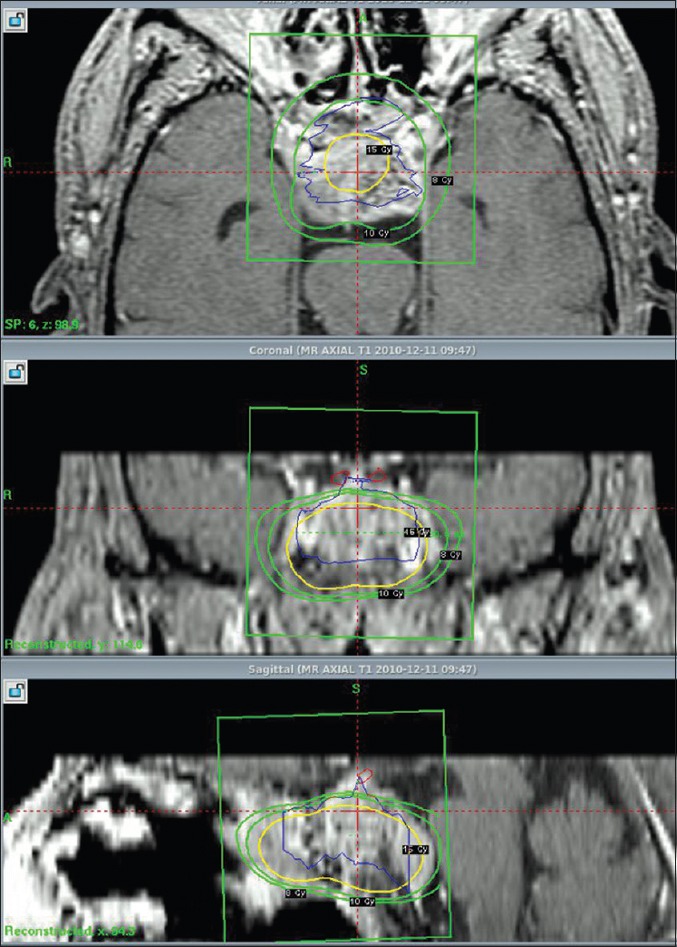
Figure 3.
coronal cuts tumor targeting in Gamma knife protocol of chordoma case
Figure 2.

Reconstruction Open Book of Gamma knife chordoma case
Patients underwent clinical follow-up examination at 3 and 6 months posttreatment and then every 6 months. Neurological status was recorded, and all complications were noted. MR images were obtained every 6 months following radiosurgery. These studies were used to follow the changes in size of treated chordoma and to assess for the presence of radiation-induced central tumoral necrosis. We calculated tumor size by obtaining direct measurements along three axes on follow-up MR images and comparing these values with pretreatment MR imaging. A tumor was considered to have decline in size if it was reduced by 3 mm or greater in one or more dimensions. We considered tumors to have increased in size if one or more dimensions increased by 3 mm or greater. All patients with chordoma were followed up from 8 to 39 months (mean 18 months).
We treated skull base chordoma with tumor control rate 50% and 25% after 18 and 36 months, respectively; however, we found that their were declined in the tumor control rate with long follow-up time. Four tumors were stable in their size just for 18 months, and then there two of these tumors were progressed in their size, the other four patients showed progression in their tumors in their 1st year of treatment without sign of central tumor necrosis [Table 1].
Table 1.
Pre- and post-gamma knife radiosurgery tumor largest diameter

For those patients showed progression in their tumors after radiosurgery, we referred to another surgical interference to improve the patients symptoms.
Discussion
Gamma knife radiosurgery has emerged as a potential treatment for some patients with chordomas. The steep dose gradient obtained with radiosurgery minimizes the amount of radiation that is delivered outside the tumor target. Hence, it is possible to deliver a much larger, and presumably more efficacious, dose to tumor without exceeding the radiation-related tolerance of normal tissues. In addition, several authors have hypothesized that a therapeutic gain may be achieved by treating slowly proliferating tumors as chordomas, with larger-sized fractions, and this treatment is only possible with radiosurgical techniques.[9,10] In this study, we review our experience in the treatment of patients with chordomas in whom GKRS stereotactic radiosurgery was performed. Management of patients with skull base chordomas is difficult, as a moderate to high rate of tumor recurrence and poor long-term prognosis have been reported in this population. Historical treatment has generally consisted of tumor resection alone or resection followed by radiation therapy; however, complete resection is not possible in a significant subset of these patients due to the extensive involvement of the cranial base as neurological structures.[11,12,13] Furthermore, conventional radiotherapy used in the treatment of residual or recurrent chordomas has been shown to be of benefit.[5,14,15,16,17,18,19,20,21]
Despite combined surgery and radiotherapy, progression of the tumor over 5 years has been reported in 24%–83% of patients,[5,14,15,16,20] with 5-year survival ranging from 38 to 79%.[5,14,19,20]
In a select subgroup of patients with residual or recurrent chordoma, Gamma knife radiosurgery can be an important treatment option. The steep dose gradient achievable with radiosurgery minimizes radiation outside the tumor target, allowing the delivery of a much larger, and presumably more efficacious, dose to the tumor without exceeding the radiation-related tolerance of normal tissues. Some authors have hypothesized that a therapeutic gain may be achieved by treating slowly proliferating tumors, such as chordomas, with larger radiation doses without harmful affection of normal neural tissues which is possible with radiosurgical techniques.[10,22]
Muthukumar et al.[23] reported on 15 patients (nine with chordoma and six with chondrosarcoma) in whom gamma knife surgery was used. Doses to the tumor margin were between 12 and 20 Gy, and the maximum tumor dose was 24–40 Gy. Clinical improvement was achieved in eight patients, three remained stable, and four died during the average 4-year follow-up period. Of the surviving 11 patients, the tumor was declined in size in five, had stabilized in size in five, and had increased in size in one.[23]
Miller et al.[24] reviewed the cases of eight patients with skull base chordoma treated with gamma knife surgery. At 2-year follow up, the authors noted a 100% local control rate and 100% patient survival rate and this may due to short follow-up period in this study.
In Liu et al., 2008,[25] who showed tumor control rate 64.2% and 21.4% after 3 and 5 years, respectively, on their 32 patients of skull base chordoma treated with gamma knife radiosurgery.
In our study: we have four patients with stable tumor size after 18 months Post GKRS and just 50% of those patients have no progression in their tumor size in 36 months post GKRS. On the other hand, the rest four patients showed progression in their tumor size in the 1st year of treatment without sign of central tumor necrosis.
The declined in the tumor control rate with long follow-up time was correlated with radioresistant nature of skull base chordoma.
On the other hands, all skull base chordoma patient complained from symptoms due to tumor mass effect which were not prospected to respond to gamma knife radiosurgery as the aim of this type of treatment was the local tumor control; hence, we advise to surgical intervention rather than gamma knife radiosurgey for this type of skull base tumors and the rule of gamma knife is better for residual small size tumors.
Conclusion
Skull base chordoma patients complained from symptoms due to tumor mass effect which was not prospected to respond to gamma knife radiosurgery alone as the aim of this type of treatment was the local tumor control, we found that there were declined in the tumor control rate with long follow-up time and this correlated with radioresistant nature of skull base chordoma. Hence, we advise a gross total resection of the tumor to decrease the volume of the tumor; however, this usually impossible due to the involvement of critical neurovascular structures so the gamma knife for the residual skull base chordomas still a good choice
In patients with advanced age, significant concomitant medical problems, high-risk tumor location or patients who are not willing to undergo an open surgical procedure, we would recommend performing GKRS as a safe and effective alternative primary treatment modality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Forsyth PA, Cascino TL, Shaw EG, Scheithauer BW, O’Fallon JR, Dozier JC, et al. Intracranial chordomas: A clinicopathological and prognostic study of 51 cases. J Neurosurg. 1993;78:741–7. doi: 10.3171/jns.1993.78.5.0741. [DOI] [PubMed] [Google Scholar]
- 2.Maira G, Pallini R, Anile C, Fernandez E, Salvinelli F, La Rocca LM, et al. Surgical treatment of clival chordomas: The transsphenoidal approach revisited. J Neurosurg. 1996;85:784–92. doi: 10.3171/jns.1996.85.5.0784. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L, Kapadia SB. The biology and pathology of selected skull base tumors. J Neurooncol. 1994;20:213–40. doi: 10.1007/BF01053041. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell A, Scheithauer BW, Unni KK, Forsyth PJ, Wold LE, McGivney DJ, et al. Chordoma and chondroid neoplasms of the spheno-occiput. An immunohistochemical study of 41 cases with prognostic and nosologic implications. Cancer. 1993;72:2943–9. doi: 10.1002/1097-0142(19931115)72:10<2943::aid-cncr2820721014>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Hug EB, Loredo LN, Slater JD, DeVries A, Grove RI, Schaefer RA, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432–9. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- 6.Brown RV, Sage MR, Brophy BP. CT and MR findings in patients with chordomas of the petrous apex. AJNR Am J Neuroradiol. 1990;11:121–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers SP, Hirsch WL, Jr, Curtin HD, Barnes L, Sekhar LN, Sen C, et al. Chordomas of the skull base: MR features. AJNR Am J Neuroradiol. 1992;13:1627–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller DB, Bloom JG. Radiotherapy for chordoma. Int J Radiat Oncol Biol Phys. 1988;15:331–9. doi: 10.1016/s0360-3016(98)90012-8. [DOI] [PubMed] [Google Scholar]
- 9.Thames HD, Jr, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: Implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–26. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 10.Withers HR, Thames HD, Jr, Peters LJ. Biological bases for high RBE values for late effects of neutron irradiation. Int J Radiat Oncol Biol Phys. 1982;8:2071–6. doi: 10.1016/0360-3016(82)90547-8. [DOI] [PubMed] [Google Scholar]
- 11.Gay E, Sekhar LN, Rubinstein E, Wright DC, Sen C, Janecka IP, et al. Chordomas and chondrosarcomas of the cranial base: Results and follow-up of 60 patients. Neurosurgery. 1995;36:887–96. doi: 10.1227/00006123-199505000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Gay E, Sekhar LN, Wright DC. Chordomas and chondrosarcomas of the cranial base. In: Kaye AH, Laws ER Jr, editors. Brain Tumors: An Encyclopedic Approach. Edinburgh: Churchill Livingstone; 1995. pp. 777–94. [Google Scholar]
- 13.Watkins L, Khudados ES, Kaleoglu M, Revesz T, Sacares P, Crockard HA, et al. Skull base chordomas: A review of 38 patients, 1958-88. Br J Neurosurg. 1993;7:241–8. doi: 10.3109/02688699309023805. [DOI] [PubMed] [Google Scholar]
- 14.Benk V, Liebsch NJ, Munzenrider JE, Efird J, McManus P, Suit H, et al. Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys. 1995;31:577–81. doi: 10.1016/0360-3016(94)00395-2. [DOI] [PubMed] [Google Scholar]
- 15.Benk V, Liebsch NJ, Munzenrider JE, Efird J, McManus P, Suit H, et al. Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys. 1995;31:577–81. doi: 10.1016/0360-3016(94)00395-2. [DOI] [PubMed] [Google Scholar]
- 16.Fagundes MA, Hug EB, Liebsch NJ, Daly W, Efird J, Munzenrider JE, et al. Radiation therapy for chordomas of the base of skull and cervical spine: Patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys. 1995;33:579–84. doi: 10.1016/0360-3016(95)02014-3. [DOI] [PubMed] [Google Scholar]
- 17.Lybeert ML, Meerwaldt JH. Chordoma. Report on treatment results in eighteen cases. Acta Radiol Oncol. 1986;25:41–3. doi: 10.3109/02841868609136376. [DOI] [PubMed] [Google Scholar]
- 18.O’Connell JX, Renard LG, Liebsch NJ, Efird JT, Munzenrider JE, Rosenberg AE, et al. Base of skull chordoma. A correlative study of histologic and clinical features of 62 cases. Cancer. 1994;74:2261–7. doi: 10.1002/1097-0142(19941015)74:8<2261::aid-cncr2820740809>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Raffel C, Wright DC, Gutin PH, Wilson CB. Cranial chordomas: Clinical presentation and results of operative and radiation therapy in twenty-six patients. Neurosurgery. 1985;17:703–10. doi: 10.1227/00006123-198511000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Romero J, Cardenes H, la Torre A, Valcarcel F, Magallon R, Regueiro C, et al. Chordoma: Results of radiation therapy in eighteen patients. Radiother Oncol. 1993;29:27–32. doi: 10.1016/0167-8140(93)90169-9. [DOI] [PubMed] [Google Scholar]
- 21.Tai PT, Craighead P, Bagdon F. Optimization of radiotherapy for patients with cranial chordoma. A review of dose-response ratios for photon techniques. Cancer. 1995;75:749–56. doi: 10.1002/1097-0142(19950201)75:3<749::aid-cncr2820750302>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Muthukumar N, Kondziolka D, Lunsford LD, Flickinger JC. Stereotactic radiosurgery for chordoma and chondrosarcoma: Further experiences. Int J Radiat Oncol Biol Phys. 1998;41:387–92. doi: 10.1016/s0360-3016(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 24.Miller RC, Foote RL, Coffey RJ, Gorman DA, Earle JD, Schomberg PJ, et al. The role of stereotactic radiosurgery in the treatment of malignant skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39:977–81. doi: 10.1016/s0360-3016(97)00377-5. [DOI] [PubMed] [Google Scholar]
- 25.Liu AL, Wang ZC, Sun SB, Wang MH, Luo B, Liu P, et al. Gamma knife radiosurgery for residual skull base chordomas. Neurol Res. 2008;30:557–61. doi: 10.1179/174313208X297878. [DOI] [PubMed] [Google Scholar]