Abstract
Objectives:
The objective of this study was to compare safety and efficacy of endoscopic third ventriculostomy (ETV) versus ventriculoperitoneal (VP) shunt in the treatment of hydrocephalus in tuberculous meningitis (TBM) and to assess clinical and radiological profiles of patients with TBM that would be better suited to either VP shunt or ETV.
Methods:
This study was a single-center randomized prospective study on 52 patients with TBM hydrocephalus in the pediatric age group (<18 years of age). Patients included in the study were randomized into undergo either VP shunt or ETV. Both groups were followed up for a minimum of 5 months and assessed for success and failure rates as well as procedural complications and neurologic sequelae.
Results:
Twenty-six patients underwent ETV with a success rate of 65.4% with six of nine failures occurring within the first 16 days after surgery (median time to failure – 3 days). In the VP shunt group, there was a success rate of 61.54% and a median time to failure of 50 days. Modified Vellore grading was found to be a significant factor in determining outcome in both ETV and VP shunt groups with high-grade TBM consistently associated with poor outcome (odds ratio = 4.2).
Conclusions:
ETV can be performed effectively in young children including infants, as well as those with communicating hydrocephalus, high cerebrospinal fluid (CSF) cell counts, and protein levels with a lower rate of failure than that of VP shunt. Hence, ETV should be attempted as the first-choice CSF diversion procedure in hydrocephalus secondary to TBM where technical expertise and experience with this procedure is available as it avoids the myriad of lifelong complications associated with shunts.
Keywords: Endoscopic third ventriculostomy, ventriculoperitoneal shunt, tuberculous meningitis
Introduction
Hydrocephalus is one of the most common complications of tuberculous meningitis (TBM) occurring in up to 85% of children with the disease and still remains a major cause of childhood morbidity and mortality in India.[1] Among the surgical procedures to treat TBM hydrocephalus, ventricular shunting, most commonly ventriculoperitoneal (VP) shunting, has been the procedure of choice so far.[1,2] However, complications of shunt surgery in patients with TBM and hydrocephalus are high with frequent shunt obstructions and shunt infections requiring repeated revisions.[3] Endoscopic third ventriculostomy (ETV) is inherently superior to the shunt in the treatment of hydrocephalus, circumventing the need for insertion of a foreign body, and avoiding shunt-related morbidity.[3] However, its efficacy in hydrocephalus due to TBM has never been proven. Hence, this study was undertaken to objectively compare the outcomes of these two procedures in the management of hydrocephalus secondary to TBM.
Aims and objectives
The aims and objectives of this study were as follows
To compare safety and efficacy of ETV versus VP shunt in the treatment of hydrocephalus in TBM
To assess clinical and radiological profiles of patients with TBM that would be better suited to either VP shunt or ETV.
Methods
This study was a randomized prospective study on 52 patients with TBM hydrocephalus in the pediatric age group (<18 years of age) conducted between December 2015 and June 2017 that were referred to our institute. All patients underwent baseline investigations including those required for the diagnosis of TBM according to the consensus definition of TBM (2010) [4] based on clinical, cerebrospinal fluid (CSF) and radiological criteria. [Tables 1 and 2]. Patients included in the study were randomized into undergo either VP shunt or ETV. Patients that had previously undergone a CSF diversion procedure and those with absent prepontine CSF spaces on computed tomography (CT) were excluded from the study.
Table 1.
Consensus definition of tuberculous meningitis (2010) Panel 1 and 2
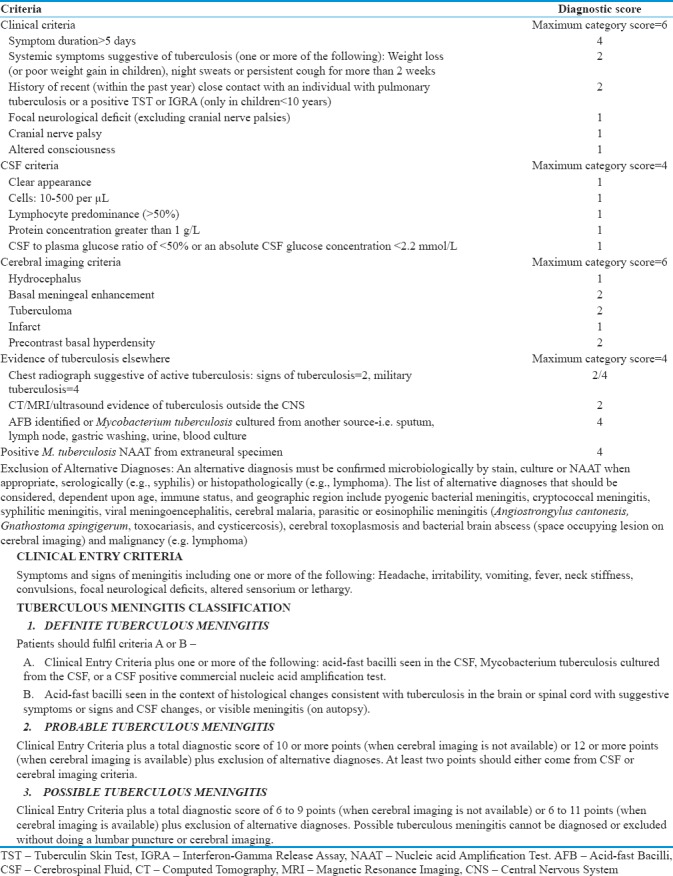
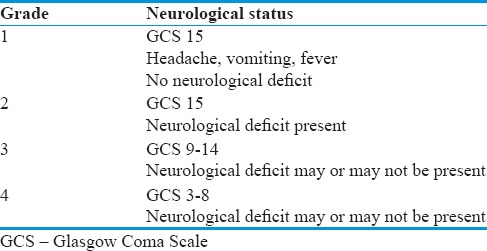
Table 2.
Modified Vellore Grading of tuberculous meningitis with hydrocephalus
After enrollment in the study, the patients were randomized into two groups using block randomization method (blocks of 4) based on computer-generated random numbers and planned for respective CSF diversion procedures.
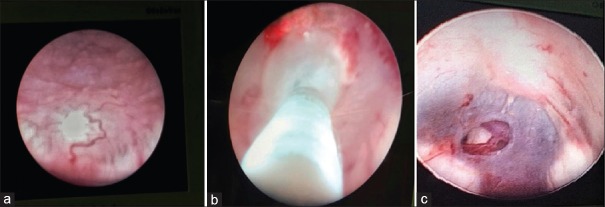
In all ETV procedures, ventricles were accessed through a precoronal burr hole in the midpupillary line using a rigid Gaab Neuroendoscope (Karl Storz, Germany). Lateral angle of the open anterior fontanelle was used as an entry point in small children. Third ventriculostomy was performed with the aid of a 3 or 4 Fr Fogarty balloon catheter and bipolar cautery. Postoperatively, lumbar spinal tap was done for 3 days in all patients who underwent the procedure [Figure 1].
Figure 1.
(a) Floor of third ventricle studded with tubercles (b) stoma being created using Fogarty's catheter (c) Basilar artery seen through stoma in the floor of the third ventricle
The standard VP shunt procedure was performed through a parietal burr hole using Chhabra VP shunt (G. Surgiwear Ltd., Uttar Pradesh, India). Type of shunt (either low pressure or medium pressure) used was based on opening flow of CSF on ventricular catheterization. All patients received antiepileptics, glucocorticoids, and anti-tuberculous therapy (ATT) as recommended by the WHO, British Infection Society, as well as the National guidelines for pediatric tuberculosis (TB).[5,6,7,8]
Ventricular tapping or insertion of an extraventricular drain (EVD) was employed as a temporizing measure in patients with higher grades of TBM and those with very poor general condition to assess the response to CSF drainage as well as manometric assessment and routine CSF analysis.[3,9,10] Both groups were followed up for a minimum of 5 months and assessed for success and failure rates as well as procedural complications and neurologic sequelae.
Results
Primary outcome
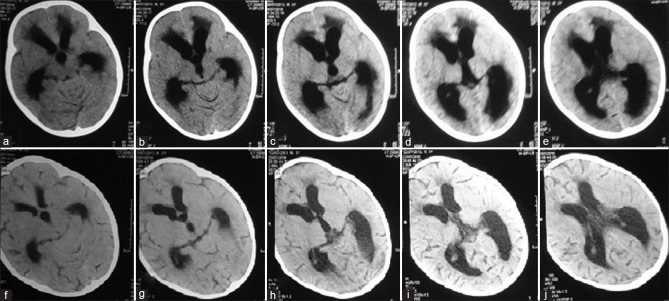
Of the 26 patients that underwent ETV, 17 patients were successful (65.4%), who showed clinical improvement, requiring no further surgical intervention. In the VP shunt group, 26 patients underwent surgery with a success rate of 61.6% and a median time to failure of 50 days [Figures 2 and 3].
Figure 2.
Preoperative (a-e) and postoperative (f-j) computed tomography scans showing resolution of hydrocephalus following endoscopic third ventriculostomy
Figure 3.
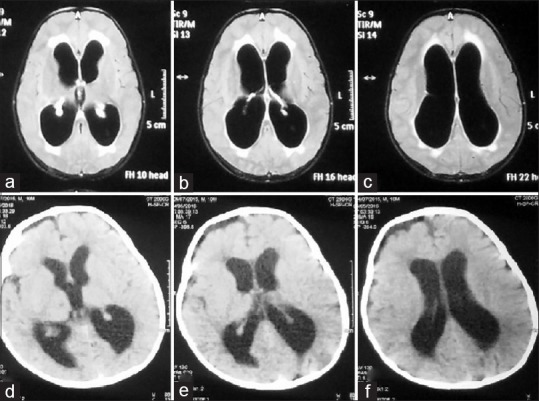
Preoperative magnetic resonance imaging scans (a-c) and postoperative computed tomography scans (d-f) showing decrease in the size of ventricles following endoscopic third ventriculostomy
Mortalities
There were two mortalities in each group, and at follow-up, 92.3% and 88.5% of patients showed clinical improvement in ETV and VP shunt groups, respectively. Two patients required multiple revision surgeries and ultimately died due to ventriculitis.
One patient died due to status epilepticus with hyponatremia, and one due to TBM-associated vasculitis with multiple infarcts.
Complications
In the ETV group, 23 of 26 cases had indistinct third ventricular floor anatomy. Six out of nine failures occurred within the first 16 days after surgery (median time to failure – 3 days) where adequate stoma could not be made due to basal exudates with indistinct third ventricular floor anatomy these patients then underwent VP shunt within 2 days of neuroendoscopy. One patient developed postoperative CSF leak which resolved on conservative management with lumbar drainage.
In the shunt group, cause of failure in ten cases was as follows:
Lower end malfunction – 6
Shunt tract infection – 3
Ventricular end malfunction – 1
Median time to failure was 50 days.
All patients requiring revision surgery were <6 years old. No other significant complications were noted.
At the last follow-up visit, 20 of the 48 (41.6%) surviving patients had residual neurological deficits, of which hemiparesis was the most common (18.75%) followed by blindness (16.67%). No patients were lost to follow-up. Noncompliance to ATT was not noted among any cases in the study.
Of the 12 cases with definite TBM according to the TBM standard case definition (2010),[4] 11 patients showed CSF GeneXpert[4,11] positive, all showing sensitivity to rifampicin with one patient showing histopathological evidence of TB on endoscopic biopsy [Table 3].
Table 3.
Comparison of outcomes and variables affecting outcome between the two groups
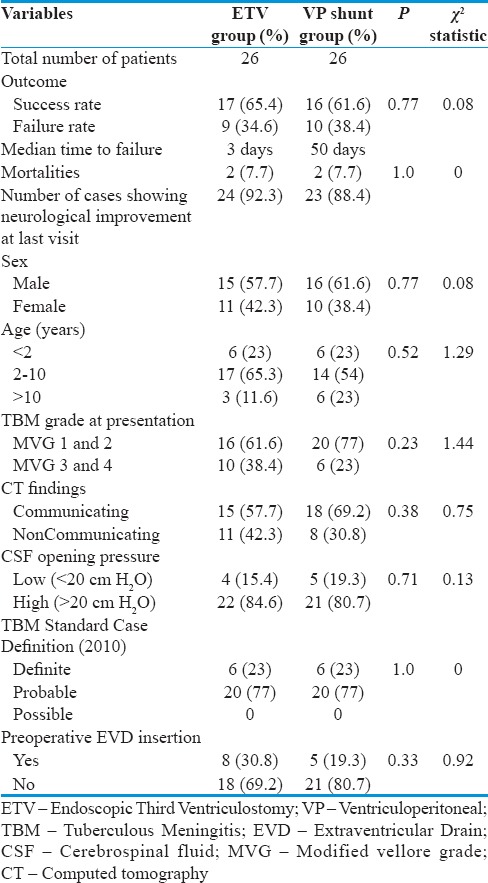
Both groups in our study were comparable with regard to variables such as age, sex, modified Vellore grading (MVG), duration of illness, preoperative EVD insertion, and CSF opening pressure as determined using Pearson's Chi-squared test.
Modified Vellore grading[1] was found to be a significant factor in determining outcome in both ETV and VP shunt groups with high-grade TBM consistently associated with poor outcome with an odds ratio (OR) of 4.2 for the failure of CSF diversion surgery [Table 4].
Table 4.
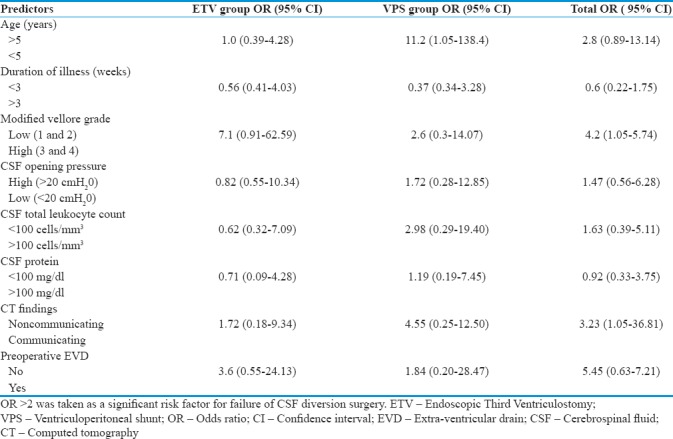
Predictors of clinical outcome by binary logistic regression analysis
Discussion
Hydrocephalus due to TBM is a complex entity and opinions regarding the ever-growing role of ETV in the management still vary widely. Although recently many have considered ETV as the first-choice treatment in the chronic burnt out phase of the disease and in obstructive hydrocephalus, its role in the acute phase of the disease and in communicating hydrocephalus is much more controversial.[12,13,14,15,16] Success rates of ETV in hydrocephalus due to TBM have varied widely. Jha et al.[17] reported an overall success rate of 64.2% in their series of 14 patients whereas Yadav et al.[12] reported a success rate of 67% and Chugh et al.[18] had 73.2% success in 26 adult patients with obstructive hydrocephalus due to TBM. Singh et al.[19] reported 77% of success rate in their series of 35 patients. The success rate in our study for ETV (65.4%) was comparable with these results. In the only other reported randomized study comparing ETV and VP shunt in TBM, they reported success in 41.7% of ETV cases and 54.2% of VP shunts in a total of 24 patients in each arm of the trial.[20]
It has been reported that the complications of shunt surgery are higher in patients with TBM than in patients with other conditions. The reasons for this are the poor general condition of these patients and also the presence of higher protein and cellular content in the CSF leading to more frequent shunt obstruction. Agrawal et al.[21] reported shunt-related complications in 11 (30%) children, and Palur et al.[10] reported that 26 of 114 (22.8%) patients had to undergo one or more shunt revisions. Sil and Chatterjee[3] reported a shunt revision rate of 43.8% in their series of 37 children who underwent shunt surgery for TBM with hydrocephalus. Shunt infection and erosion of the skin over the shunt components are the other major complications of shunt surgery in patients with poor-grade TBM and hydrocephalus.
Failure rate in the VP shunt group in our study was 40% again was similar to other studies.[3,10,21,22,23,24,25] Higher complication rates in our study may be attributable to larger number of smaller children with disseminated TB with associated malnutrition. Four such patients ultimately required multiple shunt revisions due to concomitant abdominal TB with lower end malfunction.
Late complications and failure rates in shunt surgery are well documented.[3,22,23,24] In this study, the median time to failure in the shunt group was 50 days as compared to 3 days in the ETV group showing that if successful in the initial postoperative period, ETV is much more likely to provide long-term benefit to these patients through a more physiological form of CSF diversion.
Many studies have implicated age as a factor determining the outcome in ETV with Kulkarni's ETV success score,[26] predicting a 10%–30% lower success rate for ETV in infants as compared to children between 1 and 10 years of age. However, age was not a significant risk factor for ETV failure in our study, although the age of <5 years was a significant risk factor for failure in the VP shunt group. The high rate of shunt failure in children <5 years of age in our study may be due to a high rate of disseminated TB with concomitant abdominal TB as well as associated malnutrition in these children, predisposing to shunt tract infections.
In this study, MVG was found to be a significant factor in determining outcome in both ETV and VP shunt groups. High-grade TBM was consistently associated with poor outcome (OR = 4.2). This observation was in accordance with other studies.[1,2,3,9,10,13,15,16,17,18,20,27]
Preoperative EVD placement was found to significantly increase the risk of failure of ETV (OR = 3.6) as well as in the VP shunt group (OR = 1.84) even though the median duration of preoperative EVD placement was <48 h. This may be explained by the fact that EVD was mainly inserted preoperatively in those with MVG 3 and 4 TBM. Patients with a communicating type of hydrocephalus on preoperative CT scan were found to have significantly higher failure rates of VP shunt (OR = 4.55). The role of ETV has been considered controversial in TBM with communicating hydrocephalus by some authors.[12,13,14,15,16,18] However, our study showed comparable ETV results in communicating hydrocephalus and obstructive hydrocephalus. These results are in concordance with those reported by Singh et al.[19] Studies elucidating newer concepts in the pathophysiology of CSF circulation have provided explanations for the success of ETV in communicating hydrocephalus including bringing CSF circulation to previously inaccessible and probably normal areas of absorption, thereby clearing exudates.[19,28,29] ETV may also decrease the transventricular pressure gradient and the demyelination of periventricular brain parenchyma, which could contribute to some symptoms of hydrocephalus.[18,19,30,31] Although evidence regarding the role of ETV in hydrocephalus with lower CSF pressures (<20 cm H2O) is lacking, its effectiveness may be explained due to the aforementioned changes in our understanding of CSF circulation pathophysiology[18,19,28,29,30,31] benefiting patients with communicating hydrocephalus.
High CSF total leukocyte counts (>100 cells/mm3) showed a high OR for failure of VP shunt, possibly due to high CSF cellularity causing more frequent shunt obstruction.[1] On the contrary, high CSF leukocyte counts were not found to be a risk factor for ETV failure.
Conclusions
ETV appears to be a safe and effective method for the surgical management of hydrocephalus secondary to TBM in children, with a lower rate of failure than that of VP shunt for the same disease. It can be performed effectively in young children including infants, as well as those with communicating hydrocephalus, high CSF cell counts and protein levels, despite indistinct third ventricular floor anatomy. It has been shown to have a number of inherent advantages over shunt surgery such as elimination of dependence on mechanical shunt devices with significantly reduced risk of infection, absence of tissue reaction to a foreign body, short duration of surgery with fewer incisions, avoidance of complications related to concomitant tuberculous peritonitis, elimination of the problem of CSF overdrainage, and a lower rate of long-term complications
The most consistent factor affecting failure rates in both ETV and VP shunt surgery is the MVG or the clinical grade of TBM (OR = 4.2)
Risk factors for the failure of VP shunt also include age <5 years, communicating hydrocephalus, and high CSF leukocyte counts
Although there is a relatively high rate of failure, ETV should be attempted as the first-choice CSF diversion procedure in hydrocephalus secondary to TBM where technical expertise and experience with this procedure is available as it avoids the myriad of lifelong complications associated with shunts.
Limitations of the study
The number of patients enrolled in the study was small. Larger scale, multicentric randomized trials would be required to establish well-defined guidelines for the surgical management of hydrocephalus in TBM
Contrast-enhanced magnetic resonance imaging (MRI)/CT scan could not be done preoperatively for most patients in this study due to setup constraints and the nature of the medical emergency in certain patients. This would be ideal for better selection of cases and prognostication before the surgery
Cine MRI/CSF flow studies could only be done in a limited number of cases due to a lack of availability at our center, which would objectively confirm the patency and functioning of the stoma postoperatively
Median follow-up period of 8 months was not long enough to properly assess long-term outcomes and complications
Surgeries were performed by multiple surgeons of varying experience at our institution, which may have affected outcomes.
Future guidelines and recommendations
ETV should be attempted as the first-choice CSF diversion procedure in hydrocephalus secondary to TBM where technical expertise and experience with this procedure is available as it avoids the myriad of lifelong complications associated with shunts
Early diagnosis and surgical intervention are the best way to reduce morbidity and mortality as the most consistent predictor of outcome is clinical grade of TBM
Further development of the technique of ETV through the lamina terminalis using a flexible endoscope could serve as a valuable alternative in cases with a plastered third ventricular floor due to inflammatory exudates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57:368–74. doi: 10.4103/0028-3886.55572. [DOI] [PubMed] [Google Scholar]
- 2.Kemaloglu S, Ozkan U, Bukte Y, Ceviz A, Ozates M. Timing of shunt surgery in childhood tuberculous meningitis with hydrocephalus. Pediatr Neurosurg. 2002;37:194–8. doi: 10.1159/000065398. [DOI] [PubMed] [Google Scholar]
- 3.Sil K, Chatterjee S. Shunting in tuberculous meningitis: A neurosurgeon's nightmare. Childs Nerv Syst. 2008;24:1029–32. doi: 10.1007/s00381-008-0620-x. [DOI] [PubMed] [Google Scholar]
- 4.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10:803–12. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Gupta D, Nagaraja SB, Singh V, Sethi GR, Prasad J, et al. Updated national guidelines for pediatric tuberculosis in India, 2012. Indian Pediatr. 2013;50:301–6. doi: 10.1007/s13312-013-0085-1. [DOI] [PubMed] [Google Scholar]
- 6.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63:e147–95. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemotherapy and management of tuberculosis in the United Kingdom: Recommendations 1998. Joint tuberculosis committee of the British Thoracic Society. Thorax. 1998;53:536–48. [PMC free article] [PubMed] [Google Scholar]
- 8.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–51. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 9.Tandon V, Mahapatra AK. Management of post-tubercular hydrocephalus. Childs Nerv Syst. 2011;27:1699–707. doi: 10.1007/s00381-011-1482-1. [DOI] [PubMed] [Google Scholar]
- 10.Palur R, Rajshekhar V, Chandy MJ, Joseph T, Abraham J. Shunt surgery for hydrocephalus in tuberculous meningitis: A long-term follow-up study. J Neurosurg. 1991;74:64–9. doi: 10.3171/jns.1991.74.1.0064. [DOI] [PubMed] [Google Scholar]
- 11.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–37. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav YR, Mukerji G, Parihar V, Sinha M, Pandey S. Complex hydrocephalus (combination of communicating and obstructive type): An important cause of failed endoscopic third ventriculostomy. BMC Res Notes. 2009;2:137. doi: 10.1186/1756-0500-2-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav YR, Shenoy R, Mukerji G, Parihar V. Water jet dissection technique for endoscopic third ventriculostomy minimises the risk of bleeding and neurological complications in obstructive hydrocephalus with a thick and opaque third ventricle floor. Minim Invasive Neurosurg. 2010;53:155–8. doi: 10.1055/s-0030-1263107. [DOI] [PubMed] [Google Scholar]
- 14.Yadav YR, Jaiswal S, Adam N, Basoor A, Jain G. Endoscopic third ventriculostomy in infants. Neurol India. 2006;54:161–3. [PubMed] [Google Scholar]
- 15.Yadav YR, Parihar V, Agrawal M, Bhatele PR. Endoscopic third ventriculostomy in tubercular meningitis with hydrocephalus. Neurol India. 2011;59:855–60. doi: 10.4103/0028-3886.91365. [DOI] [PubMed] [Google Scholar]
- 16.Yadav YR, Parihar VS, Todorov M, Kher Y, Chaurasia ID, Pande S, et al. Role of endoscopic third ventriculostomy in tuberculous meningitis with hydrocephalus. Asian J Neurosurg. 2016;11:325–9. doi: 10.4103/1793-5482.145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jha DK, Mishra V, Choudhary A, Khatri P, Tiwari R, Sural A, et al. Factors affecting the outcome of neuroendoscopy in patients with tuberculous meningitis hydrocephalus: A preliminary study. Surg Neurol. 2007;68:35–41. doi: 10.1016/j.surneu.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 18.Chugh A, Husain M, Gupta RK, Ojha BK, Chandra A, Rastogi M, et al. Surgical outcome of tuberculous meningitis hydrocephalus treated by endoscopic third ventriculostomy: Prognostic factors and postoperative neuroimaging for functional assessment of ventriculostomy. J Neurosurg Pediatr. 2009;3:371–7. doi: 10.3171/2009.1.PEDS0947. [DOI] [PubMed] [Google Scholar]
- 19.Singh D, Sachdev V, Singh AK, Sinha S. Endoscopic third ventriculostomy in post-tubercular meningitic hydrocephalus: A preliminary report. Minim Invasive Neurosurg. 2005;48:47–52. doi: 10.1055/s-2004-830183. [DOI] [PubMed] [Google Scholar]
- 20.Goyal P, Srivastava C, Ojha BK, Singh SK, Chandra A, Garg RK, et al. A randomized study of ventriculoperitoneal shunt versus endoscopic third ventriculostomy for the management of tubercular meningitis with hydrocephalus. Childs Nerv Syst. 2014;30:851–7. doi: 10.1007/s00381-014-2371-1. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal D, Gupta A, Mehta VS. Role of shunt surgery in pediatric tubercular meningitis with hydrocephalus. Indian Pediatr. 2005;42:245–50. [PubMed] [Google Scholar]
- 22.Sainte-Rose C, Piatt JH, Renier D, Pierre-Kahn A, Hirsch JF, Hoffman HJ, et al. Mechanical complications in shunts. Pediatr Neurosurg. 1991;17:2–9. doi: 10.1159/000120557. [DOI] [PubMed] [Google Scholar]
- 23.Robinson S, Kaufman BA, Park TS. Outcome analysis of initial neonatal shunts: Does the valve make a difference? Pediatr Neurosurg. 2002;37:287–94. doi: 10.1159/000066307. [DOI] [PubMed] [Google Scholar]
- 24.McGirt MJ, Leveque JC, Wellons JC, 3rd, Villavicencio AT, Hopkins JS, Fuchs HE, et al. Cerebrospinal fluid shunt survival and etiology of failures: A seven-year institutional experience. Pediatr Neurosurg. 2002;36:248–55. doi: 10.1159/000058428. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi DK, Balasubramaniam S, Jayaswal SA, Savant HV, Gandhi AS. Outcome analysis of Ventriculoperitoneal Shunt procedures in hydrocephalus due to Tubercular Meningitis and non-infective cases. Int J Contemp Pediatr. 2016;3:1210–5. [Google Scholar]
- 26.Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S, et al. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 2009;155:254–90. doi: 10.1016/j.jpeds.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 27.Singh D, Kumar S. Ventriculoperitoneal shunt in post tubercular hydrocephalus. Indian Pediatr. 1996;33:854–5. [PubMed] [Google Scholar]
- 28.Husain M, Jha DK, Rastogi M, Husain N, Gupta RK. Role of neuroendoscopy in the management of patients with tuberculous meningitis hydrocephalus. Neurosurg Rev. 2005;28:278–83. doi: 10.1007/s10143-005-0397-2. [DOI] [PubMed] [Google Scholar]
- 29.Hopf NJ, Grunert P, Fries G, Resch KD, Perneczky A. Endoscopic third ventriculostomy: Outcome analysis of 100 consecutive procedures. Neurosurgery. 1999;44:795–804. doi: 10.1097/00006123-199904000-00062. [DOI] [PubMed] [Google Scholar]
- 30.Greitz D. Radiological assessment of hydrocephalus: New theories and implications for therapy. Neurosurg Rev. 2004;27:145–65. doi: 10.1007/s10143-004-0326-9. [DOI] [PubMed] [Google Scholar]
- 31.Greitz D, Hannerz J. A proposed model of cerebrospinal fluid circulation: Observations with radionuclide cisternography. AJNR Am J Neuroradiol. 1996;17:431–8. [PMC free article] [PubMed] [Google Scholar]