Abstract
Cerebral perivascular spaces (PVSs), otherwise known as Virchow-Robin spaces, are interstitial fluid-filled channels, <2 mm in diameter that form around arterial perforators as they course from the cortex into the brain parenchyma. In contrast, a giant tumefactive PVS is a rare entity comprising of clusters of such channels larger than 15mm resembling a neoplastic process as the name suggests. We report a 55-year-old male who presented with unsteady gait, cognitive decline, and left lower limb weakness for 6 months. Magnetic resonance imaging revealed a noncontrast enhancing multicystic intraaxial lesion of the right mesencephalon-diencephalon junction extending into the anterior third ventricle causing obstructive hydrocephalus. A ventriculoperitoneal shunt was inserted with a complete reversal of his neurological symptoms. Such PVSs can easily be misidentified for a cystic tumor, and their unique radiological features are discussed to prevent unnecessary surgery. We also demonstrate that when they cause hydrocephalus and midbrain compression symptoms cerebrospinal fluid shunting alone can result in excellent outcomes.
Keywords: Cerebrospinal fluid shunting, giant tumefactive perivascular space, hydrocephalus, Virchow–robin space
Introduction
Intracranial giant tumefactive perivascular spaces (TPVS) are rare clusters of nonneoplastic cysts >15 mm in size.[1] They are pial-lined, interstitial fluid-filled structures that accompany penetrating arteries and are generally located at the mesencephalothalamic region.[1] Fewer than 80 cases have been reported in the literature, and surgical intervention may be necessary when they become symptomatic. We describe a patient that experienced neurocognitive decline and limb weakness that was subsequently diagnosed to have a giant TPVS of the mesencephalon-diencephalon junction with obstructive hydrocephalus.
Case Report
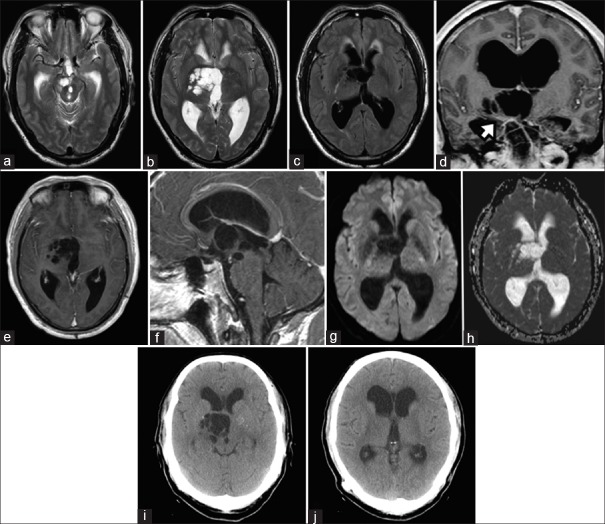
A 55-year-old male experienced frequent falls for 6 months associated with progressive memory loss. Physical examination revealed left lower limb weakness of Medical Research Council Grade 4/5. The Neurobehavioral Cognitive State Examination (NCSE) and the Montreal Cognitive Assessment (MOCA) revealed severe deficiencies in short-term memory with the latter score being 26/30. Magnetic resonance imaging (MRI) depicted an irregular noncontrast enhancing multicystic lesion of the right cerebral peduncle extending into the third ventricle that caused obstructive hydrocephalus at the level of the Foramen of Monro [Figure 1a–f]. Contrast T1-weighted sequences showed that the thalamoperforating arteries coursed through the lesion at the level of the mesencephalon-diencephalon junction [Figure 1d]. Perilesional edema could not be demonstrated on the fluid-attenuated inversion recovery sequence. Diffusion weighted-imaging and apparent diffusion coefficient sequences did not reveal signal restriction within the cysts [Figure 1g and h]. MR perfusion showed perilesional decreased cerebral blood volume. MR spectroscopy showed no increased choline content with normal choline/creatine and choline/N-acetylaspartate ratios. The radiological findings were highly suggestive of a giant TPVS. A ventriculoperitoneal shunt with a programmable valve was inserted uneventfully. During shunt placement, the cerebrospinal fluid (CSF) opening pressure was relatively high at 22 cmH2O (16 mmHg). Collected CSF specimens showed no evidence of tumor cells and no microorganisms were cultured. Six weeks after the operation, the patient experienced full neurological recovery. His MOCA score was 30/30, and all NCSE domain scores were within the normal range. A year later, the patient remained asymptomatic, and a follow-up computed tomography scan showed resolution of the transependymal edema with no significant change in TPVS and ventricular size [Figure 1i and j].
Figure 1.
Multicystic perivascular spaces at the right cerebral peduncle of the midbrain that extended to the mesencephalon-diencephalon junction (a and b) Fluid-attenuation inversion recovery imaging revealed an absence of perilesional edema, but the presence of transependymal edema secondary to hydrocephalus (c). Thalamoperforating arteries as they coursed through the perivascular spaces (d, white arrow). The perivascular spaces did not display contrast enhancement (e and f). Diffusion weighted-imaging (g) and apparent diffusion coefficient (h) sequences showed that perivascular spaces fluid content had no restricted diffusion. One-year postoperative computed tomography scans showed no change in perivascular spaces (i) and ventricular size (j)
Discussion
Cerebral PVSs, also known as Virchow–Robin spaces, are physiological interstitial fluid-filled channels typically <2 mm in diameter that extends from the subpial space and form around arterial perforators as they course from the cortex into the brain parenchyma.[1] The precise functions of these structures have yet to be delineated, but predominant theories suggest that they: (1) Facilitate fluid movement between the basal cisterns to the interstitial space, (2) Modulate immune responses by providing a conduit for macrophages and lymphocytes to reach CSF, and (3) Forms part of the glymphatic system for metabolic waste product elimination.[2] PVSs are considered dilated when they become larger than 2 mm and are frequently observed with advancing age, various neuropsychiatric disorders, multiple sclerosis, microvascular disease, and traumatic brain injury.[1] A retrospective review of 816 MRI scans performed for various indications found that 38% of adult patients had dilated PVSs.[3]
The cause for PVS dilatation is unclear, hydrodynamic disturbances in CSF and interstitial fluid flow caused by slow-growing benign tumors or preexisting hydrocephalus have been suggested.[4] Alternatively, increased vessel permeability with fluid exudation due to microvascular disease or ex vacuo periarteriolar ischemic parenchymal injury resulting in interstitial fluid leakage have also been postulated.[5,6]
Dilated PVSs can be classified into three types with respect to their anatomical arterial relationship.[7] Type I lesions are located along the lenticulostriate arteries as they cross through the anterior perforated substance into the basal ganglia. Type II PVSs surround the cortical medullary arteries as they descend into the gray-white matter junction. Type III PVSs are located in the mesencephalic region and may follow the collicular, thalamoperforating, paramedian mesencephalothalamic, and circumferential penetrating arteries.
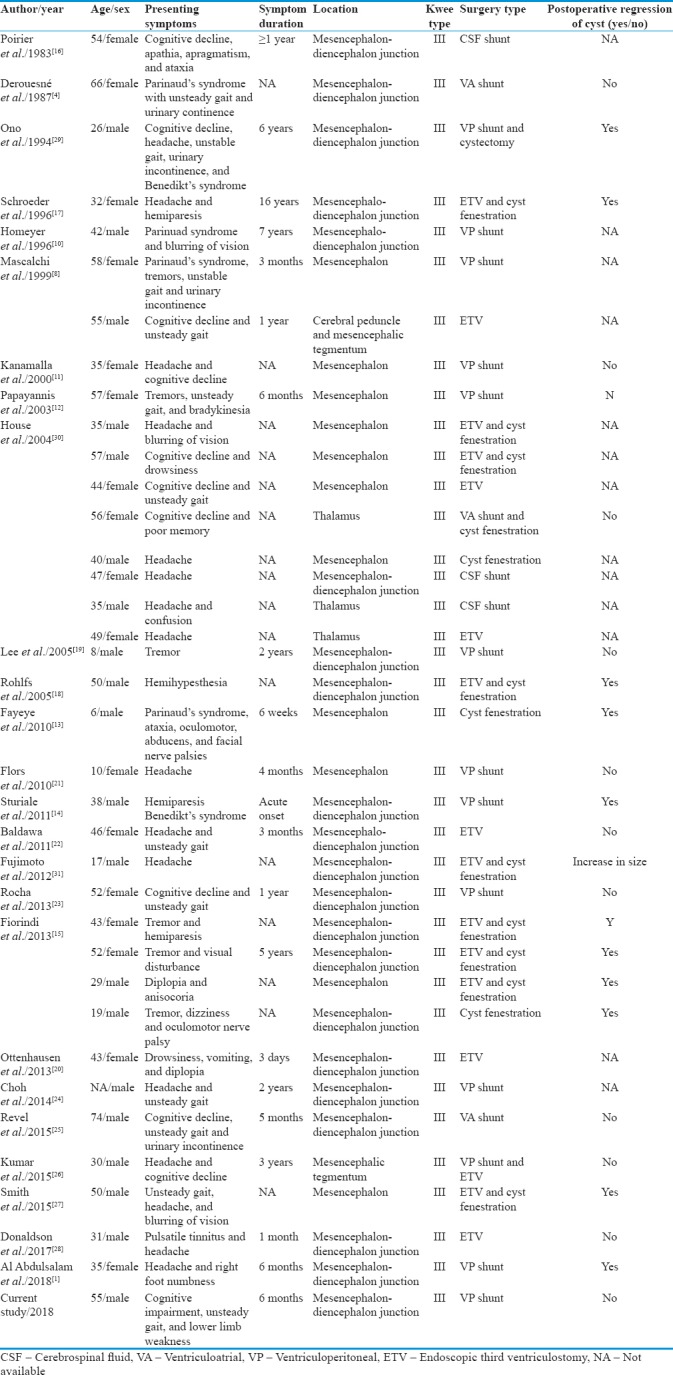
Although dilated PSVs are commonly encountered, fewer than 80 giant TPVSs (defined as being >15 mm) have been reported in the literature. Among this group of patients, 45 (61%, 45/74) had obstructive hydrocephalus and 37 (50%, 37/74) required CSF diversion. All 37 TPVSs were Type III lesions due to their proximity to the third ventricle and the Sylvian aqueduct [Table 1]. Cases reported by Salzman et al.[6] were excluded since no individual clinical or radiological features were described in their study to allow in-depth analysis.[1,4,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] Our pooled analysis showed that 49% (18/37) of TPVSs in this group had accompanying midbrain-localizing neurological signs. Apart from hemiparesis, patients were reported to have rubral tremors, oculomotor nerve palsy, Benedikt's syndrome, Parkinsonism, Parinaud's syndrome, and cerebellar ataxia.[4,8,10,12,13,14,15,16,17,18,19,20,29] The mean age of diagnosis was 41-year-old (range: 6–74) with a female-to-male ratio of 1: 1.9. Patients with giant TPVSs were considerably younger than those with dilated PVSs per se.[3]
Table 1.
Reported cases of giant tumefactive perivascular spaces with obstructive hydrocephalus treated by cerebrospinal fluid diversion
Giant TPVSs can morphologically resemble neurocysticercosis, cystic low-grade gliomas, porencephalic cysts, ventricular diverticulae, and protein deposition disorders such as mucopolysaccharidosis on MRI.[6,7] However, PVSs are typically sharply demarcated, nonenhancing, purely cystic (displaying signal intensities similar to CSF on all sequences) and are often located along characteristic perforator vessel locations as described by Kwee[6,7] In contrast, the presence of perilesional edema, cyst content exhibiting restricted diffusion, intracystic solid, or enhancing components are more indicative of a neoplastic or infectious process.[6]
The management of Type III TPVS should address both the hydrocephalus and its mass effect on the midbrain.[30] Twenty-three patients (62%) with hydrocephalus had their symptoms relieved by endoscopic third ventriculostomy (ETV) or shunt placement alone [Table 1]. In addition, among those with focal neurological symptoms, ten patients (59%) experienced sustained improvement with CSF diversion without the need for direct cyst manipulation. This finding lends support to the important role of the PVS in CSF-interstitial fluid hydrodynamics.[2,7] Modulating the fluid pressures in the ventricular and PVS compartments by CSF diversion could mean that additional cyst fenestration may be unnecessary. Fiorindi et al. further advised against cyst manipulation due to the risks of tearing its arterial perforators that perfuse the midbrain, thalamus, and basal ganglia.[15] It is evident that endoscopic cyst fenestration can lead to lesion regression on serial imaging, but whether this could lead to superior functional outcomes compared to CSF diversion alone is unclear. For this reason and due to concerns that the PVS wall would prohibit clear visualization of the third ventricular floor, we decided for shunt placement instead of ETV for our patient. Since most TPVSs do not involute with CSF diversion alone, as observed in our patient, one should vigilantly follow-up these patients as symptomatic reexpansion has been reported to occur more than 10 years after surgery.[31]
Conclusions
Giant TPVSs are rare entities and Type III lesions may present with hydrocephalus and focal neurological deficits due to their involvement of the midbrain. The clinician should be cognizant of the existence of such rare lesions by carefully evaluating the MRI so as to avoid unnecessary biopsies or excisions of these lesions. Our case illustrates that symptoms of midbrain compression can be completely reversed by CSF shunting without direct cyst decompression.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Al Abdulsalam H, Alatar AA, Elwatidy S. Giant tumefactive perivascular spaces: A Case report and literature review. World Neurosurg. 2018;112:201–4. doi: 10.1016/j.wneu.2018.01.144. [DOI] [PubMed] [Google Scholar]
- 2.Cherian I, Beltran M, Kasper EM, Bhattarai B, Munokami S, Grasso G, et al. Exploring the Virchow-Robin spaces function: A unified theory of brain diseases. Surg Neurol Int. 2016;7:S711–4. doi: 10.4103/2152-7806.192486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD, et al. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol. 1989;10:929–36. [PMC free article] [PubMed] [Google Scholar]
- 4.Derouesné C, Gray F, Escourolle R, Castaigne P. ‘Expanding cerebral lacunae’ in a hypertensive patient with normal pressure hydrocephalus. Neuropathol Appl Neurobiol. 1987;13:309–20. doi: 10.1111/j.1365-2990.1987.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 5.Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre MRI cohort study. J Neurol Neurosurg Psychiatry. 2013;84:624–9. doi: 10.1136/jnnp-2012-304434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzman KL, Osborn AG, House P, Jinkins JR, Ditchfield A, Cooper JA, et al. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol. 2005;26:298–305. [PMC free article] [PubMed] [Google Scholar]
- 7.Kwee RM, Kwee TC. Virchow-robin spaces at MR imaging. Radiographics. 2007;27:1071–86. doi: 10.1148/rg.274065722. [DOI] [PubMed] [Google Scholar]
- 8.Mascalchi M, Salvi F, Godano U, Nistri M, Taiuti R, Tosetti M, et al. Expanding lacunae causing triventricular hydrocephalus. Report of two cases. J Neurosurg. 1999;91:669–74. doi: 10.3171/jns.1999.91.4.0669. [DOI] [PubMed] [Google Scholar]
- 9.Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: Their relationship to lacunes. J Anat. 1997;191(Pt 3):337–46. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homeyer P, Cornu P, Lacomblez L, Chiras J, Derouesné C. A special form of cerebral lacunae: Expanding lacunae. J Neurol Neurosurg Psychiatry. 1996;61:200–2. doi: 10.1136/jnnp.61.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamalla US, Calabrò F, Jinkins JR. Cavernous dilatation of mesencephalic Virchow-Robin spaces with obstructive hydrocephalus. Neuroradiology. 2000;42:881–4. doi: 10.1007/s002340000440. [DOI] [PubMed] [Google Scholar]
- 12.Papayannis CE, Saidon P, Rugilo CA, Hess D, Rodriguez G, Sica RE, et al. Expanding Virchow Robin spaces in the midbrain causing hydrocephalus. AJNR Am J Neuroradiol. 2003;24:1399–403. [PMC free article] [PubMed] [Google Scholar]
- 13.Fayeye O, Pettorini BL, Foster K, Rodrigues D. Mesencephalic enlarged Virchow-Robin spaces in a 6-year-old boy: A case-based update. Childs Nerv Syst. 2010;26:1155–60. doi: 10.1007/s00381-010-1164-4. [DOI] [PubMed] [Google Scholar]
- 14.Sturiale CL, Albanese A, Lofrese G, Frassanito P, Sabatino G, Marchese E, et al. Pathological enlargement of midbrain Virchow-Robin spaces: A rare cause of obstructive hydrocephalus. Br J Neurosurg. 2011;25:130–1. doi: 10.3109/02688697.2010.504050. [DOI] [PubMed] [Google Scholar]
- 15.Fiorindi A, Delitala A, Francaviglia N, Longatti P. Neuroendoscopic options in the treatment of mesencephalic expanding cysts: Report of four cases and review of the literature. Clin Neurol Neurosurg. 2013;115:2370–6. doi: 10.1016/j.clineuro.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Poirier J, Barbizet J, Gaston A, Meyrignac C. Thalamic dementia. Expansive lacunae of the thalamo-paramedian mesencephalic area. Hydrocephalus caused by stenosis of the aqueduct of sylvius. Rev Neurol (Paris) 1983;139:349–58. [PubMed] [Google Scholar]
- 17.Schroeder HW, Gaab MR, Warzok RW. Endoscopic treatment of an unusual multicystic lesion of the brainstem: Case report. Br J Neurosurg. 1996;10:193–6. doi: 10.1080/02688699650040368. [DOI] [PubMed] [Google Scholar]
- 18.Rohlfs J, Riegel T, Khalil M, Iwinska-Zelder J, Mennel HD, Bertalanffy H, et al. Enlarged perivascular spaces mimicking multicystic brain tumors. Report of two cases and review of the literature. J Neurosurg. 2005;102:1142–6. doi: 10.3171/jns.2005.102.6.1142. [DOI] [PubMed] [Google Scholar]
- 19.Lee KJ, Joo WI, Kim MC, Choi CR. Obstructive hydrocephalus induced tremor in patient with mesencephalic lacunae. J Korean Neurosurg Soc. 2005;37:456–8. [Google Scholar]
- 20.Ottenhausen M, Meier U, Tittel A, Lemcke J. Acute decompensation of noncommunicating hydrocephalus caused by dilated Virchow-Robin spaces type III in a woman treated by endoscopic third ventriculostomy: A case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2013;74(Suppl 1):e242–7. doi: 10.1055/s-0033-1349339. [DOI] [PubMed] [Google Scholar]
- 21.Flors L, Leiva-Salinas C, Cabrera G, Mazón M, Poyatos C. Obstructive hydrocephalus due to cavernous dilation of Virchow-Robin spaces. Neurology. 2010;74:1746. doi: 10.1212/WNL.0b013e3181e04312. [DOI] [PubMed] [Google Scholar]
- 22.Baldawa SS, Easwer HV, Nair S, Menon G. Mesencephalothalamic giant virchow-robin space causing obstructive hydrocephalus. Neurosurg Q. 2011;21:214–8. [Google Scholar]
- 23.Rocha S, Pinho J, Rito M, Machado Á. Expanding Virchow-Robin spaces: Transient global amnesia and obstructive hydrocephalus. J Neuropsychiatry Clin Neurosci. 2013;25:E49–50. doi: 10.1176/appi.neuropsych.12050123. [DOI] [PubMed] [Google Scholar]
- 24.Choh NA, Shaheen F, Robbani I, Singh M, Gojwari T. Tumefactive Virchow-Robin spaces: A rare cause of obstructive hydrocephalus. Ann Indian Acad Neurol. 2014;17:345–6. doi: 10.4103/0972-2327.138524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revel F, Cotton F, Haine M, Gilbert T. Hydrocephalus due to extreme dilation of Virchow-Robin spaces. BMJ Case Rep 2015. 2015:pii: bcr2014207109. doi: 10.1136/bcr-2014-207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Gupta R, Garg A, Sharma BS. Giant mesencephalic dilated Virchow Robin spaces causing obstructive hydrocephalus treated by endoscopic third ventriculostomy. World Neurosurg. 2015;84:2074.e11–4. doi: 10.1016/j.wneu.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Smith KA, Lavin P, Chamoun R. Neuroendoscopic treatment of symptomatic giant Virchow-Robin spaces. Surg Neurol Int. 2015;6:120. doi: 10.4103/2152-7806.161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donaldson C, Chatha G, Chandra RV, Goldschlager T. Obstructive hydrocephalus secondary to enlarged Virchow-Robin spaces: A Rare cause of pulsatile tinnitus. World Neurosurg. 2017;101:815.e1–00. doi: 10.1016/j.wneu.2017.02.119. [DOI] [PubMed] [Google Scholar]
- 29.Ono Y, Suzuki M, Kayama T, Yoshimoto T. Multilobulated cystic formation in the brain stem with Benedikt's syndrome: Case report. Neurosurgery. 1994;34:726–9. [PubMed] [Google Scholar]
- 30.House P, Salzman KL, Osborn AG, MacDonald JD, Jensen RL, Couldwell WT, et al. Surgical considerations regarding giant dilations of the perivascular spaces. J Neurosurg. 2004;100:820–4. doi: 10.3171/jns.2004.100.5.0820. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto K, Kuroda J, Hide T, Hasegawa Y, Yano S, Kuratsu J, et al. Giant tumefactive perivascular spaces that expanded and became symptomatic 14 years after initial surgery. Surg Neurol Int. 2012;3:127. doi: 10.4103/2152-7806.102942. [DOI] [PMC free article] [PubMed] [Google Scholar]