Abstract
Surgery is the standard treatment for chronic subdural hematoma (CSDH), one of the common problems in neurosurgical practice. Although medical treatment was used by some authors and found some positive results, it is not accepted by many authors. The aim of this review is to give overall view of the medical management of CSDH. Action of various drugs in the pathophysiological cascade of formation of CSDH was depicted. The review of literature is done under three headings – the primary medical treatment of CSDH, adjuvant medical treatment with surgery, and the treatment of the recurrence. Various classifications of CSDH and the factors influencing the outcome of medical treatment are discussed. There is a role of medical treatment in a selected group of patients with CSDH. Well-designed, multicenter, randomized controlled trials are required to define the indications and standard protocols for the medical treatment of CSDH.
Keywords: Chronic subdural hematoma, classification of chronic subdural hematoma, conservative management, medical treatment, nonsurgical treatment
Introduction
Chronic subdural hematoma (CSDH) is a common neurosurgical problem seen in elderly showing a rise in incidence due to increasing usage of anticoagulants and rise in elderly population. The standard mode of treatment for CSDH is surgery. There are many studies of medical management of CSDH in the literature.[1,2,3,4,5,6] Till now, there is no Class I evidence regarding any drug used for medical treatment of CSDH.
It is noted that a less number of neurosurgeons prescribe medications for the treatment of CSDH. The proportion of doctors vary from one place to another. In a questionnaire survey of practice in the United Kingdom and the Republic of Ireland, it is recorded that 94% of surgeons employ conservative management in less than one-quarter of cases of CSDH. Forty-two percent of surgeons never prescribe steroids and 55% prescribe them to those managed conservatively.[7] As per a national survey in the Netherlands, opinion collected from various vascular neurologists and neurosurgeons about the treatment of CSDH. Less than one-quarter of the total doctors favored conservative treatment with corticosteroids as primary treatment. The proportion of patients primarily treated with corticosteroids are increasing year by year.[8] In another Canadian survey regarding neurosurgical practice of treatment of CSDH, <15% of neurosurgeons prefer using high-dose corticosteroids.[9]
The aim of this review is to give overall view of the medical management of CSDH. The different modes of treatment of CSDH used in the literature are tabulated in Table 1. The nonsurgical medical treatment includes many drugs, the discussion of which forms the main gist of this article.
Table 1.
Different modes of treatment for the chronic subdural hematoma

Methods
Relevant literature search was performed using – Medline (through PubMed) and Google Scholar (Google search) up to August 2016. The keywords used were “medical treatment,” “nonsurgical,” and “conservative management.” Additional articles identified from these references that contained relevant supporting information were then included in this study. The search was performed by one reviewer.
The nonsurgical medical management of CSDH was divided into three groups. These included primary medical treatment of CSDH, adjuvant medical treatment with surgery, and the treatment of the recurrence.
Mechanism of Action of Various Drugs used to Treat Chronic Subdural Hematoma
Figure 1 shows stages of development of CSDH after initial bleed in subdural space usually after trivial trauma in elderly patients. Drugs will act at different stages of development of CSDH. The point at which different drugs act on different stages of CSDH development is depicted. Many drugs primarily inhibit inflammation and act at the first step itself. Figure 2 shows the mechanism of action of steroids in CSDH.[10] Tranexamic acid will inhibit at two stages – it inhibits inflammatory reaction through Kallikrein system and it inactivates plasminogen, thereby inhibiting fibrinolytic activity.[1] Etizolam inactivates platelet-activating factor resulting in inhibition of lipid-mediated inflammation.[2] Atorvastatin is the most potent drug that helps in angiogenesis without risk of hematoma. A low dose (20 mg/day) potentiates angiogenesis resulting in absorption and resolution of CSDH.[3]
Figure 1.
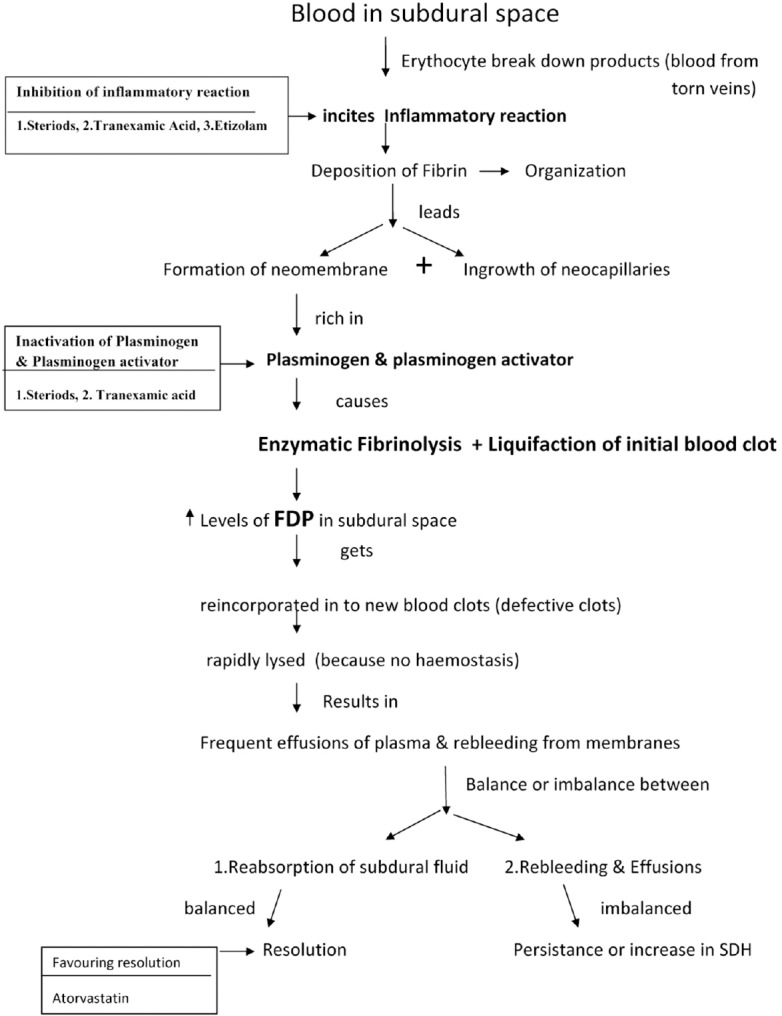
The cascade of events that occur in the formation of chronic subdural hematoma and drugs acting at various stages
Figure 2.
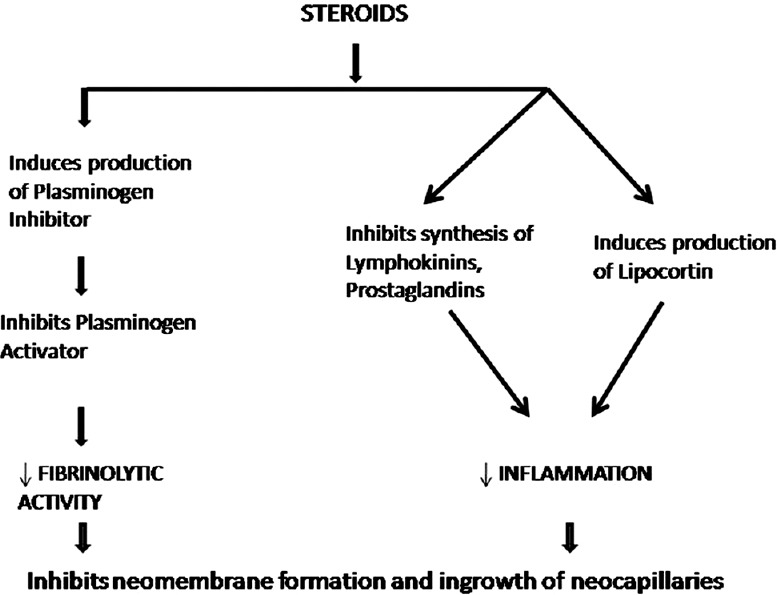
Mechanism of action of steroids in the treatment of chronic subdural hematoma
The discussion is done under three headings – the primary medical treatment of CSDH, adjuvant medical treatment with surgery, and the treatment of the recurrence.
Nonsurgical Medical Treatment of Chronic Subdural Hematoma
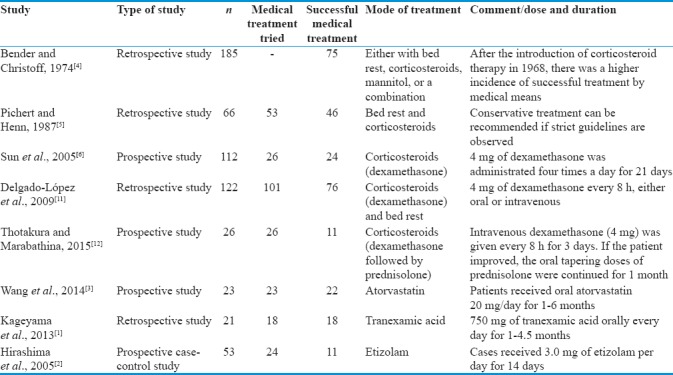
Various studies with nonsurgical medical treatment of CSDH with positive results are tabulated in Table 2. There were some case reports of positive results of CSDH with corticosteroids.[13,14,15,16] Out of all the drugs tried for CSDH, corticosteroids were the most common.
Table 2.
Various studies with nonsurgical medical treatment of chronic subdural hematoma with positive results
Some authors observed negative effects of corticosteroids on patients when used for the treatment of CSDH. Almenawer et al., in their systematic review and meta-analysis, noted higher morbidity with the adjuvant use of corticosteroids, with no significant improvement in recurrence and cure rates.[17] Prud’homme et al. presented data from a prospective, randomized pilot study of twenty CSDH patients treated with dexamethasone or placebo.[18] These preliminary results have not shown a clear beneficial effect of dexamethasone against placebo. However, dexamethasone treatment was responsible for significant complications.
Local drug-Gorei-san
It is a Kampo medicine (Japanese traditional herbal medicine), which was tried in CSDH patients by some authors of Japan and noted successful results. Miyagami and Kagawa managed 27 patients of CSDH conservatively with Gorei-san and noted its effectiveness in 23 patients.[19] They opined that Gorei-san is a useful option in the conservative treatment of CSDHs with no or minimum symptoms. Muramatsu et al. tried and successfully managed elderly patients of CSDH conservatively with Gorei-san.[20]
Adjuvant Medical Treatment in Prevention of Recurrence of Chronic Subdural Hematoma after Surgery
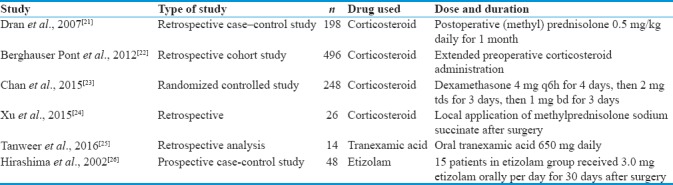
The medications that are used along with surgery to prevent the recurrence of CSDH are included in this group. Some authors used the medication after surgery; some authors started the drug before the surgery and continued it after the surgery. The various studies done regarding the usage of adjuvant medication along with surgery to prevent the CSDH recurrence are tabulated in Table 3. Many drugs were tried including corticosteroids, tranexamic acid, etizolam, angiotensin-converting enzyme (ACE) inhibitors, and other local drugs. Sun et al. favor steroid administration complementary to surgery to decrease the rate of reaccumulation and the need for re-drainage compared to surgical drainage only.[6]
Table 3.
Various studies with adjuvant medical treatment for the prevention of postoperative recurrence of chronic subdural hematoma
Angiotensin-converting enzyme inhibitor
Only a few studies were carried out on the usage of ACE inhibitors in patients of CSDH with variable results. Weigel et al., in a comparative study of 310 patients, suggested that ACE inhibitor treatment for the control of arterial hypertension lowers the risk of recurrence in patients undergoing operation for CSDH and possibly even the development of CSDH.[27] Later, in a randomized trial on the use of perindopril on residual CSDH volume, Poulsen et al. suggested that perindopril does not diminish the size of residual CSDH 6 weeks after burr hole surgery and that ACE inhibitors do not decrease the risk of CSDH recurrence.[28] In a recent article by Neidert et al., it was noted that the median initial hematoma volume in individuals on ACE inhibitors was significantly higher compared to patients without ACE inhibitors.[29] There was an increased probability of surgical reintervention in the ACE inhibitor group.
Local drugs
Gorei-san
Some authors from Japan studied the usage of the local herbal product, Gorei-san in patients with CSDH. Okamura et al., in their retrospective study of 125 patients, suggested the potential role of preoperative use of Gorei-san in preventing postoperative recurrence of CSDH.[30] Wakabayashi et al., in their study of 199 patients of CSDH, administered Gorei-san with tranexamic acid after surgery.[31] They concluded that postoperative administration of Gorei-san with the tranexamic acid can minimize the recurrence of CSDH.
Ibudilast
Ibudilast, an antagonist of platelet-activating factor receptors, was administered to patients with CSDH to assess its effectiveness in preventing recurrence by Ohta et al.,[32] they concluded that ibudilast administration may be useful in the prevention of recurrence of CSDH.
Xuefuzhuyutang
Ma et al. studied the curative effect of modified Xuefuzhuyutang on CSDH after burr-hole irrigation and drainage, and they noted that it is effective in reducing the postoperative residual volume and recurrent CSDH.[33]
Medical Treatment of the Recurrence of Chronic Subdural Hematoma
Pichert and Henn, in their study of conservative treatment of CSDH patients, treated successfully three patients of postoperative recurrent CSDH.[5] Drapkin advised to use corticosteroids for symptomatic recurrence of CSDH before reoperation.[10] Thotakura and Marabathina, in their study of nonsurgical treatment of CSDH with steroids, treated successfully one postoperative patient of CSDH with recurrence using steroids.[12]
Muramatsu et al. treated four patients of recurrent CSDH with local herbal medicine, Gorei-san successfully without surgery.[20]
Classification of Chronic Subdural Hematoma
It is important to classify CSDH to find the subgroups of patients who improve with the medical treatment and who are resistant to the treatment.
There are many classifications of CSDH based on clinical status, radiology, histopathology, and clinicopathological status. Clinical and radiological classifications are more important and useful for medical treatment protocols as patient can be classified on admission and appropriate treatment can be planned.
Markwalder classified patients of CSDH into 5 grades: Grades 0–4 based on their neurological status.[34] Some authors modified the Markwalder neurological grading using Glasgow Coma Scale in the grading.[6,35]
Conventionally, based on the computed tomography (CT) density of the hematoma, CSDH was classified into high-, mixed-, iso-, and low-density hematomas. Park et al. added layered type to the existing types.[36] They were also classified into three groups according to their magnetic resonance intensity characteristics on TIW images: low, high, and mixed intensity.[37,38]
A radiological classification of the internal architecture of the hematoma, corresponding to possible stages in the natural history of CSDH, was suggested by Nakaguchi et al. in 2001 – Homogeneous type, Laminar type, Separated type, and Trabecular type.[39]
Radiological grading was done by Thotakura and Marabathina based on the points given to the size of the lesion based on midline shift and the density of the hematoma in the CT scan (Hounsfield units).[12] The CSDH can be graded into six grades (Grades 0–5) as per this Amit-Rao grading. The size of CSDH is classified into small, medium, large, and massive hematoma based on the midline shift.
Nagahori et al. studied the outer membrane of the CSDH histopathologically and classified into four types.[40] They are Type I - noninflammatory membrane, Type II - inflammatory membrane, Type III - hemorrhagic-inflammatory membrane, and Type IV - scar-inflammatory membrane.
Yamashima and Yamamoto made a clinicopathological classification of CSDH into three types.[41] They included CSDH with a visible inner membrane (type I), acute subdural hematoma in chronic healing stage (Type II), and chronic subdural effusion of hemorrhagic type (Type III).
Factors Influencing the Outcome of Medical Treatment in Chronic Subdural Hematoma
Age
It is a well-known fact that CSDH incidence increases with the age. The atrophy of brain increases with the age and there will be more space available in the cranial cavity to accommodate more blood without much symptoms. Parlato et al. concluded in their study that age more than 70 years, brain atrophy, and absence of increase of intracranial pressure are clinical and radiological signs that allow one to choose conservative treatment.[42]
Gender
Overall, the incidence of CSDH is very less in female population compared to male population. Thotakura and Marabathina noted more successful results with steroid treatment in female patients.[12] Giuffrè et al. observed a higher incidence of estrogen receptors and progesterone receptors in men rather than in women in their study on hematoma external membrane.[43] According to the investigators, in men, whose tissues are not usually adapted to the estrogen action, the effect of estrogen on responsive tissue, such as a newly vascularized hematoma external membrane, could lead to increased formation of tissue plasminogen activator, which could maintain local hyperfibrinolysis. The probable hormonal factors that help women in preventing the formation of CSDH might also help them obtain good outcomes with medical treatment.
Neurological status
The neurological status of the patient at the time of presentation to the hospital is the most important clinical factor that decides whether medical treatment can be tried or not. The authors who tried different nonsurgical medical treatments include patients with better neurological status Markwalder grading scores 0, 1, and 2. In general, it is accepted that an unconscious patient presenting with CSDH will be taken up for surgery straight away without any thought for medical treatment. The medical treatment will take up to 3–4 months of time to act on CSDH to resolve or decrease as already shown by some authors. To show initial response, it takes 3 days to 1 week time generally. Hence, only good neurological grade patients can be treated medically. The patients who are comatose or of poor neurological grade may not be tried or treated medically.
The symptoms of patients who had spontaneous resolution of CSDH were reported to be mild headache and decrease in cognitive level. The mass effect of the hematoma in these patients was small without raised intracranial pressure.[42,44,45] They also recorded that the presence of hemiparesis was associated with the requirement of surgery. The presence of hemiparesis as an initial symptom was associated with the requirement of surgery in a study done to evaluate the effect of etizolam on the resolution of CSDH.[2]
Size of the lesion
Some authors noted that small-sized CSDH responds better with medical treatment. Although volume of the bleed represents the size of the lesion, midline shift can also be given more importance as it represents the overall mass effect and is easy to calculate on imaging. A small size of the lesion correlates with successful medical treatment of CSDH with steroids in the study by Thotakura and Marabathina.[12] Delgado-López et al. noted in their study that up to 70% of patients with chronic SDH could be treated with steroid medical therapy.[11] Fifty-five of 101 patients who were managed by dexamethasone did not have any midline shift; they correlate to low grades in the Amit-Rao radiological grading.
It can be thought that the CSDH is a spectrum of disease. The small lesions cause a less mass effect with fewer symptoms and may resolve spontaneously. The moderate- to large-sized lesions are symptomatic, most of them being with good clinical grades, may respond to medical line of treatment. The large- to massive-sized lesions cause more mass effect, some of them are with poor clinical grades and most of them require surgery.
Density of the hematoma
Thotakura and Marabathina noted that less dense hematomas (low CT attenuation values) responded better to steroid treatment than with more dense hematomas.[12] Hirashima et al. noted the significance of density of the bleed in influencing the outcome of etizolam on resolution of CSDH.[2] Low dense hematomas do better with etizolam.
Side Effects of the Drugs Used in Chronic Subdural Hematoma
Steroids
Infections, gastrointestinal bleeding, and hyperglycemia were the most relevant complications encountered after administering long-term corticosteroids. Infection was noted in 9% patients.[11] Only two patients were reported to have gastrointestinal bleeding.[4,11] Hyperglycemia noted in 50% of diabetic patients[6] and 3.8 - 40% of nondiabetic patients[11,12,18]. One patient (3.8%) had gastritis.[12] Pichert and Henn observed complications such as pulmonary embolism, steroid psychosis, or leg infection in five patients (9.4%).[5] Prud’homme et al. recorded one patient (10%) each developing hypertension, pulmonary embolus, cellulitis, pulmonary edema, and suicide.[18]
Atorvastatin
No documented atorvastatin-related side effects were observed.[3]
Tranexamic acid
Among all study patients, no adverse events were observed.[1]
Etizolam
Somnolence was noted as a side effect in 33.3% of patients.[2]
Ongoing research
Emich et al. doing a randomized controlled study to know the efficacy of dexamethasone on reduction in the reoperation rate of CSDH – the DRESH study.[46] This trial includes 820 patients who are randomized to take 6 days of dexamethasone or placebo after surgery. Jiang et al. started a randomized controlled trial, effect of atorvastatin on CSDH to know whether atorvastatin is an effective and safe alternative to surgical treatment of CSDH.[47] Iorio-Morin et al. started a randomized controlled trial, TRACS to investigate whether tranexamic acid can increase the rate of CSDH resolution following conservative management, lower the number of required surgical procedures, and decrease the rate of CSDH recurrence following surgical evacuation.[48]
Future Research
Although a lot of research was carried out on various surgical approaches and other surgical aspects, not many were there in the medical treatment of CSDH. There were many issues to be solved in the medical treatment of CSDH such as the indications of drugs in CSDH management, ideal dosage, duration, and ideal subgroup of patients.
Although certain drugs reduce the postoperative recurrence rate of CSDH, not all the patients require them as the recurrence rate is up to 30%.[49] It should be aimed to identify the subgroup of population who are at risk of recurrence of CSDH and the medications which help to prevent the recurrence.
Conclusions
There is a role of medical treatment in a selected group of patients with CSDH. Well-designed, multicenter, randomized controlled trials are required to define the indications and standard protocols for the medical treatment of CSDH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kageyama H, Toyooka T, Tsuzuki N, Oka K. Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg. 2013;119:332–7. doi: 10.3171/2013.3.JNS122162. [DOI] [PubMed] [Google Scholar]
- 2.Hirashima Y, Kurimoto M, Nagai S, Hori E, Origasa H, Endo S. Effect of platelet-activating factor receptor antagonist, etizolam, on resolution of chronic subdural hematoma – A prospective study to investigate use as conservative therapy. Neurol Med Chir (Tokyo) 2005;45:621–6. doi: 10.2176/nmc.45.621. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Li T, Tian Y, Wang S, Jin C, Wei H, et al. Effects of atorvastatin on chronic subdural hematoma: A preliminary report from three medical centers. J Neurol Sci. 2014;336:237–42. doi: 10.1016/j.jns.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31:73–9. doi: 10.1001/archneur.1974.00490380021001. [DOI] [PubMed] [Google Scholar]
- 5.Pichert G, Henn V. Conservative therapy of chronic subdural hematomas. Schweiz Med Wochenschr. 1987;117:1856–62. [PubMed] [Google Scholar]
- 6.Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: Preliminary results of using dexamethasone. Br J Neurosurg. 2005;19:327–33. doi: 10.1080/02688690500305332. [DOI] [PubMed] [Google Scholar]
- 7.Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ. The management of primary chronic subdural haematoma: A questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2008;22:529–34. doi: 10.1080/02688690802195381. [DOI] [PubMed] [Google Scholar]
- 8.Berghauser Pont LM, Dippel DW, Verweij BH, Dirven CM, Dammers R. Ambivalence among neurologists and neurosurgeons on the treatment of chronic subdural hematoma: A national survey. Acta Neurol Belg. 2013;113:55–9. doi: 10.1007/s13760-012-0130-1. [DOI] [PubMed] [Google Scholar]
- 9.Cenic A, Bhandari M, Reddy K. Management of chronic subdural hematoma: A national survey and literature review. Can J Neurol Sci. 2005;32:501–6. doi: 10.1017/s0317167100004510. [DOI] [PubMed] [Google Scholar]
- 10.Drapkin AJ. Chronic subdural hematoma: Pathophysiological basis for treatment. Br J Neurosurg. 1991;5:467–73. doi: 10.3109/02688699108998475. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Rodríguez-Salazar A, Galacho-Harriero AM, Fernández-Arconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur) 2009;20:346–59. doi: 10.1016/s1130-1473(09)70154-x. [DOI] [PubMed] [Google Scholar]
- 12.Thotakura AK, Marabathina NR. Nonsurgical treatment of chronic subdural hematoma with steroids. World Neurosurg. 2015;84:1968–72. doi: 10.1016/j.wneu.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosetto C. Post-traumatic subdural hematoma. Further observations on nonsurgical treatment. Arch Neurol. 1962;6:287–92. doi: 10.1001/archneur.1962.00450220029005. [DOI] [PubMed] [Google Scholar]
- 14.Inzelberg R, Neufeld MY, Reider I, Gari P. Non surgical treatment of subdural hematoma in a hemodialysis patient. Clin Neurol Neurosurg. 1989;91:85–9. doi: 10.1016/s0303-8467(89)80014-9. [DOI] [PubMed] [Google Scholar]
- 15.Rudiger A, Ronsdorf A, Merlo A, Zimmerli W. Dexamethasone treatment of a patient with large bilateral chronic subdural haematomata. Swiss Med Wkly. 2001;131:387. doi: 10.4414/smw.2001.09745. [DOI] [PubMed] [Google Scholar]
- 16.Decaux O, Cador B, Dufour T, Jégo P, Cazalets C, Laurat E, et al. Nonsurgical treatment of chronic subdural hematoma with steroids: Two case reports. Rev Med Interne. 2002;23:788–91. doi: 10.1016/s0248-8663(02)00676-8. [DOI] [PubMed] [Google Scholar]
- 17.Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al. Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014;259:449–57. doi: 10.1097/SLA.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 18.Prud’homme M, Mathieu F, Marcotte N, Cottin S. A pilot placebo controlled randomized trial of dexamethasone for chronic subdural hematoma. Can J Neurol Sci. 2016;43:284–90. doi: 10.1017/cjn.2015.393. [DOI] [PubMed] [Google Scholar]
- 19.Miyagami M, Kagawa Y. Effectiveness of Kampo medicine Gorei-San for chronic subdural hematoma. No Shinkei Geka. 2009;37:765–70. [PubMed] [Google Scholar]
- 20.Muramatsu M, Yoshikawa T, Hanabusa K. Effectiveness of Kampo medicine Gorei-san-ryo for chronic subdural hematoma in very elderly patients. No Shinkei Geka. 2005;33:965–9. [PubMed] [Google Scholar]
- 21.Dran G, Berthier F, Fontaine D, Rasenrarijao D, Paquis P. Effectiveness of adjuvant corticosteroid therapy for chronic subdural hematoma: A retrospective study of 198 cases. Neurochirurgie. 2007;53:477–82. doi: 10.1016/j.neuchi.2007.09.146. [DOI] [PubMed] [Google Scholar]
- 22.Berghauser Pont LM, Dammers R, Schouten JW, Lingsma HF, Dirven CM. Clinical factors associated with outcome in chronic subdural hematoma: A retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery. 2012;70:873–80. doi: 10.1227/NEU.0b013e31823672ad. [DOI] [PubMed] [Google Scholar]
- 23.Chan DY, Sun TF, Poon WS. Steroid for chronic subdural hematoma? A prospective phase IIB pilot randomized controlled trial on the use of dexamethasone with surgical drainage for the reduction of recurrence with reoperation. Chin Neurosurg J. 2015;1:2. [Google Scholar]
- 24.Xu XP, Liu C, Liu J, Pang YG, O XD, Fu J, et al. Local application of corticosteroids combined with surgery for the treatment of chronic subdural hematoma. Turk Neurosurg. 2015;25:252–5. doi: 10.5137/1019-5149.JTN.8989-13.3. [DOI] [PubMed] [Google Scholar]
- 25.Tanweer O, Frisoli FA, Bravate C, Harrison G, Pacione D, Kondziolka D, et al. Tranexamic acid for treatment of residual subdural hematoma after bedside twist-drill evacuation. World Neurosurg. 2016;91:29–33. doi: 10.1016/j.wneu.2016.03.062. [DOI] [PubMed] [Google Scholar]
- 26.Hirashima Y, Kuwayama N, Hamada H, Hayashi N, Endo S. Etizolam, an anti-anxiety agent, attenuates recurrence of chronic subdural hematoma – Evaluation by computed tomography. Neurol Med Chir (Tokyo) 2002;42:53–5. doi: 10.2176/nmc.42.53. [DOI] [PubMed] [Google Scholar]
- 27.Weigel R, Hohenstein A, Schlickum L, Weiss C, Schilling L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 2007;61:788–92. doi: 10.1227/01.NEU.0000298907.56012.E8. [DOI] [PubMed] [Google Scholar]
- 28.Poulsen FR, Munthe S, Søe M, Halle B. Perindopril and residual chronic subdural hematoma volumes six weeks after burr hole surgery: A randomized trial. Clin Neurol Neurosurg. 2014;123:4–8. doi: 10.1016/j.clineuro.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Neidert MC, Schmidt T, Mitova T, Fierstra J, Bellut D, Regli L, et al. Preoperative angiotensin converting enzyme inhibitor usage in patients with chronic subdural hematoma: Associations with initial presentation and clinical outcome. J Clin Neurosci. 2016;28:82–6. doi: 10.1016/j.jocn.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Okamura A, Kawamoto Y, Sakoda E, Murakami T, Hara T. Evaluation of recurrence factors and Gorei-san administration for chronic subdural hematoma after percutaneous subdural tapping. Hiroshima J Med Sci. 2013;62:77–82. [PubMed] [Google Scholar]
- 31.Wakabayashi Y, Yamashita M, Asano T, Yamada A, Kenai H, Kondoh Y, et al. Effect of Gorei-san with tranexamic acid for preventing recurrence of chronic subdural hematoma. No Shinkei Geka. 2012;40:967–71. [PubMed] [Google Scholar]
- 32.Ohta H, Genmoto T, Akiba D, Urasaki E, Yokota A. Clinical application of ibudilast for elder patients with chronic subdural hematoma. J UOEH. 2005;27:377–83. doi: 10.7888/juoeh.27.377. [DOI] [PubMed] [Google Scholar]
- 33.Ma DY, Zhou ZF, Li QQ, Zhang MW, Wang Q. Clinical effect of modified Xuefuzhuyutang on senile chronic subdural hematoma after operation. Zhong Yao Cai. 2014;37:1499–501. [PubMed] [Google Scholar]
- 34.Markwalder TM, Steinsiepe KF, Rohner M, Reichenbach W, Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55:390–6. doi: 10.3171/jns.1981.55.3.0390. [DOI] [PubMed] [Google Scholar]
- 35.Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R. The role of corticosteroids in the management of chronic subdural hematoma: A systematic review. Eur J Neurol. 2012;19:1397–403. doi: 10.1111/j.1468-1331.2012.03768.x. [DOI] [PubMed] [Google Scholar]
- 36.Park HR, Lee KS, Shim JJ, Yoon SM, Bae HG, Doh JW. Multiple densities of the chronic subdural hematoma in CT scans. J Korean Neurosurg Soc. 2013;54:38–41. doi: 10.3340/jkns.2013.54.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senturk S, Guzel A, Bilici A, Takmaz I, Guzel E, Aluclu MU, et al. CT and MR imaging of chronic subdural hematomas: A comparative study. Swiss Med Wkly. 2010;140:335–40. doi: 10.4414/smw.2010.12867. [DOI] [PubMed] [Google Scholar]
- 38.Lee KS, Bae WK, Bae HG, Doh JW, Yun IG. The computed tomographic attenuation and the age of subdural hematomas. J Korean Med Sci. 1997;12:353–9. doi: 10.3346/jkms.1997.12.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256–62. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 40.Nagahori T, Nishijima M, Takaku A. Histological study of the outer membrane of chronic subdural hematoma: Possible mechanism for expansion of hematoma cavity. No Shinkei Geka. 1993;21:697–701. [PubMed] [Google Scholar]
- 41.Yamashima T, Yamamoto S. Clinicopathological classification of chronic subdural hematoma. Zentralbl Neurochir. 1985;46:304–14. [PubMed] [Google Scholar]
- 42.Parlato C, Guarracino A, Moraci A. Spontaneous resolution of chronic subdural hematoma. Surg Neurol. 2000;53:312–5. doi: 10.1016/s0090-3019(00)00200-7. [DOI] [PubMed] [Google Scholar]
- 43.Giuffrè R, Palma E, Liccardo G, Sciarra F, Pastore FS, Concolino G. Sex steroid hormones in the pathogenesis of chronic subdural haematoma. Neurochirurgia (Stuttg) 1992;35:103–7. doi: 10.1055/s-2008-1052258. [DOI] [PubMed] [Google Scholar]
- 44.Naganuma H, Fukamachi A, Kawakami M, Misumi S, Nakajima H, Wakao T. Spontaneous resolution of chronic subdural hematomas. Neurosurgery. 1986;19:794–8. doi: 10.1227/00006123-198611000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Marcikic M, Hreckovski B, Samardzic J, Martinovic M, Rotim K. Spontaneous resolution of post-traumatic chronic subdural hematoma: Case report. Acta Clin Croat. 2010;49:331–4. [PubMed] [Google Scholar]
- 46.Emich S, Richling B, McCoy MR, Al-Schameri RA, Ling F, Sun L, et al. The efficacy of dexamethasone on reduction in the reoperation rate of chronic subdural hematoma-the DRESH study: Straightforward study protocol for a randomized controlled trial. Trials. 2014;15:6. doi: 10.1186/1745-6215-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang R, Wang D, Poon WS, Lu YC, Li XG, Zhao SG, et al. Effect of ATorvastatin On Chronic subdural Hematoma (ATOCH): A study protocol for a randomized controlled trial. Trials. 2015;16:528. doi: 10.1186/s13063-015-1045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iorio-Morin C, Blanchard J, Richer M, Mathieu D. Tranexamic Acid in Chronic Subdural Hematomas (TRACS): Study protocol for a randomized controlled trial. Trials. 2016;17:235. doi: 10.1186/s13063-016-1358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh HJ, Lee KS, Shim JJ, Yoon SM, Yun IG, Bae HG. Postoperative course and recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2010;48:518–23. doi: 10.3340/jkns.2010.48.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]


