Abstract
Background
Infections in early life have been linked to type 1 diabetes (T1D) risk, but no previous study has comprehensively analysed exposure to antibiotics, acetaminophen and infections during pregnancy and early childhood in relation to offspring risk of T1D.
Methods
Participants in the Norwegian Mother and Child Cohort Study (n = 114 215 children, of whom 403 children were diagnosed with T1D) reported infections and medication use through repeated questionnaires from pregnancy until the children were 18 months old. Adjusted hazard ratios (aHR) for offspring T1D were estimated through Cox regression adjusted for child’s sex, maternal age and parity, maternal T1D, smoking in pregnancy, education level, pre-pregnancy body mass index (BMI) and birthweight. Antibiotic use was also analysed in a population-based register cohort of 541 036 children of whom 836 developed T1D.
Results
Hospitalization for gastroenteritis during the first 18 months of life was associated with increased risk (aHR 2.27, 95% CI 1.21 − 4.29, P = 0.01) of T1D. Childhood infections not requiring hospitalization, or any kind of maternal infection during pregnancy, did not predict offspring risk of T1D. Antibiotic or acetaminophen use in pregnancy, or child`s use in early childhood, was not associated with risk of T1D.
Conclusions
Our study, which is population-based and the largest of its kind, did not find support for general early life infections, infection frequency or use of antibiotics or acetaminophen to play a major role in childhood T1D. Hospital admission for gastroenteritis was associated with T1D risk, but must be interpreted cautiously due to scarcity of cases.
Keywords: Antibiotics, acetaminophen, paracetamol, infection, type 1 diabetes, pregnancy, childhood, Norwegian Mother and Child Cohort
Key Messages
Antibiotic or acetaminophen (paracetamol) use during pregnancy was not associated with offspring type 1 diabetes risk.
Infections during pregnancy were not associated with offspring type 1 diabetes risk.
Antibiotic or acetaminophen use in early life was not associated with type 1 diabetes risk.
General infections during early life were not associated with type 1 diabetes risk, but there was a possible association between hospitalization for gastroenteritis in early childhood and higher risk of type 1 diabetes.
Introduction
Genetic studies in type 1 diabetes (T1D) have revealed multiple risk loci, but environmental risk factors are essentially unknown. Childhood infections have long been suspected (for a brief overview see Supplement and Supplementary Figure 1, available as Supplementary data at IJE online), but it is unclear whether specific or general infections, or infection severity, could be associated with type 1 diabetes. Previous studies have not simultaneously considered antibiotics and acetaminophen (paracetamol), which are commonly used for infections, and could be mediators in observed associations.
Antibiotics in early life influence microbiota composition, a factor proposed in type 1 diabetes development.1 The maternal gut microbiota influences postnatal immune development in mice,2 including diabetes development.3 Maternal antibiotic use has been associated with offspring asthma risk in epidemiological studies, and is suspected to be an indicator for infection propensity.4 There are few studies of antibiotics and type 1 diabetes, and they have not simultaneously considered infections as potential confounders. Use of acetaminophen during pregnancy has been linked to offspring asthma,5,6 but to our knowledge, use during pregnancy and offspring risk of type 1 diabetes have not previously been studied. It is also conceivable that acetaminophen use during febrile infections may influence immunity and possibly immune-mediated diseases in childhood. A recent study of analgesic antipyretics use found no association with islet autoimmunity, a surrogate endpoint for type 1 diabetes.7 Other earlier studies on analgesic use8–11 might include other medications and are retrospective or use medical records, which indicates an underlying condition.
We aimed to study prenatal and early life exposure to antibiotics, acetaminophen or infections, and type 1 diabetes risk, in a large, prospective, population-based birth cohort. Antibiotics were also studied in a nationwide register-based cohort. We further assessed whether a possible association between infections and type 1 diabetes would be influenced by adjustment for antibiotic or acetaminophen use.
Methods
Participants, design and outcome: prospective pregnancy cohort study
We analysed two cohorts (Supplementary Figure 2, available as Supplementary data at IJE online). The primary cohort is the Norwegian Mother and Child Cohort Study (MoBa), a Norwegian nationwide population-based pregnancy cohort that recruited ∼114 000 children (114 215 in the present study).12 Participants (41% of eligible pregnancies) were recruited around pregnancy week 17 during 1999–2008. Maternal pregnancy exposures were assessed by questionnaires that are available at [www.fhi.no/moba], administered at pregnancy weeks 17 and 30, and at child’s age 6 months (covering pregnancy weeks 0–17, 18–30 and 30 until delivery, respectively). The child’s exposures were collected from parent-recorded questionnaires administered at ages 6 and 18 months. Follow-up in MoBa is ongoing; in this sub-study, we included cases diagnosed with type 1 diabetes by 31 May 2017.
Children with type 1 diabetes (n = 403) were identified through the Norwegian Childhood Diabetes Registry (data capture until 31 May 2017),13 and the Norwegian Patient Register (NPR). Reporting to the NPR is mandatory, and the International Classification of Diseases, 10th revision (ICD-10) diagnosis E10 (type 1 diabetes) was available from all government-owned hospitals and outpatient clinics in Norway, which covers virtually all paediatric care, from 1 January 1 2008 until 31 December 31 2013. Data were linked using the Norwegian 11-digit personal identification number. The study included live-born children who survived their first year of life, with questionnaire data from pregnancy up to 6 (n = 84 418; 336 cases) and 18 months (n = 70 440; 286 cases).
Participants, design and outcome: register-based cohort study
To complement our analyses of antibiotics and type 1 diabetes, we obtained a partially independent register-based cohort with only antibiotic data. All children born between 1 January 2004 (start of follow–up) and 31 December 2012, as registered in the Medical Birth Registry of Norway (MBRN) (n = 541 036, including 836 type 1 diabetes cases) formed the cohort (Supplementary Figure 2, available as Supplementary data at IJE online), with follow-up until January 2015. The Norwegian prescription database (NorPD) provided antibiotic prescription data during pregnancy or early childhood, and insulin prescriptions to mother or child, dispensed at Norwegian pharmacies. Type 1 diabetes diagnosis was identified using ICD-10 code E10 from the NPR up to the end of 2013, with the first dispensed insulin prescription from NorPD used as a proxy for diagnosis date. We identified 726 children from the NPR (2008–13) with any registered E10 code, and 665 children had an insulin prescription, yielding 656 with both. Children with only insulin prescription (n = 9) or E10 diagnosis (n = 70) were likely misclassifications, as sourcing insulin outside health services is unlikely and children have regular check-ups which necessitates a diagnosis, and were excluded. Additionally 181 had insulin prescriptions in 2014, (data unavailable for 2014 from the NPR), which we included as cases. Children with first insulin prescription <6 months of age (n = 1) were excluded, leaving 836 cases.
Highest attained maternal education by 2014 was obtained from the government agency Statistics Norway. No information on maternal body mass index (BMI), infections or acetaminophen was available in this cohort, but this cohort has no self-selection and a larger sample size than MoBa. Children in MoBa born 2004–09 were also part of the register-based cohort.
Exposures
Our specified primary exposure for analysis was the number of parent-reported exposures (antibiotics, acetaminophen and infections), each measured separately as described below, during the whole pregnancy or the first 18 months of life. Exposures were divided into groups, with the lowest category as reference. Secondary analyses considered specific time periods (first 6 months of life, only before or after pregnancy week 17, or both) and specific categories as described below. Additionally, analyses to replicate earlier reported findings were done.
In Norway, antibiotics are only available through prescription, whereas acetaminophen is freely available at pharmacies and general stores. All medication use was coded using the anatomical therapeutic chemical (ATC) pharmaceutical classification system. Medication use was counted irrespective of indication. Antibiotics were grouped into penicillin V, extended-spectrum penicillins, other specified systemic antibiotics, or unspecified if no ATC code could be identified (Supplementary Table 1, available as Supplementary data at IJE online). We assessed potential dose-response effects by considering repeated use of antibiotics (≥2 vs 1 vs 0 courses), and number of days of acetaminophen usage. Children with very high frequency of reported acetaminophen use (>60 days in the first 6 months, n = 10) were excluded from analysis due to potential comorbidities.
Our primary analysis considered the total number of infections, and secondary analyses considered specific groups/categories of infections. Type of infection (yes/no) was specified as common cold, throat infection, sinusitis/ear infection, lower respiratory tract infections [pneumonia/bronchitis in mothers, and additionally respiratory syncytial virus (RS-virus) in the children], urinary tract infections (pyelonephritis/acute cystitis in mothers) and gastroenteritis. Additionally, mothers reported influenza during pregnancy and child’s croup. Infections were grouped by type: respiratory tract infections (pneumonia/acute bronchitis, common cold, influenza, throat, sinusitis/ear infections), gastroenteritis and urinary tract infections during pregnancy, upper respiratory tract infections (common cold, throat infection, ear infection and croup), lower respiratory tract infections and gastroenteritis in childhood. Additional groups were febrile infections in pregnancy (>38.5°C, compared with no infections) and infections requiring medical care in early childhood (the participants were asked if the child had been hospitalized during 0—6 or 6—18 months of life, or had doctor/clinic visits the first 6 months of life).
A priori, we decided to count separate reports of common cold/influenza, pneumonia/acute bronchitis and throat infection/sinusitis/ear infection as a single episode if reported within the same period of pregnancy. Children with an implausibly high infection frequency (≥10 specific infections in a 6-month period) were excluded from analysis.
Other variables
Based on previous literature14,15 and available data, we selected the following adjustment variables which were covariates in the adjusted analysis: maternal age, pregnancy smoking, type 1 diabetes, education, pre-pregnancy body mass index (BMI) and parity; child’s sex, birthweight, gestational age and mode of delivery (categorized as shown in Table 1). Infections, antibiotic and acetaminophen use were included in the adjusted models, and mutually adjusted for each other (e.g. when analysing infections, variables of antibiotic and acetaminophen use were included as covariates). BMI was unavailable in the register cohort. As a sensitivity analysis, we included breastfeeding (any versus no breastfeeding) in the childhood analyses. Variable data were retrieved from pregnancy questionnaires and MBRN.16 Maternal type 1 diabetes was ascertained from the NPR (ICD-10 code E10) in MoBa and from NorPD in the register cohort. We investigated the unadjusted association between each exposure (infections, antibiotic or acetaminophen use) and offspring type 1 diabetes. We then adjusted for covariates listed above, and lastly additionally adjusted for other exposures (e.g when analysing infections, we adjusted for antibiotic and acetaminophen use in addition to covariates). We present unadjusted estimates, and estimates adjusted for other exposures and covariates. To illustrate the assumed relationships between these variables, directed acyclic graphs (DAGs) were drawn (Supplementary Figure 3, available as Supplementary data at IJE online).
Table 1.
Characteristics of children in MoBa pregnancy cohort and population based-cohort
| MoBa; pregnancy, and until 6 months of age. n (%) | MoBa; until 18 months of age, n (%) | Register-based cohort | |
|---|---|---|---|
| Participants with exposure data | 84 407 (100.0%) | 70 430 (100.0%) | 537 460 (100.0%) |
| Girls | 41 224 (48.8%) | 34 429 (48.9%) | 261 583 (48.7%) |
| Maternal diabetes | 454 (0.5%) | 379 (0.5%) | 6 510 (1.2%)a |
| Maternal educationb | |||
| Low | 29 939 (35.5%) | 23 983 (34.1%) | 87 878 (16.4%) |
| Medium | 34 887 (41.3%) | 29 648 (42.1%) | 153 954 (28.6%) |
| High | 19 226 (22.8%) | 16 528 (23.5%) | 274 010 (51.0%) |
| Missing | 355 (0.4%) | 271 (0.4%) | 21 618 (4.0%) |
| Maternal smoking in pregnancy | |||
| No | 76 351 (90.5%) | 64 185 (91.1%) | 385 688 (71.8%) |
| Yes | 6747 (8.0%) | 5232 (7.4%) | 61 524 (11.5%) |
| Missing | 1309 (1.6%) | 1013 (1.4%) | 90 248 (16.8%) |
| Maternal parity | |||
| 0 | 39 528 (46.8%) | 33 592 (47.7%) | 226 465 (42.1%) |
| 1 | 29 318 (34.7%) | 24 047 (34.1%) | 191 902 (35.7%) |
| ≥2 | 15 561 (18.4%) | 12 791 (18.2%) | 119 093 (22.2%) |
| Maternal pre-pregnancy BMI | |||
| <25 | 56 602 (67.1%) | 47 566 (67.5%) | – |
| 25-29.9 | 17 968 (21.3%) | 14 898 (21.2%) | – |
| ≥30 | 7662 (9.1%) | 6250 (8.9%) | – |
| Missing | 2175 (2.6%) | 1716 (2.4%) | 537 460 (100%)c |
| Birthweight (grams) | |||
| <2500 g | 3756 (4.4%) | 3154 (4.5%) | 26 140 (4.9%) |
| 2500-3499 g | 32 108 (38.0%) | 26 847 (38.1%) | 227 094 (42.3%) |
| 3500-4499 g | 44 795 (53.1%) | 37 315 (53.0%) | 265 635 (49.4%) |
| ≥4500 g | 3748 (4.4%) | 3114 (4.4%) | 18 591 (3.5%) |
| Maternal age (years) | |||
| ≤24 | 8487 (10.1%) | 6541 (9.3%) | 88 739 (16.5%) |
| 25-34 | 61 114 (72.4%) | 51 379 (73.0%) | 346 882 (64.5%) |
| ≥35 | 14 806 (17.5%) | 12 510 (17.8%) | 101 839 (19.0%) |
| Gestational age | |||
| <37 weeks | 4774 (5.7%) | 3981 (5.7%) | 35 397 (6.6%) |
| ≥37 weeks | 79 276 (93.9%) | 66 149 (93.9%) | 498 263 (92.7%) |
| Missing | 357 (0.4%) | 300 (0.4%) | 3800 (0.7%) |
| Caesarean section | 12 271 (14.5%) | 10 172 (14.4%) | 90 128 (16.8%) |
| Breastfeeding | – | – | |
| Never | 945 (1.1%) | 778 (1.1%) | – |
| Yes | 83 462 (98.9%) | 69 652 (98.9%) | – |
| Missing | – | – | 537 460 (100%)c |
| Child age at end of follow-up among non-cases, median (range) | 12.3 (8.4-17.9) | 12.3 (8.4-17.9) | 6.4 (2.0–11.0) |
In the register-based cohort this is defined as maternal insulin use, which would include gestational diabetes and type 2 diabetes.
Characterized as: ≤12 years, 13–15 years, ≥16 years in the MoBa cohort; and 9 years, 10–12 years, ≥13 years in the register cohort. This is maternal education at ∼week 18 of pregnancy in the MoBa cohort, and highest attained education in the register-based cohort. This is due to differences in how the data are provided from Statistics Norway and how the questions are asked in the MoBa questionnaires.
Not available in the register-based cohort.
Statistical analyses
We used Cox proportional hazard regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), after confirmation that the models did not violate the proportional hazards assumption by assessment of Schönfeld residuals. Several models were fit on the same exposure: exposure as a continuous variable (which gives maximum statistical power under the log-linearity assumption), as a categorical variable (to assess linearity and possible threshold effects) and as a binary variable (yes/no, to compare with other studies). Robust variance estimator was used to account for potentially correlated data among siblings. In analyses of pregnancy exposures, we counted time from birth to diagnosis of type 1 diabetes. To ensure exposures occurred before type 1 diabetes in childhood analyses, we excluded cases with diagnosis before 6 and 18 months of age in these time periods. Therefore, we counted time from 6 or 18 months to type 1 diabetes diagnosis for exposures in the first 6 and 18 months of life, respectively. Children were followed until diagnosis or administrative censoring (May 2017 in MoBa, January 2015 in the register-based cohort), whichever occurred first. To investigate possible effects of misclassification, a bias analysis was conducted using the episens package.17 As a sensitivity analysis, a conditional logistic regression using mother as grouping variable (sibling-matched design) was done in the register-based cohort. Stata version 14 (StataCorp LP, TX, USA) was used for the statistical analyses.
Ethics
Written informed consent was obtained from all MoBa participants. The establishment and data collection in MoBa has obtained a license from the Norwegian Data Protection Authority, and the present study was approved by the Regional Committee for Medical Research Ethics in South-Eastern Norway (REK). The register-based cohort study was approved by REK, and has an independent licence from the Norwegian Data Protection Authority.
Results
Distributions of covariates for the cohorts are shown in Table 1. The prevalence of exposures in the MoBa cohort are shown in Supplementary Table 2, available as Supplementary data at IJE online.
Among children in MoBa (n = 84 407), median age attained at study end (May 2017) was 12.3 years (range 8. –17.9), and 403 (204 girls, 51%) were diagnosed with type 1 diabetes, with median diagnosis age of 7.4 years (range 0.7–15.2 years). In the register-based cohort (n = 537 460), median age attained at study end (January 2015) was 6.4 years (range 2.0–11.0), and 836 (391 girls, 46.7%) were classified as type 1 diabetes cases, with a median diagnosis age of 4.4 years (range 0.5–10.5 years).
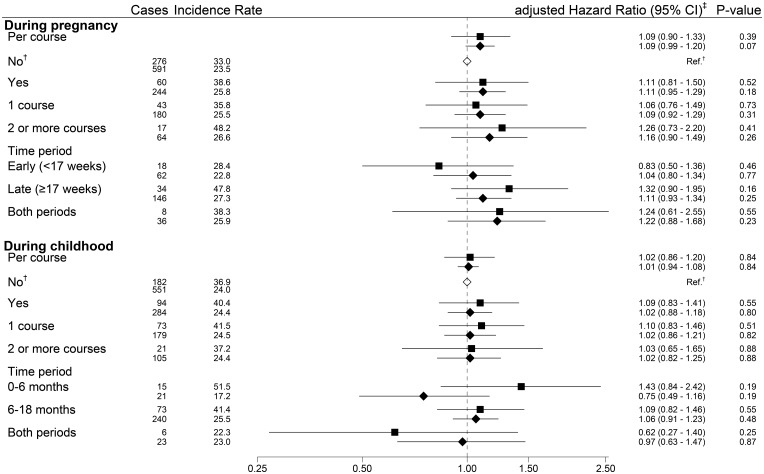
Antibiotic use during pregnancy, the first 6 or 18 months of life did not seem to be associated to offspring risk of type 1 diabetes in either cohort, and we observed no dose-dependent associations (Figure 1). To investigate previous reports that children taking ≥5 or >7 antibiotic courses had increased risk we tested both these cut-offs but failed to replicate these findings (data not shown). Sibling-matched analysis of antibiotic use gave similar results (Supplementary Table 5, available as Supplementary data at IJE online).
Figure 1.
Forest plot of antibiotic use and type 1 diabetes risk in both cohorts.* *Results from the MoBa cohort (n = 84 407) are in the top lines, marked with a square, and results from the register cohort (n = 537 460) are in the bottom line, marked with a diamond. †Common reference for all exposure categories in the relevant time period. ‡Hazard ratios estimated through Cox regression adjusted for child’s sex, maternal age and parity, maternal type 1 diabetes, smoking in pregnancy, education level, pre-pregnancy BMI, prematurity, birthweight, mode of delivery, infections and acetaminophen use during the relevant time period (pregnancy, or first 6 or 18 months of life) in the MoBa cohort. The register cohort was adjusted for the same variables, with the exception of maternal pre-pregnancy BMI, smoking, acetaminophen use and infections. Adjusting for maternal smoking in the register-based cohort did not appreciably change our estimates; we present data not adjusted for maternal smoking, as there was a high proportion with missing data in the register-based cohort. Children with complete data on covariates (n = 67 718 for analysis at 0-18 months of age) were included in adjusted model for the MoBa cohort.
Acetaminophen exposure in pregnancy or in the first 18 months of life did not show an increased type 1 diabetes risk (Table 2), although estimates for use in pregnancy were increased (aHR 1.22, −CI 0.97 − 1.53, P = 0.08). Using acetaminophen ≥3 days the first 6 months of life showed an association with type 1 diabetes (aHR 0.65, 95% CI 0.43 − 0.98, P = 0.04 Supplementary Table 3, available as Supplementary data at IJE online).
Table 2.
Risk of type 1 diabetes in offspring according to acetaminophen use during pregnancy and early childhood in the MoBa cohort
| Cases n = 336a | Incidence rate/100 000b | Unadjusted |
Adjustedc |
|||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | aHR | 95% CI | P-value | |||
| Use during pregnancy | ||||||||
| No | 165 | 30.9 | Ref. | Ref | ||||
| Yes | 171 | 37.4 | 1.22 | 0.98–1.51 | 0.08 | 1.22 | 0.97–1.53 | 0.08 |
| Number of days usedd | ||||||||
| No days | 160 | 31.2 | Ref. | Ref. | ||||
| 1 day | 43 | 36.1 | 1.16 | 0.83–1.63 | 0.38 | 1.21 | 0.86–1.71 | 0.27 |
| 2-4 days | 43 | 35.9 | 1.16 | 0.82–1.63 | 0.40 | 1.23 | 0.87–1.74 | 0.25 |
| >5 days more days | 36 | 32.6 | 1.05 | 0.73–1.52 | 0.77 | 1.02 | 0.70–1.50 | 0.92 |
| Per day of use | 1.00 | 0.99–1.01 | 0.93 | 1.00 | 0.98–1.01 | 0.31 | ||
| Period of use | ||||||||
| Early (<17 weeks) | 61 | 38.1 | 1.24 | 0.92–1.67 | 0.17 | 1.27 | 0.93–1.73 | 0.13 |
| Late (>17 weeks) | 40 | 36.0 | 1.17 | 0.83–1.66 | 0.37 | 1.13 | 0.79–1.62 | 0.51 |
| Both periods | 70 | 37.5 | 1.22 | 0.92–1.63 | 0.17 | 1.23 | 0.92–1.66 | 0.17 |
| Child use 0-18 months of age | n = 286 | |||||||
| No | 109 | 36.3 | Ref. | Ref. | ||||
| Yes | 217 | 38.4 | 1.02 | 0.78–1.33 | 0.88 | 0.98 | 0.74–1.28 | 0.86 |
| Number of episodese | ||||||||
| No use | 79 | 37.6 | Ref. | Ref. | ||||
| 1 episode | 43 | 37.2 | 0.99 | 0.68–1.44 | 0.95 | 0.95 | 0.65–1.40 | 0.80 |
| 2 episodes | 52 | 38.1 | 1.01 | 0.71–1.44 | 0.94 | 0.99 | 0.69–1.41 | 0.94 |
| 3 episodes | 42 | 39.5 | 1.05 | 0.72–1.53 | 0.79 | 1.02 | 0.69–1.49 | 0.93 |
| 4 or more episodes | 60 | 38.2 | 1.03 | 0.73–1.44 | 0.87 | 0.96 | 0.67–1.37 | 0.82 |
| Per episode | 1.01 | 0.93–1.09 | 0.96 | 0.99 | 0.92–1.06 | 0.78 | ||
| Period of use | ||||||||
| No use | 103 | 34.9 | Ref. | Ref. | ||||
| 0-6 months of age | 47 | 41.3 | 1.15 | 0.72–1.82 | 0.56 | 1.15 | 0.72–1.82 | 0.57 |
| 6-18 months of age | 111 | 41.1 | 1.14 | 0.85–1.54 | 0.38 | 1.09 | 0.80–1.48 | 0.58 |
| 0-18 months | 65 | 34.9 | 0.98 | 0.70–1.37 | 0.90 | 0.93 | 0.66–1.32 | 0.70 |
Not all participants had complete data on all exposures, so totals in the groups do not necessarily add up to 336.
Risk presented as incidence proportion (cumulative incidence) of type 1 diabetes during follow-up.
Hazard ratios estimated through Cox regression adjusted for child’s sex, maternal age and parity, maternal type 1 diabetes, smoking in pregnancy, education level, pre-pregnancy BMI, prematurity, birthweight, infections and antibiotic use.
Range 0–280, missing = 9297. The estimates presented are days of use as a categorical variable (1 day, 2-4 days or ≥ 5 days) and as a continuous variable (per day of use), with no use as reference.
A count of child’s reported acetaminophen use (reported at 0-6, 6-8, 9-11, 12-14 and 15-18 months of life), irrespective of days used. The estimates presented are number of episodes as a categorical variable (one, two, three, four episodes or more) and as a continuous variable (per episode), with no episodes as reference.
Infections during pregnancy did not seem to be associated with offspring type 1 diabetes risk (Table 3). Children hospitalized for gastrointestinal infections the first 18 months of life did have increased type 1 diabetes risk (aHR 2.27, 95% CI 1.21 − 4.29, P = 0.01), but hospitalizations for upper and lower respiratory tract infections were not associated with type 1 diabetes (Table 3). Results were similar when restricting analyses to infections in the first 6 months of life (Supplementary Table 3, available as Supplementary data at IJE online), except that admission to hospital for any infection was associated with type 1 diabetes risk. Overall numbers of infections or other specific infections were not associated with type 1 diabetes in the first 18 (Table 3) or 6 months of life (Supplementary Table 3, available as Supplementary data at IJE online). Adjusting for breastfeeding did not appreciably change our estimates.
Table 3.
Risk of type 1 diabetes in offspring according to infections during pregnancy and early life in the MoBa cohort
| Infections during pregnancy | Cases n = 336a | Incidence rate/100 000b | Unadjusted |
Adjustedc |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | aHR | 95% CI | P-value | |||
| Any infectiond | ||||||||
| None | 88 | 30.6 | Ref. | Ref. | ||||
| 1 episode | 122 | 38.6 | 1.26 | 0.96–1.66 | 0.10 | 1.29 | 0.97–1.71 | 0.08 |
| 2 or more episodes | 126 | 32.4 | 1.06 | 0.81–1.40 | 0.66 | 0.98 | 0.73–1.33 | 0.92 |
| Per infection | 1.01 | 0.93–1.09 | 0.84 | 0.98 | 0.90–1.07 | 0.65 | ||
| Any infection with fever | ||||||||
| No | 88 | 30.6 | Ref. | Ref. | ||||
| Yes | 39 | 29.9 | 0.98 | 0.67–1.43 | 0.91 | 0.80 | 0.52–1.24 | 0.32 |
| Infection period | ||||||||
| None | 88 | 30.6 | Ref. | Ref. | ||||
| Early (<17 weeks) | 80 | 37.6 | 1.23 | 0.91–1.67 | 0.18 | 1.26 | 0.92–1.72 | 0.16 |
| Late (≥17 weeks) | 68 | 32.2 | 1.05 | 0.77–1.45 | 0.75 | 1.06 | 0.76–1.47 | 0.73 |
| Both periods | 100 | 35.6 | 1.17 | 0.88–1.56 | 0.29 | 1.10 | 0.81–1.50 | 0.55 |
| Gastroenteritis‖ | ||||||||
| No | 257 | 33.4 | Ref. | Ref. | ||||
| Yes | 79 | 35.5 | 1.07 | 0.83–1.38 | 0.62 | 1.05 | 0.81–1.37 | 0.70 |
| Respiratory tract infectione | ||||||||
| No | 122 | 31.6 | Ref. | Ref. | ||||
| 1 episode | 133 | 38.9 | 1.23 | 0.96–1.58 | 0.09 | 1.23 | 0.95–1.58 | 0.12 |
| 2 or more episodes | 81 | 30.7 | 0.98 | 0.74–1.30 | 0.87 | 0.91 | 0.67–1.23 | 0.55 |
| Urinary tract infectionf | ||||||||
| No | 297 | 33.5 | Ref. | Ref. | ||||
| Yes | 39 | 37.2 | 1.10 | 0.79–1.54 | 0.56 | 1.04 | 0.70–1.55 | 0.85 |
| Infections during early life | (n = 286)a | |||||||
| Any infectiond | ||||||||
| 0-4 infections | 70 | 37.3 | Ref. | Ref. | ||||
| 5-6 infections | 58 | 39.3 | 1.06 | 0.75–1.51 | 0.74 | 1.00 | 0.70–1.43 | 1.00 |
| 7-9 infections | 76 | 41.0 | 1.11 | 0.80–1.54 | 0.82 | 1.12 | 0.80–1.60 | 0.52 |
| ≥10 infections | 71 | 34.8 | 0.95 | 0.68–1.32 | 0.76 | 0.92 | 0.64–1.30 | 0.62 |
| Per infection episode | 0.98 | 0.96–1.01 | 0.18 | 0.98 | 0.95–1.01 | 0.95 | ||
| Hospital admission | ||||||||
| No | 247 | 37.4 | Ref. | Ref. | ||||
| Yes | 29 | 45.1 | 1.20 | 0.82–1.77 | 0.35 | 1.24 | 0.82–1.87 | 0.31 |
| Gastroenteritis‖ | ||||||||
| No infections | 115 | 39.9 | Ref. | Ref. | ||||
| One infection | 95 | 41.7 | 1.05 | 0.80–1.38 | 0.72 | 1.04 | 0.78–1.37 | 0.81 |
| Two or more infections | 65 | 31.4 | 0.80 | 0.59–1.09 | 0.15 | 0.78 | 0.57–1.07 | 0.13 |
| Per infection episode | 0.93 | 0.84–1.02 | 0.12 | 0.92 | 0.83–1.02 | 0.13 | ||
| Hospital admission | ||||||||
| No | 266 | 37.3 | Ref. | Ref. | ||||
| Yes | 10 | 81.1 | 2.19 | 1.16–4.10 | 0.02 | 2.27 | 1.21–4.29 | 0.01 |
| Upper respiratory tract infectione | ||||||||
| 0-3 infections | 67 | 37.9 | Ref. | Ref. | ||||
| 4-5 infections | 64 | 36.6 | 0.97 | 0.69–1.38 | 0.88 | 0.93 | 0.65–1.33 | 0.70 |
| 6-7 infections | 55 | 37.2 | 0.99 | 0.69–1.42 | 0.97 | 1.00 | 0.70–1.45 | 0.99 |
| ≥8 infections | 89 | 39.5 | 1.06 | 0.77–1.45 | 0.72 | 1.05 | 0.75–1.47 | 0.78 |
| Per infection episode | 0.99 | 0.96–1.02 | 0.50 | 0.99 | 0.96–1.02 | 0.47 | ||
| Hospital admission | ||||||||
| No | 260 | 37.3 | Ref. | Ref. | ||||
| Yes | 16 | 56.6 | 1.51 | 0.91–2.50 | 0.11 | 1.55 | 0.93–2.56 | 0.09 |
| Lower respiratory tract infectiong | ||||||||
| No | 240 | 38.8 | Ref. | Ref. | ||||
| Yes | 34 | 33.1 | 0.85 | 0.59–1.21 | 0.36 | 0.82 | 0.56–1.21 | 0.31 |
| Per infection episode | 0.88 | 0.71–1.09 | 0.25 | 0.87 | 0.69–1.09 | 0.22 | ||
| Hospital admission | ||||||||
| No | 270 | 38.8 | Ref. | Ref. | ||||
| Yes | 6 | 21.1 | 0.54 | 0.24–1.21 | 0.14 | 0.54 | 0.23–1.24 | 0.14 |
Not all participants had complete data on infections, so the totals in the groups do not necessarily add up total number of cases.
Risk presented as incidence proportion (cumulative incidence) of type 1 diabetes during follow-up.
Hazard ratios estimated through Cox regression adjusted for child’s sex, maternal age and parity, maternal type 1 diabetes, smoking in pregnancy, education level, pre-pregnancy BMI, prematurity, birthweight, acetaminophen and antibiotic use during the relevant time period (pregnancy, or 0–18 months of life). Children with complete data on covariates (n = 81 341 for infections in pregnancy and n = 67 693 in 0–18 months of life) were included in adjusted model.
Includes gastroenteritis, respiratory tract infections, and urinary tract infections. Range 0–11 infections during pregnancy and 0–58 infections during early life. The estimates presented are maternally reported infections as a categorical variable (none, one or ≥two infection episodes during pregnancy, and 0–4, 5–6, 7–9 and ≥10 infections during early life) with the lowest category as reference, and as a continuous variable (per infection episode), with no infections as reference. ‖Range 0–3 infections during pregnancy and 0–17 infections during early life.
Includes common cold/influenza, throat infection/sinusitis/ear infection and pneumonia/acute bronchitis during pregnancy (range 0–9 infections). Includes common cold/influenza, throat infection/sinusitis/ear infection during 0—18 months of life (range 0—49 infections).
Defined as acute cystitis or pyelonephritis. Range 0–3 infections during pregnancy.
Defined as pneumonia/acute bronchitis. Range 0–16 infections during 0–18 months of life.
We present unadjusted estimates and estimates where exposures are mutually adjusted for each other (e.g when analysing infections, the estimates are adjusted for acetaminophen and antibiotic use). Estimates where the exposures were not mutually adjusted for each other were not appreciably different (data not shown). As the estimates did not change appreciably after adjustment, potential effects of infections, antibiotic use and acetaminophen use do not seem to be mediated or confounded by each other or adjusting variables. The bias analysis (see Supplementary material, available at IJE online) shows that extensive misclassification is necessary to appreciably change reported estimates (Supplementary Table 4, available as Supplementary data at IJE online).
Discussion
In this large-scale pregnancy cohort, general infections, acetaminophen use and antibiotic use during pregnancy and early life did not seem to be associated with offspring type 1 diabetes. Increased type 1 diabetes risk was observed after hospitalization for gastroenteritis in early childhood.
Strengths and limitations of the study
Strengths include study size, population-based design and prospective, comprehensive questionnaire data collected during pregnancy and childhood. Findings are likely generalizable to the population. Our study covers a wide infection range with self-reported infections, visits to a clinic/doctor or hospitalization. As common infections are generally self-limited, self-reported infections might better capture symptomatic infections compared with medical records, as it is uncommon in Norway to seek medical care during non-severe infections. Likewise, self-reported use of acetaminophen better captures typical use when compared with prescribed acetaminophen, as acetaminophen is available prescription-free at general stores and a prescription indicates an underlying condition. We were able to adjust for possible confounders, and investigated whether exposures mediated or confounded each other. Comparing unadjusted and adjusted results rules out strong confounding by studied variables. We regard the risk of misclassification of type 1 diabetes as minimal. Diagnostic criteria are clear, we have data from two nationwide registers, and other conditions, such as maturity onset diabetes of the young (MODY) or insulin-dependent type 2 diabetes, are very rare in young children and are screened for in the Norwegian Childhood Diabetes Register.16 The endpoint studied is type 1 diabetes, not islet autoantibodies which is a commonly used surrogate endpoint, but not everyone with islet autoantibodies progresses to type 1 diabetes.
Limitations include potential self-selection to MoBa (but not in the register-based cohort), exposure misclassification and lack of data on infectious agents. Participating mothers in MoBa are slightly older, with a healthier lifestyle than the general population, and the same applies to those who continued to participate. Self-selection is more likely to impact on disease incidences, which in our study are similar to national type 1 diabetes data.13 Potential misclassifications cannot be ruled out as participants might misunderstand or make errors, but this is expected to be randomly distributed and should be non-differential. Grouping infections minimizes possible misclassifications between similar infections. Although we cannot entirely exclude possible errors, the short recall period likely minimizes potential information bias. We are unable to validate the self-reported data, but believe parents would accurately report rare exposures (e.g hospitalizations) as these are memorable, and common exposures should be easily identified. An earlier study, comparing parent-reported hospitalization in MoBa with admissions toone major hospital, found 197 of 212 admissions correctly reported.18 The bias analysis shows that estimates do not appreciably change unless there is implausible high misclassification.
This study is unsuitable to capture specific, asymptomatic or subclinical infections, given our restriction to questionnaire data not confirmed by clinicians or laboratory test results. We cannot refute that a specific infectious agent, or asymptomatic infections, could influence type 1 diabetes risk. We have not investigated short time periods of antibiotic use, nor use of specific antibiotics, as this could easily lead to spurious results due to small numbers. We also cannot be certain that antibiotics were necessary, or that courses were fully followed. Antibiotics given at a hospital are not registered in the NorPD, but most intravenous courses are completed with oral administration (which would be registered), and use in newborns is registered in the MBRN. Antibiotic prescriptions in the register-based cohort were dispensed, but we cannot confirm usage. Antibiotics require prescription in Norway, which is restrictive in their use.19 This makes it unlikely that antibiotics are prescribed or used needlessly, and makes it probable that courses are used.
Comparison with other studies in the field
There are important inter-study differences in exposure data. Prospective studies tend to use medical records, whereas retrospective studies tend to use questionnaires. Registry studies tend to be larger, but often lack data on confounders. There are also exposure differences, such as hospitalization vs self-reported symptoms. An exposure might be a risk factor for islet autoimmunity, or for progression from islet autoimmunity to clinical type 1 diabetes.
Our findings of no association with general antibiotic use are consistent with previous studies in pregnancy 8,10,20–22 and childhood.20,21,23–26 Similar observations in both cohorts strongly suggest that antibiotic use in general does not influence type 1 diabetes risk. This does not exclude that antibiotic types infrequently used, repeatedly use or used in specific populations could be associated with later type 1 diabetes or potential time window effects. A recent study linked broad-spectrum antibiotic use in the first 2 years of life with type 1 diabetes risk in children delivered by caesarean section.26 Kilkkinen et al.22 and Mikkelsen et al.25 show increased risk in children with repeated courses (>7 and ≥ 5, respectively), but we did not replicate these findings. This could be due to few children taking so many courses, using broad-spectrum antibiotics or caesarean section. A Dutch study reported that children (<19 years) were prescribed more antibiotics prior to type 1 diabetes diagnosis,27 but this study investigated a different period and is not directly comparable.
Studies covering over-the-counter medications such as acetaminophen require a prospective design and are not possible to study in register-based studies. Clinical trials do not usually include pregnant women or young children, which makes observational studies necessary to elucidate possible risks. With a high proportion of participants using acetaminophen, our study indicates that acetaminophen use in pregnancy does not influence offspring type 1 diabetes risk. Earlier studies on pregnancy analgesic use report somewhat higher risk estimates for type 1 diabetes.8,10 The differences in risk might be due to a different exposure or recall bias. Nevertheless, as our study found a slightly increased estimate (HR 1.22, 95% CI 0.98 - 1.53, P = 0.08) after acetaminophen exposure, and increased risk for offspring type 1 diabetes has been observed after analgesic use in preceding studies, further studies are recommended. We found that using acetaminophen for ≥3 days in the first 6 months of life had a protective effect in a subanalysis, which must be interpreted with caution and replicated independently. A recent study found no association between analgesic antipyretic use and development of islet autoimmunity,7 which is in line with our results, but do not report on number of days used.
Earlier studies on general infections in pregnancy and early life, and childhood type 1 diabetes, have published seemingly contradicting results (for an overview, see Supplement and Supplementary Figure 1, available as Supplementary data at IJE online). Retrospective studies are susceptible to recall bias, so we compare our results on infections only with other prospective studies. Most studies tend to show no increased risk after infections in pregnancy,10,20,28 which fits our results. Two studies have reported increased risk in early childhood after infections noted in hospital records,20 and after viral infections during the first 6 months of life using medical insurance claims data.29 There is also a recent study using inpatient claims on infections during childhood, which report increased risk.28 These findings fit well with our results on hospitalization, although the studies are heterogeneous in nature. Two studies found essentially no increase in risk using medical records from primary care23 and prospective diaries,21 which is similar to our results on general infections. Two smaller studies found decreased risk after at least one infection the first 6 months30 and first year of life,31 but we did not replicate these findings (data not shown). These differences could be due to differences in exposure, age, adjustment for different confounding variables or differences between countries.
Infections could trigger autoimmunity, increase progression of autoimmunity or act as a precipitating factor for clinical diagnosis. All cases but one were diagnosed with type 1 diabetes at >5 years of age, which suggests that hospitalization for gastroenteritis is not a precipitating factor for clinical type 1 diabetes diagnosis. Infection severity could be important for later type 1 diabetes, as only hospitalization was associated, and only the most serious cases would be hospitalized. This represents strong inflammation, which could predispose to later type 1 diabetes. More severe gastroenteritis could also increase intestinal permeability, which could lead to infectious agents infiltrating the pancreas and, if not cleared, persistent inflammation which could increase type 1 diabetes risk. Hospitalization for gastroenteritis could also be linked to changes in the microbiota, which could predispose towards later autoimmunity and type 1 diabetes. Few children were hospitalized, which makes estimates uncertain, and these findings should be replicated, as this association could be due to chance or uncontrolled bias. We also cannot exclude the possibility that hospitalization resulted from an excessive immune response or infection susceptibility due to an underlying, already present subclinical autoimmunity.
To conclude, use of antibiotics, acetaminophen and general infections in pregnancy or early childhood did not predict childhood type 1 diabetes. Hospital admission for gastroenteritis in early childhood was associated with increased risk for type 1 diabetes, but must be interpreted cautiously due to scarcity of cases.
Funding
K.S. was supported by an unrestricted grant from the Oak Foundation, Geneva, Switzerland. Costs of all data acquisition, including laboratory assays in MoBa (the sub-study PAGE; Prediction of Autoimmune Diabetes and Celiac Disease in Childhood by Genes and Perinatal Environment), was supported by grant no. 2210909/F20 from the Norwegian Research Council (L.S.). The study was in part supported by funds from the European Research Council, the K.G. Jebsen Foundation and the Research Council of Norway (to P.R.N.). The Norwegian Mother and Child Cohort Study are supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558) and NIH/NINDS (grant no.1 UO1 NS 047537–01 and grant no.2 UO1 NS 047537–06A1). The Norwegian Childhood Diabetes Registry is funded by the South-Eastern Norway Regional Health Authority.
Supplementary Material
Acknowledgements
Data from the Norwegian Patient Register have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian Patient Register is intended nor should be inferred. We are grateful to all the participating families in Norway who take part in this ongoing cohort study. We are grateful to Nicolai Andre Lund-Blix for help with breastfeeding data.
Author Contributions
Concept and design: L.C.S., G.T., K.S., K.M. Guarantors: K.S., G.T. Literature search: G.T., K.S., L.C.S. Acquisition of pregnancy cohort data: L.C.S., K.S. Acquisition of register data: L.C.S., K.S. Acquisition of childhood incident type 1 diabetes data: T.S., G.J., P.R.N. Data cleaning and preparation: K.S., G.T., L.C.S., K.M. Planning statistical analyses: L.C.S., G.T., K.S., K.M. Statistical analyses: K.S., G.T., L.C.S. Interpretation of data: all authors.
Conflict of interest: No potential conflict of interest relevant to this article was reported. The authors alone are responsible for the content and writing of the paper.
References
- 1. Davis-Richardson AG, Ardissone AN, Dias R.. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol 2014;5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T.. The maternal microbiota drives early postnatal innate immune development. Science 2016;351:1296–302. [DOI] [PubMed] [Google Scholar]
- 3. Hu Y, Peng J, Tai N. et al. Maternal antibiotic treatment protects offspring from diabetes development in nonobese diabetic mice by generation of tolerogenic APCs. J Immunol 2015;195:4176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stokholm J, Sevelsted A, Bonnelykke K, Bisgaard H.. Maternal propensity for infections and risk of childhood asthma: a registry-based cohort study. Lancet Respir Med 2014;2:631–37. [DOI] [PubMed] [Google Scholar]
- 5. Magnus MC, Karlstad O, Haberg SE, Nafstad P, Davey Smith G, Nystad W.. Prenatal and infant paracetamol exposure and development of asthma: the Norwegian Mother and Child Cohort Study. Int J Epidemiol 2016;45:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eyers S, Weatherall M, Jefferies S, Beasley R.. Paracetamol in pregnancy and the risk of wheezing in offspring: a systematic review and meta-analysis. Clin Exp Allergy 2011;41:482–89. [DOI] [PubMed] [Google Scholar]
- 7. Lundgren M, Steed LJ, Tamura R. et al. Analgesic antipyretic use among young children in the TEDDY study: no association with islet autoimmunity. BMC Pediatr 2017;17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Visalli N, Sebastiani L, Adorisio E. et al. Environmental risk factors for type 1 diabetes in Rome and province. Arch Dis Child 2003;88:695–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Majeed AA, Mea, Hassan K.. Risk factors for type 1 diabetes mellitus among children and adolescents in Basrah. Oman Med J 2011;26:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKinney PA, Parslow R, Gurney K, Law G, Bodansky HJ, Williams DR.. Antenatal risk factors for childhood diabetes mellitus; a case-control study of medical record data in Yorkshire, UK. Diabetologia 1997;40:933–39. [DOI] [PubMed] [Google Scholar]
- 11. McKinney PA, Parslow R, Gurney KA, Law GR, Bodansky HJ, Williams R.. Perinatal and neonatal determinants of childhood type 1 diabetes. A case-control study in Yorkshire, U.K. Diabetes Care 1999;22:928–32. [DOI] [PubMed] [Google Scholar]
- 12. Magnus P, Birke C, Vejrup K. et al. Cohort Profile Update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016;45:382–88. [DOI] [PubMed] [Google Scholar]
- 13. Skrivarhaug T, Stene LC, Drivvoll AK, Strom H, Joner G.. Norwegian Childhood Diabetes Study Group. Incidence of type 1 diabetes in Norway among children aged 0-14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian Childhood Diabetes Registry. Diabetologia 2014;57:57–62. [DOI] [PubMed] [Google Scholar]
- 14. Knip M, Simell O.. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med 2012;2:a007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stene LC, Tuomilehto J, Rewers MJ.. Global epidemiology of type 1 diabetes In: Ekoé JM, Rewers MJ, Williams R, Zimmet P (eds). The Epidemiology of Diabetes Mellitus. 2nd edn Chichester, UK: Wiley, 2008. [Google Scholar]
- 16. Irgens HU, Molnes J, Johansson BB. et al. Prevalence of monogenic diabetes in the population-based Norwegian Childhood Diabetes Registry. Diabetologia 2013;56:1512–19. [DOI] [PubMed] [Google Scholar]
- 17. Orsini N, Bellocco R, Bottai M, Wolk A, Greenland S.. A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies. Stata J 2008;8:29–48. [Google Scholar]
- 18. Størdal K, Lundeby KM, Brantsæter AL. et al. Breast-feeding and infant hospitalization for infections: large cohort and sibling analysis. J Pediatr Gastroenterol Nutr 2017;65:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stordal K, Marild K, Blix HS.. Use of antibiotics among children during 2005–2016. Tidsskr Nor Laegeforen 2017;137, Oct 2. doi: 10.4045/tidsskr.17.0272. [DOI] [PubMed] [Google Scholar]
- 20. EURODIAB Substudy 2 Study Group. Infections and vaccinations as risk factors for childhood type I (insulin-dependent) diabetes mellitus: a multicentre case-control investigation. Diabetologia 2000;43:47–53. [DOI] [PubMed] [Google Scholar]
- 21. Welander A, Montgomery SM, Ludvigsson J, Ludvigsson JF.. Infectious disease at gluten introduction and risk of childhood diabetes mellitus. J Pediatr 2014;165:326–31.e1. [DOI] [PubMed] [Google Scholar]
- 22. Kilkkinen A, Virtanen SM, Klaukka T. et al. Use of antimicrobials and risk of type 1 diabetes in a population-based mother-child cohort. Diabetologia 2006;49:66–70. [DOI] [PubMed] [Google Scholar]
- 23. Cardwell CR, Carson DJ, Patterson CC.. No association between routinely recorded infections in early life and subsequent risk of childhood-onset Type 1 diabetes: a matched case-control study using the UK General Practice Research Database. Diabetic Med 2008;25:261–67. [DOI] [PubMed] [Google Scholar]
- 24. Hviid A, Svanstrom H.. Antibiotic use and type 1 diabetes in childhood. Am J Epidemiol 2009;169:1079–84. [DOI] [PubMed] [Google Scholar]
- 25. Mikkelsen KH, Knop FK, Vilsbøll T, Frost M, Hallas J, Pottegård A.. Use of antibiotics in childhood and risk of Type 1 diabetes: a population-based case-control study. Diabet Med 2017;34:272–77. [DOI] [PubMed] [Google Scholar]
- 26. Clausen TD, Bergholt T, Bouaziz O. et al. Broad-spectrum antibiotic treatment and subsequent childhood type 1 diabetes: a Nationwide Danish Cohort Study. PLoS One 2016;11:e0161654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fazeli Farsani S, Souverein PC, van der Vorst MM, Knibbe CA, de Boer A, Mantel-Teeuwisse AK.. Population-based cohort study of anti-infective medication use before and after the onset of type 1 diabetes in children and adolescents. Antimicrob Agents Chemother 2014;58:4666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee HY, Lu CL, Chen HF, Su HF, Li CY.. Perinatal and childhood risk factors for early-onset type 1 diabetes: a population-based case-control study in Taiwan. Eur J Public Health 2015;25:1024–29. [DOI] [PubMed] [Google Scholar]
- 29. Beyerlein A, Donnachie E, Jergens S, Ziegler AG.. Infections in early life and development of type 1 diabetes. JAMA 2016;315:1899–901. [DOI] [PubMed] [Google Scholar]
- 30. Pundziūtė-Lyckå A, Urbonaitė B, Dahlquist G.. Infections and risk of Type I (insulin-dependent) diabetes mellitus in Lithuanian children. Diabetologia 2000;43:1229–34. [DOI] [PubMed] [Google Scholar]
- 31. Gibbon C, Smith T, Egger P, Betts P, Phillips D.. Early infection and subsequent insulin dependent diabetes. Arch Dis Child 1997;77:384–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.