Abstract
Background
The relationship between ultrasongraphically derived estimates of fetal growth and educational attainment in the postnatal period is unknown. Results from previous studies focusing on cognitive ability, however, suggest there may be gestation-specific associations. Our objective was to model growth in fetal weight (EFW) and head circumference (HC) and identify whether growth variation in different periods was related to academic attainment in middle childhood.
Methods
Data come from the Born in Bradford (BiB) cohort study, which has performed data linkage to both routine antenatal scans and national academic attainment tests at age 6–7 years. Multilevel linear spline models were used to model EFW and HC. Random effects from these were related to Key Stage 1 (KS1) results in reading, writing, mathematics, science and a composite of all four (age 6–7 years), using ordinal logistic and logistic regression. Associations were adjusted for potential confounders, facilitated by directed acyclic graphs. Missing covariate data were imputed using multiple imputation.
Results
In all, 6995 and 8438 children had complete KS1, and EFW and HC data, respectively. Positive associations were observed between both fetal weight in early pregnancy (14 weeks) and EFW growth in mid-pregnancy (14-26 weeks) and the individual KS1 outcomes. Furthermore, after adjustment for previous size and confounders, a 1-z score increase in growth in mid-pregnancy was associated with an 8% increased odds of achieving the expected standard for all KS1 outcomes [odds ratio (OR): 1.08, 95% confidence interval (CI): 1.02; 1.13]. Similar results were observed for HC, with generally larger effect sizes. Smaller associations were observed with growth in the early-third trimester, with no associations observed with growth in the later-third trimester.
Conclusions
We observed consistent positive associations between fetal size and growth in early and mid-gestation and academic attainment in childhood. The smaller and null associations with growth in the early-third and later-third trimester, respectively, suggests that early-mid gestation may be a sensitive period for future cognitive development.
Keywords: Fetal growth, education, attainment, Born in Bradford, longitudinal, spline
Key Messages
This longitudinal study found that fetal growth is associated with academic attainment in mid-childhood.
Increased EFW and HC in early gestation and an increased growth of these in mid-gestation were associated with increased odds of achieving a higher level of academic attainment at age 6-7 years.
Smaller/no associations were observed between growth in later gestation and academic attainment.
Introduction
There is a large literature focusing on the relationship between size at birth (a proxy for fetal growth) and cognitive outcomes in children,1–4 with studies generally reporting a positive association between the two. Assessing fetal growth using summary measures at the end of the intrauterine period however, not only masks the pattern of growth that has occurred in fetal life, i.e. various growth trajectories can ultimately result in the same birthweight, but also lacks consideration of critical periods of growth during gestation. For example, growth in early pregnancy, during which rapid cell hyperplasia occurs, may be a more critical period for later cognitive development as compared with growth during later pregnancy, which may be characterized by reduced velocity and cell hypertrophy.5 Fetal head growth (represented by head circumference),5,6 for example, is rapid in early gestation7 and it is well recognized that the early fetal periods are vital in neural development.8 There is mixed evidence regarding the association between directly observed estimates of fetal growth (obtained via prenatal ultrasonography) and cognitive and developmental outcomes (assessed using standardized cognitive tests) and it is unclear what, if any, period of gestation confers the greatest association.9–12
Although intelligence tests provide an index of global cognitive ability, academic attainment is a more relevant marker of a child’s functional outcomes13 Poor academic attainment in childhood has been associated with low educational attainment in adolescence14 and adulthood,15 with implications for longer-term wealth16 and socioeconomic status (SES).17 Research into the relationship between fetal growth and academic attainment has also focused on weight at birth, with some studies reporting positive associations in childhood.18,19 However a recent instrumental variable analysis using a number of single nucleotide polymorphisms (SNPs; therefore not subject to confounding) determining birthweight revealed no association with academic attainment in adolescence.20 There is, however, a paucity of research into the effects of ultrasonographically assessed estimates of fetal growth on future academic attainment. Understanding the effects of fetal growth on educational outcomes may shed light on sensitive periods of fetal growth in relation to long-term functional outcomes and the targeting of antenatal strategies to improve long-term outcomes. We hypothesized that, in line with the studies which have observed that early pregnancy may be a potential critical period for later neurodevelopment,9,10 growth in earlier gestation would have a greater association with childhood educational outcomes than growth in later gestation. Specifically, a greater rate of growth would be associated with higher educational attainment. Our objective therefore was to relate early fetal size and growth, measured using estimated fetal weight (EFW; representing growth of the whole fetus) and head circumference (HC; representing fetal brain growth), across different periods of gestation, to academic attainment routinely recorded in middle childhood as part of the national curriculum in England.
Methods
Born in Bradford (BiB) is a longitudinal multi-ethnic [largely bi-ethnic (White British and Pakistani-origin)] birth cohort study aiming to examine the impact of environmental, psychological and genetic factors on maternal and child health and well-being, and has been described in detail elsewhere.21 In brief, the full BiB cohort recruited 12 450 women comprising 13 773 pregnancies and 13 858 children between 2007 and 2010. The methods of recruitment and outcomes for BiB have been published elsewhere.21,22 The cohort is broadly characteristic of the city’s maternal population. Women were typically recruited when they attended the Bradford Royal Infirmary (BRI) (Bradford, UK) for a routine oral glucose tolerance test (GTT) at 26–28 weeks of gestation. Women who consented to the study at this visit also completed an interviewer-administered questionnaire that included items about demographic characteristics, lifestyle and health status/history. Furthermore, BiB has conducted extensive data linkage to external data sources, e.g. routine health records stored on ‘SystmOne’ (clinical system used by almost all GP practices in Bradford) as well as records of educational attainment from local education authorities. This has been facilitated with the use of common identifiers contained within the linked datasets (i.e. deterministic linkage). For hospital data this was done using hospital and NHS numbers. For education data this was achieved using full name, date of birth, gender and postcode. This analysis is based on live-born singleton children with complete exposure (fetal growth) and outcome (academic attainment) data.
Exposure: ultrasongraphically derived estimates of fetal growth
The expected date of delivery (EDD) was based on the date of the woman’s last menstrual period (LMP). The LMP was used as a guide for the planning the dating scan, at which the EDD and LMP-determined gestational age were confirmed by ultrasound scan [median gestational age of the dating scan in BiB was 11.7 weeks (IQR: 10.3–13.0)]. If there was a 2-week discrepancy between the LMP and ultrasound-derived dates, dates based on the ultrasound were used.
Estimated fetal weight (EFW) and HC were extracted from sonographic notes, compiled as part of the NHS screening programme23 and the BiB protocol. Fetal biometry was measured in accordance with the guidelines recommended by the British Medical Ultrasound Society (BMUS).24
Outcome: educational attainment in childhood
Key Stage 1 (KS1) attainment: in England, all state-funded and some independent schools follow a national curriculum comprising four ‘key stages’. Key Stage 1 covers coursework completed in the first 2 years of school (age 5–7 years) in four key domains: reading, writing, mathematics and science. The KS1 statutory assessments comprise teacher evaluations of the student’s academic attainment in each domain according to uniform criteria, facilitated by the completion of a standardized KS1 test in most subject areas.25 The assessment framework can then be used to determine whether a child is working below, at or above the expected standard. KS126–29 attainment has been used as an outcome measure in a number of observational studies and clinical trials. The testing framework for these tests changed in the 2015–16 academic year- and we have described the pre- and post-2016 frameworks in the Supplementary material (available as Supplementary data at IJE online).
Statistical analysis
Fetal growth trajectories: growth trajectories were derived for live-born singleton births of known sex who were free from congenital malformations and who contributed at least one measurement during the fetal period (n = 10 921 for EFW model and n = 12 748 for HC).
In an attempt to stabilize the increasing variability in fetal size with gestational age, the modelling of fetal trajectories was done on the (natural) log-transformed dimensions, as has been advocated in previous studies of fetal growth.30–33 Linear spline mixed effects models were used for the modelling of fetal growth, which has been done previously for both fetal growth32 and growth in childhood.34–36 Further details about the model-fitting process can be found in the Supplementary material (available as Supplementary data at IJE online). The best-fitting model split the gestational period into three periods. The resulting random effects, representing deviations from average predicted size at approximately 14 weeks for EFW or 18 weeks for HC (z1) and the deviance from the average predicted weekly growth rate [as fetal dimensions were modelled on the (natural) log scale weekly growth was in terms of % change] in each of the three periods (z2, z3, z4), were subsequently converted into (sex-specific) z-scores so that the sizes of the coefficients were directly comparable.
Linking fetal growth to educational outcomes: the analysis was limited to those with complete exposure and outcome data. Final models were therefore based on 6995 and 8438 children for the EFW and HC analyses, respectively.
As the KS1 marking structure changed during the period of data collection, it was necessary to derive a new set of variables accounting for these changes (more information about the derivation of these new variables can be found in the Supplementary material, available as Supplementary data at IJE online). Accordingly, for the mathematics, reading and writing outcome data, new ordinal variables were created with the values: 1 = ‘below expected standard’; 2 = ‘at expected standard’; 3 = ‘above expected standard’. Fetal growth z-scores were related to these three outcome variables using ordinal logistic regression, checking for non-violation of the proportional odds assumption. Similarly a dichotomous variable was derived for science, with a value of 0 = ‘below standard’ and a value of 1 = ‘working at or above standard’. A final binary outcome variable was also created, which represented whether a child had attained the expected standard across all four KS1 domains. Logistic regression models were used to relate the fetal z-scores to these binary outcomes. For all models, clustered standard errors were used to account for the clustering of children within schools.
In the first step, separate unadjusted analyses were run which related z1, z2, z3, z4 to the respective outcomes and these estimates were reported. In the second step (for z2, z3, z4 only), we adjusted for size (z-score) at the beginning of that respective period and reported only the estimate of the latter z-score. For example, we adjusted z3 for size at the beginning of the z3 period and reported only the estimate for z3 (as the coefficient for size at the beginning of that period is interpreted as the direct effect of size which does not operate via its effect on z3, which we were not interested in).37 Finally we adjusted for a number of confounding variables identified via literature searches and after holding consultative meetings with experts in the fields related to the exposure and outcome. Results of these searches/meetings were encapsulated in the form of directed acyclic graphs (DAGs: more information provided in Supplementary material, available as Supplementary data at IJE online). The final DAG is included in Supplementary Figure 1 (available as Supplementary data at IJE online) and resulted in the adjustment for sex, ethnicity, maternal education, level of deprivation, smoking during pregnancy, alcohol intake during pregnancy, maternal height and parity. Variables on the causal pathway between exposure and outcome were not adjusted for.
In order to address missingness in covariate data for the adjusted analyses, we used multiple imputation by chained equations (MICE)38 combining estimates using Rubin’s rules.39 The number of imputations required to achieve convergence of parameter estimates was determined as 100*fraction missing information (FMI).40 In order to identify whether bias was introduced by limiting our analysis to those with complete exposure and outcome data, supplementary analyses comparing maternal and neonatal data of those included and excluded were performed. All analyses were conducted using Stata software version 14 (Stata Corp., College Station, TX).
Results
Fetal weight and academic attainment: the best-fitting linear spline model for fetal weight trajectories had knot points at 26 and 32.5 weeks for males and 26 and 32 weeks for females. The average patterns of change in fetal weight predicted by these linear spline models, for boys and girls in this population, together with 95% reference ranges, are shown in Supplementary Table 1 (available as Supplementary data at IJE online). For both males and females, the rate of increase was highest initially in early pregnancy and then decreased with increasing gestational age.
A total of 6995 children had complete fetal weight and KS1 data, of whom 51.6% (n = 3608/6995) were male and 48.8% (n = 2778/5699) were of Pakistani origin. The mean birth weight of the sample was 3210g [standard deviation (SD) = 546g], with a median gestational age at birth of 40 weeks [interquartile range (IQR): 39-41 weeks]. Further sample characteristics are reported in Table 1. Differences between the sample with complete exposure and outcome data and those missing either exposure or outcome are shown in Supplementary Table 2 (available as Supplementary data at IJE online).
Table 1.
Descriptive statistics for those with both fetal trajectory and education data
| Complete fetal weight and KS1 data (n = 6995) |
Complete fetal HC and KS1 data (n = 8438) |
|||
|---|---|---|---|---|
| Total n | Total n | |||
| Infant characteristics | ||||
| Sex [male, n (%)] | 6995 | 3608 (51.6) | 8438 | 4356 (51.6) |
| Ethnicity | 5699 | 6897 | ||
| White British [n (%)] | 2184 (38.3) | 2702 (39.2) | ||
| Pakistani [n (%)] | 2778 (48.8) | 3311 (48.0) | ||
| Other [n (%)] | 737 (12.9) | 884 (12.8) | ||
| Birthweight (g) | 6995 | 3210 (546) | 8438 | 3228 (538) |
| Gestational age at birth (weeks) | 6995 | 40 (39–41) | 8438 | 40 (39-41) |
| Achieving expected KS1 standard [n (%)] | ||||
| Maths | 6995 | 5519 (78.9) | 8438 | 6841 (81.1) |
| Science | 6995 | 5720 (81.8) | 8438 | 6990 (82.8) |
| Reading | 6995 | 5456 (78.0) | 8438 | 6750 (80.0) |
| Writing | 6995 | 5140 (73.5) | 8438 | 6398 (75.8) |
| Maternal characteristics | ||||
| Age at delivery (years) | 6995 | 27.5 (5.6) | 8438 | 27.5 (5.6) |
| Education [≤GCSE, n (%)] | 5695 | 3168 (55.6) | 6891 | 3827 (55.5) |
| Index of multiple deprivation decile (within Bradford) [most deprived decile, n (%)] | 5695 | 1114 (19.6) | 6894 | 1320 (19.2) |
| Multiparity [yes, n (%)] | 6808 | 4381 (64.4) | 8112 | 5098 (62.9) |
| Maternal booking weight (kg) | 6767 | 65.0 (56-75) | 8151 | 65.0 (56–75) |
| Maternal height (cm) | 5630 | 161.3 (6.4) | 6817 | 161.4 (6.5) |
| Smoking during pregnancy [yes, n (%)] | 5696 | 979 (17.2) | 6892 | 1145 (16.6) |
| Alcohol during pregnancy [yes, n (%)] | 5696 | 1632 (28.7) | 6893 | 2093 (30.4) |
| Pregnancy characteristics | ||||
| Gestational diabetes [yes, n (%)] | 6950 | 634 (9.1) | 8385 | 645 (7.7) |
| Preeclampsia [yes, n (%)] | 6692 | 187 (2.8) | 8096 | 209 (2.6) |
| Caesarean section [yes, n (%)] | 6995 | 1551 (22.2) | 8438 | 1825 (21.6) |
Continuous variables are summarized as mean (SD) or median (IQR). GCSE: General Certificate of Secondary Education; Index of Multiple Deprivation (IMD) is based on seven domains of deprivation (income, employment, education, health, crime, housing, living environment) which are combined to produce one overall index. Maternal booking weight is weight of mother at approximately 10–14 weeks’ gestation.
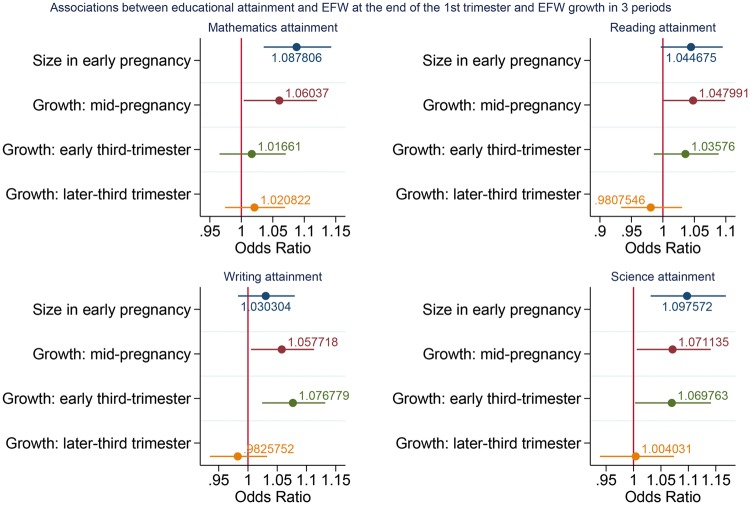
In unadjusted analyses, small but consistent positive associations were observed between size in early pregnancy (approximately 14 weeks) and attainment in mathematics, reading and science, with odds ratios (ORs) ranging from 1.06 [95% confidence interval (CI): 1.01; 1.11) to 1.11 (95% CI: 1.04; 1.18). After adjustment for confounding variables, these odds ratios were only slightly attenuated, with the largest association observed between size in early pregnancy and attainment in science (adjusted OR: 1.10, 95% CI: 1.03; 1.17) (Supplementary Table 3, available as Supplementary data at IJE online; and Figure 1). For the composite outcome, a 1-z-score increase in size in early pregnancy was associated with 6% greater odds of achieving the expected standard across the four KS1 outcomes (adjusted OR: 1.06, 95% CI: 1.01; 1.12) (Supplementary Table 3 and Supplementary Figure 2, available as Supplementary data at IJE online).
Figure 1.
Adjusteda coefficients (95% CIs) relating a 1-z-score increase in EFW size and growth and academic attainment (n = 6995). aAll coefficients adjusted for sex, maternal age, Index of Multiple Deprivation, smoking during pregnancy, alcohol during pregnancy, parity, maternal education and ethnicity. Growth in mid-pregnancy additionally adjusted for size in early pregnancy; growth in early-third trimester adjusted for the above and growth in mid-pregnancy; growth in later-third trimester adjusted for the above and growth in early-third trimester.
Similar associations were observed between growth in the mid-pregnancy and all four KS1 outcomes, with odds ratios for a higher KS1 attainment ranging from 1.05 (95% CI: 1.00; 1.10) to 1.07 (95% CI: 1.01; 1.14) for a 1-z-score increase in fetal growth (Supplementary Table 3, available as Supplementary data at IJE online; and Figure 1). A 1-z-score increase in growth during this period was also associated with 8% greater odds of achieving the expected standard across all four KS1 outcomes (adjusted OR: 1.08, 95% CI: 1.02; 1.13) (Supplementary Figure 2, available as Supplementary data at IJE online). Positive estimates were also observed for growth in the early-third trimester, whereas estimates for later-third trimester tended to the null.
Fetal HC and academic attainment: the best-fitting linear spline model for fetal HC trajectories had knot points at 25 and 32 weeks for both males and females. The average patterns of change in fetal weight and predicted by these linear spline models, for boys and girls in this population together with 95% reference ranges, are shown in Supplementary Table 4 (available as Supplementary data at IJE online).
In all, 8434 children had complete fetal HC and Key Stage 1 data, of whom 51.6% (n = 4356/8434) were male and 48.0% (n = 3311/6897) were of Pakistani origin. The mean birthweight of the sample was 3228 g (SD = 538 g), with a median gestational age at birth of 40 weeks (IQR: 39-41 weeks). Further sample characteristics are reported in Table 1. Differences between the sample with complete exposure and outcome data and those missing either exposure or outcome are shown in Supplementary Table 5 (available as Supplementary data at IJE online).
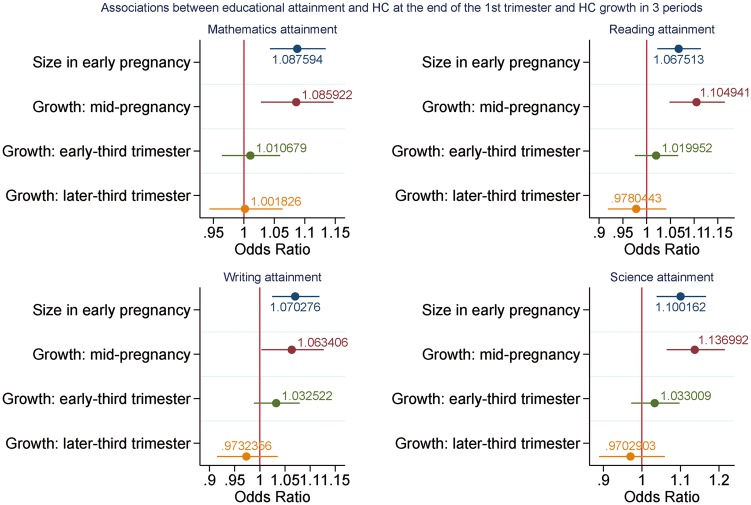
Similar to the EFW analyses, small consistent positive associations were observed between size in early pregnancy (18 weeks) and all four KS1 outcomes. For example, univariate analyses revealed that a 1-z-score increase in size in early pregnancy was associated with increased odds ratios for higher attainment ranging from 1.07 (95% CI: 1.03; 1.12) to 1.12 (95% CI: 1.06; 1.18) (Supplementary Table 6, available as Supplementary data at IJE online). Adjustment for confounding variables saw these estimates attenuate only slightly, with ORs ranging from 1.07 (95% CI: 1.02; 1.11) when reading was the outcome, to 1.10 (95% CI: 1.04; 1.17) for science (Supplementary Table 4; andand Figure 2). For the composite outcome, a 1-z-score increase in size in early pregnancy was associated with 9% greater odds of achieving the expected standard across the four KS1 outcomes (OR: 1.09, 95% CI: 1.04; 1.14) (Supplementary Table 6 and Supplementary Figure 2, available as Supplementary data at IJE online).
Figure 2.
Adjusteda coefficients (95% CIs) relating a 1-z-score increase in HC size and growth and academic attainment (n = 8438). aAll coefficients adjusted for sex, maternal age, Index of Multiple Deprivation, smoking during pregnancy, alcohol during pregnancy, parity, maternal education and ethnicity. Growth in mid-pregnancy additionally adjusted for size in early pregnancy; growth in early-third trimester adjusted for the above and growth in mid-pregnancy; growth in later-third trimester gestation adjusted for the above and growth in early-third trimester.
Positive associations were also observed between growth in mid-pregnancy and all four KS1 outcomes, with adjusted odds ratios for a 1-z-score increase in fetal growth ranging from 1.06 for KS1 writing (95% CI: 1.00; 1.13) to 1.14 for KS1 science (95% CI: 1.06; 1.21) (Supplementary Table 6, available as Supplementary data at IJE online; and Figure 2). A 1-z-score increase in growth during this period was also associated with 10% greater odds of achieving the expected standard across all four of the KS1 outcomes (OR: 1.10, 95% CI: 1.04; 1.17) (Supplementary Figure 2, available as Supplementary data at IJE online). Much smaller effect estimates were observed for growth in the early-third trimester, and estimates for the later-third trimester were around the null.
Discussion
This study investigated the association between fetal growth and academic attainment in childhood (age 6–7 years) in a largely bi-ethnic population of children participating in the Born in Bradford cohort. We observed small but positive associations between size and growth before 27 weeks’ gestation and all educational outcomes, with these associations remaining after adjustment for confounders and previous size and/or growth. For example, a 1-z-score increase in EFW growth rate between 14 and 26 weeks (approximately) was associated with a 5–7% increase in the odds of achieving a higher level of KS1 attainment. Similarly for HC, we observed a positive association between: both size in early pregnancy (18 weeks) and growth between 18 and 25 weeks; and KS1 level of attainment, manifesting in, for example, a 14% increase in the odds of achieving the expected standard for KS1 science per z-score increase in HC growth between 18 and 25 weeks. However, associations between growth in the early-third trimester and academic outcomes were smaller (especially for HC) and no associations were observed with growth in the later-third trimester (for either EFW or HC).
Unlike most studies that have utilized standardized intelligence tests to assess cognitive outcomes, we have used routinely collected measures of curriculum-based attainment as our outcome. Educational outcome measures are more important markers of long-term health, wealth and well-being, as they have been shown to have a direct impact on future educational,17,41–43 occupational44 and economic42 outcomes, than intelligence quotient (IQ) alone.13 For example, it has been shown that pupil scores in mathematics and reading at age 7 are associated with adult socioeconomic status (SES) (represented as an index of occupation, housing tenure and income) and educational attainment in middle age, even after adjustment for SES at birth.17 Possible explanations linking better academic attainment in childhood to later SES have included a greater access to higher salary occupations due to an increased educational duration and thus number of qualifications,17 improved financial decision making as a result of greater numeracy skills45 and a reduced likelihood of ill health46 which limits SES. In acknowledging these associations between early educational attainment and future SES, the positive association observed here between fetal growth and academic attainment in middle childhood can be seen as providing a possible mechanism for the observed associations between size at birth (i.e. fetal growth) and adult occupational and economic outcomes.47–49
Previous studies have reported mixed results regarding the association between fetal growth and childhood cognitive development.9–12 Harvey et al. (1982) observed that reduced head growth before 26 weeks predicted poorer cognitive ability at age 5 years, but a reduced head growth thereafter was not associated with a poorer outcome.9 Roth et al. (1999) also observed that growth in later gestation was not related to cognitive development at age 1 year, though as abdominal circumference was used as the indicator of fetal growth, this finding may be unsurprising.50 Similar to our results, Walker et al. (2007) observed a positive association between HC in early gestation (14 weeks) and cognitive outcomes, but unlike our study, did not observe any association with HC growth in any period thereafter (14–25 weeks, 14–35 weeks).10 These studies indicate that growth in earlier gestation may be more important for later cognitive development, although sample sizes were generally low. More recently however, Henrichs et al. (2010) investigated the association between fetal weight and HC growth and infant cognitive development in a large prospective cohort from The Netherlands.12 Unlike the former studies, they observed an association with growth from ‘mid- to late pregnancy’. However, as the authors’ definitions of ‘mid’ and ‘late’ pregnancy were 18–25 weeks and ≥25 weeks, respectively, it is difficult to determine the exact growth periods. Nonetheless an association with mid to late gestation would appear different from our study, which split the prenatal period into mid-pregnancy, early-third and later-third trimester and observed smaller or null associations in the latter two periods compared with size in early pregnancy or growth in mid-pregnancy.
Whereas associations with growth in the early- or later-third trimester tended towards the null, we did observe consistent positive associations between: HC size in early pregnancy and growth in mid-pregnancy; and childhood academic attainment. As head circumference has been shown to correlate with brain volume, growth in fetal HC may be cautiously interpreted as an indicator of fetal brain development.5,6 Early gestation has been shown to be an important period for brain development, with rapid neuronal multiplication of the forebrain observed at 10–18 weeks, immediately followed by multiplication of glial cells.51 Furthermore, it is at around 16 weeks of gestation, after reaching their final destination, that neurons begin branching in order to establish connections.52 It may therefore be speculated that an improved growth rate during the first trimester (represented here by an increased size in early pregnancy) and into the second trimester could represent an increased neuronal growth rate and thus cerebral development, thus providing a potential mechanism explaining the association between growth in early pregnancy and later cognitive outcome. However, it is important to note that substantial brain development occurs in the postnatal period,51 providing some explanation for the relatively small effect sizes observed in our study.
A key strength of this study is the use of routinely collected data for both exposure and outcome. Specifically, we have modelled fetal growth using serial ultrasound measurements collected at antenatal hospital visits, and related this to KS1 attainment measured as part of the English national curriculum providing excellent population coverage using standardized measures. The KS1 assessment is statutory, validated and based on both subjective (teacher observation) and objective (test) components, together providing a robust measure of a child’s academic attainment. The ability to combine these resources alongside the rich data collected within BiB provides invaluable opportunities to conduct highly relevant yet novel research and to identify associations that would otherwise be difficult to determine. For example, KS1 results, as opposed to standardized intelligence tests, represent a more relevant outcome which is clearly pertinent to the longer-term educational attainment, health and well-being of the child.
Another strength is the use of spline models to split gestation into distinct periods and thus provide the opportunity to identify whether growth in certain periods is more or less strongly associated with educational outcomes. Furthermore, the use of DAGs to model the underlying network of associations between our exposure and outcome allowed for the appropriate adjustment for a set of putative confounders, in order to obtain a less biased estimate of the association between exposure and outcomes. We are, however, cautious not to refer to any association as ‘causal’, as we cannot exclude the possibility of the presence of residual confounding nor the extent of any measurement error, especially in light of the study which observed no association between genetic SNPs for birthweight and educational attainment.20 Nonetheless, appropriate adjustment was only possible due to the extensive data collection which was performed at recruitment into the study. In those participants who did have missing covariate data however, multiple imputation was used to impute missing values. This meant any differences in the size or strength of evidence for associations observed in the adjusted analysis were not a result of differential samples (compared with the sample in the unadjusted analyses) or reduced power as a result of a reduced sample size. However, as analyses were limited to those with complete exposure and outcome data, the sample size was slightly reduced. Whereas this will have reduced statistical power, we do not feel that we have introduced a substantial bias into the analysis. For example, Supplementary Tables 1 and 2 (available as Supplementary data at IJE online) show that differences between those with and without complete exposure and outcome data were, on the whole, small.
The number of ultrasound measurements per woman ranged from one to nine, with a higher number of measurements potentially representing increased surveillance due to adverse clinical indications. This may therefore have biased associations by selecting a sample potentially demonstrating suboptimal fetal growth. We investigated this by adjusting fetal trajectories for the number of scans, which did not result in changes in the fetal growth trajectories. We therefore do not believe there is substantial confounding (by indication). We did not exclude those born preterm from the analyses. However, growth trajectories at later gestational ages are more heavily influenced by those infants who were not born preterm and who were thus able to contribute data to the model. This may have introduced a bias into the analysis linking growth in later gestation to educational outcomes. In sensitivity analyses excluding preterm births, minor attenuations of estimates were observed but these did not warrant changes to our interpretation of results (Supplementary Tables 7 and 8, available as Supplementary data at IJE online).
We limited our sample to those not diagnosed with a congenital anomaly. This diagnosis was made either during pregnancy or in the early postnatal period. It has recently been shown, however, that a significant number of diagnoses are made after 1 year of life,53 and thus we may have not completely excluded all infants with congenital anomalies. A limitation of using linear splines to model fetal growth is that it imposes a biologically implausible linearity assumption. In addition, there is no consensus on the best method to choose the number and position of knot points.54 As has been done in other studies,34,35,55 we initially fitted a fractional polynomial to obtain a smooth curve and used its parameters to inform the number and placing of knot points for the spline model. Although fractional polynomials, nonlinear splines and other non-linear models may have resulted in better-fitting models, the coefficients from these models would have been harder to interpret. Finally, whereas other studies have sed KS1 attainment as outcomes,26,27 they have employed different analysis strategies and it is thus difficult to compare the sizes of association observed here with these studies.
We have shown that fetal size and growth in early-mid gestation are positively associated with academic attainment at age 6–7 years. Whereas only relatively small associations were observed, the fact that earlier educational attainment is a strong predictor of later educational and socioeconomic outcomes means that the associations observed here could have significant long-term implications. It has long been known, within the Developmental Origins of Health and Disease (DOHaD) paradigm, that optimizing maternal health and fetal development confers positive associations on future cardiometabolic health in the offspring; however, the associations observed here, if confirmed in future studies, suggest that promotion of maternal and pregnancy health may lead also to improvements in educational attainment.
Funding
BiB receives core infrastructure funding from the Wellcome Trust (WT101597MA) and a joint grant from the UK Medical Research Council (MRC) and Economic and Social Science Research Council (ESRC) (MR/N024397/1). J.W. was supported by the NIHR Collaboration for Leadership in Applied Health Research and Care Yorkshire and Humber (NIHR CLAHRC YH) [www.clahrc-yh.nihr.ac.uk]. W.J. is supported by a UK Medical Research Council (MRC) New Investigator Research Grant (MR/P023347/1), and acknowledges support from the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University and the University of Leicester. The views and opinions expressed are those of the author(s), and not necessarily those of the NHS, the NIHR or the Department of Health.
Supplementary Material
Acknowledgements
Born in Bradford is only possible because of the enthusiasm and commitment of the children and parents in Born in Bradford. The authors are grateful to all participants, health professionals and researchers who have made Born in Bradford happen. We are particularly grateful to all the school nurse teams in Bradford for their support and enthusiasm for this study.
Author Contributions
T.N., W.J. and E.P. conceived of the study. T.N. performed all data analysis, with the help of W.J, E.P. and S.J. Intellectual input was received from J.W., S.J., E.D., N.C., S.O. and P.B. T.N. wrote the first draft of the manuscript, with all authors contributing important revisions. T.N. will act as guarantor for the paper.
Conflict of interest: The authors report no conflicts of interest.
References
- 1. Saigal S, Ouden L. D, Wolke D.. School-age outcomes in children who were extremely low birth weight from four international population-based cohorts. Pediatrics 2003;112:943–50. [DOI] [PubMed] [Google Scholar]
- 2. Shenkin S, Starr J, Deary P.. Birth weight and cognitive ability in childhood: a systematic review. Psychol Bull 2004;130:989.. [DOI] [PubMed] [Google Scholar]
- 3. Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J.. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009;124:717–28. [DOI] [PubMed] [Google Scholar]
- 4. Gale CR, O’Callaghan FJ, Bredow M, Martyn CN.. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 2006;118:1486–92. [DOI] [PubMed] [Google Scholar]
- 5. Cole TJ. Growth and organ development In: Breastfeeding: Early Influences on Later Health. Dordrecht, The Netherlands: Springer Netherlands, 2009. [Google Scholar]
- 6. Cooke RWI, Lucas A, Yudkin PLN, Pryse-Davies J.. Head circumference as an index of brain weight in the fetus and newborn. Early Hum Dev 1977;1:145–49. [DOI] [PubMed] [Google Scholar]
- 7. Tanner J. Foetus into Man: Physical Growth From Conception to Maturity. Cambridge, MA: Harvard University Press, 1990. [Google Scholar]
- 8. Desposito F, Cunniff C, Frias J.. Folic acid for the prevention of neural tube defects. Pediatrics 1999;104:325–27. [DOI] [PubMed] [Google Scholar]
- 9. Harvey D, Prince J, Bunton J, Parkinson C, Campbell S.. Abilities of children who were small-for- gestational-age babies. Pediatrics 1982;69:296–300. [PubMed] [Google Scholar]
- 10. Walker SP, Thame MM, Chang SM, Bennett F, Forrester TE.. Association of growth in utero with cognitive function at age 6-8 years. Early Hum Dev 2007;83:355–60. [DOI] [PubMed] [Google Scholar]
- 11. Ehrenstein OS, Von Mikolajczyk RT, Zhang J.. Timing and trajectories of fetal growth related to cognitive development in childhood. Am J Epidemiol 2009;170:1388–95. [DOI] [PubMed] [Google Scholar]
- 12. Henrichs J, Schenk JJ, Barendregt CS. et al. Fetal growth from mid- to late pregnancy is associated with infant development: the Generation R Study. Dev Med Child Neurol 2010;52:644–51. [DOI] [PubMed] [Google Scholar]
- 13. Borghans L, Golsteyn BHH, Heckman JJ, Humphries JE.. What grades and achievement tests measure. Proc Natl Acad Sci U S A 2016;113:13354–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneider W, Wolke D, Schlagmüller M, Meyer R.. Pathsways to school achievement in very preterm and full term children. Eur J Psychol Educ 2004;19:385–406. [Google Scholar]
- 15. Nomura Y, Halperin JM, Newcorn JH. et al. The risk for impaired learning-related abilities in childhood and educational attainment among adults born near-term. J Pediatr Psychol 2009;34:406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basten M, Jaekel J, Johnson S, Gilmore C, Wolke D.. Preterm birth and adult wealth: mathematics skills count. Psychol Sci 2015;26:1608–19. [DOI] [PubMed] [Google Scholar]
- 17. Ritchie SJ, Bates TC.. Enduring links from childhood mathematics and reading achievement to adult socioeconomic status. Psychol Sci 2013;24:1301–08. [DOI] [PubMed] [Google Scholar]
- 18. Chatterji P, Kim D, Lahiri K.. Birth weight and academic achievement in childhood. Health Econ 2014;23:1013–35. [DOI] [PubMed] [Google Scholar]
- 19. McNicholas F, Healy E, White M. et al. Medical, cognitive and academic outcomes of very low birth weight infants at age 10–14 years in Ireland. Ir J Med Sci 2014;183:525–32. [DOI] [PubMed] [Google Scholar]
- 20. Lin SL, Leung GM, Schooling CM.. The effect of birth weight on academic performance: Instrumental variable analysis. Am J Epidemiol 2017;185:853–59. [DOI] [PubMed] [Google Scholar]
- 21. Wright J, Small N, Raynor P. et al. Cohort Profile: The Born in Bradford multi-ethnic family cohort study. Int J Epidemiol 2013;42:978–91. [DOI] [PubMed] [Google Scholar]
- 22. Raynor P, Duley L, Small N. et al. Born in Bradford, a cohort study of babies born in Bradford, and their parents: protocol for the recruitment phase. BMC Public Health 2008;8:327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirwan D. and the NHS Fetal Anomaly Screening Programme. 18 + 0 to 20 + 6 Weeks Fetal Anomaly Scan National Standards and Guidance for England 2012. http://www.bmfms.org.uk/UserFiles/documents/nhs_fasp_fa_standards_and_gudance_2010.pdf.
- 24. Loughna P, Chitty L, Evans T, Chudleigh T.. Fetal size and dating: charts recommended for clinical obstetric practice. Ultrasound 2009;17:160–66. [Google Scholar]
- 25. Standards and Testing Agency. Interim Teacher Assessment Frameworks at the End of Key Stage 1 Key Stage 1 Interim Teacher Assessment Framework at the End of Key Stage 1 –Reading; 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/576179/2017_interim_teacher_assessment_frameworks_at_the_end_of_key_stage_1_V2_PDFa.pdf.
- 26. Kenyon S, Pike K, Jones DR. et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labor: 7 year follow up of the ORACLE II trial. Lancet 2008;372: 1319–27. [DOI] [PubMed] [Google Scholar]
- 27. Chan E, Quigley MA.. School performance at age 7 years in late preterm and early term birth: a cohort study. Arch Dis Child Fetal Neonatal Ed 2014;99:F451–57. [DOI] [PubMed] [Google Scholar]
- 28. Nathan L, Stackhouse J, Goulandris N, Snowling MJ.. Educational consequences of developmental speech disorder: Key Stage I National Curriculum assessment results in English and mathematics. Br J Educ Psychol 2004;74:173–86. [DOI] [PubMed] [Google Scholar]
- 29. Strand S. Ethnic group, sex and economic disadvantage: associations with pupils’ educational progress from Baseline to the end of Key Stage 1. Br Educ Res J 1999;25:179–202. [Google Scholar]
- 30. Owen P, Donnet ML, Ogston SA, Christie AD, Howie PW, Patel NB.. Standards for ultrasound fetal growth velocity. BJOG and 1996;103:60–69. [DOI] [PubMed] [Google Scholar]
- 31. Drooger JC, Troe JWM, Borsboom GJJM. et al. Ethnic differences in prenatal growth and the association with maternal and fetal characteristics. Ultrasound Obstet Gynecol 2005;26:115–22. [DOI] [PubMed] [Google Scholar]
- 32. Lampl M, Kusanovic JP, Erez O. et al. Early rapid growth, early birth: accelerated fetal growth and spontaneous late preterm birth. Am J Hum Biol 2009;21:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Royston P. DIY fractional polynomials. In: Proceedings of 16th UK Stata User Groups Meeting, 9-10 September 2010 London: Timberlake, 2010.
- 34. Ben-Shlomo Y, McCarthy A, Hughes R, Tilling K, Davies D, Davey Smith G.. Immediate postnatal growth is associated with blood pressure in young adulthood: the Barry Caerphilly Growth Study. Hypertension 2008;52:638–44. [DOI] [PubMed] [Google Scholar]
- 35. Howe LD, Tilling K, Galobardes B, Davey Smith G, Gunnell D, Lawlor DA.. Socioeconomic differences in childhood growth trajectories: at what age do height inequalities emerge? J Epidemiol Community Health 2012;66:143–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson EL, Fraser A, Martin RM. et al. Associations of postnatal growth with asthma and atopy: the PROBIT Study. Pediatr Allergy Immunol 2013;24:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Westreich D, Greenland S.. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013;177:292–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Royston P, White I.. Multiple Imputation by Chained Equations (MICE): implementation in Stata. J Stat Softw 2011;45:1-20. [Google Scholar]
- 39. Rubin D. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley, 2004. [Google Scholar]
- 40. White IR, Royston P, Wood AM.. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 41. McGee R, Prior M, Williams S, Smart D, Sanson A.. The long-term significance of teacher-rated hyperactivity and reading ability in childhood: findings from two longitudinal studies. J Child Psychol Psychiatry 2002;43:1004–17. [DOI] [PubMed] [Google Scholar]
- 42. Chetty R, Friedman J, Hilger N, Saez E, Schanzenbach D, Yagan D.. How does your kindergarten classroom affect your earnings? Evidence from Project STAR. Q J Econ 2011;126:1593–660. [DOI] [PubMed] [Google Scholar]
- 43. Watts TW, Duncan GJ, Siegler RS, Davis-Kean PE.. What’s past is prologue. Educ Res 2014;43:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abbott-Chapman J, Martin K, Ollington N, Venn A, Dwyer T, Gall S.. The longitudinal association of childhood school engagement with adult educational and occupational achievement: findings from an Australian national study. Br Educ Res J 2014;40:102–20. [Google Scholar]
- 45. Agarwal S, Mazumder B.. Cognitive abilities and household financial decision making. Am Econ J Appl Econ 2013;5:193–207. [Google Scholar]
- 46. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K.. Low health literacy and health outcomes : an updated systematic review. Ann Intern Med 2011;155:97–106. [DOI] [PubMed] [Google Scholar]
- 47. Black SE, Devereux PJ, Salvanes KG.. From the cradle to the labor market? The effect of birth weight on adult outcomes. Q J Econ 2007;122:409–39 [Google Scholar]
- 48. Currie J, Hyson R.. Is the impact of health shocks cushioned by socioeconomic status? the case of low birthweight. Am Econ Rev 1999;89:245–50. [Google Scholar]
- 49. Oreopoulos P, Stabile M, Walld R, Roos LL.. Short-, medium-, and long-term consequences of poor infant health: an analysis using siblings and twins. J Hum Resour 2008;43:88–138. [Google Scholar]
- 50. Roth S, Chang TC, Robson S, Spencer JAD, Wyatt JS, Stewart AL.. The neurodevelopmental outcome of term infants with different intrauterine growth characteristics. Early Hum Dev 1999;55:39–50. [DOI] [PubMed] [Google Scholar]
- 51. Dobbing J, Sands J.. Quantitative growth and development of human brain. Arch Dis Child 1973;48:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sidman RL, Rakic P.. Neuronal migration, with special reference to developing human brain: a review. Brain Res 1973;62:1–35. [DOI] [PubMed] [Google Scholar]
- 53. Bishop C, Small N, Mason D. et al. Improving case ascertainment of congenital anomalies: findings from a prospective birth cohort with detailed primary care record linkage. BMJ Paediatr Open 2017;1:e000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tilling K, MacDonald-Wallis C, Lawlor DA, Hughes RA, Howe LD.. Modelling childhood growth using fractional polynomials and linear splines. Ann Nutr Metab 2014;65:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tilling K, Davies NM, Nicoli E. et al. Associations of growth trajectories in infancy and early childhood with later childhood outcomes. Am J Clin Nutr 2011;94(Suppl 6):1808-13S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.