Abstract
The antileishmanial activity of the essential oil (EO) from Chenopodium ambrosioides L. has been demonstrated in vitro and in animal models, attributed to the major components of the EO. This study focused on the effects of the three major EO compounds carvacrol, caryophyllene oxide (Caryo), and the antileishmanial endoperoxide ascaridole (Asc) on mitochondrial functions in Leishmania tarentolae promastigotes (LtP). EO and Caryo were able to partially inhibit the leishmanial electron transport chain, whereas other components failed to demonstrate a direct immediate effect. Caryo demonstrated inhibition of complex III activity in LtP and in isolated complex III from other species. The formation of superoxide radicals was studied in Leishmania by electron spin resonance spectroscopy in the presence of iron chelators wherein selected compounds failed to trigger a significant immediate additional superoxide production in LtP. However, upon prolonged incubation of Leishmania with Asc and especially in the absence of iron chelators (allowing the activation of Asc), an increased superoxide radical production and significant impairment of mitochondrial coupling in Leishmania was observed. Prolonged incubation with all EO components resulted in thiol depletion. Taken together, the major components of EO mediate their leishmanicidal activity via different mitochondrial targets and time profiles. Further studies are required to elucidate possible synergistic effects of carvacrol and Asc and the influence of minor compounds.
Keywords: ascaridole, carvacrol, caryophyllene oxide, Chenopodium ambrosioides L, Leishmania, mitochondria
Abbreviations
- AA
antimycin A
- Asc
ascaridole
- BH‐bc1
complex bc 1 from submitochondrial particles of bovine heart
- BH‐SMP
submitochondrial particles from bovine heart
- BSA
bovine serum albumin
- Car
carvacrol
- Caryo
caryophyllene oxide
- CCCP
carbonyl cyanide‐m‐chlorophenylhydrazone
- CMFDA
5‐chloromethylfluorescein diacetate
- CMH
1‐hydroxy‐3‐methoxycarbonyl‐2,2,5,5‐tetramethylpyrrolidine‐HCl
- CM●
radical of CMH
- cyt c3+
cytochrome c 3+
- DCPIP
2,6‐dichlorophenol–indophenol
- DFO
desferrioxamine mesylate
- DMSO
dimethyl sulfoxide
- DTPA
diethylenetriaminepentaacetic acid
- DTT
dithiothreitol
- dUQ
decylubiquinone
- dUQH2
decylubiquinol
- EDTA
ethylenediamine tetraacetic acid
- EO
essential oil from Chenopodium ambrosioides L.
- ESR
electron spin resonance
- ETC
electron transport chain
- IC50
median inhibitory concentration
- LaP
Leishmania amazonensis promastigotes
- LtP
Leishmania tarentolae promastigotes
- LtP‐Mit
mitochondrial fraction from Leishmania tarentolae promastigotes
- NADH
reduced nicotinamide–adenine dinucleotide
- NMR
nuclear magnetic resonance
- Oligo
oligomycin
- PBS
phosphate‐buffered saline
- RCR
respiratory control ratio
- ScY
Saccharomyces cerevisiae yeast
- ScY‐bc1
complex bc 1 from submitochondrial particles of Saccharomyces cerevisiae
- ScY‐SMP
submitochondrial particles from Saccharomyces cerevisiae
- SMP
submitochondrial particles
- Tris
tris(hydroxymethyl)aminomethane
- YEM
yeast extract medium
1. INTRODUCTION
Chenopodium ambrosioides L. is an aromatic herb native to Central and South America. It has been distributed throughout the tropical parts of the world and is considered as invasive (Duke, Bogenshutz, du‐Cellier, & Duke, 2002; Trivellato‐Grassi et al., 2013). The plant is annual or perennial; it can grow up to 1 m in height, and one of its main characteristics is the strong aromatic odor. It has been employed by empirical herbalists and healers, particularly against parasites (Franca, Lago, & Marsden, 1996; Quinlan, Quinlan, & Nolan, 2002). Some significant biological properties have been demonstrated, including antitumor (Nascimento et al., 2006), antimicrobial (Jardim, Jham, Dhingra, & Freire, 2008; Liu, Liu, Zhang, Li, & Cheng, 2013; Pandey, Singh, Palni, & Tripathi, 2013), antiparasitic (Guerra, Torres, & Martínez, 2001; Kiuchi et al., 2002; Monzote et al., 2004), anti‐inflammatory, and antinociceptive (Trivellato‐Grassi et al., 2013) effects.
In a series of previous studies, we observed the antileishmanial potential of essential oil (EO) from C. ambrosioides L. in different in vitro and in vivo models (Monzote et al., 2006; Monzote, Garcia, et al., 2014). In parallel, in the chromatogram obtained by gas chromatography/mass spectrometry, we identified three major compounds of the EO (Figure 1), namely, carvacrol (Car) with 62%, ascaridole (Asc) with 22%, and caryophyllene oxide (Caryo) with 5% of total content (Monzote et al., 2006). These three compounds were also identified in EO of Chinese C. ambrosioides L., however, in different percentages (Chu, Hu, & Liu, 2011). In addition to these volatile components, this EO contains also nonvolatile (solvent extractable) pharmacologically active compounds (Shah & Khan, 2017). Car, Caryo, and Asc showed antileishmanial activity, although they were less selective for Leishmania in comparison with mammalian host cells than EO for Leishmania compared with effects on mammalian host cells (Monzote et al., 2006; Monzote, Garcia, et al., 2014). Asc, which is also present in tea tree oil, demonstrated a skin‐sensitizing effect in mammals (Chittiboyina, Avonto, & Khan, 2016; Krutz et al., 2015). By the use of iron chelators, it was shown that activation of the endoperoxide Asc in EO by iron is essential for its antiparasitic actions. Nevertheless, differences in the activity profile of Asc and EO have been observed in the system of macrophages/Leishmania.
Figure 1.
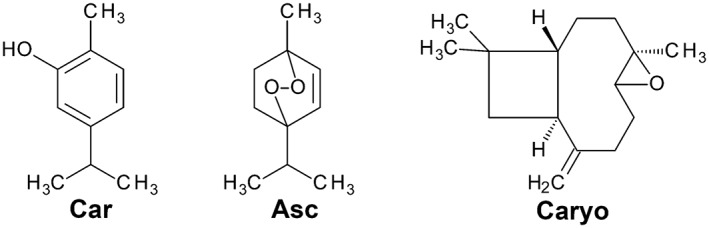
Chemical structure of main compounds of essential oil from Chenopodium ambrosioides L.: carvacrol (Car, 62%), ascaridole (Asc, 22%), and caryophyllene oxide (Caryo, 5%) according to Monzote et al. (2006)
In addition, it has been demonstrated that one possible toxicological mechanism behind the actions of EO and its major components against mammalian cells is related to mitochondrial dysfunction (Monzote, Stamberg, Staniek, & Gille, 2009). In Leishmania, there have been indications that EO and its major compounds also influence mitochondrial functions (Monzote, Garcia, et al., 2014), but specific mechanisms and targets have not been identified so far.
Herein, we study the effects of EO's main compounds (Asc, Car, and Caryo) on electron transport chain (ETC) complexes I–III in different models (yeast, Leishmania, and mammals) at the molecular level. Short‐term effects as well as long‐term effects of Asc have been investigated with the objective to elucidate the role of mitochondrial effects in the EO actions in Leishmania.
2. METHODS
2.1. Chemicals
Diethylenetriaminepentaacetic acid (DTPA, sodium salt), dimethyl sulfoxide (DMSO), ethylenediamine tetraacetic acid (EDTA), glucose, HCl, KCN, KH2PO4, Na2HPO4, NaH2PO4, NaCl, NaN3, sodium dodecyl sulphate, succinate, sucrose, and tris(hydroxymethyl)aminomethane (Tris) were obtained from Merck (Germany). Bovine serum albumin (BSA), decylubiquinone (dUQ), 2,6‐dichlorophenol–indophenol (DCPIP), dithiothreitol (DTT), cytochrome c 3+ (cyt c 3+), glutathione, hemin, hydroxyapatite, phenylmethylsulfonyl fluoride, penicillin–streptomycin solution, reduced nicotinamide–adenine dinucleotide (NADH), resazurin, sorbitol, Schneider's medium, brain heart infusion (BHI) medium, oligomycin (Oligo), antimycin A (AA), carbonyl cyanide‐m‐chlorophenylhydrazone (CCCP), paraffin oil, and Triton X‐100 were purchased from Sigma (USA). Triethanolamine hydrochloride was from Fluka (Switzerland), whereas Desferal (desferrioxamine mesylate [DFO]) was from Novartis Pharma (Germany), and 1‐hydroxy‐3‐methoxycarbonyl‐2,2,5,5‐tetramethylpyrrolidine‐HCl (CMH) was from Noxygen (Germany). Idebenone and zymolyase were from Takeda and Seikagaku Corporation (Japan), respectively. Yeast extract powder was supplied by Amresco (USA). 5‐Chloromethylfluorescein diacetate (CMFDA) was purchased from Abcam (USA).
2.2. EO from C. ambrosioides L. and its main compounds
In this study, we used an aliquot of the original sample (stored at −20 °C) for which the type of collection, extraction of EO, and chemical characterization were described previously (Monzote et al., 2006). Briefly, aerial part of C. ambrosioides L. plant in flowering stage was collected in July, and voucher specimen number (ROIG4639) was assigned at the Experimental Station of Medicinal Plants “Dr Juan Tomás Roig,” Cuba. The EO was extracted from fresh material by hydrodistillation in a Clevenger apparatus over 4 hr to yield approximately 1% oil (Monzote et al., 2006). Asc was obtained by chemical synthesis by addition of singlet oxygen to α‐terpinene using rose bengal as a photosensitizer (Monzote et al., 2009). Structure and stability of the product was studied by nuclear magnetic resonance (NMR), and a purity of around 95% was determined. 1H NMR (400.13 MHz, CDCl3): δ [ppm] 6.49 (d, J = 8.6 Hz, 1H, H‐3), 6.31 (d, J = 8.6 Hz, 1H, H‐2), 2.03 (m, 2H, H‐5a, H‐6a), 1.92 (sept, J = 7.0 Hz, 1H, H‐7), 1.51 (m, 2H, H‐5b, H‐6b), 1.37 (s, 3H, 4‐Me), 1.00 (d, J = 7.0 Hz, 6H, H‐8). δ = 7.29 ppm = solvent (CDCl3) and δ = 1.60 ppm = H2O. 13C NMR (100.61 MHz, CDCl3): δ [ppm] 17.12 (isopropyl CH3), 17.20 (isopropyl CH3), 21.37 (4‐Me), 25.58 (C‐6), 29.51 (C‐5), 32.11 (isopropyl C), 74.32 (C‐4), 79.76 (C‐1), 133.03 (C‐3), 136.37 (C‐2; assignments according to Cavalli, Tomi, Bernardini, & Casanova, 2004). The accordance of this NMR data with our previously published data (Monzote et al., 2009) confirms the stability and purity of Asc. Car and Caryo were from Sigma (USA), with a purity of >98% and >95%, respectively. All products were diluted with DMSO.
2.3. Parasite culture
Leishmania tarentolae promastigotes (LtP) strain P10 from Jena Bioscience (Germany) was used. Parasites were cultured at 26 °C either in yeast extract medium (YEM; 20.7 g/L yeast extract powder, 0.2 g/L KH2PO4, 1.2 g/L K2HPO4, and 2.9 g/L glucose) or in BHI medium (37 g/L) supplemented with 5 mg/L hemin and 50,000 U/L penicillin—50 mg/L streptomycin.
2.4. Preparation of mitochondrial fractions
2.4.1. Isolation of mitochondrial fractions from LtP
LtP culture (2,700 ml) was centrifuged at 478 g over 10 min at 4 °C (Sorvall RC26 Plus, USA). The supernatant was discarded, and the cell pellet was resuspended in buffer (10 mM Tris–HCl, 0.3 M sucrose, 0.2 mM EDTA, and 0.2% BSA, pH 7.4). Following two repeated washes (478 g, 10 min at 4 °C), the resulting cell pellet was incubated in lysis buffer (5 mM Tris–HCl, pH 7.4) for 10 min at 25 °C and subsequently homogenized in a Dounce homogenizer. Cell debris was removed by centrifugation (1,005 g, 10 min, 4 °C). The supernatant was again centrifuged (13,176 g, 20 min, 4 °C) to sediment the mitochondrial fraction (LtP‐Mit). The mitochondria were resuspended in 1 ml of buffer (250 mM sucrose, 50 mM KH2PO4, and 0.2 mM EDTA, pH 7.2) and stored in liquid nitrogen until use.
2.4.2. Isolation of submitochondrial particles from bovine heart
Bovine heart submitochondrial particles (BH‐SMP) were obtained from bovine heart mitochondria by sonication (Nohl & Hegner, 1978) and stored in liquid nitrogen until use.
2.4.3. Isolation of SMP from yeast
Yeast mitochondria were prepared from Saccharomyces cerevisiae yeast (ScY) strain DBY 747 (Daum, Bohni, & Schatz, 1982). Cells were harvested by centrifugation (1,464 g, 5 min, 20 °C), and the pellet was resuspended in buffer I (10 mM Tris and 10 mM DTT, pH 9.4). Following 15 min of incubation at 37 °C, cells were centrifuged (1,464 g, 5 min, 20 °C) and resuspended in buffer II (1.2 M sorbitol and 20 mM KH2PO4, pH 7.4). Finally, after a repeated centrifugation (1,464 g, 5 min, 20 °C), the weight of the cell pellets was determined. To prepare spheroblasts, pellets were suspended in buffer II complemented with 2 mg zymolyase/g yeast cells. After incubation for 45 min at 28 °C, spheroblasts were collected by centrifugation (1,464 g, 5 min, 20 °C), resuspended in 30 ml of buffer II, sedimented again (5 min at 1,464 g and 20 °C), and homogenized in 30 ml of buffer III (600 mM sorbitol and 20 mM Tris, pH 7.4) using a Wheaton Dounce tissue grinder. Cells and cell debris were removed by two centrifugations (1,464 g, 5 min, 4 °C). Mitochondria were finally collected from the supernatant by centrifugation (11,952 g, 10 min, 4 °C) and used to prepare ScY submitochondrial particles (ScY‐SMP). Mitochondrial pellets were suspended in 5 ml of buffer I (without DTT) and diluted to 25 ml with 10 mM Tris (pH 7.5). The suspension was kept on ice for 20 min followed by a centrifugation (39,500 g, 10 min, 4 °C). The pellet was resuspended in 20 ml of sucrose buffer (250 mM sucrose and 10 mM Tris, pH 7.4) and sonicated 18 times for 20 s (Branson sonifier at maximum intensity) with interruptions of 10 s for heat dissipation. Subsequently, the suspension was centrifuged (5,400 g, 10 min, 4 °C) to remove mitochondria. ScY‐SMP were sedimented from the supernatant by centrifugation (195,000 g, 60 min, 4 °C). The obtained ScY‐SMP pellet was homogenized in 1.5 ml of buffer (250 mM sucrose, 0.2 mM EDTA, and 50 mM KH2PO4, pH 7.2) and stored in liquid nitrogen.
2.4.4. Isolation of cytochrome bc 1 complex
Cytochrome bc 1 complex was obtained from ScY‐SMP and BH‐SMP following published methodology (Geier, Schägger, Brandt, Colson, & von Jagow, 1992) with minor modifications as described below. SMP were resuspended in 1.1% Triton X‐100 and 200 mM phenylmethylsulfonyl fluoride, pH 7.2, and centrifuged for 1 hr at 100,000 g. The bc 1 complex in the sediment was solubilized with 2.2% Triton X‐100, 600 mM NaCl, 10 mM KH2PO4, and 10 mM EDTA, pH 7.2. After centrifugation at 100,000 g for 1 hr, the supernatant was mixed with 50 ml of hydroxyapatite, equilibrated with 0.5% Triton X‐100, 250 mM NaCl, and 100 mM NaHPO4, pH 7.2. After washing the hydroxyapatite with 50 ml of equilibration buffer (0.05% Triton X‐100, 100 mM NaHPO4, and 250 mM NaCl), the bc 1 complexes from ScY‐SMP and BH‐SMP (ScY‐bc 1 and BH‐bc 1, respectively) were eluted from hydroxyapatite with 250 mM KH2PO4 and 0.25% Triton X‐100, pH 7.2, and stored in liquid nitrogen.
2.5. Determination of protein and cell concentrations
The protein concentration of mitochondrial preparations was determined by the Biuret method (Lowry, Rosebrough, Farr, & Randall, 1951). The number of LtP was determined by optical density at 600 nm (HITACHI U‐1100 Spectrophotometer, Japan). The cell broth was diluted 1:10 with culture medium and measured against a blank of culture medium. The cell number was calculated using the formula CCell (106/ml) = OD600nm * 0.969 * 124 (Fritsche, Sitz, Weiland, Breitling, & Pohl, 2007). Two replicates of each culture were performed.
2.6. Viability of LtP treated with EO main components
LtP (200 μl, 2 × 106 parasites/ml) in YEM/phosphate‐buffered saline (PBS; 1:1 vol/vol, including antibiotics and 6 μM hemin) were distributed in 96‐well plates (nontreated plates). Compound stocks (in DMSO, max. 2% final concentration) were added, and five 1:3 serial dilutions were performed. Control rows with YEM/PBS (no activity) and with untreated LtP (vehicle, DMSO; 100% activity) were loaded, and the plate was incubated at 26 °C for 48 hr. Then resazurin (50 μl in PBS) was added (20 μM final concentration), and after 4 hr of incubation at 26 °C, the fluorescence of resazurin was measured at 560 nm excitation and 590 nm emission using a plate reader (PerkinElmer Enspire, Germany). Each compound was tested in triplicate.
2.7. Activity of EO compounds on ETC complexes in LtP‐Mit and BH‐SMP
2.7.1. Influence on NADH:ubiquinone oxidoreductase (complex I) and succinate:ubiquinone oxidoreductase (complex II) activities
NADH:ubiquinone oxidoreductase (complex I) and succinate:ubiquinone oxidoreductase (complex II) activities were determined in 96 well‐plates using DCPIP as the electron acceptor. In each well, 200 μl of premix containing buffer (250 mM sucrose, 20 mM triethanolamine, and 1 mM EDTA, pH 7.4), DCPIP (60 μM), BSA (3.5 mg/ml for complex I and 1 mg/ml for complex II), idebenone (50 μM), and LtP‐Mit (complex I 0.17 μg/ml protein and complex II: 51 μg/ml protein) or BH‐SMP (complexes I and II: 8 μg/ml protein) were added. In addition, in the first wells, 97 μl of premix and 3 μl of EO compounds were added. Then six serial 1:3 dilutions were carried out transferring 100 μl in each step. The reaction was started by adding 50 μl of start‐mix per well, giving final concentrations of KCN (1 mM), for complex I:NADH (300 μM) or complex II:succinate (4 mM). After start of the reaction, images of the plates were recorded with a Canon EOS 300D camera (PLReader software, red–green channel) each 10 min for LtP‐Mit and 3 min for BH‐SMP (20 absorbance measurements). For each EO compound, percentage of inhibition was obtained with respect to controls (set to 100%) treated with maximum volume of vehicle (DMSO) introduced by test compound stocks.
2.7.2. Influence on ubiquinol:cytochrome c oxidoreductase activity
To measure the ubiquinol:cyt c 3+ oxidoreductase (complex III) activity, the reduction of 100 μM cyt c 3+ at 550 nm using 540 nm as reference was monitored in the presence of the artificial substrate decylubiquinol (dUQH2, 75 μM), which was prepared from dUQ by reduction (Müllebner et al., 2010). The dUQH2:cyt c 3+ oxidoreductase activities of 40 μg protein/ml of LtP‐Mit or 3.2 μg protein/ml of BH‐SMP were measured in 1 ml of buffer containing 250 mM sucrose, 50 mM KH2PO4, 0.2 mM EDTA (pH 7.2), 2 mM KCN, and 4 mM NaN3. Respective EO compounds were added 50 s after starting the time scan, and the reaction was started after 100 s with dUQH2 and was monitored for additional 150 s. The activity of noninhibited dUQH2:cyt c 3+ oxidoreductase activity was measured in the presence of the vehicle for the respective inhibitor (DMSO). All inhibitor concentrations were tested in triplicate. The reduction rates for cyt c 3+ were calculated from the time trace of the absorption difference at 550 − 540 nm (ε550–540 nm = 19 mmol−1 L cm−1). Reduction rates in the presence of DMSO (maximum amount that was introduced by test compound stocks) were set to 100%, and the remaining activities in the presence of EO compounds were expressed in %. Three replicates were measured for each concentration.
2.8. Influence of EO compounds on isolated bc 1 complex
To determine the influence of EO compounds on purified bc 1 complex, the dUQH2:cyt c 3+ oxidoreductase activities were measured as described. In this case, concentrations of 21.3 μg/ml of ScY‐bc 1 or 1.6 μg/ml of BH‐bc 1 were used.
2.9. Oxygen consumption of LtP
2.9.1. Clark electrode
Direct inhibitors of mitochondrial ETC instantaneously influence mitochondrial oxygen consumption. To assay the effect of EO compounds on oxygen consumption of LtP, a Clark‐type oxygen electrode (Hansatech, Germany) and software MCREC were used. LtP at approximately 108 cells/ml in YEM (25 °C) were added and treated with increasing concentrations of EO compounds between 10 and 200 μM and for EO 5.6–89.6 μg/ml. Each concentration was assayed in quadruplicates, and the results were expressed as percentage of oxygen consumption in comparison with the untreated control LtP. The highest concentration of the vehicle (1% DMSO) caused only 2% inhibition. The uncoupling effect in short‐term and long‐term (0, 6, and 24 hr) incubations with Asc (200 μM) was studied in Oligo‐inhibited (5 μM) and uncoupler‐stimulated (0.5 μM CCCP) LtP in Schneider's medium supplemented with 6 μM hemin. Four replicates were performed for each concentration.
2.9.2. OxoPlates
U‐shaped 96‐well OxoPlates (OP96U PreSens, Germany) with integrated fluorescence oxygen sensors were used for parallel LtP experiments. Oxygen concentrations were measured using a PerkinElmer Enspire fluorescence plate reader using excitation wavelength 540 nm and two emission wavelengths (reference dye 590 nm, IRef, and O2‐sensing dye 650 nm, IInd). The oxygen concentration (μM O2) was calculated according to manufacturer's instructions as described previously (Monzote et al., 2016). Measurements with LtP were performed in air‐saturated BHI medium. After calibration of the OxoPlates, they were loaded with 200 μl medium (medium controls for drift corrections), 50 μl medium in wells for untreated control cells, or 50 μl medium with EO compounds (Car, Asc, and Caryo) or uncouplers of mitochondrial respiration in wells for treated cells. Immediately before the measurement, 150 μl of well‐aerated cell suspensions (13 × 107 LtP per ml) was added to the respective wells. Finally, on the top of each well, 50 μl of paraffin oil was layered. Two minutes after mixing, the fluorescence measurements at 27 °C were started, and eight measurements at 5‐min intervals were performed. From the linear part of the O2 decay, the slopes were calculated and corrected for the medium drift for further statistic evaluation. All measurements were performed at least in triplicate. For bioenergetic characterization, the ATP synthase inhibitor Oligo (5 μM) and as positive control CCCP (0.7–5 μM) were used. Respiratory control ratio (RCR) was calculated from respiration rate in the presence of Oligo and compounds/uncoupler divided by the rate in the presence of Oligo.
2.10. Measurement of superoxide radicals
Detection of superoxide radicals was performed using CMH as reaction partner and measuring the formed stable nitroxyl radicals by electron spin resonance (ESR) spectroscopy. Measurements were performed in PBS (136 mM NaCl, 1.15 mM KH2PO4, 14 mM Na2HPO4, and 2.7 mM KCl, pH 7.4) containing 15 mM glucose, 400 μM CMH, and 100 μM DFO (desferrioxamine mesylate) and 25 μM DTPA. Usually, these assays are performed in the presence of iron chelators (DFO and DTPA) to prevent CMH interaction with free Fe3+ leading to CM● unrelated to superoxide radicals. Before the measurements, LtP suspensions were washed twice with PBS to remove the medium. Typical experiments contained 5 × 108 LtP cells/ml. Stock solutions of EO compounds dissolved in DMSO were added. The influence of Asc (6–700 μM) on the CMH oxidation rate by LtP was assessed immediately and after prolonged incubation time (1 hr between Asc and CMH addition). The influence of iron‐dependent Asc activation in LtP on CMH oxidation was studied in the absence of iron chelators DFO and DTPA. Prior to the ESR measurement, 400 μM CMH was added. Afterwards, 17 μl of suspension was aspirated in gas‐permeable Teflon tubes (ID 0.7 mm). This capillary tube was placed in the resonator of the ESR instrument (Bruker EMX, split ring MD5, Germany), and 10 sequential measurements were performed. The following instrument settings were used: microwave frequency 9.682 GHz, modulation frequency 100 kHz, modulation amplitude 1 G, time constant 0.082 s, center field 3,446 G, scan rate 71 G/min, sweep width 100 G, scan time 84 s, and attenuation CMH: 7.96 × 103. From the ESR spectra, the middle peak intensity was retrieved, and concentrations of oxidized CMH were obtained by comparison with a standard curve prepared from 3‐carboxy‐proxyl solutions with defined concentrations. Four replicate measurements were performed for each condition.
2.11. Measurement of low molecular thiols in LtP
LtP were centrifuged twice (1,800 g, 10 min, 20 °C), the cell pellets were resuspended in PBS/glucose (15 mM), and the cell number was adjusted to 1 × 107/ml. Aliquots (250 μl) were added to 96‐well plates (black, nontreated plates), along with compound stocks (keeping a maximum of 2% DMSO). Following a 5‐hr incubation at 26 °C, plates were centrifuged (1,800 g, 10 min, 20 °C), and 200 μl of supernatant was removed. To the remaining cell suspension, 100 μl of CMFDA in PBS was added giving a final concentration of 5 μM. After gentle shaking, the cells were incubated for 15 min at 37 °C in the dark. The fluorescence of the methyl fluorescein (MF)‐thiol adduct (Sarkar, Mandal, Singh, Sundar, & Chatterjee, 2009) was measured at 15, 30, 45, and 60 min at 485 nm (excitation wavelength) and 530 nm (emission wavelength) using a plate reader (PerkinElmer Enspire, Germany). The OD600 nm was determined for cell count, and the measured fluorescence intensity was normalized to the cell number. Three replicate measurements were performed for each condition.
2.12. Statistical analysis
The median of inhibitory concentration (IC50) value was determined from nonlinear concentration–response curves using Origin® Program Version 6.1 and expressed as the mean ± standard deviation. Statistically significant differences of p < .05 were identified using Student's t test.
3. RESULTS
3.1. Antileishmanial activity of EO components
Viability assays for LtP resulted in IC50 values for Asc of 24.5 ± 3.0 μM, Car of 11.6 ± 3.4 μM, and Caryo of 36.0 ± 17.6 μM (n = 3). For EO, an IC50 value of 19.1 ± 6.1 μg/ml was determined. This indicates that all major EO components possibly contribute to the antileishmanial action of EO.
3.2. Inhibition of oxygen consumption
EO inhibited the leishmanial oxygen consumption in concentrations above 20 μg/ml (IC50 being 66.6 ± 6.4 μg/ml). The assayed components Asc, Car, and Caryo are sufficiently lipophilic to be immediately taken up by Leishmania; it suggests that they can reach leishmanial mitochondria within minutes; accordingly, we examined their impact on LtP mitochondria. Therefore, mitochondrial function was evaluated by measurement of oxygen consumption of LtP in the presence of EO components (Figure 2). Car and Asc failed to strongly inhibit oxygen consumption, whereas Caryo strongly inhibited LtP oxygen consumption, IC50 being 98.0 ± 2.0 μM. This effect could contribute to the inhibition by EO because it contains about 5% Caryo.
Figure 2.
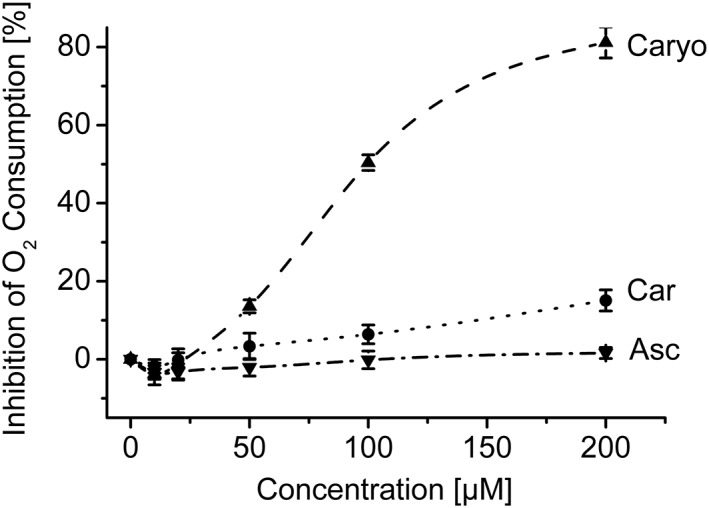
Influence of major components of essential oil from Chenopodium ambrosioides L. on the cellular oxygen consumption of Leishmania tarentolae promastigotes (LtP). Oxygen consumption of LtP (72–100 × 106 cells/ml) was assessed by a Clark‐type electrode in air‐saturated medium containing 14.6 mM glucose. Increasing concentrations of compounds were added subsequently using DMSO as vehicle. At 1% DMSO (highest final concentration), O2 consumption of LtP was inhibited by 1.74 ± 9.46%. Data are means ± standard deviation of four independent experiments. Asc = Ascaridole; Car = carvacrol; Caryo = caryophyllene oxide
3.3. Inhibition of mitochondrial complexes
In general, no strong inhibition was observed for complexes I and II (Table 1). However, complex III inhibition of LtP‐Mit by Caryo confirmed its interference at this site. In contrast, for BH‐SMP, the inhibitory effect of Caryo was weaker. Asc and Car showed no strong inhibition in the studied concentration ranges suggesting that they have no specific targets in the ETC of Leishmania and mammals (Table 1).
Table 1.
Influence of major EO components on the LtP‐Mit in comparison with BH‐SMP on mitochondrial activities of complexes I–III
| Compound | IC50 (μM; % of inhibition at highest concentration tested) | |||||
|---|---|---|---|---|---|---|
| Complex I | Complex II | Complex III | ||||
| LtP‐Mit | BH‐SMP | LtP‐Mit | BH‐SMP | LtP‐Mit | BH‐SMP | |
| Asc | >100 (1.1) | >100 (5.1) | >100 (17.6) | >100 (21.2) | 131.3 ± 3.5 | >100 (17.7) |
| Car | >100 (16.4) | >100 (13.3) | >100 (8.8) | >100 (21.5) | 150.1 ± 2.3 | >100 (22.8) |
| Caryo | >100 (7.2) | >100 (39.7) | >100 (12.3) | >100 (16.3) | 54.8 ± 4.0 | >100 (10.0) |
Note. IC50 > value: In these cases, the IC50 was not determined because only an inhibition <50% was observed at highest concentration. In parentheses, % of inhibition at highest concentration tested. Results were expressed as mean ± SD or as percentage of three independent experiments. Asc = ascaridole; BH‐SMP = submitochondrial particles from bovine heart; Car = carvacrol; Caryo = caryophyllene oxide; EO = essential oil; LtP‐Mit = mitochondrial crude fraction of Leishmania tarentolae; SD = standard deviation.
In a next step, we extended our experiments to isolated ScY‐bc 1 and BH‐bc 1 (Table 2). Among major compounds of EO, Caryo exhibited the strongest inhibition, whereas Asc and Car were less effective (Table 2), suggesting that the immediate effects of EO major components on mitochondria in all studied species are mediated by Caryo and not by the major antileishmanially active compound Asc.
Table 2.
Immediate inhibitory activity of main compounds of EO from Chenopodium ambrosioides L. on ScY‐bc 1 and BH‐bc 1
| Compound | IC50 ± SD (μM) | |
|---|---|---|
| ScY‐bc 1 | BH‐bc 1 | |
| Asc | 675 ± 6 | 381 ± 9 |
| Car | 941 ± 41 | 421 ± 4 |
| Caryo | 179 ± 1 | 197 ± 1 |
Results were expressed as median inhibitory concentration (IC50) ± SD of five independent experiments. Asc = ascaridole; BH‐bc 1 = cytochrome bc 1 complex purified from Bos taurus; Car = carvacrol; Caryo = caryophyllene oxide; EO = essential oil; ScY‐bc 1 = cytochrome bc 1 complex purified from Saccharomyces cerevisiae; SD = standard deviation.
3.4. Influence on cellular superoxide radical production
In a next experiment, we studied superoxide radical formation in LtP by an ESR method using cyclic hydroxyl amine (CMH), which is converted upon one‐electron transfer reaction with superoxide radicals to a stable nitroxyl radical (CM●, Figure 3a). As can be seen (Figure 3b), LtP significantly triggered CMH oxidation in comparison with buffer. Also, the positive control with AA, a well‐known trigger of mitochondrial superoxide formation, showed an increase of CM● formation in LtP. Asc, Car, and Caryo only slightly increased CM● formation. At first view, it appears puzzling that Asc, which is known to trigger formation of carbon‐centered radicals (Geroldinger et al., 2017), does not trigger superoxide formation in this assay.
Figure 3.
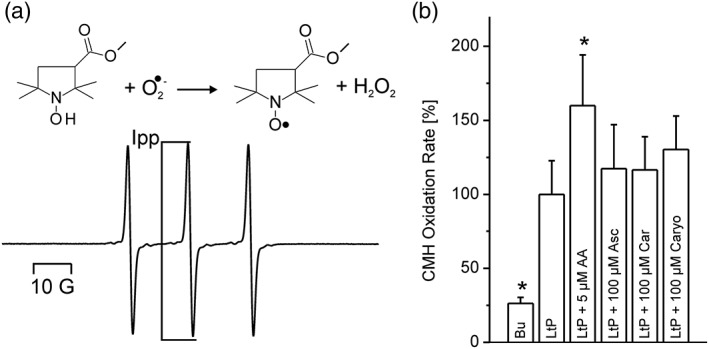
Detection of superoxide radicals in Leishmania tarentolae promastigotes (LtP) and the influence of major compounds of essential oil from Chenopodium ambrosioides L. Superoxide radicals in LtP converted the cyclic hydroxylamine CMH to a stable nitroxyl radical shown in (a). The intensity of the middle peak (Ipp) is proportional to the amount of superoxide formed. (b) The influence of major components of essential oil (100 μM) and antimycin A (AA, 5 μM) on the radical formation in LtP. Buffer (Bu) indicates samples without LtP. The reaction with 400 μM CMH was performed in suspensions containing 5 × 108 LtP/ml in phosphate‐buffered saline with 15 mM glucose, 100 μM DFO, and 25 μM DTPA. Data represent mean ± standard deviation of quadruplicate experiments. *Significant differences versus LtP on the level p < .1. Asc = Ascaridole; Car = carvacrol; Caryo = caryophyllene oxide
3.5. Activation of Asc and cellular superoxide radical production
The dose‐dependent increase of CMH oxidation triggered by Asc becomes increasingly effective upon prolonged incubation and in the absence of iron chelators (Figure 4) suggesting that superoxide radical formation by Asc does not occur immediately and is enhanced by the availability of iron allowing for Asc activation.
Figure 4.
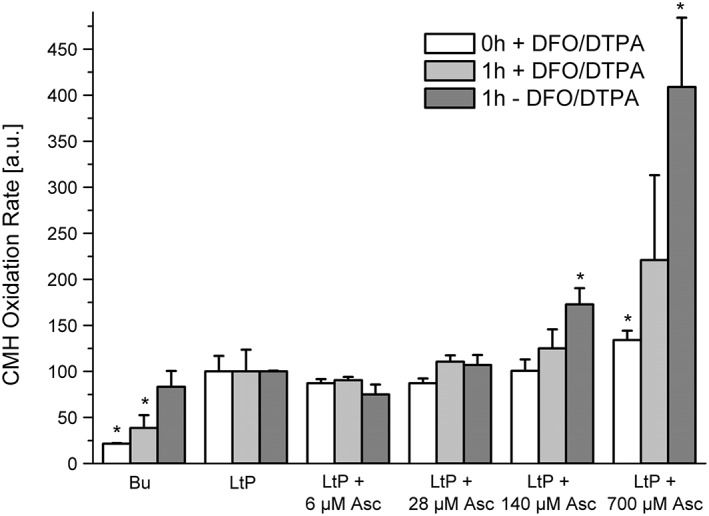
Formation of superoxide radicals in Leishmania tarentolae promastigotes (LtP) triggered by ascaridole (Asc) increased with Asc concentration, incubation time, and iron availability. Superoxide radical formation was assessed by reaction of 400 μM CMH in suspensions containing 5 × 108 LtP/ml in phosphate‐buffered saline with 15 mM glucose and 100 μM DFO and 25 μM DTPA (white bars). Immediate effects (0‐hr incubation) of Asc in LtP in the presence of chelators (DFO/DTPA). After 1‐hr incubation (light grey) and after 1‐hr incubation in the absence of iron chelators (dark grey). Data represent mean ± standard deviation of quadruplicate experiments. *Significant differences versus LtP on the level p < .05. Bu = buffer controls without LtP
3.6. Influence on mitochondrial coupling
Although Asc is not a direct inhibitor of ETC in LtP (Figure 2), a decrease in the membrane potential of Leishmania amazonensis promastigotes (LaP), as assessed by JC‐1, was observed after 72 hr of incubation (Monzote, Garcia, et al., 2014), and Asc was shown to produce radicals in LtP (Geroldinger et al., 2017). Accordingly, the long‐term effects of Asc on mitochondrial coupling in LtP were examined by measuring the respiration of LtP with a Clark‐type electrode. Upon addition of Oligo (inhibitor of ATP synthase), oxygen consumption stimulated by ATP production was blocked (Figure 5), and inclusion of the uncoupler CCCP yielded the maximally uncoupled respiration. The quotient of both rates, the RCR, is an indication of the ability of LtP to respond to increased ATP demands due to stress conditions. Therefore, a high RCR is indicative of a healthy cell, and a decreased RCR reflects an impaired stress response.
Figure 5.
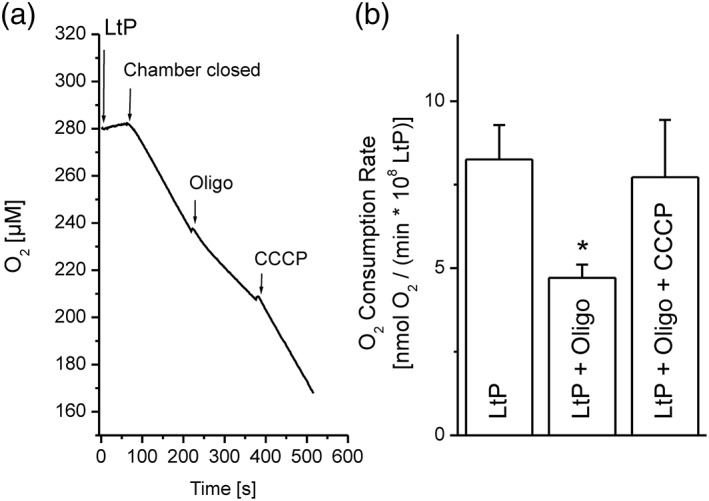
Mitochondrial coupling in Leishmania tarentolae promastigotes (LtP). (a) Typical experiment to assess mitochondrial coupling in LtP. LtP cells (110 ± 13 × 106/ml) in Schneider's medium supplemented with 6 μM hemin were equilibrated with air within the reaction chamber of a Clark‐type electrode. After closing the chamber, the basal cellular O2 consumption was measured. By addition of 5 μM oligomycin (Oligo), an inhibitor of ATP synthase, oxygen consumption coupled to ATP production was blocked. Finally, the uncoupler CCCP (0.5 μM) was added to obtain the capacity of mitochondrial electron transport in LtP at non‐coupled respiration. (b) O2 consumption rates obtained from the respective slopes in the left graph. Data represent mean ± standard deviation of four independent experiments. *Significant differences versus LtP on the level p < .05
In a first experimental series, we tested the immediate uncoupling effect of EO major components in 96‐well OxoPlates. Neither Car nor Asc (Figure 6a,b) showed an uncoupling effect under these conditions. Caryo at the highest concentration (200 μM) totally inhibited the oxygen consumption on top of the inhibition by Oligo, confirming the interference of Caryo in the leishmanial ETC (Figure 6c). In contrast, the positive control with the uncoupler CCCP showed a clear increase of Oligo‐inhibited respiration in LtP (Figure 6d), demonstrating the functionality of this assay. As expected, Asc showed only little effects on mitochondrial coupling in LtP immediately after addition (Figure 7). However, upon prolonged incubation with Asc, the RCR declined with respect to vehicle‐treated LtP suggesting an impaired cellular energy metabolism.
Figure 6.
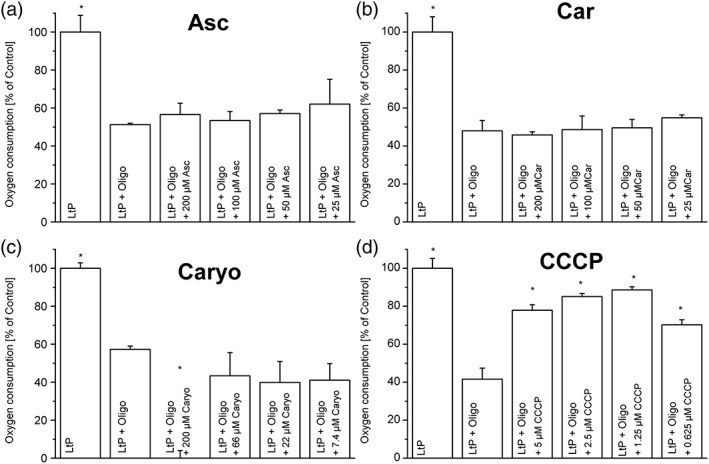
Mitochondrial uncoupling in Leishmania tarentolae promastigotes (LtP) in the presence of major components of essential oil from Chenopodium ambrosioides L. Uncoupling was assessed by stimulation of oxygen consumption in the presence of the ATP synthase inhibitor oligomycin (Oligo). LtP cells (1 × 108/ml) in brain heart infusion medium were supplemented with Oligo (5 μM) and decreasing concentrations of ascaridole (Asc), carvacrol (Car), caryophyllene oxide (Caryo), and the uncoupler CCCP. Oxygen consumption was assessed in 96‐well OxoPlates for 40 min at 26 °C. Oxygen consumption rates were normalized to the respiration of non‐inhibited Leishmania (LtP = 100%). Data represent mean ± standard deviation of 3–4 experiments. *Significant differences versus LtP + Oligo on the level p < .05
Figure 7.
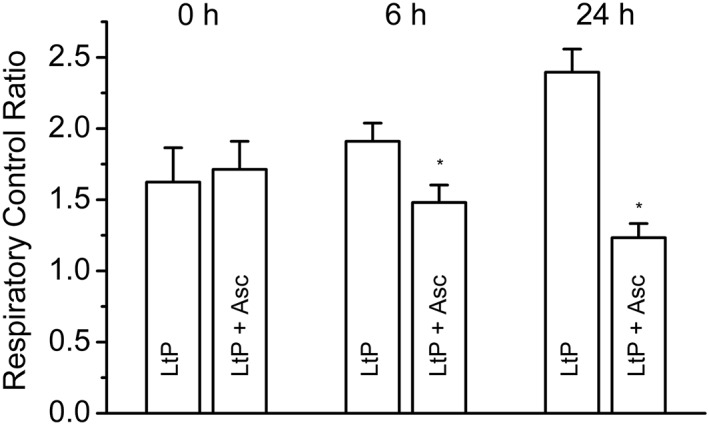
Effects of ascaridole (Asc) on mitochondrial coupling in Leishmania tarentolae promastigotes (LtP) upon prolonged incubation. Different cell batches with 1 × 108 LtP/ml in Schneider's medium plus 6 μM hemin were incubated in culture tubes either with DMSO (vehicle for Asc; LtP) or with 200 μM Asc (LtP + Asc). From these culture stocks, aliquots were taken for O2 consumption measurements at 0, 6, and 24 hr. Mean cell counts during measurements were adjusted with medium to approximately 1–2 × 108 LtP/ml. Respiratory control ratios were calculated as the ratios of O2 consumption rates in the presence of 5 μM oligomycin plus 0.5 μM CCCP to oligomycin‐inhibited O2 consumption rates as shown in Figure 5. Data represent mean ± standard deviation of four independent experiments. *Significant differences versus LtP on the level p < .05
3.7. Influence on low molecular thiols
As an indicator of oxidative stress, the status of low molecular thiols was assessed by the CMFDA method (Sarkar et al., 2009) wherein CMFDA is intracellularly deacetylated to CMF, which is then converted (by glutathione S‐transferase activity) to a low molecular fluorescent thiol‐MF adduct. The rate of this adduct formation is expected to be proportional to the intracellular low molecular thiol level. Control experiments without LtP yielded no significant rates, whereas untreated control LtP showed strong fluorescence evolution. Incubation with EO components showed a decrease for all products, especially for Caryo, which might be a link to its mitochondrial effects (Figure 8).
Figure 8.
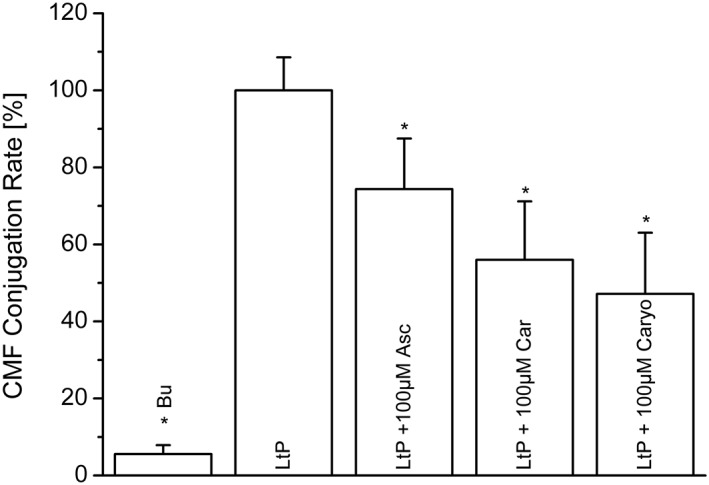
Influence of major components of essential oil from Chenopodium ambrosioides L. on the low molecular thiol status of Leishmania tarentolae promastigotes (LtP) after 5 hr incubation at 26 °C in phosphate‐buffered saline/glucose medium. Low molecular thiol status was assessed by measuring the rate of fluorescence evolution over 1 hr from the conjugation of CMF (arising from 5 μM CMFDA) to low molecular thiols in LtP (1 × 107 cells/ml) in phosphate‐buffered saline/glucose. Results represent mean ± standard deviation of three experiments. *Significant differences versus LtP on the level p < .05. Asc = Ascaridole; Bu = buffer; Car = carvacrol; Caryo = caryophyllene oxide
4. DISCUSSION
EO prepared from Cuban C. ambrosioides L. plants was analyzed by gas chromatography/mass spectrometry showing that Asc, Car, and Caryo are the main components of the EO (Monzote et al., 2006). Independent studies on EO from Chinese C. ambrosioides L. also listed Asc, Car, and Caryo as detected components, though in different amounts (Chu et al., 2011). It was shown that the EO composition from C. ambrosioides L. can vary widely (Jesus et al., 2017; Soares et al., 2017). Our mechanistic studies of Asc, Car, and Caryo address only a part of pharmacological activities of our EO and of other EOs from C. ambrosioides L. Previous studies demonstrated that EO from C. ambrosioides L. was effective against L. amazonensis infections that cause cutaneous leishmaniasis in mice (Monzote, Pastor, Scull, & Gille, 2014). In a combinatorial study, it was shown that especially combinations of Asc:Car = 1:4 were most effective against lesion development in this cutaneous leishmaniasis model. In vitro studies using the L. tarentolae model showed that Asc is activated in Leishmania to a carbon‐centered radical species preferably by low molecular iron (Fe2+) from the labile iron pool in Leishmania (Geroldinger et al., 2017). This was corroborated by the finding that IC50 values for EO and Asc are strongly increased for L. amazonensis in the presence of iron chelators (Monzote, Garcia, et al., 2014). The selectivity of Asc/EO for Leishmania is based on their much higher labile iron pool compared with the labile iron pool of macrophage/monocyte host cells (Geroldinger et al., 2017). These data indicate that Asc is a nonmitochondrial radical generator in Leishmania. Bioenergetic studies suggested the mitochondrial ETC to be one of several direct or indirect molecular targets of main compounds of EO from C. ambrosioides L. in Leishmania parasites (Monzote, Garcia, et al., 2014). In mammalian mitochondria, except for Caryo, no direct effect of EO on mitochondrial ETC with respect to oxygen consumption was observed (Monzote et al., 2009).
In this study, the influence of EO main compounds on complexes of the mitochondrial ETC as possible targets for antileishmanial drugs was explored. Biological model systems have greatly facilitated the understanding of drug actions. In our study, besides whole LtP cells, mitochondrial fractions of LtP, ScY, and BH were used to study mitochondrial functions in LtP in comparison with other eukaryotic organisms, including mammals. L. tarentolae is a parasite of geckos of the species Tarentola annularis, belonging to the genus Sauroleishmania (Lainson & Shaw, 1987), and is not pathogenic for humans (Raymond et al., 2012). L. tarentolae has been widely used in pharmacological studies for (a) the screening of natural and synthetic products (Taylor et al., 2010), (b) the purification and characterization of proteins that are used for the screening of drugs with potential antileishmanial activity (Fritsche et al., 2007; Yakovich, Ragone, Alfonzo, Sackett, & Werbovetz, 2006), and (c) the amplification of genes involved in the resistance to certain antileishmanial drugs such as amphotericin B (Singh, Papadopoulou, & Ouellette, 2001) and sodium stibogluconate (Haimeur & Ouellette, 1998).
The in vivo efficiency of EO, Asc, and combinations of Asc/Car against cutaneous leishmaniasis is only partially reflected by in vitro viability assays. Although in LaP, IC50 values clearly demonstrate a high benefit of Asc; in LtP, IC50 values for Asc, Car, and Caryo are in a similar range. In a previous study, the major components of EO gave following IC50 values: Asc, 0.6 ± 0.06 μM; Car, 101 ± 30 μM; and Caryo, 22.2 ± 10.4 μM against LaP (Monzote, Garcia, et al., 2014), which are particularly different for Asc and Car. Although qualitatively the iron‐dependent activation of Asc/EO was confirmed in LaP (Monzote, Garcia, et al., 2014) and LtP (Geroldinger et al., 2017), the quantitative outcome of viability assays may strongly depend on the cell number to drug ratio, detection method and even on assay medium and premature activation of Asc in media. Our current studies explore this systematically. From the listed IC50 values for LtP in this work, it can be concluded that it is at least likely that all three major components (and also possibly nonstudied trace compounds) are involved in the antileishmanial action of EO.
Direct effects of drugs on mitochondria include inhibition of individual ETC complexes, transporters, and disturbance of the highly sensitive coupling of proton translocation with ATP production. Indirect effects, which do not occur immediately after drug exposure but after several hours, may be caused by drug metabolites (rather rare), the intrinsic pathway of apoptosis triggered by nonmitochondrial targets, lipid peroxidation of mitochondrial membranes, and other processes.
A hallmark of drug actions on mitochondria is inhibition of the mitochondrial ETC (Chan, Truong, Shangari, & O'Brien, 2005). Therefore, oxygen uptake by LtP and its inhibition by EO major compounds were studied (Figure 2). Both EO and Caryo produced a significant inhibition of LtP oxygen consumption. This suggests that Caryo in EO directly acts on mitochondria of Leishmania. In contrast, Car and Asc did not show immediate effects on LtP oxygen consumption. Although the assay time for measuring oxygen consumption was about 0.5 hr after drug exposure, viability measurements were performed after 48/72 hr of incubation indicating that our oxygen consumption assays address immediate inhibition effects.
To determine if Asc, Car, and Caryo also induced inhibition of individual ETC complexes in Leishmania, we compared compound actions in a crude LtP‐Mit with BH‐SMP (Table 1). The results observed herein demonstrated that no relevant activity of EO compounds on complexes I and II was found in Leishmania, whereas complex III was preferably inhibited by Caryo in both, LtP‐Mit and BH‐SMP, with slight preference for LtP‐Mit.
This observation raised the question whether the inhibition by Caryo is a universal effect on complex III of other species. Therefore, purified cytochrome bc 1 complex from yeast was used, compared with bc 1 complex from bovine heart, and the inhibition by compounds Asc, Car, and Caryo was assayed. Again, the strongest inhibition was caused by Caryo, whereas Asc and Car did not show a strong inhibitory effect. This confirmed the results from the mitochondrial fractions, that is, that Caryo directly targets complex III in different eukaryotic mitochondria. Because IC50 values of Caryo in both bc 1 complexes were similar, it is likely that host cells and Leishmania are susceptible to Caryo. This is a possible mechanism how EO could influence the viability of host cell macrophages.
Due to the inherent relationship between generation of reactive oxygen species and respiratory chain inhibition, complex III was described as the main source of superoxide radicals in both mammals and Leishmania species (Carvalho et al., 2010; Dawson, Gores, Nieminen, Herman, & Lemasters, 1993; Garcia‐Ruiz, Colell, Morales, Kaplowitz, & Fernandez‐Checa, 1995). Mitochondrial inhibition is sometimes (depending on the site of inhibition) accompanied by increased mitochondrial superoxide production. In addition, impairment of the mitochondrial ETC by lipid peroxidation and protein oxidation may trigger mitochondria to produce more superoxide radicals. Also NADPH oxidases, P450 oxidases, or xanthine oxidases as well as low molecular weight iron and ascorbate (which are both present in Leishmania) are known nonmitochondrial superoxide radical sources. We studied superoxide production in LtP by the CMH/ESR method, which is highly specific for superoxide except the interference with Fe3+ (Dikalov, Skatchkov, & Bassenge, 1997). Therefore, these assays are usually performed in the presence of iron chelators to prevent this side reaction. This, however, has the limitation that under these assay conditions, we can only assess the effects of nonactivated Asc because Asc needs iron for its pharmacological action. In these experiments (Figure 3), both negative buffer control (lacking LtP) and positive control (in the presence of the complex III inhibitor AA) showed that the detection system is working. All three EO compounds slightly increased the superoxide radical formation in LtP in the assays time frame of about 15 min.
In the genus Leishmania, different low molecular weight thiol antioxidants are present: glutathione, trypanothione, cysteine, and ovothiol (Romao et al., 2006). In addition, Leishmania can synthesize ascorbate as a powerful antioxidant (Manhas, Anand, Tripathi, & Madhubala, 2014). Therefore, we assessed the redox state of thiols in the presence of Asc, Car, and Caryo by a CMFDA assay (Sarkar et al., 2009) resulting in a glutathione S‐transferase (Fyfe, Westrop, Silva, Coombs, & Hunter, 2012) catalyzed conjugation of MF to low molecular weight thiols. As shown, these findings are in line with superoxide radical measurements. Low molecular thiols are decreased by EO compounds upon 5 hr incubation (Figure 8) but possibly by different mechanisms.
In our study, Car shows neither mitochondrial inhibition nor mitochondrial uncoupling. Car is a phenol like the well‐known uncoupler 2,4‐dinitrophenol. However, the pKa value of 2,4‐dinitrophenol is around 4, whereas for normal (non‐nitro‐substituted) phenols like Car, the pKa value is around 10 (Rappoport & Frankel, 1967). This makes an action of Car as protonophore not very likely under physiological conditions. Others have shown that Car, upon prolonged incubation with superoxide radicals, forms rather stable ESR signals, which cannot be assigned to simple phenoxyl radicals (Deighton, Glidewell, Deans, & Goodman, 1993). The complex ESR signals suggest the presence of large conjugated systems, which could arise from oligomerized Car oxidation products. These trace products could have potential redox‐cycling properties causing additional harm to Leishmania. In our experiments, we used nonoxidized Car and therefore did not study these effects.
To address the situation including Asc activation, we performed experiments for Asc with prolonged incubation times and in the presence and the absence of iron chelators (Figure 4). The results clearly show that activation of Asc takes time and is strongly enhanced in the absence of iron chelators. In addition, it was demonstrated that Asc has no immediate direct effect on mitochondria but increases superoxide radical formation after activation.
This prompted us to study Asc effects on mitochondrial coupling (Figures 5, 6, 7). A major mitochondrial function in LtP is the generation of ATP, which can be impaired by inhibition of the ETC or by uncoupling of ETC from ATP synthase function. The latter is often triggered by breaking down the proton gradient across the inner mitochondrial membrane (driving ATP synthesis) by proton‐shuttling drugs or increased proton permeability of the inner mitochondrial membrane. Increased proton permeability can be mediated by radical‐triggered membrane lipid peroxidation. Coupling reflects the ability of mitochondria to adapt ATP production to ATP demands. Stress conditions such as treatment with antileishmanial drugs may directly or indirectly increase the ATP demand, usually triggering mitochondria to produce more ATP. Conversely, a decreased mitochondrial coupling impairs this stress response mechanism.
As shown, Asc has no immediate direct uncoupling effect, but after prolonged incubation (probably via Asc activation), mitochondrial coupling is impaired in LtP.
EO from C. ambrosioides L. is a complex mixture with a variety of possible pharmacological mechanisms. In addition, there are numerous possibilities for pharmacological interactions as demonstrated for Asc and Car in a previous publication (Monzote, Pastor, et al., 2014). The three main compounds are responsible for some but certainly not for all effects (Figure 9). Among these compounds, only Caryo has direct inhibitory effects on complex III in Leishmania and other eukaryotic cells. Car has no inhibiting effects on ETC but similarly impairs LtP viability. Asc has the most complex mechanism. It has no direct inhibiting effect on mitochondrial ETC and no immediate uncoupling effect in LtP. However, upon activation by iron, Asc impairs mitochondrial coupling and triggers superoxide radical formation in LtP. This suggests that impairment of mitochondrial coupling in Leishmania by prolonged incubation with Asc (Figure 7) is not the primary mode of action but a downstream event of rather selective activation of Asc in Leishmania (Geroldinger et al., 2017). Further studies are required to elucidate possible synergistic effects of Car and Asc.
Figure 9.
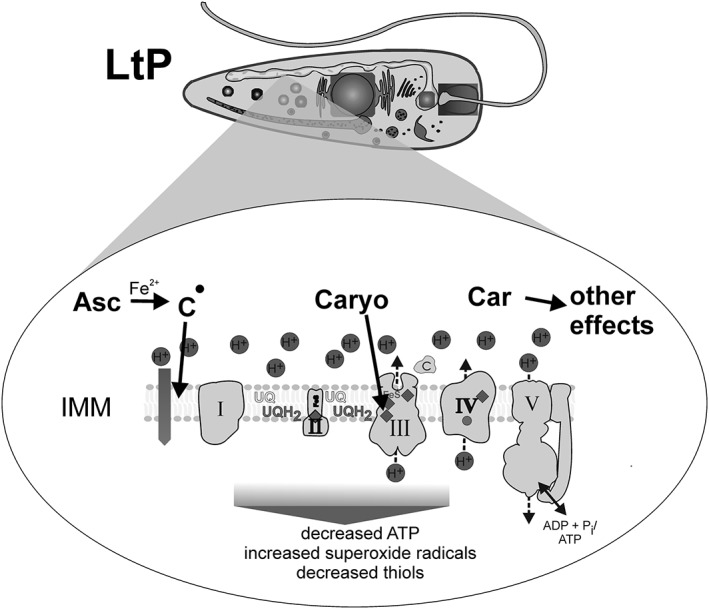
Scheme of the mitochondrial effects of major components of essential oil from Chenopodium ambrosioides L. on Leishmania tarentolae promastigotes (LtP). Asc = Ascaridole; Car = carvacrol; Caryo = caryophyllene oxide; IMM, inner mitochondrial membrane
These findings suggest that Asc, Car, and Caryo mediate their leishmanicidal activity via different targets in mitochondria and in other parts of the cell and that different mitochondrial effects are seen after different times of exposure.
CONFLICT OF INTEREST
All authors have no conflict of interest to declare.
ACKNOWLEDGEMENTS
The award of an Ernst Mach scholarship to Lianet Monzote by the Austrian Exchange Service (OEAD) and support of this work in the Scientific & Technological Cooperation with India Project No. IN 04/2017 by the OEAD is gratefully acknowledged. Special thanks to the Austrian Science Fund (FWF) for supporting this study under Grant P 27814‐B22.
Monzote L, Geroldinger G, Tonner M, et al. Interaction of ascaridole, carvacrol, and caryophyllene oxide from essential oil of Chenopodium ambrosioides L. with mitochondria in Leishmania and other eukaryotes. Phytotherapy Research. 2018;32:1729–1740. 10.1002/ptr.6097
REFERENCES
- Carvalho, L. , Luque‐Ortega, J. R. , Manzano, J. I. , Castanys, S. , Rivas, L. , & Gamarro, F. (2010). Tafenoquine, an antiplasmodial 8‐aminoquinoline, targets Leishmania respiratory complex III and induces apoptosis. Antimicrobial Agents and Chemotherapy, 54, 5344–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli, J. F. , Tomi, F. , Bernardini, A. F. , & Casanova, J. (2004). Combined analysis of the essential oil of Chenopodium ambrosioides by GC, GC‐MS and 13C‐NMR spectroscopy: Quantitative determination of ascaridole, a heat‐sensitive compound. Phytochemical Analysis, 15, 275–279. [DOI] [PubMed] [Google Scholar]
- Chan, K. , Truong, D. , Shangari, N. , & O'Brien, P. J. (2005). Drug‐induced mitochondrial toxicity. Expert Opinion on Drug Metabolism & Toxicology, 1, 655–669. [DOI] [PubMed] [Google Scholar]
- Chittiboyina, A. G. , Avonto, C. , & Khan, I. A. (2016). What happens after activation of ascaridole? Reactive compounds and their implications for skin sensitization. Chemical Research in Toxicology, 29, 1488–1492. [DOI] [PubMed] [Google Scholar]
- Chu, S. S. , Hu, J. F. , & Liu, Z. L. (2011). Composition of essential oil of Chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais . Pest Management Science, 67, 714–718. [DOI] [PubMed] [Google Scholar]
- Daum, G. , Bohni, P. C. , & Schatz, G. (1982). Import of proteins into mitochondria. Cytochrome b 2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. Journal of Biological Chemistry, 257, 13028–13033. [PubMed] [Google Scholar]
- Dawson, T. L. , Gores, G. J. , Nieminen, A. L. , Herman, B. , & Lemasters, J. J. (1993). Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. American Journal of Physiology, 264, C961–C967. [DOI] [PubMed] [Google Scholar]
- Deighton, N. , Glidewell, S. M. , Deans, S. G. , & Goodman, B. A. (1993). Identification by EPR spectroscopy of carvacrol and thymol as the major sources of free‐radicals in the oxidation of plant essential oils. Journal of the Science of Food and Agriculture, 63, 221–225. [Google Scholar]
- Dikalov, S. , Skatchkov, M. , & Bassenge, E. (1997). Spin trapping of superoxide radicals and peroxynitrite by 1‐hydroxy‐3‐carboxy‐pyrrolidine and 1‐hydroxy‐2,2,6, 6‐tetramethyl‐4‐oxo‐piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochemical and Biophysical Research Communications, 231, 701–704. [DOI] [PubMed] [Google Scholar]
- Duke, J. , Bogenshutz, M. , du‐Cellier, J. , & Duke, A. (2002). Handbook of medicinal herbs (2 edn). Boca Raton: CRC Press, FL. [Google Scholar]
- Franca, F. , Lago, E. L. , & Marsden, P. D. (1996). Plants used in the treatment of leishmanial ulcers due to Leishmania (Viannia) braziliensis in an endemic area of Bahia, Brazil. Revista da Sociedade Brasileira de Medicina Tropical, 29, 229–232. [DOI] [PubMed] [Google Scholar]
- Fritsche, C. , Sitz, M. , Weiland, N. , Breitling, R. , & Pohl, H. D. (2007). Characterization of the growth behavior of Leishmania tarentolae: A new expression system for recombinant proteins. Journal of Basic Microbiology, 47, 384–393. [DOI] [PubMed] [Google Scholar]
- Fyfe, P. K. , Westrop, G. D. , Silva, A. M. , Coombs, G. H. , & Hunter, W. N. (2012). Leishmania TDR1 structure, a unique trimeric glutathione transferase capable of deglutathionylation and antimonial prodrug activation. Proceedings of the National Academy of Science of USA, 109, 11693–11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Ruiz, C. , Colell, A. , Morales, A. , Kaplowitz, N. , & Fernandez‐Checa, J. C. (1995). Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor‐kappa B: Studies with isolated mitochondria and rat hepatocytes. Molecular Pharmacology, 48, 825–834. [PubMed] [Google Scholar]
- Geier, B. M. , Schägger, H. , Brandt, U. , Colson, A. M. , & von Jagow, G. (1992). Point mutation in cytochrome b of yeast ubihydroquinone:cytochrome‐c oxidoreductase causing myxothiazol resistance and facilitated dissociation of the iron–sulfur subunit. European Journal of Biochemistry, 208, 375–380. [DOI] [PubMed] [Google Scholar]
- Geroldinger, G. , Tonner, M. , Hettegger, H. , Bacher, M. , Monzote, L. , Walter, M. , … Gille, L. (2017). Mechanism of ascaridole activation in Leishmania . Biochemical Pharmacology, 132, 48–62. [DOI] [PubMed] [Google Scholar]
- Guerra, M. , Torres, D. , & Martínez, L. (2001). Validación del uso tradicional de plantas medicinales cultivadas en Cuba. Revista Cubana de Medicina Tropical, 6, 48–51. [Google Scholar]
- Haimeur, A. , & Ouellette, M. (1998). Gene amplification in Leishmania tarentolae selected for resistance to sodium stibogluconate. Antimicrobial Agents and Chemotherapy, 42, 1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardim, C. M. , Jham, G. N. , Dhingra, O. D. , & Freire, M. M. (2008). Composition and antifungal activity of the essential oil of the Brazilian Chenopodium ambrosioides L. Journal of Chemical Ecology, 34, 1213–1218. [DOI] [PubMed] [Google Scholar]
- Jesus, R. S. , Piana, M. , Freitas, R. B. , Brum, T. F. , Alves, C. F. S. , Belke, B. V. , … Bauermann, L. F. (2017). In vitro antimicrobial and antimycobacterial activity and HPLC‐DAD screening of phenolics from Chenopodium ambrosioides L. Brazilian Journal of Microbiology, in press, 10.1016/j.bjm.2017.02.012, 49, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi, F. , Itano, Y. , Uchiyama, N. , Honda, G. , Tsubouchi, A. , Nakajima‐Shimada, J. , & Aoki, T. (2002). Monoterpene hydroperoxides with trypanocidal activity from Chenopodium ambrosioides . Journal of Natural Products, 65, 509–512. [DOI] [PubMed] [Google Scholar]
- Krutz, N. L. , Hennen, J. , Korb, C. , Schellenberger, M. T. , Gerberick, G. F. , & Blomeke, B. (2015). Activation of the endoperoxide ascaridole modulates its sensitizing capacity. Toxicological Sciences, 147, 515–523. [DOI] [PubMed] [Google Scholar]
- Lainson, R. , & Shaw, J. J. (1987). Evolution, classification and geographical distribution of Leishmania In Peters W., & Killick‐Kendrick R. (Eds.), The leishmaniasis in biology and medicine (pp. 1–120). London: Academic Press. [Google Scholar]
- Liu, W. , Liu, Y. , Zhang, X. Z. , Li, N. , & Cheng, H. (2013). In vitro bactericidal activity of Jinghua Weikang Capsule and its individual herb Chenopodium ambrosioides L. against antibiotic‐resistant Helicobacter pylori . Chinese Journal of Integrative Medicine, 19, 54–57. [DOI] [PubMed] [Google Scholar]
- Lowry, O. H. , Rosebrough, A. L. , Farr, A. L. , & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275. [PubMed] [Google Scholar]
- Manhas, R. , Anand, S. , Tripathi, P. , & Madhubala, R. (2014). Deletion of vitamin C biosynthesis enzyme, arabino‐1, 4‐lactone oxidase in Leishmania donovani results in increased pro‐inflammatory responses from host immune cells. Molecular Microbiology, 91, 1227–1239. [DOI] [PubMed] [Google Scholar]
- Monzote, L. , Garcia, M. , Pastor, J. , Gil, L. , Scull, R. , Maes, L. , … Gille, L. (2014). Essential oil from Chenopodium ambrosioides and main components: Activity against Leishmania, their mitochondria and other microorganisms. Experimental Parasitology, 136, 20–26. [DOI] [PubMed] [Google Scholar]
- Monzote, L. , Lackova, A. , Staniek, K. , Steinbauer, S. , Pichler, G. , Jager, W. , & Gille, L. (2016). The antileishmanial activity of xanthohumol is mediated by mitochondrial inhibition. Parasitology, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzote, L. , Montalvo, A. M. , Almanonni, S. , Scull, R. , Miranda, M. , & Abreu, J. (2006). Activity of the essential oil from Chenopodium ambrosioides grown in Cuba against Leishmania amazonensis . Chemotherapy, 52, 130–136. [DOI] [PubMed] [Google Scholar]
- Monzote, L. , Pastor, J. , Scull, R. , & Gille, L. (2014). Antileishmanial activity of essential oil from Chenopodium ambrosioides and its main components against experimental cutaneous leishmaniasis in BALB/c mice. Phytomedicine, 21, 1048–1052. [DOI] [PubMed] [Google Scholar]
- Monzote, L. , Sariego, R. I. , Montalvo Álvarez, A. M. , Garrido, L. N. , Scull, L. R. , & Abreu, P. J. (2004). Propiedades antiprotozoarias de aceites esenciales extraídos de plantas cubanas. Revista Cubana de Medicina Tropical, 56, 230–233. [Google Scholar]
- Monzote, L. , Stamberg, W. , Staniek, K. , & Gille, L. (2009). Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium ambrosioides on mitochondria. Toxicology and Applied Pharmacology, 240, 337–347. [DOI] [PubMed] [Google Scholar]
- Müllebner, A. , Patel, A. , Stamberg, W. , Staniek, K. , Rosenau, T. , Netscher, T. , & Gille, L. (2010). Modulation of the mitochondrial cytochrome bc 1 complex activity by chromanols and related compounds. Chemical Research in Toxicology, 23, 193–202. [DOI] [PubMed] [Google Scholar]
- Nascimento, F. R. , Cruz, G. V. , Pereira, P. V. , Maciel, M. C. , Silva, L. A. , Azevedo, A. P. , … Guerra, R. N. (2006). Ascitic and solid Ehrlich tumor inhibition by Chenopodium ambrosioides L. treatment. Life Sciences, 78, 2650–2653. [DOI] [PubMed] [Google Scholar]
- Nohl, H. , & Hegner, D. (1978). Do mitochondria produce oxygen radicals in vivo? European Journal of Biochemistry, 82, 563–567. [DOI] [PubMed] [Google Scholar]
- Pandey, A. K. , Singh, P. , Palni, U. T. , & Tripathi, N. N. (2013). Application of Chenopodium ambrosioides Linn. essential oil as botanical fungicide for the management of fungal deterioration in pulse. Biological Agriculture and Horticulture, 29, 197–208. [Google Scholar]
- Quinlan, M. B. , Quinlan, R. J. , & Nolan, J. M. (2002). Ethnophysiology and herbal treatments of intestinal worms in Dominica, West Indies. Journal of Ethnopharmacology, 80, 75–83. [DOI] [PubMed] [Google Scholar]
- Rappoport, Z. , & Frankel, M. (1967). CRC handbook of tables for organic compound identification (3rd ed.). [Google Scholar]
- Raymond, F. , Boisvert, S. , Roy, G. , Ritt, J. F. , Legare, D. , Isnard, A. , … Corbeil, J. (2012). Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species. Nucleic Acids Research, 40, 1131–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romao, P. R. , Tovar, J. , Fonseca, S. G. , Moraes, R. H. , Cruz, A. K. , Hothersall, J. S. , … Cunha, F. Q. (2006). Glutathione and the redox control system trypanothione/trypanothione reductase are involved in the protection of Leishmania spp. against nitrosothiol‐induced cytotoxicity. Brazilian Journal of Medical and Biological Research, 39, 355–363. [DOI] [PubMed] [Google Scholar]
- Sarkar, A. , Mandal, G. , Singh, N. , Sundar, S. , & Chatterjee, M. (2009). Flow cytometric determination of intracellular non‐protein thiols in Leishmania promastigotes using 5‐chloromethyl fluorescein diacetate. Experimental Parasitology, 122, 299–305. [DOI] [PubMed] [Google Scholar]
- Shah, H. , & Khan, A. A. (2017). Phytochemical characterisation of an important medicinal plant, Chenopodium ambrosioides Linn. Natural Product Research, 31, 2321–2324. [DOI] [PubMed] [Google Scholar]
- Singh, A. K. , Papadopoulou, B. , & Ouellette, M. (2001). Gene amplification in amphotericin B‐resistant Leishmania tarentolae . Experimental Parasitology, 99, 141–147. [DOI] [PubMed] [Google Scholar]
- Soares, M. H. , Dias, H. J. , Vieira, T. M. , de Souza, M. G. M. , Cruz, A. F. F. , Badoco, F. R. , … Crotti, A. E. M. (2017). Chemical composition, antibacterial, schistosomicidal, and cytotoxic activities of the essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants (Chenopodiaceae). Chemistry & Biodiversity, 14. [DOI] [PubMed] [Google Scholar]
- Taylor, V. M. , Munoz, D. L. , Cedeno, D. L. , Velez, I. D. , Jones, M. A. , & Robledo, S. M. (2010). Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Experimental Parasitology, 126, 471–475. [DOI] [PubMed] [Google Scholar]
- Trivellato‐Grassi, L. , Malheiros, A. , Meyre‐Silva, C. , Buss, Z. S. , Monguilhott, E. D. , Frode, T. S. , … de Souza, M. M. (2013). From popular use to pharmacological validation: A study of the anti‐inflammatory, anti‐nociceptive and healing effects of Chenopodium ambrosioides extract. Journal of Ethnopharmacology, 145, 127–138. [DOI] [PubMed] [Google Scholar]
- Yakovich, A. J. , Ragone, F. L. , Alfonzo, J. D. , Sackett, D. L. , & Werbovetz, K. A. (2006). Leishmania tarentolae: Purification and characterization of tubulin and its suitability for antileishmanial drug screening. Experimental Parasitology, 114, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]


