Abstract
Introduction:
Electronic cigarettes (e-cigarettes) are promoted as a less risky alternative to conventional cigarettes and have grown in popularity. Experimental and clinical evidence suggests that they could increase the risk of myocardial infarction.
Methods:
The National Health Interview Surveys of 2014 (n=36,697) and 2016 (n=33,028) were used to examine the cross-sectional association between e-cigarette use (never, former, some days, daily) and cigarette smoking (same categories) and myocardial infarction in a single logistic regression model that also included demographics (age, gender, BMI) and health characteristics (hypertension, diabetes, and hypercholesterolemia) using logistic regression. Data were collected in 2014 and 2016 and analyzed in 2017 and 2018.
Results:
Daily e-cigarette use was independently associated with increased odds of having had a myocardial infarction (OR=1.79, 95% CI=1.20, 2.66, p=0.004) as was daily conventional cigarette smoking (OR=2.72, 95% CI=2.29, 3.24, p<0.001). Former and some day e-cigarette use were not significantly associated with having had a myocardial infarction (p=0.608 and p=0.392) whereas former (OR=1.70, p<0.001) and some day cigarette smoking (OR=2.36, p<0.001) were. Odds of a myocardial infarction were also increased with history of hypertension (OR=2.32, p<0.001); high cholesterol (OR=2.36, p<0.001); and diabetes (OR=1.77, p<0.001); and age (OR=1.65 per 10 years, p<0.001). Women (OR=0.47, p<0.001) had lower odds of myocardial infarction.
Conclusions:
Daily e-cigarette use, adjusted for smoking conventional cigarettes as well as other risk factors, is associated with increased risk of myocardial infarction.
INTRODUCTION
Electronic cigarettes (e-cigarettes), which deliver an aerosol of (usually) nicotine and other flavors by heating a liquid, are often promoted as a safer alternative to conventional cigarettes, which generate the nicotine aerosol by burning tobacco.1,2 Both e-cigarettes and conventional cigarettes deliver ultrafine particles that are one to two orders of magnitude smaller than a human hair,1–5 which in smoke and air pollution increase risk of cardiovascular disease and acute myocardial infarction (MI) with a nonlinear dose response curve.6,7 MI risk drops when people stop smoking conventional cigarettes or stop being exposed to secondhand smoke.8,9 E-cigarette and traditional cigarette smoking in healthy smokers with no known cardiovascular disease exhibit similar inhibition of endothelial function as measured by flow-mediated dilation of arteries,10 shift in cardiac autonomic balance toward sympathetic predominance,10,11 and increased oxi dative stress,10,11 which are associated with increased cardiac risk.12,13 There is also increased oxidative stress in both e-cigarette users and conventional cigarette smokers.10 Laboratory studies done with e-cigarette extracts found that e-cigarette use increases the release of inflammatory mediators from keratinocyte, alveolar epithelial cell lines, and neutrophils.14 E-cigarette aerosol also induces platelet activation, aggregation, and adhesion.15 In mice, chronic whole body exposure to e-cigarette aerosol accelerates aortic stiffness, significantly impairs aortic endothelial function, and may lead to impaired cardiac function.16 These observations led the authors to hypothesize that e-cigarette use would be associated with increased risk of acute MI.
METHODS
Study Population
The National Health Interview Survey (NHIS), a survey of people aged ≥18 years, is conducted by the U.S. Census Bureau using in person interviews in a random sampling of U.S. households.17 Data from the 2014 and 2016 NHIS were used.
Measures
Subjects who answered yes to the question Have you EVER been told by a doctor or other health professional that you had a heart attack (also called myocardial infarction)? were classified as having had an MI.
The full model includes current and former e-cigarette use and cigarette use as separate variables in the same model together with demographic (sex, age, BMI, race/ethnicity) and clinical covariates for MI (hypertension, diabetes mellitus, high cholesterol). Dual users were indicated by the concurrent values of the e-cigarette and cigarette variables rather than as a separate category.
Subjects who answered no to Have you ever used an e-cigarette, even one time? were classified as never users. Subjects who answered yes were then asked, Do you now use e-cigarettes every day, some days, or not at all? Subjects who responded not at all were classified as former users and those who selected some days and every day were classified as some day users and daily users, respectively.
Subjects were classified as never smokers if they answered no to the question Have you smoked at least 100 cigarettes in your ENTIRE LIFE? Subjects were classified as former smokers if they had smoked >100 cigarettes but answered not at all to Do you NOW smoke cigarettes every day, some days or not at all? The remaining subjects were classified as some day smokers and daily smokers.
Demographic characteristics in the analysis were sex and age at time of survey. These data were obtained by asking the subjects, Are you Male or Female? And, How old are you? BMI was obtained and calculated based on each subject’s height (How tall are you without shoes?) and weight (How much do you weigh without shoes?). Race/ethnicity was classified as Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic Asian, or other.
This study assessed the diagnosis of hypertension, high cholesterol, and diabetes mellitus from those who answered yes to the questions: Have you EVER been told by a doctor or other health professional that you had… (1) hypertension, also called high blood pressure, high cholesterol, or (2) diabetes or sugar diabetes? respectively. For diabetes, people who responded no or borderline or prediabetes were coded as no. People who refused, were not asked, or did not know were coded as missing.
Statistical Analysis
Descriptive statistics (means and SDs for continuous variables and frequency tables for categorical variables) were computed and one-way ANOVA and chi-square were used to test for differences between never, former, and current e-cigarette users.
Logistic regression was used to estimate the odds of having had an MI as a function of e-cigarette use, cigarette smoking, and the other covariates listed above in a single logistic regression. This approach concurrently estimates both the effects of e-cigarette and cigarette use at the same time while controlling for the other product use. The reference condition for both e-cigarette use and cigarette smoking is people who never used e-cigarettes or cigarettes.
There was no evidence of multicollinearity in the fully adjusted models (all variance inflation factors ≤1.45). Interaction between cigarette and e-cigarette use was tested using a variable that was set to 1 for respondents who currently used both cigarettes and e-cigarettes (0 otherwise); this interaction term was not significant (p=0.214), so the final logistic regression model does not include an interaction. The lack of a significant interaction suggests that the effects of e-cigarettes and conventional cigarettes on having had an MI are independent of each other.
Subjects with missing data (0.58%) were not included in the final analysis, leaving an analytic sample size of 69,046 for the multivariable analysis.
Analyses were performed using Stata, version 14.2, accounting for the complex survey design of NHIS and following NHIS procedures for combining the 2014 and 2016 data sets.18 Data were collected in 2014 and 2016 and analyzed in 2017 and 2018.
RESULTS
Demographic and health characteristics for subjects who used e-cigarettes are shown in Table 1. (Appendix Tables 1 and 2 [available online] contain the results for 2014 and 2016 separately.) The analysis of combined data showed that 25.8% of current (some days or daily) e-cigarette users were former smokers and 66.2% of current e-cigarette users were current (some days or daily) cigarette smokers.
Table 1.
Sample Characteristics of NHIS 2014 and 2016 Combined
| E-cigarette use | |||||
|---|---|---|---|---|---|
| Variable | Never, % (n) | Former, % (n) | Some days, % (n) | Daily, % (n) | p-value |
| n | 60,100 | 7,093 | 1,483 | 776 | |
| Myocardial infarction | 3.9 (2,309) | 3.2 (225) | 4.1 (61) | 6.1 (47) | 0.015 |
| Cigarette smoking | <0.001 | ||||
| Never | 66.0 (39,649) | 19.9 (1,413) | 9.2 (136) | 5.3 (41) | |
| Former | 23.9 (14,358) | 21.0 (1,486) | 12.4 (184) | 51.5 (399) | |
| Some days | 2.6 (1,581) | 11.5 (816) | 17.5 (260) | 11.1 (86) | |
| Daily | 7.4 (4,437) | 47.6 (3,370) | 60.9 (902) | 32.1 (249) | |
| Health status | |||||
| Hypertension | 35.6 (21,387) | 26.6 (1,887) | 28.7 (426) | 33.4 (259) | <0.001 |
| Diabetes mellitus | 11.1 (6,642) | 6.8 (485) | 8.3 (123) | 9.2 (71) | <0.001 |
| High cholesterol | 31.1 (18,655) | 22.8 (1,612) | 23.7 (350) | 31.1 (240) | <0.001 |
| Demographics | |||||
| Woman | 56.1 (33,698) | 48.2 (3,422) | 48.7 (722) | 47.3 (367) | <0.001 |
| Age, year (±SD) | 51.6 (±18.48) | 39.9 (±15.12) | 41.4 (±15.24) | 44.2 (±15.30) | <0.001 |
| BMI (±SD) | 30.39 (±14.40) | 29.29 (±11.95) | 29.31 (±12.48) | 29.68 (±12.54) | <0.001 |
| Race/ethnicity | <0.001 | ||||
| Hispanic | 14.9 (8,935) | 10.0 (709) | 9.4 (139) | 5.5 (43) | |
| White | 65.0 (39,071) | 76.0 (5,387) | 76.7 (1,138) | 83.3 (646) | |
| Black | 13.1 (7,871) | 8.9 (631) | 8.4 (125) | 5.2 (40) | |
| Asian | 5.8 (3,481) | 3.1 (222) | 2.7 (40) | 3.4 (26) | |
| Other | 1.23 (742) | 2.0 (144) | 2.8 (41) | 2.7 (21) | |
Note: Boldface indicates statistical significance (p<0.005). P-values test the null hypothesis that there are no differences across the groups using analysis of variance or chi-square, as appropriate.
NHIS, National Health Interview Survey.
Current e-cigarette users were less likely to be daily users (34.4% or 776/2,259) than were current cigarette smokers (76.5% or 8,969/11,718, p<0.001).
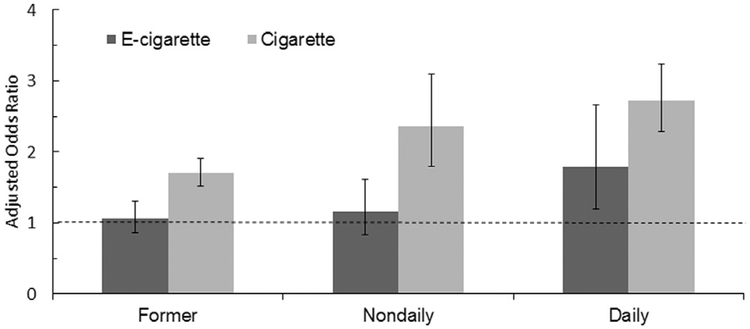
Both unadjusted and adjusted models of the combined data showed that odds of having had an MI is about 1.7 for daily e-cigarette users compared with subjects who had never used e-cigarettes, suggesting that the effect was independent of cigarette smoking status (never, former, some days, daily), demographic factors, and other health conditions (Table 2 and Figure 1, Appendix Tables 3 and 4 [available online] contain the analyses of the 2014 and 2016 data separately). Neither former nor some day e-cigarette use are associated with increased risk of MI (p=0.608 and p=0.392).
Table 2.
Univariate and Multivariable Associations Between E-cigarette Use and Myocardial Infarction of NHIS 2014 and 2016 Combined
| Unadjusted model | Adjusted model | |||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | p-value | OR (95% CI) | p-value |
| E-cigarette use | ||||
| Never | ref | ref | ||
| Former | 0.79 (0.67, 0.94) | 0.009 | 1.06 (0.86, 1.30) | 0.608 |
| Some days | 1.06 (0.79, 1.44) | 0.665 | 1.16 (0.83, 1.62) | 0.392 |
| Daily | 1.69 (1.19, 2.39) | 0.003 | 1.79 (1.20, 2.66) | 0.004 |
| Cigarette smoking | ||||
| Never | ref | |||
| Former | 1.70 (1.51, 1.91) | <0.001 | ||
| Some days | 2.36 (1.80, 3.09) | <0.001 | ||
| Daily | 2.72 (2.29, 3.24) | <0.001 | ||
| Hypertension | 2.32 (2.05, 2.64) | <0.001 | ||
| Diabetes mellitus | 1.77 (1.57, 2.00) | <0.001 | ||
| High cholesterol | 2.36 (2.12, 2.63) | <0.001 | ||
| Woman | 0.47 (0.42, 0.52) | <0.001 | ||
| Age (per 10 years) | 1.65 (1.56, 1.71) | <0.001 | ||
| BMI | 1.00 (1.00, 1.00) | 0.959 | ||
| Race/ethnicity | ||||
| White | ref | |||
| Hispanic | 0.82 (0.69, 0.98) | 0.031 | ||
| Black | 0.91 (0.78, 1.05) | 0.207 | ||
| Asian | 0.58 (0.42, 0.80) | 0.001 | ||
| Other race | 1.50 (0.95, 2.38) | 0.079 | ||
| n | 69,395 | 69,046 | ||
Note: Boldface indicates statistical significance (p<0.05).
NHIS, National Health Interview Survey.
Figure 1. AORs of e-cigarette and cigarette users.
Notes: Daily e-cigarette use was associated with a statistically significant increase in the odds of having had a myocardial infarction (OR=1.79, 95% CI=1.20, 2.66; p=0.004) controlling for cigarette smoking behavior and demographic and clinical risk factors for myocardial infarction compared to never e-cigarette users who never smoked. By comparison, the odds of having had a myocardial infarction were OR=2.72 (95% CI=2.29, 3.24) for daily cigarette smoking, controlling for e-cigarette use, and the other risk factors.
By comparison, in the adjusted model, being a daily cigarette smoker is associated with odds of having had an MI of 2.72, controlling for e-cigarette use. Being a some day or former cigarette smoker is also associated with increased odds of having had an MI.
DISCUSSION
This is the first study to examine the relationship between e-cigarette use and MI. The fact that the use of e-cigarettes and conventional cigarettes are both included in the same logistic regression means that they both independently contribute to the risk of having had an MI after adjusting for other risk factors (including the use of the other product). All the ORs in Table 2 estimate the independent risks compared with people who have never used e-cigarettes or cigarettes.
Because the different products are independently associated with risk of having had an MI, it is possible to use the results in Table 2 to estimate other behaviors, including dual use and switching from cigarettes to e-cigarettes. For example, the total odds of having had an MI among a former cigarette smoker who currently uses e-cigarettes daily is (odds of MI among former smokers) × (odds of MI among daily e-cigarette user) = 1.70 × 1.79 = 3.04 compared with a never smoker who has never used e-cigarettes. Thus, odds of having had a heart attack for an individual who switched from daily smoking to daily e-cigarette use would change by a factor of
By contrast, the total odds of having had an MI among a current daily dual user who both smokes cigarettes daily and also uses e-cigarettes daily is (odds of MI among daily smoker) × (odds of MI among daily e-cigarette user) = 2.72 × 1.70 = 4.62 compared with a never smoker who has never used e-cigarettes. In other words, dual use of e-cigarettes and conventional cigarettes appears to be more dangerous than using either product alone. Finally, the total odds of having had a heart attack for an individual who switched from daily smoking to daily e-cigarette use compared with quitting smoking “cold turkey” would be
These results are consistent with biological studies, which showed that e-cigarette use is associated with endothelial dysfunction, oxidative stress, inflammation, platelet activation, and activation of the sympathetic nervous system.10,11,14 These results suggest that e-cigarettes represent an independent risk factor for MI on top of the effects of smoking.
The point estimate of the odds of MI associated with daily e-cigarette use (1.79, 95% CI=1.20, 2.66) is lower than current cigarette smoking (2.72, 95% CI=2.29, 3.24) in the adjusted models (Table 2). The difference may also reflect differences in the (unknown) intensity of use, but this may not be a major factor because smoking even a single cigarette a day has about half the risk of being a pack-a-day smoker.19 This difference may be because e-cigarettes are not as dangerous as cigarettes, statistical uncertainty, or the fact that some e-cigarette users likely had MIs before e-cigarettes became available, which will bias the estimated OR to the null.20
In contrast to the lasting effect associated with being a former cigarette smoker, there was not a significant increase in MI risk for former or some day e-cigarette users (Table 2). It may be that the risks of e-cigarette use dissipate rapidly when someone stops using them, that some people briefly experiment with e-cigarettes and stop using them before any lasting damage is done, or that e-cigarettes have not been available long enough to cause permanent damage to the cardiovascular system.
The associations between the traditional risk factors and MI observed in this study are comparable to prior reports, which increases the confidence one can have in the findings on the association between e-cigarette use and MI. The increased odds of an MI associated with being a current (OR=2.64, 95% CI=2.24, 3.12, combining daily and nondaily current smokers) and former smoker (OR=1.70, 95% CI=1.51, 1.91) in this analysis are similar to the results (ORs and CIs) from two earlier case-control studies: 2.9 (95% CI=2.4, 3.4) and 2.0 (95% CI=1.1, 3.8) in the Rosenthal et al. study21 and 2.95 (95% CI=2.77,3.14) and 1.87 (95% CI=1.55, 2.24) in the INTERHEART study conducted in 52 countries.22
The odds associated with other risk factors including hypercholesterolemia, diabetes mellitus, and hypertension on acute MI found in this study are also consistent with previous studies. The United Kingdom prospective diabetes study showed that the estimated hazard ratio for coronary artery disease comparing the upper third relative to the lower third was 1.52 (95% CI=1.15, 2.01) for hemoglobin A1c, which is a diagnostic marker for diabetes mellitus.23 Almdal and colleagues24 studied the effect of type 2 diabetes mellitus on ischemic heart disease in a population-based study with 20 years of follow-up and found that diabetes mellitus increases the risk of acute MI or stroke by 1.5- to 2-fold in men and 1.5- to6.5-fold in women. In European Prospective Investigation into Cancer and Nutrition Potsdam study, the odds of MI increases by 1.64-fold (95% CI=1.05, 2.56) for diabetes mellitus and 1.84-fold (95% CI=1.27, 2.65) for hypertension.25 Glazer et al.26 reported that risk of MI is high (OR=2.1, 95% CI=1.8, 2.5) in individuals with a high level of total cholesterol.
Limitations
The NHIS is a cross-sectional study, so it only permits identifying associations rather than causal relationships. NHIS relies on self-report, so there is also the possibility of recall bias. However, NHIS is conducted in person and the question “Have you EVER been told by a doctor or other health professional that you had a heart attack (also called myocardial infarction)?” specifically asks if the respondent was told by a doctor or other health professional that he or she had a heart attack, which presumably reflects clinical validation of the diagnosis by his or her doctor. In addition, studies in Finland27 and Minnesota28 found 81% and 98% agreement between self-reported MI and medical records.
It is not known when the MIs occurred relative to e-cigarette use, and it is likely that some of the heart attacks subjects reported occurred before e-cigarettes became available in the U.S. (around 2009). This situation will bias the OR estimates toward the null,20 meaning that the study results likely underestimate the true risks associated with e-cigarette use.
One could argue that e-cigarette usage may simply be indicative of a smoking cessation strategy in traditional cigarette smokers who had their MI while they were smoking traditional cigarettes. The logistic regression explicitly allows for the eventuality that some people switched from cigarettes to e-cigarettes before the data were collected because it included both smoking behavior and e-cigarette use in the same model, including status as a former smoker. If someone switched from cigarettes to e-cigarettes in order to quit smoking after an MI and the increased risk was due to being a former smoker, that risk would be captured in the former smoker variable rather than appearing as an artifact in one of the e-cigarette variables. Moreover, for this situation to induce a spurious association between e-cigarette use and having had an MI, people who had MIs and subsequently quit smoking would have to have done so preferentially with e-cigarettes as a smoking cessation device more than smokers who had not had an MI. No data to support this assumption could be identified.
Although the definition for smoker is well established, there is not yet a consensus on how to define established e-cigarette users and there is no information on amount, time, or duration of e-cigarette use. As a result, the e-cigarette users likely include experimenters who only used an e-cigarette a few times as well as regular users. Former smokers and former e-cigarette users are defined differently, with former smokers having had to have smoked 100 or more cigarettes in the past and former e-cigarettes users only having had to have used one e-cigarette in the past. This difference in how users are defined will likely bias estimates of the risks associated with e-cigarette use toward the null.
Other limitations include the fact that there is no information on the size of the MI. As with any study, there is always the possibility of unknown confounding from variables not included in the analysis, such as family history of MI, physical activity level, and statin use. The fact that the results from smoking and the other risk factors are consistent with previous longitudinal studies as well as experimental studies on the acute cardiovascular effects of e-cigarette use increases the confidence one can have in conclusions regarding e-cigarettes.
CONCLUSIONS
Daily e-cigarette use is associated with increased odds of MI independent of and in addition to the risks associated with smoking and other risk factors. Dual use of e-cigarettes and conventional cigarettes—the most common use pattern among e-cigarette users—is more dangerous than using either product alone. From these findings, recreational use of e-cigarettes or use of e-cigarettes for smoking cessation should not be recommended.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Glantz’s work was supported by grants R01DA043950 from the National Institute of Drug Abuse and P50CA180890 from the National Cancer Institute and the Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or the Food and Drug Administration. The funding agencies played no role in in study design, collection, analysis, and interpretation of data, writing the report, or the decision to submit for publication. No financial disclosures were reported by the authors of this paper.
AT had the idea for the study and completed the analysis and drafted the paper. IP and NT assisted with the literature review and helped prepare the first draft of the manuscript. SAG guided the analysis and helped revise and prepare the final manuscript.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j. amepre.2018.05.004.
REFERENCES
- 1.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986. 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatnagar A Cardiovascular perspective of the promises and perils of e-cigarettes. Circ Res. 2016;118(12):1872–1875. 10.1161/CIRCRESAHA.116.308723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88(7):1295–1308. 10.1007/s00204-014-1294-7. [DOI] [PubMed] [Google Scholar]
- 5.Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–1326. 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope CA 3rd, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120(11):941–948. 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD, Bard RL, Burnett RT, et al. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68(3):224–230. 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- 8.Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96(4):1089–1096. 10.1161/01.CIR.96.4.1089. [DOI] [PubMed] [Google Scholar]
- 9.Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126(18):2177–2183. 10.1161/CIRCULATIONAHA.112.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnevale R, Sciarretta S, Violi F, et al. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150(3):606–612. 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Moheimani RS, Bhetraratana M, Yin F, et al. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol. 2017;2(3):278–284. 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. 10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- 13.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107(22):2805–2809. 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 14.Higham A, Rattray NJ, Dewhurst JA, et al. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir Res. 2016;17(1):56 10.1186/s12931-016-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W, Rubenstein DA. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets. 2016;27(7):694–702. 10.3109/09537104.2016.1158403. [DOI] [PubMed] [Google Scholar]
- 16.Olfert IM, DeVallance E, Hoskinson H, et al. Chronic exposure to electronic cigarette (E-cig) results in impaired cardiovascular function in mice. J Appl Physiol (1985). 2018;124(3):573–582. 10.1152/japplphysiol.00713.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC). NHIS—National Health Interview Survey Homepage. www.cdc.gov/nchs/nhis/index.htm. Accessed September 20, 2017.
- 18.Centers for Disease Control and Prevention (CDC). 2016. National Health Interview Survey (NHIS) Public Use Data Release: Survey Description (page 99). http://http.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2016/srvydesc.pdf. Published 2017. Accessed September 20, 2017.
- 19.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360:j5855 10.1136/bmj.j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gail MH. Encyclopedia of Biostatistics: Bias Toward the Null. New York, NY: John Wiley & Sons; 2005. 10.1002/0470011815.b2a03016. [DOI] [Google Scholar]
- 21.Rosenberg L, Kaufman DW, Helmrich SP, Shapiro S. The risk of myocardial infarction after quitting smoking in men under 55 years of age. N Engl J Med. 1985;313(24):1511–1514. 10.1056/NEJM198512123132404. [DOI] [PubMed] [Google Scholar]
- 22.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocar-dial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647–658. 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 23.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316(7134):823–828. 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164(13):1422–1426. 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 25.Heidemann C, Hoffmann K, Klipstein-Grobusch K, et al. Potentially modifiable classic risk factors and their impact on incident myocardial infarction: results from the EPIC-Potsdam study. Eur J Cardiovasc Prev Rehabil. 2007;14(1):65–71. 10.1097/01.hjr.0000238392.19847.4c. [DOI] [PubMed] [Google Scholar]
- 26.Glazer NL, Smith NL, Heckbert SR, Doggen CJ, Lemaitre RN, Psaty BM. Risk of myocardial infarction attributable to elevated levels of total cholesterol among hypertensives. Am J Hypertens. 2005;18(6):759–766. 10.1016/j.amjhyper.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Tretli S, Lund-Larsen PG, Foss OP. Reliability of questionnaire information on cardiovascular disease and diabetes: cardiovascular disease study in Finnmark county. J Epidemiol Community Health. 1982;36(4):269–273. 10.1136/jech.36.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.