Abstract
Aedes mosquitoes are considered highly successful global invasive species and vectors of several pathogens of relevance for public health. Their midgut’s microbiota can play an important role in affecting not only their vectorial competence but also their fitness, physiology, food digestion, metabolism, immunity and adaptation to new environmental conditions. Using high-throughput sequencing we compared the microbiota of Aedes albopictus collected in Italy with those reported in populations from France and Vietnam. We also analysed Aedes koreicus gut microbiota for the first time. We found remarkable individual difference along with common bacterial taxa in both species. Ae. albopictus collected in Italy had a lower richness and a different composition of microbiota in respect to specimens collected in France and Vietnam. It also showed a core microbiota formed mainly of bacteria of the genus Pseudomonas. Overall, the two Aedes species (Ae. albopictus and Ae. koreicus) collected in Italy, showed a large core microbiota with 75.98% of the identified Operational Taxonomic Units. Furthermore, Ae. albopictus had 2.5% prevalence of Wolbachia and 0.07% of Asaia spp, while Ae. koreicus had 14.42% of Asaia spp. and no Wolbachia. This study provides new informations on the spatial variation of the midgut bacterial communities in mosquitoes of medical relevance within areas of recent invasion and provide the basis for further studies aimed at assessing the effects of such variation on vectorial capacity for a range of pathogens.
Introduction
Biological invasion of alien species represents a rising global issue. In fact, invasive species often exert a negative impact on local biodiversity and economy, including the impact on human and animal health as consequence of pathogen transmission1,2.
The mosquito species Aedes (Stegomyia) albopictus (Skuse, 1894) and Aedes (Finlaya) koreicus (Edwards, 1917) are native to South East Asia and are now invading several European countries posing an increasing threat to human and animal public health3. Ae. albopictus, considered as one of the top 100 most invasive species of the world4, is a competent vector for at least 26 arboviruses5, including dengue, chikungunya and Zika, and it was first detected in Europe in 19796 and in the Province of Trento (Italy) in 19967. Ae. koreicus, recorded in Italy for the first time in 20118 transmits the parasitic nematode Dirofilaria immitis9, has been proven to transmit experimentally the Japanese Encephalitis virus10, while the competence to transmit viruses such as dengue, chikungunya and Zika requires experimental validation.
It should be taken into consideration that colonization of new habitats often represents a challenge for the invasive species itself, since the adaptation to the new environment is highly costly due to the variety of new selective pressures encountered by the introduced species11.
The structure of the gut microbiota is a sensitive indicator of health status in humans and animals, where a high microbiota diversity is usually associated with healthy conditions of the host12. Recently, it has been shown that environmental disturbances can also cause a loss of diversity in the gut microbiota of wild species13, leading to the hypothesis that gut microbiota diversity can be used as a measure of fitness of a wild species invading a new habitat. In case of arthropods, the role of microbial communities inhabiting gut are increasingly under study. As in mammals, microbiota takes part in the characteristic physiology of the species and plays a key role in food digestion, metabolism, immunity and adaptation to new environmental conditions14,15, even if most insect guts generally contain few microbial species14. When the invasive species is an arthropod vector of pathogens, testing its vectorial capacity and ability to spread into new environment is essential in term of actual disease risk estimate. Infact, in blood-feeding insect vectors microbiota plays another important function, namely it affects vectorial competence, i.e. their ability to transmit pathogens to their target species. Indeed, gut microbiota modulates actively the proliferation and development of pathogens, and any pathogen entering the host must interact both with the midgut and the resident bacteria16,17. All mosquito-transmitted pathogens complete their life-cycle in the gut where they are exposed to barriers and immunity mediators activated by the microbiota17,18.
Recent studies have highlighted the ability of insect-associated bacteria to modulate vector competence for arboviruses and other pathogens through mechanisms such as immune response activation, resource competition, or production of anti-viral molecules17. Therefore, monitoring the gut microbiota diversity during colonization of new environments can represent a novel approach in terms of evaluation of the vectorial capacity and the actual disease hazard estimate, combining indications on potential vector competence and fitness. Vectorial capacity is in fact defined as the capability for disease transmission by a vector to a host, as influenced by behavioral, ecological and environmental factors, such as population density, host preference, feeding habits or frequency, duration of latent period, or longevity.
The development of metataxonomic analysis based on High Throughput Sequencing (HTS) has made possible to detect a deeper level of microbial diversity in animal hosts and recent studies demonstrated that this technique can be successfully applied to mosquito’s gut19,20. Since the environment (and the adaptation to it) is known to influence the association between host and microbiota community composition in mosquitoes19,21,22, we hypothesized that gut microbiota from introduced populations should be similar to each other, but different to autochthonous populations, at least in terms of richness. To assess this assumption we compared the midgut microbiota from Ae. albopictus samples collected in northern Italy with data recently published on introduced populations from France and autochthonous populations from Vietnam20. Furthermore, we analyzed and compared wild-caught samples of Aedes species (Aedes albopictus and Aedes koreicus) collected in the same region, in order to compare their microbial community and assess potential hazards of pathogens transmission in our territory. Finally this is the first study reporting data on the gut microbiota of Ae. koreicus.
Results
Using publicly available data, we compared the gut microbiota of a population of Ae. albopictus from the Trentino region, an area located in northern Italy, to French samples of the same species, and to samples from Vietnam, that belongs to the area endemically occupied by this species, before its recent global spread. We also characterized the structure, composition and diversity of the gut microbiota of Ae. albopictus and Ae. koreicus from the study area.
Comparative analysis of Italian, French and Vietnamese Ae. albopictus microbiota
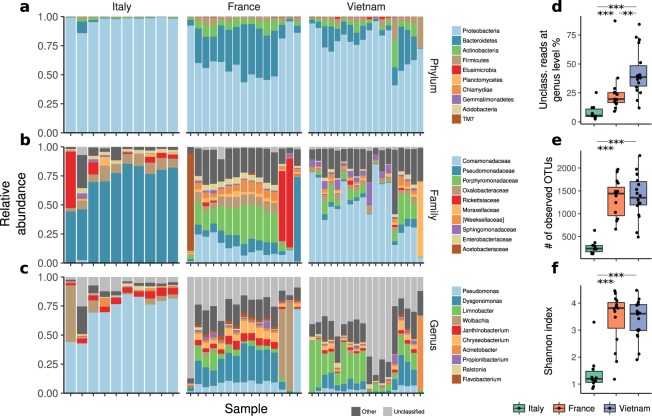
The taxonomic structure at the Phylum, Family and Genus levels showed clear diversification between the Italian, French and Vietnamese populations (Fig. 1a–c and Supplementary Tables S1–S3).
Figure 1.
Taxonomical composition of Italian, French and Vietnamese Ae. albopictus microbiota (a) by Phylum, (b) by family, (c) by genus. Box-plot of the % of unclassified reads at genus level per country (d); numbers of observed OTUs per country (e) and Shannon index per country (f). **p < 0.05, ***p < 0.01.
At the Phylum level, the composition of midgut bacterial community in the Italian population was almost exclusively composed by Proteobacteria (mean 97%, sd 4.1%, Supplementary Table S1), while the French and Vietnamese populations, although still containing a high proportion of Proteobacteria (mean 62.7% sd 17.5% and mean 76.1% sd 14.7%, respectively, Supplementary Table S1), also contained variable fractions of Bacteroidetes (France mean 27.7%, sd 15.2%, Vietnam mean 12.7%, sd 10%, Italy mean 1.5% sd 3%, Supplementary Table S1), Actinobacteria (France mean 6.6%, sd 3.3%, Vietnam mean 5.3%, sd 5.3%, Italy mean 0.9% sd 0.7%, Supplementary Table S1), Firmicutes (France mean 3%, sd 1,6%, Vietnam mean 5%, sd 7%, Italy mean 0.4% sd 0.4%, Supplementary Table S1) and other representatives with negligible quantity. These differences were confirmed at the Family and Genus level (Supplementary Tables S2 and S3). While the Italian population was dominated by the Family Pseudomonadaceae (71%, sd 15%, Supplementary Table S2), Genus Pseudomonas (71%, sd 15%, Supplementary Table S3), the French and Vietnamese populations showed a much more complex structure, with high prevalence of bacteria from the family Comamodanaceae (12%, sd 7.8%, and 49.2%, sd 22.3%, respectively, Supplementary Table S2), in particular from the genus Dysgonomonas for the French samples (18.2%, sd 11%, Supplementary Table S3) and Limnobacter for the Vietnamese samples (17.4%, sd 13.2%, Supplementary Table S3). This family was almost absent from the Italian samples (see Fig. 1b,c and Supplementary Table S2).
The simplified microbiota structure in the Italian population was also confirmed by considering the number of unclassified reads (Fig. 1d), which is a measure of the fraction of the microbiota that is composed by organisms that are not present in the reference taxonomic databases. This fraction was much higher in the French and Vietnamese populations, suggesting the presence of a large fraction of not yet characterized organisms, compared to the Italian population. In particular, we found statistically significant differences in terms of unclassified reads at genus level (Fig. 1d) between Italy and France (Wilcoxon rank sum test, FDR corrected P-value = 6 × 10−4), Italy and Vietnam (P = 8.7 × 10−6) and France and Vietnam (P = 10−3). In addition, the Italian population showed a significantly reduced α-diversity when compared to France and Vietnam both in terms of number of observed OTUs (Fig. 1e, P = 9.2 × 10−7 for both France and Vietnam) and of Shannon entropy (Fig. 1f, P = 6.1 × 10−5 for France and P = 2.1 × 10−5 for Vietnam), while France and Vietnam were not statistically different (Fig. 1e, P = 0.87, and Fig. 1f, P = 0.58, respectively; Supplementary Table S4).
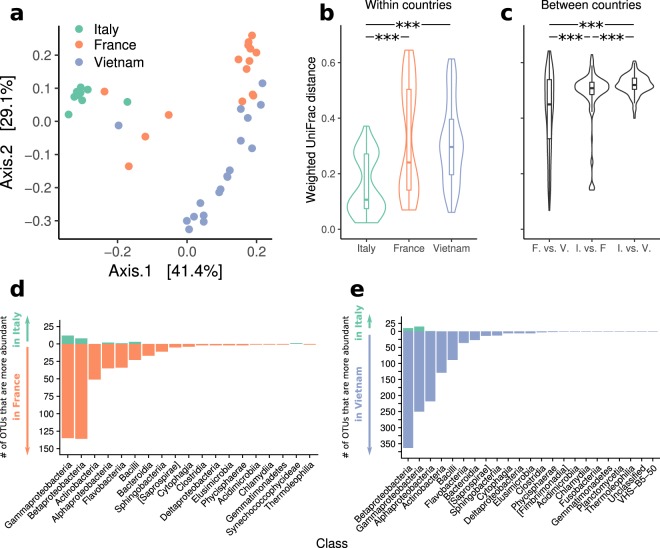
To further investigate if the three different populations corresponded to clearly differentiated structure of the gut microbiota of Ae. albopictus, we computed the -diversity of the samples using the unweighted Unifrac, weighted Unifrac and Bray-Curtis dissimilarity. A plot of the first two axes of the PCoA of the distances amongst the samples calculated using the weighted UniFrac distance showed three distinct clusters, corresponding to the Italian, French and Vietnamese samples (Fig. 2a).
Figure 2.
(a) Principal coordinates analyses (PCoA) of weighted UniFrac distances, (b) within countries distances (c) between countries distances. Number of OTUs that are significantly more abundant in (d) France or Italy and (e) Vietnam or Italy, grouped by class (P < 0.01 DESeq’s Wald significance test, Benjamini & Hochberg FDR correction).
This result is confirmed by the PCoA plots using the unweighted UniFrac distance and the Bray-Curtis dissimilarity (see Supplementary Figures S1 and S2). A permutational multivariate analysis of variance using distances (PERMANOVA) showed that the three different populations (Italian, French and Vietnamese) were significantly different (P < 10−4 for the unweighted, weighted UniFrac distances and for Bray-Curtis dissimilarity, see also Fig. 2c). To confirm that the Italian population was clearly distinct from and less diverse than the French and Vietnamese ones, we compared the distributions of the distances within and between the three populations (Fig. 2b,c). Variability within Italy and France and within Italy and Vietnam was significantly different (P = 7.6 × 10−7 and P = 3.3 × 10−10 respectively, see Fig. 2b), but not within France and Vietnam (P = 0.96). Similarly, distances between countries highlighted the closeness between France and Vietnam, compared to Italy and France and Italy and Vietnam (P = 1.4 × 10−5 P = 7.6 × 10−13 respectively, Fig. 2c). We then examined which were the taxa differently distributed between Italy and France and Italy and Vietnam. As expected, we found that in most cases the difference between the Italian population and the others was due to taxa significantly more abundant in France and Vietnam than in Italy (P < 0.01, Wald significance test, Benjamini & Hochberg FDR correction, Fig. 2d,e for a summary at the class level, and Supplementary Tables S5 and S6). However, in Gamma- and Betaproteobacteria only a few OTUs were significantly more abundant in the Italian population, suggesting that the structure of the microbiota of these samples is the result of a general rearrangement of the relative abundances of the different species, rather than being just a simplified version of the French or Vietnamese ones.
Midgut microbiota of Ae. albopictus vs. Ae. koreicus
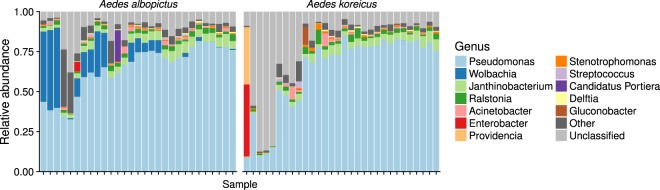
Ae. koreicus is a species very recently introduced in Italy. To investigate if stress factors due to the adaptation to an alien environment (which appears to be very strong in Ae. albopictus) dominated over the genetic differentiation of these two species in determining the structure of the midgut microbiota, we compared samples from the Italian populations of Ae. albopictus and Ae. koreicus. After rarefaction (without replacement) 2 153 OTUs were clustered into 9 Families and 12 Genus (Supplementary Tables S7–S9). Despite extensive variation between individuals of the same host species in the composition of their gut microbiota (Fig. 3), the comparison between Ae. albopictus and Ae. koreicus revealed a large shared core (75.98% of the reads), complemented by a smaller fraction specific to each of the two species. At Phylum level Proteobacteria and Firmicutes dominated the community (Supplementary Table S7). At Family level Pseudomonadaceae was the most abundant so far, followed by Rickettsiaceae, Oxalobacteraceae, Moraxellaceae, Enterobacteraceae, Xanthomonadaceae, Halomonadaceae, Acetobacteraceae and Comamodanaceae (Supplementary Table S8).
Figure 3.
Taxonomical composition of midgut microbiota of Ae. albopictus and Ae. koreicus at genus level (Supplementary Table S9).
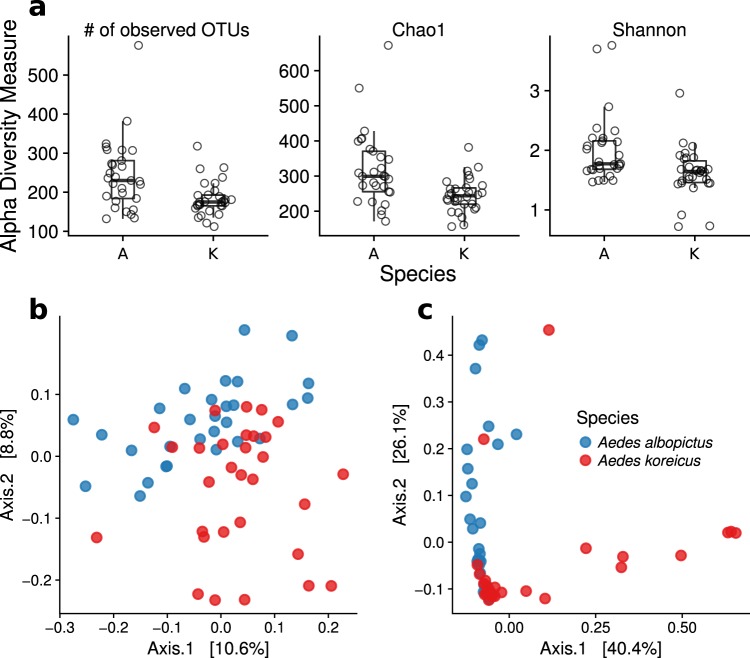
The number of observed OTUs, the Chao1 estimator and Shannon entropy indicated a significantly higher α-diversity in Ae. albopictus compared to Ae. koreicus (Fig. 4a and Supplementary Table S10, P = 0.0027, 8.4 × 10−4 and 9.5 × 10−4 for number of OTUs, Chao1 and Shannon entropy, respectively, Wilcoxon rank-sum test, uncorrected p-values). Moreover, beside their different richness, the samples from the two species had a significantly different gut microbiota as resulted from a PERMANOVA test (9 999 permutations) on the β-diversity (P < 10−4, P < 10−4 and P = 9 × 10−4 for unweighted UniFrac and weighted UniFrac, respectively, Fig. 4b,c).
Figure 4.
Comparison of α- and β-diversity for Ae. albopictus (A) and Ae. koreicus (K). (a) Observed number of OTUs, Chao1 estimator and Shannon entropy. In all cases the difference was statistically significant (P = 0.0027, 8.4 × 10−4 and 9.5 × 10−4 for number of OTUs, Chao1 and Shannon entropy, respectively, Wilcoxon rank-sum test). (b) Principal coordinates analyses (PCoA) of unweighted (b) and weighted (c) UniFrac distances.
The core microbiota was largely composed by species of the genus Pseudomonas that alone accounted for an average of 64.63% and 63.38% of the reads in Ae. albopictus and in Ae. koreicus, respectively (core OTUs are listed in Supplementary Table S11). In 7 samples of Ae. koreicus there was a large contribution (up to the 14.42%) from an unidentified genus of the family Acetobacteraceae (see Supplementary Table S8). According to BLAST, the closest relative genus belonged to Asaia (97% percentual identity). We found that only 3 OTUs were specific to Ae. albopictus, corresponding to 9.81% of the total Ae. albopictus abundance (OTUs listed in Supplementary Table S12), while no OTUs were specific to Ae. koreicus. Using indicator species analysis we confirmed the three OTUs specific to Ae. albopictus, additionally identifying 8 OTUs of Phylum Proteobacteria specific to Ae. albopictus, and three specific to Ae. koreicus (Supplementary Table S13).
Two out of this three Ae. albopictus-specific OTUs were classified as belonging to the genus Wolbachia which resulted to be the dominant component in three samples from this species. No OTUs classified as Wolbachia were found in Ae. koreicus. In order to characterize more precisely the differences in the taxonomic composition of the samples from the two species, we identified all those OTUs with significant different relative abundance (Wilcoxon rank-sum test P < 0.05, FDR corrected). In addition to the species-specific OTUs identified above, we obtained 7 relatively rare OTUs with relative abundances ranging from a few percent of the total microbiota to less than 10−3 (Supplementary Table S14).
Discussion
Invasion by alien exotic mosquito species, such as Ae. albopictus and Ae. koreicus, is now occurring in an increasing number of european countries (see https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps). These processes therefore enhance the probability of new diseases outbreak events within new areas. Therefore the needs is now to improve scientific knowledge on all the factors affecting mosquitoes vectorial capacity under a “pathobiome” approach23. Northern Italy is the only site in Europe where both Ae. albopictus and Ae. koreicus are considered established. Therefore, we took this unique opportunity to compare their gut microbiota not only for the potential health hazard they represent, but also because of the lack of knowledge on the midgut microbiota composition of Ae. koreicus. This latter species, although introduced in northern Italy few years ago8, is well established in an area of 3000 km2 and its spreading is still ongoing24.
The mosquito’s gut microbiota composition varies as a result of complex interactions between environmental characteristics and developmental stages. Nonetheless the common characteristics of the habitat of Aedes spp. coupled with the high inter-individual diversity, makes it difficult to determine the geographic origin based on microbiota composition19,25. Moreover when alien species invade a new area they are usually less parasitised than native or well established species as seen within a previous study from Minard et al. showing that the midgut microbiota of the French tiger mosquitoes had a lower richness than the Vietnamese one20. This suggests that adaptation to a different environment had a profound impact on the resident intestinal microbiota of this species, with potential impacts on its biology, and consequently on its vector competence. According to these findings, our hypothesis was that if the differences were driven by the adaptation to a new environment, we should expect French and Italian populations to have similar richness, given the comparable macro climate (temperate), type of breeding sites (urban settlements and human-made containers) and introduction period (end of nineties-beginning of millennium). Surprisingly, we found that the degree of microbial diversity in the midgut microbiota of Italian female Ae. albopictus was lower not only compared with Vietnamese, but also with French ones, suggesting the existence of other population-specific factors. These could be either a particular condition of the Italian population, or a larger and continuous higher rate of introduction of new individuals in France, probably due to international commercial trades. The higher variability shown by French and Vietnamese samples compared to Italian ones, can also be linked to specific microclimatic features of the sampling sites, such as vegetation and naturality, but also to the type of hosts available. We tried to minimize or exclude most of the confounding factors, which could affect variability among populations (previous blood meal, type of habitat and seasonality). All the sampled wild adult female mosquitoes analyzed in these studies were unfed; however, no further molecular tests were performed to exclude previous blood meals. Although Aedes albopictus colonizes edges of forests and breed in natural habitats in its country of origin (tree holes, bamboo stumps, and bromeliads), this species has adapted well to suburban and urban environments with larvae now breeding in artificial containers such as tires, cemetery urns, and water storage tanks. All the mosquito samples were collected in urban and suburban environments, with the exception of one out of four sites in Vietnam. The year of collection was different for the Italian (2015, this study) and the French and Vietnamese samples (201220), but the season of collection was optimal for this species for all countries. To the best of our knowledge, there are no studies on the role of geographic location and year as factors affecting individual gut microbiota and we believe that such factors should be considered when comparing seasonality abundances or other ecological factors affecting population dynamic. In order to better assess the reasons of the differences among Ae. albopictus populations further comparisons with local mosquito species could be taken into account in the future.
The genus Pseudomonas dominated the microbiota of the Italian samples similarly to what reported in wild females of other species such as Ae. aegypti in Brazil26, Culex pipiens in Belgium27, and in Anopheles stephensi and An. culicifacies in southern Iran28,29. The interaction of this bacteria with pathogens transmitted by mosquitoes was observed in the case of Anopheles and Plasmodium with both positive and negative effects30,31. Moreover it has been reported that the presence of Pseudomonas helps in facing the oxidative stress resulting from the catabolism of blood meal32 which could cause a serious damage to insect cells. This bacteria has also been recently indicated as a good candidate to paratransgenesis since it grows in ordinary culture media and it is suitable for genetic transformation27.
The large core microbiota among the two Aedes species suggests a common environmental exposure in the breeding sites. In fact, the two mosquito species both share the same artificial habitats in urban settlements. Nonetheless, Ae. albopictus showed a higher richness and a different composition, as highlighted by α- and β-diversity. This could be partly explained by the fact that Ae. albopictus colonized the area about 15 years before Ae. koreicus.
Along with Pseudomonadaceae, other bacterial families were identified in our Aedes samples (like Enterobacteriaceae, Acetobacteraceae, Rickettsiaceae and Moraxellaceae). These were already found in different species of mosquitoes collected from geographically distant locations, supporting the hypothesis that many components of the midgut are cosmopolitan, well established as commensals, and have an important role in insect life cycle33–38. The role of Enterobacteriaceae is considered important in the digestion of blood in hematophagous Diptera and is frequently recovered in female mosquitoes of various species38–41. Some species of this family could also be used for a paratransgenic approach; for example Enterobacter cloacae has been found to block the development of Plasmodium falciparum in An. gambiae and sporogonic development of P. vivax in An. albimanus42,43 and to induce the expression of mosquito immune components in midgut of An. stephensi44. Other representatives from this family (genus Serratia) have a role in the suppression of the immune response of Ae. aegypti, therefore increasing its susceptibility to chikungunya and dengue viruses45,46.
A special attention should be focused on the contrasting presence of Wolbachia and members of the Acetobacteraceae family, most probably Asaia spp, in the two mosquito species. These two symbionts are the only bacteria known to be located in the reproductive organs of mosquito species, therefore vertically transmitted. The relationship between Wolbachia and mosquitoes is one of the most extensively studied among microbes harbored by insects47–51 as it is commonly found in mosquitoes midgut (with the notable exception of Ae. aegypti52), but also in somatic cells and gonads of Orthoptera53 and Rhyncota, such as bedbugs54. The most known effects of Wolbachia presence is the cytoplasmatic incompatibility between infected males and uninfected females, and its ability to inhibit the horizontal transmission or reduce the vector competence of some mosquito-borne pathogens like Plasmodium spp., dengue, yellow fever, West Nile fever and chikungunya viruses52,55–62. Once the vector potential of Ae. koreicus will be assessed, the lack of this endosymbiont could have important implications for the management of public health risk. Since the vectorial competence of Ae. koreicus for chikungunya virus was recently assessed by Ciocchetta et al.63 more studies are now necessary to better assess the consequences of the lack of this endosymbiont on the enhancement of its vectorial capacity.
Asaia spp. is a group of acetic acid bacteria that can be found in different niches like nectar-bearing flowers. Recently, this genus has been reported to be stably associated with different mosquito species, often being the dominant microorganism of the mosquito microbiota. Asaia has been widely reported in lab-reared64, but also in wild caught Ae. albopictus species41 and it seems to compete with Wolbachia in the ability to infect reproductive sites. Rossi et al. shown the infection of Asaia in the gut, but not in the reproductive organs of naturally infected Ae. albopictus and this was further confirmed by the absence of Asaia from the egg surface of this mosquito species65. Our findings reinforce the theory that mosquito-species naturally uninfected with Wolbachia (i.e. An. gambiae and An. stephensi66, Ae. aegypti67, Ae. koreicus (this study)) host Asaia spp. in various anatomical districts (midgut and ovary) and vice-versa, the occurrence of Asaia spp. in mosquitoes infected with Wolbachia is low. Most of the studies regarding the role of Asaia in mosquitoes, are related to several Anopheles species. In particular, it has been shown that Asaia spp. accelerates68 or has a beneficial role69 on larval development, with positive consequences on survivorship and competition with other mosquito species. In our study area, the two Aedes species overlap, therefore the presence of these bacteria needs to be further investigated. The consequences of microbiota composition in term of vector competence require more experimental studies since it has been previously assessed that the midgut bacteria play an important role in increasing or decreasing the vectorial capacity of the mosquito. Moreover it has been proven that vector-borne diseases can spread outside their endemic areas provided that the vector is present. An example are the chikungunya outbreaks in Italy in 200770 and 201771. Northern Italy is experiencing a regular introduction of infected chikungunya and dengue viruses human cases every year, while West Nile and Usutu viruses are endemic72–74. The presence of a species such as Ae. koreicus which has better chances to survive to colder temperatures24, its proved competence in transmitting pathogenic viruses such as chikungunya and the lacks of Wolbachia which potentially inhibits the circulation of these viruses, enhances the chances of spreading of exotic pathogens.
Conclusions
In conclusion, the invasion of alien mosquito species into new areas challenges their gut microbiota in terms of reduction of richness and diversity, with possible consequences on the mosquito fitness and disease hazard. Local sampling conditions, though, could affect the gut microbiota composition and further research is necessary to investigate the effect of environmental factors on all developmental stages. With this study we provided an improvement of our knowledge of the highly diverse community of micro-organisms present in the midgut of two invasive mosquito species of public health concern.
Methods
Samples collection
Mosquito collections were performed in July and August 2015 in the Autonomous Province of Trento (Trentino-Alto Adige region, Italy), which is an area of recent invasion by the two studied species. This territory covers an area of 6 200 km2 with more than 70% lying above 1 000 m a.s.l. Only non-fed females were considered for the microbiota analysis. Thirty females belonging to the species Ae. albopictus and 30 to Ae. koreicus were included. Most of the specimens were collected by aspiration and CO2-baited BG sentinel traps, with the exception of 3 samples of Ae. koreicus which were captured by human landing technique. All the sampling sites were located in urban and suburban habitats. Mosquitoes were kept alive until the arrival in laboratory and then killed with a brief exposure to low temperature (−20°). Prior to dissection under stereomicroscope, specimens were identified to species level on the basis of morphological features24,75.
Midgut isolation and DNA extraction
Before dissection, mosquitoes were surface sterilized in ethanol 70% for few minutes and rinsed with sterile water for molecular analysis. A sterile drop of PBS (100 µl) was used on the slide to facilitate the extraction of midgut. Only mosquitoes without visible traces of blood in the midgut were chosen for the study. Sterilized instruments were used for each dissection while the stereomicroscope was frequently cleaned with absolute alcohol. Midguts were put in sterile tubes and incubated overnight with ATL buffer and proteinase K at 56 °C with a gentle shaking (Thermo-shaker Grant Bio) prior processing. DNA extraction was performed using the Qiamp DNA Investigator kit (Qiagen) protocol “tissues” following the manufacturer’s protocol. Final elution volume was 25 µl (ATE buffer). DNA was quantified with fluorometer Qubit 2.0 (Invitrogen).
Amplicon library preparation and sequencing
We sequenced genomic DNA of 10 Ae. albopictus samples targeting a domain of ~280-bp of the V5-V6 hypervariable region of 16 S rRNA gene using the specific primer 784 F (5′-AGGATTAGATACCCTGGTA-3′) and 1061 R (5′-CRRCACGAGCTGACGAC-3′)76 with overhang Illumina adapters. Genomic DNA of 30 Ae. albopictus and 30 Ae. koreicus samples were sequenced targeting a domain of ~460-bp of the V3-V4 hypervariable region of 16 S rRNA gene using the specific primer 341 F (5′ CCTACGGGNGGCWGCAG 3′) and 805Rmod (5′ GACTACNVGGGTWTCTAATCC 3′)77. PCR conditions, library preparation and sequencing followed the same protocol for all sequencing. Total genomic DNA was subjected to PCR amplification. All PCRs were conducted in 25 µl of volume and prepared under sterile conditions. Each PCR reaction contained 2.5 μL of 10 × Fast Start High Fidelity Reaction Buffer (Roche), 0.5 μL of 10 mM dNTP mix (Fermentas, UK), 1 μL of 10 μM forward and reverse primer, 0.25 μL of 5 U/μL Fast Start High Fidelity Enzyme blend (Roche), DNA (12 ng/μL) and sterile water to reach the volume. Reaction without template served as negative control. All PCR amplifications were carried out using a Veriti-96 Well Thermal Cycler (Applied Biosystem) and the following steps were set: melting step; 94 °C for 3 minutes (one cycle), annealing step; 94 °C for 15 seconds, 55 °C for 45 seconds, 72 °C for 1 minute and 10 seconds (35 cycles), extension step; 72 °C for 8 minutes (1 cycle). The PCR products were checked on 1.5% agarose gel and cleaned from free primers and primer dimer using the Agencourt AMPure XP system (Beckman Coulter, Brea, CA, USA) following the manufacturer’s protocol. Subsequently dual indices and Illumina sequencing adapters Nextera XT Index Primer (Illumina) were attached by 7 cycles PCR (16 S Metagenomic Sequencing Library Preparation, Illumina). The final libraries, after purification by the Agencourt AMPure XP system (Beckman), were analysed on a Typestation 2200 platform (Agilent Technologies, Santa Clara, CA, USA) and quantified using the Quant-IT PicoGreen dsDNA assay kit (Thermo Fisher Scientific) by the Synergy2 microplate reader (Biotek). Finally all the libraries were pooled in an equimolar way in a final amplicon library and quantified using the KAPA Library quantification kit by the real time qPCR LighCycler 480 (Roche). Barcoded library were sequenced on an lllumina® MiSeq (PE300) platform (MiSeq Control Software 2.0.5 and Real-Time Analysis software 1.16.18).
Data analysis: Aedes albopictus, V5-V6 hypervariable region
Since the same region of the 16 S rRNA gene and the same primers of Minard and colleagues20 were used to sequence our 10 samples of Ae. albopictus, it was possible to analyze the sequence data from the two studies using the same protocols. Forty samples from Minard et al.20 were downloaded from the Sequence Read Archive (SRA, https://www.ncbi.nlm.nih.gov/sra) under the study ERP006543 (BioProject PRJEB6896). After paired-end alignment and primer trimming a total of 1 624 783 reads from Italy and 8 015 969 reads from France and Vietnam were quality filtered with an EE threshold of 0.5% and a minimum length of 250 bp. Reads with less than 60% similarity to the Greengenes database (May 2013 version, clustered at 85% identity) were removed using VSEARCH. Merging together all the remaining sequences we obtained 9 361 654 high quality reads. Reads were pre-processed using the MICCA pipeline (http://www.micca.org, version 1.6)78. After forward and reverse primer trimming, reads with an expected error rate (EE)79 higher than 0.5% and shorter than 400 bp were discarded. Reads with less than 60% similarity to the Greengenes database (May 2013 version, clustered at 85% identity)80, were removed using VSEARCH version 1.9.581. 6 101 971 high quality reads were clustered de novo at 97% identity using the “de novo greedy” Operational Taxonomic Unit (OTU) picking method implemented in MICCA. The representative sequences were classified using the consensus classifier82 against the Greengenes database (May 2013 version) clustered at 97% identity. The sequences were aligned using the NAST83 multiple sequence aligner and a phylogenetic tree was inferred using the FastTree software available in the MICCA pipeline. Downstream analyses were performed in R environment using the phyloseq84, vegan85 and picante86 libraries. Midgut samples were rarefied (without replacement) (see Supplementary Fig. 3S) at 90 000 reads per sample. A randomization sampling test was performed in order to rule out any bias in the taxonomic classification87 (Supplementary Fig. 4S). The average proportion error was calculated as the relative difference between the relative abundance of each taxon in the whole library (i.e., with the maximum number of reads,9,361,654) and in the random sample (20 random samples for each sample depth). As expected, the consistency of the estimations of taxa abundances grows as the sample depth increases.
Data analysis: Aedes albopictus and Aedes koreicus, V3-V4 hypervariable region
High Throughput Sequencing resulted in a total of 7 186 454 reads (after paired-end read alignment and merging). Pre-processing, filtering, clustering, taxonomic classification, multiple sequence alignment and phylogenetic tree inference was applied as for the V5-V6 samples. Samples were rarefied (without replacement) (see Supplementary Fig. 5S) at 45 000 reads per sample and one sample with 11 416 reads was removed. The core microbiota of all samples is composed by OTUs seen more than 10 times in at least 50% of the samples in each species. The species specific microbiota is composed by OTUs seen more than 10 times in at least 50% of the samples in one species and in less than 5% of samples in the other. Indicator species analysis was performed using the R package indicspecies88. P-values were FDR corrected.
Bacterial community diversity
To quantify the bacterial richness in each mosquito species three different α-diversity estimators were used: the number of observed OTUs, the Chao 1 estimator and the Shannon entropy. To highlight the differences in the midgut microbiota of Ae. albopictus among countries and between Ae. koreicus and Ae. albopictus, we calculated the β-diversity using the unweighted and weighted Unifrac distances89 and the Bray-Curtis (BC) dissimilarity90.
Principal Coordinates Analysis (PCoA) was used to visualize differences between microbial communities in samples according to weighted and unweighted Unifrac measures and BC values. Statistical significance of the between-groups distances was assessed using PERMANOVA (Permutational Multivariate Analysis Of Variance Using Distance Matrices)91 (with 9 999 permutations), implemented in the adonis function in the vegan R-package. We defined as ‘core’ the set of OTUs with at least 10 reads in at least 50% of the samples from each species, and as ‘specific’ the set of OTUs with at least 10 reads in at least 50% of the samples from one species, but absent from the other (i.e. present in less than 5% of the samples). OTUs lesser than 0.05%, in at least 20% of the samples were removed.
Electronic supplementary material
Acknowledgements
We thanks dr Tobias Weil from Edmund Mach Foundation for his critical review and comments. This work was carried out in the framework of Project LExEM (Laboratory of Excellence for Epidemiology and Modelling, http://www.lexem.eu), funded by a ‘Grandi Progetti’ grant from the Autonomous Province of Trento (Italy).
Author Contributions
F.R., V.T., D.A.l., C.D., A.R. conceived the experiment and drafted the paper. M.P. prepared the amplicon library and the sequencing database. D.A.l. and C.D. analysed the data. F.B. and D.A.r. collected the samples in the field.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Materials files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fausta Rosso and Valentina Tagliapietra contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34640-z.
References
- 1.Hulme PE. Invasive species challenge the global response to emerging diseases. Trends Parasitol. 2014;30:267–270. doi: 10.1016/j.pt.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Early R, et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016;7:12485. doi: 10.1038/ncomms12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin. Microbiol. Infect. 2013;19:685–692. doi: 10.1111/1469-0691.12189. [DOI] [PubMed] [Google Scholar]
- 4.Medlock JM, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J. Am. Mosq. Control Assoc. 1998;14:340–343. [PubMed] [Google Scholar]
- 7.Roiz D, Rosà R, Arnoldi D, Rizzoli A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis. 2010;10:811–816. doi: 10.1089/vbz.2009.0098. [DOI] [PubMed] [Google Scholar]
- 8.Capelli G, et al. First report in Italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasit. Vectors. 2011;4:188. doi: 10.1186/1756-3305-4-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montarsi F, et al. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species forEurope. Parasit. Vectors. 2015;8:177. doi: 10.1186/s13071-015-0800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutsevich, A. V., Monchadskii, A. S. & Shtakel’berg, A. A. Mosquitoes, family Culicidae. (Academy of Sciences of the USSR • Zoological Institute, 1971).
- 11.Suarez AV, Holway DA, Tsutsui ND. Genetics and behavior of a colonizing species: the invasive Argentine ant. Am. Nat. 2008;172(Suppl 1):S72–84. doi: 10.1086/588638. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barelli C, et al. Habitat fragmentation is associated to gut microbiota diversity of an endangered primate: implications for conservation. Sci. Rep. 2015;5:14862. doi: 10.1038/srep14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel P, Moran NA. The gut microbiota of insects - diversity in structure and function. FEMS Microbiol. Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 15.Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 16.Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011;27:514–522. doi: 10.1016/j.pt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jupatanakul N, Sim S, Dimopoulos G. The insect microbiome modulates vector competence for arboviruses. Viruses. 2014;6:4294–4313. doi: 10.3390/v6114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saraiva RG, Kang S, Simões ML, Angleró-Rodríguez YI, Dimopoulos G. Mosquito gut antiparasitic and antiviral immunity. Dev. Comp. Immunol. 2016;64:53–64. doi: 10.1016/j.dci.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Osei-Poku J, Mbogo CM, Palmer WJ, Jiggins FM. Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol. Ecol. 2012;21:5138–5150. doi: 10.1111/j.1365-294X.2012.05759.x. [DOI] [PubMed] [Google Scholar]
- 20.Minard, G. et al. French invasive Asian tiger mosquito populations harbor reduced bacterial microbiota and genetic diversity compared to Vietnamese autochthonous relatives. Front. Microbiol. 6 (2015). [DOI] [PMC free article] [PubMed]
- 21.Zouache K, et al. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol. Ecol. 2010;75:377–389. doi: 10.1111/j.1574-6941.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim C-H, Lampman RL, Muturi EJ. Bacterial Communities and Midgut Microbiota Associated with Mosquito Populations from Waste Tires in East-Central Illinois. J. Med. Entomol. 2015;52:63–75. doi: 10.1093/jme/tju011. [DOI] [PubMed] [Google Scholar]
- 23.Vayssier-Taussat M, et al. Shifting the paradigm from pathogens to pathobiome: new concepts in the light of meta-omics. Front. Cell. Infect. Microbiol. 2014;4:29. doi: 10.3389/fcimb.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montarsi F, et al. Distribution and habitat characterization of the recently introduced invasive mosquito Aedes koreicus [Hulecoeteomyia koreica], a new potential vector and pest in north-eastern Italy. Parasit. Vectors. 2013;6:292. doi: 10.1186/1756-3305-6-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boissière A, et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012;8:e1002742. doi: 10.1371/journal.ppat.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David MR, Santos LMBD, Vicente ACP, Maciel-de-Freitas R. Effects of environment, dietary regime and ageing on the dengue vector microbiota: evidence of a core microbiota throughout Aedes aegypti lifespan. Mem. Inst. Oswaldo Cruz. 2016;111:577–587. doi: 10.1590/0074-02760160238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raharimalala FN, Boukraa S, Bawin T, Boyer S, Francis F. Molecular detection of six (endo-) symbiotic bacteria in Belgian mosquitoes: first step towards the selection of appropriate paratransgenesis candidates. Parasitol. Res. 2016;115:1391–1399. doi: 10.1007/s00436-015-4873-5. [DOI] [PubMed] [Google Scholar]
- 28.Chavshin AR, et al. Identification of bacterial microflora in the midgut of the larvae and adult of wild caught Anopheles stephensi: a step toward finding suitable paratransgenesis candidates. Acta Trop. 2012;121:129–134. doi: 10.1016/j.actatropica.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Chavshin A, et al. Isolation and identification of culturable bacteria from wild Anopheles culicifacies, a first step in a paratransgenesis approach. Parasit. Vectors. 2014;7:419. doi: 10.1186/1756-3305-7-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchioffo MT, et al. Modulation of malaria infection in Anopheles gambiae mosquitoes exposed to natural midgut bacteria. PLoS One. 2013;8:e81663. doi: 10.1371/journal.pone.0081663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahia AC, et al. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ. Microbiol. 2014;16:2980–2994. doi: 10.1111/1462-2920.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Gilbreath TM, 3rd, Kukutla P, Yan G, Xu J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One. 2011;6:e24767. doi: 10.1371/journal.pone.0024767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crotti E, et al. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ. Microbiol. 2009;11:3252–3264. doi: 10.1111/j.1462-2920.2009.02048.x. [DOI] [PubMed] [Google Scholar]
- 34.Demaio J, Pumpuni CB, Kent M, Beier JC. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am. J. Trop. Med. Hyg. 1996;54:219–223. doi: 10.4269/ajtmh.1996.54.219. [DOI] [PubMed] [Google Scholar]
- 35.Pidiyar VJ, Jangid K, Patole MS, Shouche YS. Studies on cultured and uncultured microbiota of wild culex quinquefasciatus mosquito midgut based on 16s ribosomal RNA gene analysis. Am. J. Trop. Med. Hyg. 2004;70:597–603. doi: 10.4269/ajtmh.2004.70.597. [DOI] [PubMed] [Google Scholar]
- 36.Terenius O, et al. 16S rRNA gene sequences from bacteria associated with adult Anopheles darlingi (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2008;45:172–175. doi: 10.1093/jmedent/45.1.172. [DOI] [PubMed] [Google Scholar]
- 37.Zouache K, et al. Persistent Wolbachia and cultivable bacteria infection in the reproductive and somatic tissues of the mosquito vector Aedes albopictus. PLoS One. 2009;4:e6388. doi: 10.1371/journal.pone.0006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gusmão DS, et al. Culture-dependent and culture-independent characterization of microorganisms associated with Aedes aegypti (Diptera: Culicidae) (L.) and dynamics of bacterial colonization in the midgut. Acta Trop. 2010;115:275–281. doi: 10.1016/j.actatropica.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Rani A, Sharma A, Rajagopal R, Adak T, Bhatnagar RK. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 2009;9:96. doi: 10.1186/1471-2180-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaio AO, et al. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (diptera: culicidae) (L.) Parasit. Vectors. 2011;4:105. doi: 10.1186/1756-3305-4-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minard G, Mavingui P, Moro CV. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors. 2013;6:146. doi: 10.1186/1756-3305-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cirimotich CM, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Ceron L, Santillan F, Rodriguez MH, Mendez D, Hernandez-Avila JE. Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J. Med. Entomol. 2003;40:371–374. doi: 10.1603/0022-2585-40.3.371. [DOI] [PubMed] [Google Scholar]
- 44.Eappen AG, Smith RC, Jacobs-Lorena M. Enterobacter-activated mosquito immune responses to Plasmodium involve activation of SRPN6 in Anopheles stephensi. PLoS One. 2013;8:e62937. doi: 10.1371/journal.pone.0062937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apte-Deshpande AD, Paingankar MS, Gokhale MD, Deobagkar DN. Serratia odorifera mediated enhancement in susceptibility of Aedes aegypti for chikungunya virus. Indian J. Med. Res. 2014;139:762–768. [PMC free article] [PubMed] [Google Scholar]
- 46.Apte-Deshpande A, Paingankar M, Gokhale MD, Deobagkar DN. Serratia odorifera a Midgut Inhabitant of Aedes aegypti Mosquito Enhances Its Susceptibility to Dengue-2 Virus. PLoS One. 2012;7:e40401. doi: 10.1371/journal.pone.0040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen JH, Ralph Barr A. New Hypothesis of the Cause of Cytoplasmic Incompatibility in Culex pipiens L. Nature. 1971;232:657–658. doi: 10.1038/232657a0. [DOI] [PubMed] [Google Scholar]
- 48.Charan SS, Pawar KD, Severson DW, Patole MS, Shouche YS. Comparative analysis of midgut bacterial communities of Aedes aegypti mosquito strains varying in vector competence to dengue virus. Parasitol. Res. 2013;112:2627–2637. doi: 10.1007/s00436-013-3428-x. [DOI] [PubMed] [Google Scholar]
- 49.Stouthamer R, Breeuwer JA, Hurst GD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 50.Turley AP, Moreira LA, O’Neill SL, McGraw EA. Wolbachia Infection Reduces Blood-Feeding Success in the Dengue Fever Mosquito. Aedes aegypti. PLoS Negl. Trop. Dis. 2009;3:e516. doi: 10.1371/journal.pntd.0000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dobson SL, Marsland EJ, Rattanadechakul W. Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2001;38:382–387. doi: 10.1603/0022-2585-38.3.382. [DOI] [PubMed] [Google Scholar]
- 52.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 53.Martínez-Rodríguez P, Hernández-Pérez M, Bella JL. Detection of Spiroplasma and Wolbachia in the bacterial gonad community of Chorthippus parallelus. Microb. Ecol. 2013;66:211–223. doi: 10.1007/s00248-013-0226-z. [DOI] [PubMed] [Google Scholar]
- 54.Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blagrove MSC, Arias-Goeta C-B, Failloux A, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proceedings of the National Academy of Sciences. 2011;109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blagrove MSC, Arias-Goeta C, Di Genua C, Failloux A-B, Sinkins SP. A Wolbachia wMel Transinfection in Aedes albopictus Is Not Detrimental to Host Fitness and Inhibits ChikungunyaVirus. PLoS Negl. Trop. Dis. 2013;7:e2152. doi: 10.1371/journal.pntd.0002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Hurk AF, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bian G, Zhou G, Lu P, Xi Z. Replacing a Native Wolbachia with a Novel Strain Results in an Increase in Endosymbiont Load and Resistance to Dengue Virus in a Mosquito Vector. PLoS Negl. Trop. Dis. 2013;7:e2250. doi: 10.1371/journal.pntd.0002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glaser RL, Meola MA. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One. 2010;5:e11977. doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mousson L, et al. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl. Trop. Dis. 2012;6:e1989. doi: 10.1371/journal.pntd.0001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novakova E, et al. Mosquito Microbiome Dynamics, a Background for Prevalence and Seasonality of West NileVirus. Front. Microbiol. 2017;8:526. doi: 10.3389/fmicb.2017.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciocchetta S, et al. The new European invader Aedes (Finlaya) koreicus: a potential vector of chikungunya virus. Pathog. Glob. Health. 2018;112:107–114. doi: 10.1080/20477724.2018.1464780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chouaia B, et al. Molecular evidence for multiple infections as revealed by typing of Asaia bacterial symbionts of four mosquito species. Appl. Environ. Microbiol. 2010;76:7444–7450. doi: 10.1128/AEM.01747-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rossi P, et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit. Vectors. 2015;8:278. doi: 10.1186/s13071-015-0888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes GL, et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad. Sci. USA. 2014;111:12498–12503. doi: 10.1073/pnas.1408888111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruang-Areerate T, Kittayapong P. Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc. Natl. Acad. Sci. USA. 2006;103:12534–12539. doi: 10.1073/pnas.0508879103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitraka E, Stathopoulos S, Siden-Kiamos I, Christophides GK, Louis C. Asaia accelerates larval development of Anopheles gambiae. Pathog. Glob. Health. 2013;107:305–311. doi: 10.1179/2047773213Y.0000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chouaia B, et al. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol. 2012;12(Suppl 1):S2. doi: 10.1186/1471-2180-12-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rezza G, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 71.Cella E, et al. The new Chikungunya virus outbreak in Italy possibly originated from a single introduction from Asia. Pathog. Glob. Health. 2018;112:93–95. doi: 10.1080/20477724.2017.1406565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gobbi F, et al. Surveillance for West Nile, dengue, and chikungunya virus infections, Veneto Region, Italy, 2010. Emerg. Infect. Dis. 2012;18:671–673. doi: 10.3201/eid1804.110753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gobbi, F. et al. Human and entomological surveillance of West Nile fever, dengue and chikungunya in Veneto Region, Italy, 2010-2012. BMC Infect. Dis. 14 (2014). [DOI] [PMC free article] [PubMed]
- 74.Marcantonio M, et al. First assessment of potential distribution and dispersal capacity of the emerging invasive mosquito Aedes koreicus in Northeast Italy. Parasit. Vectors. 2016;9:63. doi: 10.1186/s13071-016-1340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Becker, N. et al. Mosquitoes and Their Control. (Springer Science & Business Media, 2010).
- 76.Mizrahi-Man O, Davenport ER, Gilad Y. Taxonomic classification of bacterial 16S rRNA genes using short sequencing reads: evaluation of effective study designs. PLoS One. 2013;8:e53608. doi: 10.1371/journal.pone.0053608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klindworth A, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albanese, D., Fontana, P., De Filippo, C., Cavalieri, D. & Donati, C. MICCA: a complete and accurate software for taxonomic profiling of metagenomic data. Sci. Rep. 5 (2015). [DOI] [PMC free article] [PubMed]
- 79.Edgar RC, Flyvbjerg H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics. 2015;31:3476–3482. doi: 10.1093/bioinformatics/btv401. [DOI] [PubMed] [Google Scholar]
- 80.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bokulich, N. A. et al. A standardized, extensible framework for optimizing classification improves marker-gene taxonomic assignments, 10.7287/peerj.preprints.934v1 (2015).
- 83.DeSantis TZ, et al. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 2006;34:W394–W399. doi: 10.1093/nar/gkl244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McMurdie PJ, Holmes S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oksanen J, et al. vegan: Community Ecology Package. R package version. 2017;2:4–3. [Google Scholar]
- 86.Kembel SW, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- 87.Jovel, J. et al. Characterization of the Gut Microbiome Using 16S or Shotgun Metagenomics. Front. Microbiol. 7 (2016). [DOI] [PMC free article] [PubMed]
- 88.De Cáceres M, Legendre P, Moretti M. Improving indicator species analysis by combining groups of sites. Oikos. 2010;119:1674–1684. doi: 10.1111/j.1600-0706.2010.18334.x. [DOI] [Google Scholar]
- 89.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bray JR, Roger Bray J, Curtis JT. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957;27:325–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 91.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Materials files).