Abstract
Purpose of review
Rhodiola rosea extracts have been used as a dietary supplement in healthy populations, including athletes, to non-specifically enhance the natural resistance of the body to both physical and behavior stresses for fighting fatigue and depression. We summarize the information with respect to the new pharmacological activities of Rhodiola rosea extracts and its underlying molecular mechanisms in this review article.
Recent findings
In addition to its multiplex stress-protective activity, Rhodiola rosea extracts have recently demonstrated its anti-aging, anti-inflammation, immunostimulating, DNA repair and anti-cancer effects in different model systems. Molecular mechanisms of Rhodiola rosea extracts’s action have been studied mainly along with one of its bioactive compounds, salidroside. Both Rhodiola rosea extracts and salidroside have contrast molecular mechanisms on cancer and normal physiological functions. For cancer, Rhodiola rosea extracts and salidroside inhibit the mTOR pathway and reduce angiogenesis through down-regulation of the expression of HIF-1α/HIF-2α. For normal physiological functions, Rhodiola rosea extracts and salidroside activate the mTOR pathway, stimulate paracrine function and promote neovascularization by inhibiting PHD3 and stabilizing HIF-1α proteins in skeletal muscles. In contrast to many natural compounds, salidroside is water-soluble and highly bioavailable via oral administration and concentrated in urine by kidney excretion.
Summary
Rhodiola rosea extracts and salidroside can impose cellular and systemic benefits similar to the effect of positive lifestyle interventions to normal physiological functions and for anti-cancer. The unique pharmacological properties of Rhodiola rosea extracts or salidroside deserve further investigation for cancer chemoprevention, in particular for human urinary bladder cancer.
Keywords: chemoprevention, Rhodiola, Salidroside, hypoxia, mTOR and DNA repair
1. Introduction
The majority of common cancers, such as bladder, breast, colon, kidney, lung, and prostate cancers occur mainly in older people [1–3]. Due to recent medical advances in treatments and diagnostic tools and the expansion of human life-span, the prevalence of cancer survivors is rapidly increasing in aging population [2, 3]. It is estimated that the number of cancer survivors grows up to 19 million by 2024 in the United States and that the global cancer burden increases 50% by 2020 [1]. This situation results in an exponential increase in health care costs and reduced access to good cancer care both in lesser and more developed nations, which is becoming a serious socioeconomic problem globally. Therefore, finding economically effective approaches to manage cancer in aging population becomes increasingly more pertinent as the population continues to age.
Cancer chemoprevention is a cost-effective approach to inhibit or delay the development and progression of cancer at its various stages by the use of natural, synthetic, or biological agents in order to reduce cancer incidence, morbidity, and mortality, as well as to improve overall quality of life [4, 5]. Cancer chemoprevention could yield significant reductions in cancer morbidity and mortality and disease burdens only by delaying the process of cancer development and progression a few years in the elderly [6]. Intake of beverages, fruits and vegetables in routine diets was reported to be associated with reduced cancer risk [7]. In addition, some traditional medicines (e.g. herbs) have been used for centuries with proven safety and health benefits [8–10]. Therefore, phytochemicals that are derived from dietary foods and herbs become safe and reliable compounds for cancer chemoprevention. The development of novel cancer preventive or treatment agents from these low cost phytochemicals may also contribute to the goal of ensuring that all people have access to cancer care compared to expensive and low accessible targeted therapies and precision medicine [11]. Idea cancer chemopreventive agents should have pleiotropic health benefits with minimal toxicities in the surrounding normal tissue [12]. In addition, cancer chemopreventive agents could be used in combination with chemotherapeutic agents for prevention of recurrence, optimizing health, enhancing quality of life, and managing cancer treatment related symptoms (e.g., fatigue, pain, neuropathy, lymphedema, difficult sleeping, weight gain, cognitive dysfunction, sexual dysfunction, fear of recurrence, stress and etc.) [13].
Rhodiola rosea L is a relatively rare and high-value medicinal plant and grows at high altitudes (up to 2280 m) in the arctic and mountainous regions throughout Europe, Asia and North America [14]. Rhodiola Rosea L. has been traditionally used as an “adaptogen” for enhancing physical and mental performance and fighting stress in healthy population for centuries [14]. Currently, Rhodiola Rosea extracts are used as a dietary supplement throughout Europe, Asia, and the United States for similar indications [15, 16]. Results from recent studies have revealed its wide variety of other medicinal properties and/or biological activities, which include anti-aging, anti-inflammation, anti-stresses, antioxidant, anti-viral and anti-cancer effects, as well as increasing immunity, enhancing DNA repair and modulating adaptation to hypoxia and angiogenesis [14, 17–21]. The unique biological activities of Rhodiola Rosea extracts or its active compounds for both enhancing normal physiological functions and anti-cancer effects support their promise for further cancer chemoprevention investigation. This review summarizes the information on biological activities of Rhodiola Rosea extracts and its main bioactive compound salidroside and their underlying mechanisms of action.
2. Rhodiola rosea L. and its active components
The genus Rhodiola (Crassulacea) consists of nearly 200 species [15, 16], in which at least 20 species are used in the traditional medicine of Russia, Scandinavia and Asia countries (e.g. China and India) for various health-promoting effects [15, 16]. The best known is Rhodiola rosea now cultivated also in Europe and North America, and present on the market as dietary supplement [15, 16]. Other Rhodiola species, including Rhodiola heterodonta, Rhodiola quadrifida, Rhodiola kirilowii, Rhodiola imbricata, Rhodiola algida, and Rhodiola crenulata also have been used in traditional herbal medicines in different regions of the world, such as the mountainous regions of Southwest China and the Himalayas, the alpine regions of Asia and Europe and Arctic regions of North America, but less studied [15, 16]. Traditionally, Rhodiola was an herbal medicine for a treatment for headaches, hysteria, “hernias”, and discharges, as well as for improving high-altitude sickness and as an astringent [14]. Recently, numerous Rhodiola extracts have been sold as a dietary supplement or as an adaptogen to increase attention and endurance in fatigue and to prevent/reduce stress induced impairments and disorders related to neuro-endocrine and immune system [14]. Athletes and Russian astronauts have used to prevent fatigue and improve performance as Rhodiola is allowed by sports regulators [22]. In addition, Rhodiola extracts were also indicated for age related conditions and depression [5, 21, 23]. Rhodiola has a history of centuries of folk use and has been the subject of many clinical studies. No side effects or interactions have been reported [24, 25].
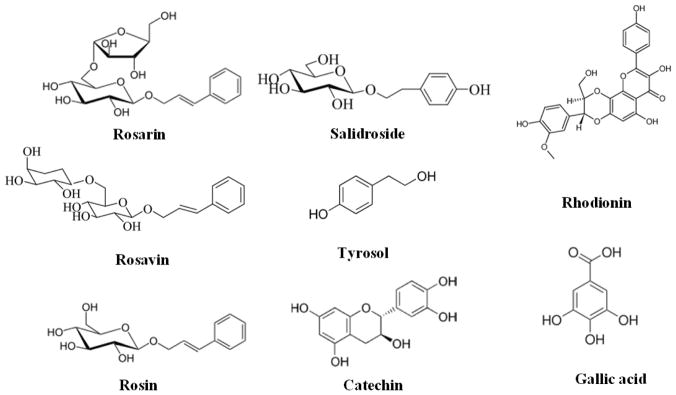
More than 140 compounds have been isolated from roots and rhizomes of Rhodiola species, including monoterpene alcohols and their glycosides, cyanogenicglycosides, arylglycosides, phenylethanoids, phenylpropanoids and their glycosides, flavonoids, flavonlignans, proanthocyanidins and gallic acid derivatives. The pharmacological and medicinal properties of Rhodiola are species-dependent phenomena [26, 27]. A variety of co-occurring phytochemical constituents in the plant may also be responsible for their unique pharmacological activity of different Radiola species. [14, 28–35]. Therefore, eight compounds, including rosarin, rosavin, rosin, salidroside, tyrosol, rhodionin, catechin and gallic acid (Figure 1), have been suggested as reference markers for distinguishing Rhodiola species from other plants [36]. Salidroside, is present in all species of the Rhodiola genus and in a wide variety of species outside this genus, while the rosavins (rosavin, rosin, rosarin) are specific components of Rhodiola Rosea [14, 28–35]. The naturally occurring ratio of rosin and its derivatives and salidroside was estimated to be approximately 3:1. In order to mimic this ratio, standardized Rhodiola rosea extracts contain a minimum of 3% rosin and its derivatives and 0.8–1% salidroside for the most experimental studies in the literatures. Of all the Rhodiola species, Rhodiola rosea L. has been the predominant subject of phytochemical, animal, and human studies [37–50]. About 51% of all animal studies and 94% of all human studies on Rhodiola used Rhodiola rosea species.
Fig. 1.
Compounds of Reference Markers for Rhodiola species
SHR-5 is a standardized Rhodiola rosea extract and manufactured according to Good Manufacturing Practice by the Swedish Herbal Institute (the SHI, Gothenburg, Sweden). SHR-5 has passed extensive toxicological studies and has been certified safe for both animals and humans [51, 52]. The typical amounts of Rhodiola rosea extract are 200 – 600 mg per day in capsules or tablets [14]. SHR-5 has been commercialized since 1985 and numerous clinical trials have been carried out with SHR-5 [25, 43–44, 51, 52]. Several clinical trials have shown that SHR-5 improved mental performance and attention in cognitive function in fatigue after single and repeated administration [44, 53, 54], and prevented physical, emotional, and mental exhaustion in patients with fatigue syndrome [43]. SHR-5 was also shown to be effective in the treatment of mild to moderate depression [42] and generalized anxiety [54, 55] in clinical trials. Due to the rapidly growing demand for Rhodiola-based products on the market during a past few years, Rhodiola has been considered to be endangered plant species in many countries [16].
3. The anti-stress properties of Rhodiola rosea L
Rhodiola rosea extracts act as an adaptogen to provide nonspecific resistance to physical, chemical and biological stresses [56–60]. The stress-protective effects of Rhodiola rosea extracts have been shown to be engaged with the hypothalamic-pituitary-adrenal (HPA) axis [61] and several key mediators of stress responses, such as heat shock proteins (HSP) [56, 62], stress-activated c-JUN N-terminal protein kinase 1(JNK1) [63], Forkhead box O (FOXO) transcription factor DAF-1[39], cortisol [63], nitric oxide [63] and beta-endorphine [64]. Xia et al [65] reported that Rhodiola rosea extracts reduced the serum levels of corticotropin-releasing hormone and corticosterone via down-regulating the expression of c-FOS in the hypothalamus of rats subjected to stress. It was also showed that Rhodiola rosea extract treatment of pond snail Limnaea stagnalis larvae resulted in the resistance to both 600 μM menadione and heat shock under 43°C [66]. Rhodiola rosea extract also enhanced the stress resistance in the silkworm, against heat stress (37 °C) and starvation [67]. In C. elegans, 10–25 microg/ml Rhodiola rosea extracts increased stress resistance against a relatively short period of time of heat shock (35 °C for 3 hours) as well as chronic heat treatment at 26 °C by activating DAF-16/FOXO via promoting its nuclear translocation [39]. Rhodiola rosea extract also protected C2C12 myotubes from oxidative stress by increasing the expression of HSP70 and HSP72 and the release of neuropeptide Y (NPY) [56, 14]. Salidroside as a predominant compound in Rhodiola rosea extracts protected against beta-amyloid (Abeta) peptide induced oxidative stress by inhibiting its mediated phosphorylation of JNK and p38 MAP kinase, but not ERK1/2, which suggested the usefulness of salidroside for treating or preventing neurodegenerative diseases.
The chemical structures of the main bioactive compounds in Rhodiola rosea extracts, rosin and its derivatives and salidroside, contain phenolic hydroxyl groups and unsaturated bonds. These compounds were shown to be effective at scavenging reactive oxygen species (ROS). In addition, Rhodiola rosea extracts and salidroside was able to increase the expression of antioxidant enzymes (e.g. GPx) and activate the nuclear erythroid 2-related factor 2 (Nrf2) pathways in rats to protect against bleomycin-induced pulmonary fibrosis in rats [68] and to reverse ultraviolet B induced DNA damages in HaCaT cells [69], respectively.
Chronic or long-term stress can lead to symptoms like anxiety, depression, sleep problems and weak immune system, as well as disease status such as cardiovascular and metabolic diseases [71, 72]. There is increasing data that indicate an intriguing relationship between stress resistance and slowed-aging although the causal effects between two remain unclear [70, 71]. Stress resistance is believed to be related to hallmarks of aging, including altered intra- and intercellular communication, dysregulated nutrient sensing, mitochondrial health, cell senescence, stem cell exhaustion, genomic instability (DNA damage), telomere attrition, and certain patterns of gene expression [70–72]. Moreover, accumulating evidences have supported that chronic stress promote cancer progression in many experimental models [73–76]. Based on these results, the unique property of Rhodiola rosea extracts for enhancing resistance to general stresses deserves its further investigation in both anti-aging and cancer prevention.
4. The anti-aging effect of Rhodiola rosea L
Rhodiola rosea extracts can extend lifespan in a range of model organisms, such as fruit flies, worms, and yeast [23, 38, 39, 67, 77–82], without affecting its daily food intake, body weight, or fecundity. Rhodiola rosea extract also delayed the age-related decline of physical activity and immune functions, and increased stress resistance [78–81]. Rhodiola rosea extract SHR-5 was shown to increase the mean and maximum life-span of the fruit fly up to 24% and 31%, respectively [23]. Rhodiola rosea extracts can extend lifespan at different caloric levels. The effect of R. rosea extracts on lifespan was independent of caloric restriction-related signaling pathways, including SIR2 proteins, insulin and insulin-like growth factor signaling, and the TOR in fruit flies [77, 82], but dependent on diet composition (in particular protein-to-carbohydrate ratios or sucrose contents) and expression of Msn2/Msn4 and Yap1 regulatory proteins [80]. The lifespan extension of Rhodiola rosea extracts was not seen in diets with high protein-to-carbohydrate ratios [77, 82]. In addition, the physiological state of an organism affected the beneficial effect of Rhodiola rosea extracts on longevity [81]. Individuals with moderate robustness appears to benefit most from the intake of Rhodiola rosea extracts [81]. Aqueous Rhodiola rosea extracts also exhibited a concentration-dependent effect on long-term survival and stress resistance of budding yeast Saccharomyces cerevisiae: Low concentrations increased yeast lifespan, whereas high concentrations shortened yeast lifespan [80]. Nevertheless, mechanisms for the anti-aging effects of Rhodiola rosea extracts are still largely unknown. In addition, the anti-aging properties of Rhodiola rosea extracts are necessary to be tested in animals before its translation into human studies.
5. The anti-cancer effects of Rhodiola rosea L
There are several studies demonstrating the anticancer activities of Rhodiola rosea extracts. Udintsev SN et al [83] showed that Rhodiola rosea extracts inhibited the growth of transplanted solid Ehrlich adenocarcinoma and Pliss lymphosarcoma, decreased their metastases to the liver, and extended survival time of rats bearing the tumors. In a model which angiogenesis was induced in the skin of Balb/c mice by grafting of syngeneic L-1 sarcoma cells [84], R. quadrifida extract and salidroside highly significantly reduced tumor-induced angiogenesis. In addition, Rhodiola rosea extracts in combination with the anti-tumor agent cyclophosphamide resulted in enhanced anti-tumor and anti-metastatic efficacy of drug treatment, as well as reduced drug-induced toxicity [85]. In cell culture studies, Rhodiola rosea inhibits cell proliferation and induces cell apoptosis in various cells and cell lines, including human urinary bladder cancer cell lines [86], breast cancer cell lines [87, 88], colorectal cancer cells [89], gastric cancer cells [90], glioma cells [91], lung cancer cells [92], and sarcoma [93]. Among them, estrogen receptor negative breast cancer cell line MDA-MB-231 and lung cancer cell line A549 appeared to be more sensitive to the cytotoxic effect of salidroside with IC50 of 10.7 and 14.3 μM, respectively [94]. However, given data on the in vivo anti-tumor activity of Rhodiola rosea extracts and salidroside against different cancers are currently very limited, it is still unclear whether specific types of cancer may be particularly responsive to the anti-cancer effect of Rhodiola rosea extracts and salidroside. We and others also showed that Rhodiola rosea extracts and salidroside induced autophagy in bladder, gastric and colorectal cancer cell lines [86, 89, 90]. Tu et al [95] showed that R. crenulata extracts inhibited the proliferation, motility, and invasion of breast cancer cell lines with minimal effect on normal human mammary epithelial cells. Mishra KP et al [96] reported that aqueous R. crenulata extract inhibited growth of an erythro leukemic cell line K-562 by inducing apoptosis and cell cycle arrest at G2/M phase. Anecdotal evidence from a clinical study [45] showed that oral administration of Rhodiola rosea extracts to patients with superficial bladder carcinoma (T1, G1–2) reduced the average frequency of relapses by half. Nevertheless, none of these above studies have linked the anticancer effects of Rhodiola to its antiaging effect. It is also unknown whether active compounds (Salidroside or Rosavin) or the ratio of active compounds in Rhodiola rosea extracts will be determining factors for its anti-cancer activities.
6. Mechanisms of Rhodiola rosea L and its active components
Several molecular mechanisms of action that could potentially responsible for the observed stress resistance, anti-aging and anti-cancer effects of Rhodiola rosea extracts and its active compounds have been identified in in vitro cell culture systems and in vivo animal models. The biological mechanisms of each of active compounds have both similar to and different from that of the Rhodiola rosea extracts [97]. Rhodiola rosea extracts and the main bioactive compound salidroside appears to have multi-targeted effects. Here, we summarized the specific signaling pathways and molecular networks associated with health beneficial effects of Rhodiola rosea extracts and salidroside.
6.1. The mTOR pathway
There have been numerous reports of the mTOR pathway promoting aging in different model organisms [98, 99]. In addition, components of the mTOR pathway are major targets for developing new agents for cancer prevention and treatment [100, 101]. We have shown that Rhodiola rosea extracts SHR-5 and its active compound salidroside inhibited the mTOR pathway in bladder cancer cell lines via activation of AMP-activated protein kinase (AMPK)-α [86]. The growth inhibitory effects of Rhodiola rosea extract and salidroside on human bladder cancer cells were, partly, dependent on TSC2 expression [86]. Our results suggested that the Rhodiola rosea extract and salidroside may play an important role in regulation of TCS2 expression for the anti-proliferative effect in bladder cancer cell lines. More importantly, we have shown that 93% of homozygous mutant Ha-ras male transgenic mice which drank 0.625% Rhodiola rosea extract SHR-5 in the drinking water survived up to 6 months, whereas 42% of which drank normal water died due to mutant H-ras transgene driven bladder specific tumor development [102]. In addition, the mean bladder weights (as a surrogate for tumor burden) in male mice drinking Rhodiola rosea extract SHR-5 decreased by 69% compared to those who drank normal water [102]. This result indicates a potent in vivo antitumor activity of the Rhodiola rosea extract SHR-5 in a transgenic model of urinary bladder cancer. Fan et al [89] also reported that salidroside induced autophagy and apoptosis and inhibited the phosphorylation of PI3K, Akt and mTOR in human colorectal cancer cells.
On the contrary, salidroside can activate the mTOR pathway to promote bone marrow mesenchymal stem cells differentiation into neural cells [103] and to attenuate cobalt chloride-induced ultrastructural damage of the mitochondria and ROS production in PC12 differentiated cells [104], suggesting that salidroside may protect brain neurons from ischemic injury through activation of the mTOR pathway. In addition, salidroside was shown to promote angiogenic differentiation of human bone marrow-derived endothelial progenitor cells [105] and to protect against oxidative endothelial injury by activation of the mTOR pathway [105–107]. Salidroside also inhibited CT-26 and Lewis lung carcinoma tumor-induced cachexia and the loss of tumor-free body weight, adipose and gastrocnemius muscles, as well as extended the survival of the treated mice by increasing the expression of phosphorylated mTOR both in C2C12 myotubes and in gastrocnemius muscle of the mice [108]. These results suggested that salidroside has a text-dependent effect on the mTOR pathway. It is likely that in normal cells, salidroside activate the mTOR pathway to protect and repair neurons, vasculatures and muscles, whereas in cancer cells it inhibited the mTOR pathway for reducing their growth.
6.2. DNA repair
Salikhova et al [109] described that Rhodiola rosea extracts functioned as an anti-mutagens to reduce chromosome aberrations, micronuclei formation and unscheduled DNA synthesis, which were induced by cyclophosphamide and N-nitroso-N-methylurea. Li et al [110] observed that salidroside specifically failed to inhibit H2O2-induced DNA strand breaks in hematopoietic stem cells (HSCs) from mice with poly(ADP-ribose)polymerase-1 (PARP-1), a component of the base excision repair pathway, deletion, but not those with deficiency in homologous recombination, nonhomologous end-joining, nucleotide excision repair, or fanconi anemia pathway. In addition, they observed that salidroside enhanced the PARP-1 activity to prevent quiescent HSCs from oxidative stress-induced cycling and subsequent exhaustion in native animals and self-renewal defect in transplanted recipients [111]. Further study by the same group demonstrated the binding of salidroside to the tryptophan-glycine-arginine-rich domain of PARP-1[111].
6.3. Hypoxia and Angiogenesis
Rhodiola is well known for its functions in enhancing adaptation to high-altitude and hypoxic conditions. The master regulators of the adaptive response to hypoxia are believed to be heterodimeric transcription factors consisting of O2-regulated α subunits (HIF1A/HIF-1α or EPAS1/HIF-2α), and a constitutively expressed ARNT/HIF-1β subunit [112]. Qi et al [113] reported that an aqueous extract of a Tibetan herb, Rhodiola algida var. tangutica down-regulated the expression of HIF-1α and -2α under hypoxic conditions in breast cancer MCF7 cells. In an in vitro studies using co-culturing of mouse endothelial and L-1 sarcoma cells, Rhodiola extracts inhibited proliferation and migration of endothelial cells [84]. Rhodiola extracts and salidroside and rosavin were also shown to inhibit in vivo neovascularization that was induced by the implantation of syngeneic tumor cells [84] or human kidney cancer homogenate [114].
In contrast to the limited studies of Rhodiola on hypoxia and cancer, there are more studies focused on Rhodiola extracts and its active compounds (mainly salidroside) for protecting against hypoxia-induced damage of normal functions and on the underlying mechanisms of actions [115–123]. Salidroside was shown to increase the expression of erythropoietin (EPO) mRNA by inducing the accumulation of HIF-1α protein, but not HIF-2α in human embryonic kidney fibroblast (HEK293T) and human hepatocellular carcinoma (HepG2) cells [115, 118, 119]. HIF-1α/HIF-2α) are tightly regulated by the HIF-prolyl hydroxylases (PHD) [124]. Zhang et al [125] have demonstrated that salidroside specifically reduced the mRNA and protein expression PHD3, but not PHD1 and PHD2 through its binding to estrogen receptor alpha (ERα) and inhibition of ERα mediated PHD3 transcription. In addition, intramuscular administration of salidroside resulted in a robust increase in neovascularization and blood perfusion recovery in a mouse model of hind-limb ischemia through promoting skeletal muscle cell migration and FGF2/FGF2R and PDGF-BB/PDGFR-β pathways mediated paracrine function [125].
6.4. Immunity
Rhodiola rosea extracts have been documented with immunostimulating activity both in vitro in human peripheral blood mononuclear cells (PBMCs) and in vivo in animals [126–133]. Rhodiola rosea extracts increased total CD3+ and memory CD4+ T cell pools, but decreased the number and function of cytotoxic CD8+ T cells in a mouse model of caecal ligation and puncture-induced sepsis [131]. Rhodiola rosea extracts increased production of Th1 cytokines (i.e. IFN-γ, IL-2 and IL-12), reduced spleen and thymus lymphocyte apoptosis and enhanced their survival through downregulation of tumor necrosis factor-α-induced protein 8-like-2 expression in septic rats [131]. Salidroside not only improved total T cell (CD3+) and Th1 cells (CD4+), but also promoted the production of immunoglobulins (total IgG, IgG1 and IgG2α) for the delayed-type hypersensitivity response to vaccine in aged, 21 months old rats [126]. In addition, Rhodiola rosea extracts and salidroside exerted vaccine adjuvant effect by stimulating the proliferation of concavalin A treated T cells in ovalbumin-immunized mice [127]. Furthermore, salidroside and the antigen ovalbumin have been encapsulated into liposome for investigating its usefulness as an effective sustained-release vaccine delivery system [128, 129]. The salidroside liposome adjuvant was shown to stimulate dendritic cells on mixed leukocyte reaction and improve the antigen presenting ability and maturation of dendritic cells in vitro [128]. This adjuvant also led to a marked Th1 immunostimulant activity in mice by increasing lymphocyte proliferation and serum concentrations of IgG, IL-2, and IFN-γ [128].
6.5 Inflammation
Rhodiola rosea extracts first demonstrated its anti-inflammation activity in formaldehyde-induced arthritis, carrageenan- and nystatin-induced paw oedema at a concentration of 250 mg/kg body weight in rat model [49]. Rhodiola rosea extracts more effectively inhibited cyclooxygenase-2 (COX-2) and Phospholipase A2 activity than COX1 activity [49]. Salidroside also exerted its anti-inflammatory effect by inhibiting the production of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) through the blocking of the NF-κB and MAPK signaling pathways both in vitro in RAW 264.7 macrophages and in vivo in mice challenged with lipopolysaccharide [134, 135]. Further studies revealed that salidroside inhibited the activation of NLRP3 inflammasome and subsequent caspase-1 cleavage as well as IL-1β secretion both in vivo and in vitro through up-regulation of SIRT1 expression [136]. In addition, salidroside can inhibit the JAK2-STAT3 pathway by suppressing nuclear localization of STAT3 [137].
7. Pharmacokinetics
The pharmacokinetics studies of Rhodiola rosea extracts have been mainly focused on one of its active compound salidroside. The pharmacokinetic parameters of salidroside and its bio-distribution and metabolism have been studied in mice, rats and beagle dogs by using liquid chromatography–electrospray ionization-mass spectrometry (LC–ESI-MS), on-line solid-phase extraction integrated with high-performance liquid chromatography (HPLC)-ESI-MS and other methods [138–143]. Oral or intravenous administration of Rhodiola extracts or salidroside at a single dose ranging from 12 mg/kg to 75 mg/kg can achieve plasma concentrations of 4.3 to 96.2 microgram/mL, which are dependent on animal species, dosages and administration routes [139–143]. In a study by Wang et al [144] for investigating the potential anti-diabetic effects of salidroside, mice drank 0.3 mg/ml salidroside containing water daily and were fed with high fat diet for 48 days. At the end of the experiment, the plasma salidroside concentration was measured to be 52.8 ± 9.4 μg/ml (~175 μM). This result suggested that the frequency of salidroside administration may also determine its achievable plasma concentrations. Salidroside exhibited rapid oral absorption and elimination by kidney clearance [142, 143]. The oral bioavailability of salidroside varied upon different dosages, ranging from 32.1% to 98% [142, 143]. Salidroside was shown to be well distributed into the skeletal muscle, fat, ovary and testis. Salidroside has high urinary excretion [142, 143]. Approximately 64.00% and 23.80% of the intravenously or orally administrated salidroside were excreted through urine in the form of salidroside, respectively, whereas their biliary excretion were about 2.86% and 0.02% of the dose, respectively [142, 143]. The peak urine concentration of salidroside in this study was estimated to be more than 448.4 μg/ml urine [142]. Salidroside was extensively metabolized by the liver to its aglycone p-tyrosol and distributed to various organs [142].
8. Conclusions and Perspectives
Cancer is an age-related disease. Interventions that slow down aging may also delay cancer. In addition, with advancing age, patients with cancer have a dramatically increase in the number of clinical relevant comorbidities, which increases complication rates and directly affect the choice of treatments and the quality of patients’ life. Given potential anti-aging agents are required to be non-toxic to healthy individuals for long term use, certain anti-aging agents, for examples, metformin, aspirin/ and resveratrol, could be considered ideal cancer chemopreventive candidates in the elderly [5].
Rhodiola rosea L, a popular herb plant, is native to the high altitude regions of Asia, Europe and Northern Hemisphere. Rhodiola rosea extracts have a long history of use as an “Adaptogen” to non-specifically enhance the resistance of the body to both physical and emotional stresses for fighting fatigue and depression. Accumulating evidences have demonstrated that Rhodiola rosea extracts have strong anti-aging effects in different model organisms, such as fruit flies, worms, and yeast. The mechanisms of Rhodiola rosea extracts’ anti-aging effects remain unclear. Currently, there is strong evidence that stress resistance contributes to both slowed aging and cancer progression in model systems, even though the current data are unable to establish a clear causal relationship. Further works are needed to be focused on elucidating the relationship between the anti-multiplex stress properties of Rhodiola rosea extracts and its anti-aging and anti-cancer effects.
Current knowledge for the mechanisms of Rhodiola rosea extracts’ action was mostly derived from the studies with one of its main bioactive compound, salidroside. Rhodiola rosea extracts and salidroside appear to have differential mechanistic effects on cancer and normal physiological functions. For cancer cells, Rhodiola rosea extracts and salidroside inhibit the mTOR pathway and angiogenesis and down-regulation of HIF-1α/HIF-2α expression. For normal physiological function, Rhodiola rosea extracts and salidroside stimulate paracrine function and promote neovascularization by inhibiting PHD3 and stabilizing HIF-1α proteins. In addition, Rhodiola rosea extracts and salidroside have demonstrated immunostimulating activity anti-inflammation functions both in vitro in cell cultures and in vivo in animals. Salidroside can function to have vaccine adjuvant effects.
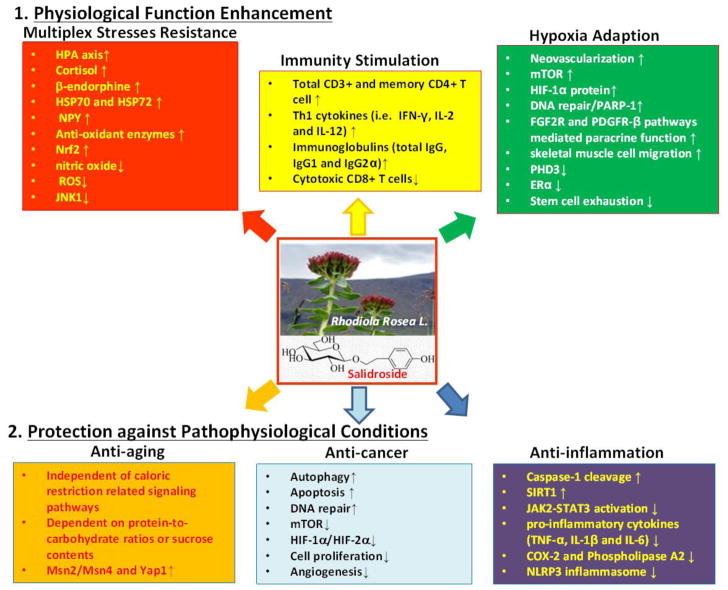
In conclusion, Rhodiola rosea extracts and salidroside are unique chemopreventive agents, which not only have anti-cancer and anti-inflammation activity, but also strengthen or stimulate normal physiological functions, such immunity, stress response and DNA repair. Rhodiola rosea extracts and salidroside could confer cellular and systemic benefits of metabolism similar to the effect of positive lifestyle interventions. The pharmacological properties of Rhodiola rosea extracts and salidroside and their mechanisms of action are summarized as Fig. 2. In contrast to the poor bioavailability of most natural compounds, salidroside is a water-soluble compound, which has rapid oral absorption and high bioavailability. Salidroside is excreted by the kidney and highly concentrated in urine. Urinary bladder could be an idea organ site for cancer chemoprevention by using Rhodiola rosea extracts and salidroside.
Fig. 2.
Schematic presentation of pharmacological properties of Rhodiola Rosea extracts and salidroside and corresponding mechanisms of action. Rhodiola Rosea extracts and salidroside act to stimulate or enhance normal physiological functions and protect against pathophysiological development and progression.
Acknowledgments
This work was supported in part by NIH award 1R01CA193967-01A1and 1R21CA152804-01A1 (to X. Zi.). Victor Pham is currently supported by NSF graduate research fellowship program DGE-1321846.
Footnotes
Conflict of Interest Statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1**.American Cancer Society. Global Cancer Facts & Figures. (3) 2017 Jun 28; https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-3rd-edition.pdf.
- 2.Johansen NJ, Saunders CM. Value-Based Care in the Worldwide Battle Against Cancer. Cureus. 2017;9:e1039. doi: 10.7759/cureus.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global Burden of Urologic Cancers, 1990–2013. Eur Urol. 2017;71:437–446. doi: 10.1016/j.eururo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Kotecha R, Takami A, Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016 Aug 9;7(32):52517–52529. doi: 10.18632/oncotarget.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Yokoyama NN, Denmon A, Uchio EM, Jordan M, Mercola D, Zi X. When Anti-Aging Studies Meet Cancer Chemoprevention: Can Anti-Aging Agent Kill Two Birds with One Blow? Curr Pharmacol Rep. 2015;1:420–433. doi: 10.1007/s40495-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Fahey JW, Kensler TW. Health span extension through green chemoprevention. Virtual Mentor. 2013;15:311–8. doi: 10.1001/virtualmentor.2013.15.4.stas1-1304. [DOI] [PubMed] [Google Scholar]
- 7.Vieira AR, Abar L, Chan D, Vingeliene S, Polemiti E, Stevens C, Greenwood D, Norat T. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017 doi: 10.1093/annonc/mdx171. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Madka V, Rao CV. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr Cancer Drug Targets. 2013;13:542–57. doi: 10.2174/15680096113139990036. [DOI] [PubMed] [Google Scholar]
- 9.Samanta SK, Sehrawat A, Kim SH, Hahm ER, Shuai Y, Roy R, Pore SK, Singh KB, Christner SM, Beumer JH, Davidson NE, Singh SV. Disease Subtype-Independent Biomarkers of Breast Cancer Chemoprevention by the Ayurvedic Medicine Phytochemical Withaferin A. J Natl Cancer Inst. 2016:109. doi: 10.1093/jnci/djw293. pii: djw293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Xu X, Li X, Liu S, Simoneau AR, He F, Wu XR, Zi X. Kava chalcone, flavokawain A, inhibits urothelial tumorigenesis in the UPII-SV40T transgenic mouse model. Cancer Prev Res (Phila) 2013;6:1365–75. doi: 10.1158/1940-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart BW, Bray F, Forman D, Ohgaki H, Straif K, Ullrich A, Wild CP. Cancer prevention as part of precision medicine: ‘plenty to be done’. Carcinogenesis. 2016;37:2–9. doi: 10.1093/carcin/bgv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adhami VM, Mukhtar H. Human cancer chemoprevention: hurdles and challenges. Top Curr Chem. 2013;329:203–20. doi: 10.1007/128_2012_342. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Parmakhtiar B, Simoneau AR, Xie J, Fruehauf J, Lilly M, Zi X. Lycopene enhances docetaxel’s effect in castration-resistant prostate cancer associated with insulin-like growth factor I receptor levels. Neoplasia. 2011;13:108–19. doi: 10.1593/neo.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17:481–93. doi: 10.1016/j.phymed.2010. [DOI] [PubMed] [Google Scholar]
- 15.Booker A, Jalil B, Frommenwiler D, Reich E, Zhai L, Kulic Z, Heinrich M. The authenticity and quality of Rhodiola rosea products. Phytomedicine. 2016;23:754–62. doi: 10.1016/j.phymed.2015. [DOI] [PubMed] [Google Scholar]
- 16*.Xin T, Li X, Yao H, Lin Y, Ma X, Cheng R, Song J, Ni L, Fan C, Chen S. Survey of commercial Rhodiola products revealed species diversity and potential safety issues. Sci Rep. 2015;5:8337. doi: 10.1038/srep08337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna K, Mishra KP, Ganju L, Singh SB. Golden root: A wholesome treat of immunity. Biomed Pharmacother. 2017;87:496–502. doi: 10.1016/j.biopha.2016.12.132. [DOI] [PubMed] [Google Scholar]
- 18.Radomska-Leśniewska DM, Skopiński P, Bałan BJ, Białoszewska A, JóŸwiak J, Rokicki D, Skopińska-Różewska E, Borecka A, Hevelke A. Angiomodulatory properties of Rhodiola spp. and other natural antioxidants. Cent Eur J Immunol. 2015;40:249–62. doi: 10.5114/ceji.2015.52839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosanovic D, Tian X, Pak O, Lai YJ, Hsieh YL, Seimetz M, Weissmann N, Schermuly RT, Dahal BK. Rhodiola: an ordinary plant or a promising future therapy for pulmonary hypertension? a brief review. Pulm Circ. 2013;3:499–506. doi: 10.1086/674303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishaque S, Shamseer L, Bukutu C, Vohra S. Rhodiola rosea for physical and mental fatigue: a systematic review. BMC Complement Altern Med. 2012;12:70. doi: 10.1186/1472-6882-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amsterdam JD, Panossian AG. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine. 2016;23:770–83. doi: 10.1016/j.phymed.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Parisi A, Tranchita E, Duranti G, Ciminelli E, Quaranta F, Ceci R, Cerulli C, Borrione P, Sabatini S. Effects of chronic Rhodiola Rosea supplementation on sport performance and antioxidant capacity in trained male: preliminary results. J Sports Med Phys Fitness. 2010;50:57–63. [PubMed] [Google Scholar]
- 23.Jafari M, Felgner JS, Bussel II, Hutchili T, Khodayari B, Rose MR, Vince-Cruz C, Mueller LD. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. 2007;10:587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- 24.Mao JJ, Xie SX, Zee J, Soeller I, Li QS, Rockwell K, Amsterdam JD. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine. 2015;22:394–9. doi: 10.1016/j.phymed.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panossian A, Hovhannisyan A, Abrahamyan H, Gabrielyan E, Wikman G. Pharmacokinetic and pharmacodynamic study of interaction of Rhodiola rosea SHR-5 extract with warfarin and theophylline in rats. Phytother Res. 2009;23:351–7. doi: 10.1002/ptr.2631. [DOI] [PubMed] [Google Scholar]
- 26.Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6:293–302. [PubMed] [Google Scholar]
- 27.Yousef GG, Grace MH, Cheng DM, Belolipov IV, Raskin I, Lila MA. Comparative phytochemical characterization of three Rhodiola species. Phytochemistry. 2006;67:2380–91. doi: 10.1016/j.phytochem.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Ali Z, Fronczek FR, Khan IA. Phenylalkanoids and monoterpene analogues from the roots of Rhodiola rosea. Planta Med. 2008;74:178–81. doi: 10.1016/j.phytochem.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Ma G, Li W, Dou D, Chang X, Bai H, Satou T, Li J, Sun D, Kang T, Nikaido T, Koike K. Rhodiolosides A-E, monoterpene glycosides from Rhodiola rosea. Chem Pharm Bull (Tokyo) 2006;54:1229–33. doi: 10.1248/cpb.54.1229. [DOI] [PubMed] [Google Scholar]
- 30.Ming DS, Hillhouse BJ, Guns ES, Eberding A, Xie S, Vimalanathan S, Towers GH. Bioactive compounds from Rhodiola rosea (Crassulaceae) Phytother Res. 2005;19:740–3. doi: 10.1002/ptr.1597. [DOI] [PubMed] [Google Scholar]
- 31.Tolonen A, Pakonen M, Hohtola A, Jalonen J. Phenylpropanoid glycosides from Rhodiola rosea. Chem Pharm Bull (Tokyo) 2003;51:467–70. doi: 10.1248/cpb.51.467. [DOI] [PubMed] [Google Scholar]
- 32.Rhodiola rosea Monograph. Altern Med Rev. 2002;7:421–3. No authors listed. [PubMed] [Google Scholar]
- 33**.Ganzera M, Yayla Y, Khan IA. Analysis of the marker compounds of Rhodiola rosea L. (golden root) by reversed phase high performance liquid chromatography. Chem Pharm Bull (Tokyo) 2001;49:465–7. doi: 10.1248/cpb.49.465. [DOI] [PubMed] [Google Scholar]
- 34.Yu WS, Chen XM, Li H, Yang L. Polyphenols from Rhodiola crenulata. Planta Med. 1993;59:80–2. doi: 10.1055/s-2006-959610. [DOI] [PubMed] [Google Scholar]
- 35.Mao Y, Li Y, Yao N. Simultaneous determination of salidroside and tyrosol in extracts of Rhodiola L. by microwave assisted extraction and high-performance liquid chromatography. J Pharm Biomed Anal. 2007;45:510–5. doi: 10.1016/j.jpba.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Grech-Baran M, Sykłowska-Baranek K, Pietrosiuk A. Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures. Phytochem Rev. 2015;14:657–674. doi: 10.1007/s11101-014-9368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schriner SE, Abrahamyan A, Avanessian A, Bussel I, Maler S, Gazarian M, Holmbeck MA, Jafari M. Decreased mitochondrial superoxide levels and enhanced protection against paraquat in Drosophila melanogaster supplemented with Rhodiola rosea. Free Radic Res. 2009;43:836–43. doi: 10.1080/10715760903089724. [DOI] [PubMed] [Google Scholar]
- 38.Bayliak MM, Lushchak VI. The golden root, Rhodiola rosea, prolongs lifespan but decreases oxidative stress resistance in yeast Saccharomyces cerevisiae. Phytomedicine. 2011;18:1262–8. doi: 10.1016/j.phymed.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 39**.Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- 40.Yin D, Yao W, Chen S, Hu R, Gao X. Salidroside, the main active compound of Rhodiola plants, inhibits high glucose-induced mesangial cell proliferation. Planta Med. 2009;75:1191–5. doi: 10.1055/s-0029-1185717. [DOI] [PubMed] [Google Scholar]
- 41.van Diermen D, Marston A, Bravo J, Reist M, Carrupt PA, Hostettmann K. Monoamine oxidase inhibition by Rhodiola rosea L roots. J Ethnopharmacol. 2009;122:397–401. doi: 10.1016/j.jep.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 42**.Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmström C, Panossian A. Clinical trial of Rhodiola rosea L extract SHR-5 in the treatment of mild to moderate depression. Nord J Psychiatry. 2007;61:343–8. doi: 10.1080/08039480701643290. [DOI] [PubMed] [Google Scholar]
- 43**.Olsson EM, von Schéele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75:105–12. doi: 10.1055/s-0028-1088346. [DOI] [PubMed] [Google Scholar]
- 44.Shevtsov VA, Zholus BI, Shervarly VI, Vol’skij VB, Korovin YP, Khristich MP, Roslyakova NA, Wikman G. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine. 2003;10:95–105. doi: 10.1078/094471103321659780. [DOI] [PubMed] [Google Scholar]
- 45.Bocharova OA, Matveev BP, Baryshnikov AIu, Figurin KM, Serebriakova RV, Bodrova NB. The effect of a Rhodiola rosea extract on the incidence of recurrences of a superficial bladder cancer (experimental clinical research) Urol Nefrol (Mosk) 1995;2:46–7. Article in Russian. [PubMed] [Google Scholar]
- 46.Kwon YI, Jang HD, Shetty K. Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension. Asia Pac J Clin Nutr. 2006;15:425–32. [PubMed] [Google Scholar]
- 47.Blomkvist J, Taube A, Larhammar D. Perspective on Roseroot (Rhodiola rosea) Studies. Planta Med. 2009;75:1187–90. doi: 10.1055/s-0029-1185720. [DOI] [PubMed] [Google Scholar]
- 48.Mishra KP, Ganju L, Chanda S, Karan D, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome stimulates Toll-like receptor 4, granzyme-B and Th1 cytokines in vitro. Immunobiology. 2009;214:27–31. doi: 10.1016/j.imbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Pooja, Bawa AS, Khanum F. Anti-inflammatory activity of Rhodiola rosea--“a second-generation adaptogen”. Phytother Res. 2009;23:1099–102. doi: 10.1002/ptr.2749. [DOI] [PubMed] [Google Scholar]
- 50.Skopńska-Rózewska E, Wójcik R, Siwicki AK, Sommer E, Wasiutyński A, Furmanowa M, Malinowski M, Mazurkiewicz M. The effect of Rhodiola quadrifida extracts on cellular immunity in mice and rats. Pol J Vet Sci. 2008;11(2):105–11. [PubMed] [Google Scholar]
- 51.Ross SM. Rhodiola rosea (SHR-5), Part I: a proprietary root extract of Rhodiola rosea is found to be effective in the treatment of stress-related fatigue. Holist Nurs Pract. 2014;28:149–54. doi: 10.1097/HNP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 52.Ross SM. Rhodiola rosea (SHR-5), Part 2: A standardized extract of Rhodiola rosea is shown to be effective in the treatment of mild to moderate depression. Holist Nurs Pract. 2014;28:217–21. doi: 10.1097/HNP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 53.Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H. Rhodiola rosea in stress induced fatigue--a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine. 2000:365–71. doi: 10.1016/S0944-7113(00)80055-0. [DOI] [PubMed] [Google Scholar]
- 54.Spasov AA, Wikman GK, Mandrikov VB, Mironova IA, Neumoin VV. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7:85–9. doi: 10.1016/S0944-7113(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 55.Bystritsky A, Kerwin L, Feusner JD. A pilot study of Rhodiola rosea (Rhodax) for generalized anxiety disorder (GAD) J Altern Complement Med. 2008;14:175–80. doi: 10.1089/acm.2007.7117. [DOI] [PubMed] [Google Scholar]
- 56.Hernández-Santana A, Pérez-López V, Zubeldia JM, Jiménez-del-Rio M. A Rhodiola rosea root extract protects skeletal muscle cells against chemically induced oxidative stress by modulating heat shock protein 70 (HSP70) expression. Phytother Res. 2014;28:623–8. doi: 10.1002/ptr.5046. [DOI] [PubMed] [Google Scholar]
- 57.Uyeturk U, Terzi EH, Gucuk A, Kemahli E, Ozturk H, Tosun M. Prevention of torsion-induced testicular injury by Rhodiola rosea. Urology. 2013;82:254e1–6. doi: 10.1016/j.urology.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 58.Uyeturk U, Terzi EH, Kemahli E, Gucuk A, Tosun M, Çetinkaya A. Alleviation of kidney damage induced by unilateral ureter obstruction in rats by Rhodiola rosea. J Endourol. 2013;27:1272–6. doi: 10.1089/end.2013.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Si PP, Zhen JL, Cai YL, Wang WJ, Wang WP. Salidroside protects against kainic acid-induced status epilepticus via suppressing oxidative stress. Neurosci Lett. 2016;618:19–24. doi: 10.1016/j.neulet.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Zhen YF, Pu-Bu-Ci-Ren, Song LG, Kong WN, Shao TM, Li X, Chai XQ. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav Brain Res. 2013;244:70–81. doi: 10.1016/j.bbr.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 61.Verpeut JL, Walters AL, Bello NT. Citrus aurantium and Rhodiola rosea in combination reduce visceral white adipose tissue and increase hypothalamic norepinephrine in a rat model of diet-induced obesity. Nutr Res. 2013;33:503–12. doi: 10.1016/j.nutres.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asea A, Kaur P, Panossian A, Wikman KG. Evaluation of molecular chaperons Hsp72 and neuropeptide Y as characteristic markers of adaptogenic activity of plant extracts. Phytomedicine. 2013;20:1323–9. doi: 10.1016/j.phymed.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Panossian A, Hambardzumyan M, Hovhanissyan A, Wikman G. The adaptogens rhodiola and schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress-activated protein kinase, nitric oxide and cortisol. Drug Target Insights. 2007;2:39–54. [PMC free article] [PubMed] [Google Scholar]
- 64.Lee WJ, Chung HH, Cheng YZ, Lin HJ, Cheng JT. Rhodiola-water extract induces β-endorphin secretion to lower blood pressure in spontaneously hypertensive rats. Phytother Res. 2013;27:1543–7. doi: 10.1002/ptr.4900. [DOI] [PubMed] [Google Scholar]
- 65.Xia N, Li J, Wang H, Wang J, Wang Y. Schisandra chinensis and Rhodiola rosea exert an anti-stress effect on the HPA axis and reduce hypothalamic c-Fos expression in rats subjected to repeated stress. Exp Ther Med. 2016;11:353–359. doi: 10.3892/etm.2015.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boon-Niermeijer EK, van den Berg A, Wikman G, Wiegant FA. Phyto-adaptogens protect against environmental stress-induced death of embryos from the freshwater snail Lymnaea stagnalis. Phytomedicine. 2000;7:389–99. doi: 10.1016/S0944-7113(00)80060-4. [DOI] [PubMed] [Google Scholar]
- 67.Chen C, Song J, Chen M, Li Z, Tong X, Hu H, Xiang Z, Lu C, Dai F. Rhodiola rosea extends lifespan and improves stress tolerance in silkworm, Bombyx mori. Biogerontology. 2016;17:373–81. doi: 10.1007/s10522-015-9622-8. [DOI] [PubMed] [Google Scholar]
- 68.Tang H, Gao L, Mao J, He H, Liu J, Cai X, Lin H, Wu T. Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones. 2016;21:239–49. doi: 10.1007/s12192-015-0654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan XY, Pang XW, Zhang GQ, Guo JY. Salidroside’s Protection Against UVB-Mediated Oxidative Damage and Apoptosis Is Associated with the Upregulation of Nrf2 Expression. Photomed Laser Surg. 2017;35:49–56. doi: 10.1089/pho.2016.4151. [DOI] [PubMed] [Google Scholar]
- 70**.Hamilton KL, Miller BF. What is the evidence for stress resistance and slowed aging? Exp Gerontol. 2016;82:67–72. doi: 10.1016/j.exger.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Epel ES, Lithgow GJ. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S10–6. doi: 10.1093/gerona/glu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hulsurkar M, Li Z, Zhang Y, Li X, Zheng D, Li W. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene. 2017;36:1525–1536. doi: 10.1038/onc.2016.319. [DOI] [PubMed] [Google Scholar]
- 74*.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, Renne G, Gandini S, Pouton CW, Ferrari D, Möller A, Stacker SA, Sloan EK. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 2013;6:1262–72. doi: 10.1158/1940-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76*.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 77.Schriner SE, Coskun V, Hogan SP, Nguyen CT, Lopez TE, Jafari M. Extension of Drosophila Lifespan by Rhodiola rosea Depends on Dietary Carbohydrate and Caloric Content in a Simplified Diet. J Med Food. 2016;19:318–23. doi: 10.1089/jmf.2015.0105. [DOI] [PubMed] [Google Scholar]
- 78.Zhang B, Li Q, Chu X, Sun S, Chen S. Salidroside reduces tau hyperphosphorylation via up-regulating GSK-3β phosphorylation in a tau transgenic Drosophila model of Alzheimer’s disease. Transl Neurodegener. 2016;5:21. doi: 10.1186/s40035-016-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fitzenberger E, Deusing DJ, Wittkop A, Kler A, Kriesl E, Bonnländer B, Wenzel U. Effects of plant extracts on the reversal of glucose-induced impairment of stress-resistance in Caenorhabditis elegans. Plant Foods Hum Nutr. 2014;69:78–84. doi: 10.1007/s11130-013-0399-0. [DOI] [PubMed] [Google Scholar]
- 80.Bayliak MM, Burdyliuk NI, Izers’ka LI, Lushchak VI. Concentration-Dependent Effects of Rhodiola Rosea on Long-Term Survival and Stress Resistance of Yeast Saccharomyces Cerevisiae: The Involvement of YAP 1 and MSN2/4 Regulatory Proteins. Dose Response. 2013;12:93–109. doi: 10.2203/dose-response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gospodaryov DV, Yurkevych IS, Jafari M, Lushchak VI, Lushchak OV. Lifespan extension and delay of age-related functional decline caused by Rhodiola rosea depends on dietary macronutrient balance. Longev Healthspan. 2013;2:5. doi: 10.1186/2046-2395-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schriner SE, Lee K, Truong S, Salvadora KT, Maler S, Nam A, Lee T, Jafari M. Extension of Drosophila lifespan by Rhodiola rosea through a mechanism independent from dietary restriction. PLoS One. 2013;8:e63886. doi: 10.1371/journal.pone.0063886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Udintsev SN, Shakhov VP. Decrease in the growth rate of Ehrlich’s tumor and Pliss’ lymphosarcoma with partial hepatectomy. Vopr Onkol. 1989;35:1072–5. Article in Russian. [PubMed] [Google Scholar]
- 84.Skopińska-Rózewska E, Malinowski M, Wasiutyński A, Sommer E, Furmanowa M, Mazurkiewicz M, Siwicki AK. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol J Vet Sci. 2008;11:97–104. [PubMed] [Google Scholar]
- 85.Udintsev SN, Schakhov VP. Decrease of cyclophosphamide haematotoxicity by Rhodiola rosea root extract in mice with Ehrlich and Lewis transplantable tumors. Eur J Cancer. 1991;27:1182. doi: 10.1016/0277-5379(91)90323-6. [DOI] [PubMed] [Google Scholar]
- 86.Liu Z, Li X, Simoneau AR, Jafari M, Zi X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012;51:257–67. doi: 10.1002/mc.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao G, Shi A, Fan Z, Du Y. Salidroside inhibits the growth of human breast cancer in vitro and in vivo. Oncol Rep. 2015;33:2553–60. doi: 10.3892/or.2015.3857. [DOI] [PubMed] [Google Scholar]
- 88.Bassa LM, Jacobs C, Gregory K, Henchey E, Ser-Dolansky J, Schneider SS. Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine. 2016;23:87–94. doi: 10.1016/j.phymed.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 89.Fan XJ, Wang Y, Wang L, Zhu M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36:3559–3567. doi: 10.3892/or.2016.5138. [DOI] [PubMed] [Google Scholar]
- 90.Huo J, Qin F, Cai X, Ju J, Hu C, Wang Z, Lu W, Wang X, Cao P. Chinese medicine formula “Weikang Keli” induces autophagic cell death on human gastric cancer cell line SGC-7901. Phytomedicine. 2013;20:159–65. doi: 10.1016/j.phymed.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Yao Y, Wang H, Guo Y, Zhang H, Chen L. Effects of salidroside on glioma formation and growth inhibition together with improvement of tumor microenvironment. Chin J Cancer Res. 2013;25:520–6. doi: 10.3978/j.issn.1000-9604.2013.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J, Li JZ, Lu AX, Zhang KF, Li BJ. Anti-cancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol Lett. 2014;7:1159–1164. doi: 10.3892/ol.2014.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun C, Wang Z, Zheng Q, Zhang H. Salidroside inhibits migration and invasion of human fibrosarcoma HT1080 cells. Phytomedicine. 2012;19:355–63. doi: 10.1016/j.phymed.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 94.Hu X, Lin S, Yu D, Qiu S, Zhang X, Mei R. A preliminary study: the anti-proliferation effect of salidroside on different human cancer cell lines. Cell Biol Toxicol. 2010;26:499–507. doi: 10.1007/s10565-010-9159-1. [DOI] [PubMed] [Google Scholar]
- 95.Tu Y, Roberts L, Shetty K, Schneider SS. Rhodiola crenulata induces death and inhibits growth of breast cancer cell lines. J Med Food. 2008;11:413–23. doi: 10.1089/jmf.2007.0736. [DOI] [PubMed] [Google Scholar]
- 96.Mishra KP, Padwad YS, Dutta A, Ganju L, Sairam M, Banerjee PK, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome inhibits proliferation of an erythroleukemic cell line K-562 by inducing apoptosis and cell cycle arrest at G2/M phase. Immunobiology. 2008;213:125–31. doi: 10.1016/j.imbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Panossian A, Hamm R, Wikman G, Efferth T. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: an interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine. 2014;21:1325–48. doi: 10.1016/j.phymed.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 98.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016;23:990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Z, Yokoyama NN, Blair CA, Li X, Avizonis D, Wu XR, Uchio E, Youssef R, McClelland M, Pollak M, Zi X. High Sensitivity of an Ha-RAS Transgenic Model of Superficial Bladder Cancer to Metformin Is Associated with ~240-Fold Higher Drug Concentration in Urine than Serum. Mol Cancer Ther. 2016;15:430–8. doi: 10.1158/1535-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Z, Antalek M, Nguyen L, Li X, Tian X, Le A, Zi X. The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines. Nutr Cancer. 2013;65(Suppl 1):68–77. doi: 10.1080/01635581.2013.785011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Z, Li X, Liu S, Xu X, Tian X, Simoneau AR, et al. Rhodiola rosea L. extract, SHR-5, and metformin exhibit potent activity against bladder carcinogenesis in the UPII-mutant Ha-ras transgenic model. Cancer Research. 2012;72(8 Supplement):618. [Google Scholar]
- 103.Chen YN, Liu H, Zhao HB, Liu Y, Bai J, Zhu XJ, Wang Y. Salidroside via ERK1/2 and PI3K/AKT/mTOR signal pathway induces mouse bone marrow mesenchymal stem cells differentiation into neural cells. Yao Xue Xue Bao. 2013;48:1247–52. [PubMed] [Google Scholar]
- 104.Zhong X, Lin R, Li Z, Mao J, Chen L. Effects of Salidroside on cobalt chloride-induced hypoxia damage and mTOR signaling repression in PC12 cells. Biol Pharm Bull. 2014;37:1199–206. doi: 10.1248/bpb.b14-00100. [DOI] [PubMed] [Google Scholar]
- 105.Tang Y, Vater C, Jacobi A, Liebers C, Zou X, Stiehler M. Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signalling pathways. Br J Pharmacol. 2014;171:2440–56. doi: 10.1111/bph.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu MC, Shi HM, Wang H, Gao XF. Salidroside protects against hydrogen peroxide-induced injury in HUVECs via the regulation of REDD1 and mTOR activation. Mol Med Rep. 2013;8:147–53. doi: 10.3892/mmr.2013.1468. [DOI] [PubMed] [Google Scholar]
- 107.Zheng XT, Wu ZH, Wei Y, Dai JJ, Yu GF, Yuan F, Ye LC. Induction of autophagy by salidroside through the AMPK-mTOR pathway protects vascular endothelial cells from oxidative stress-induced apoptosis. Mol Cell Biochem. 2017;425:125–138. doi: 10.1007/s11010-016-2868-x. [DOI] [PubMed] [Google Scholar]
- 108.Chen X, Wu Y, Yang T, Wei M, Wang Y, Deng X, Shen C, Li W, Zhang H, Xu W, Gou L, Zeng Y, Zhang Y, Wang Z, Yang J. Salidroside alleviates cachexia symptoms in mouse models of cancer cachexia via activating mTOR signalling. J Cachexia Sarcopenia Muscle. 2016;7:225–32. doi: 10.1002/jcsm.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salikhova RA, Aleksandrova IV, Mazurik VK, Mikhaĭlov VF, Ushenkova LN, Poroshenko GG. Effect of Rhodiola rosea on the yield of mutation alterations and DNA repair in bone marrow cells. Patol Fiziol Eksp Ter. 1997;4:22–4. Article in Russian. [PubMed] [Google Scholar]
- 110**.Li X, Sipple J, Pang Q, Du W. Salidroside stimulates DNA repair enzyme Parp-1 activity in mouse HSC maintenance. Blood. 2012;119:4162–73. doi: 10.1182/blood-2011-10-387332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X, Erden O, Li L, Ye Q, Wilson A, Du W. Binding to WGR domain by salidroside activates PARP1 and protects hematopoietic stem cells from oxidative stress. Antioxid Redox Signal. 2014;20:1853–65. doi: 10.1089/ars.2013.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar H, Choi DK. Hypoxia Inducible Factor Pathway and Physiological Adaptation: A Cell Survival Pathway? Mediators Inflamm. 2015:584758. doi: 10.1155/2015/584758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qi YJ, Cui S, Lu DX, Yang YZ, Luo Y, Ma L, Ma Y, Wuren T, Chang R, Qi L, Ben BJ, Han J, Ge RL. Effects of the aqueous extract of a Tibetan herb, Rhodiola algida var. tangutica on proliferation and HIF-1α, HIF-2α expression in MCF-7 cells under hypoxic condition in vitro. Cancer Cell Int. 2015;15:81. doi: 10.1186/s12935-015-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Radomska-Leśniewska DM, Skopiński P, Bałan BJ, Białoszewska A, JóŸwiak J, Rokicki D, Skopińska-Różewska E, Borecka A, Hevelke A. Angiomodulatory properties of Rhodiola spp. and other natural antioxidants. Cent Eur J Immunol. 2015;40:249–62. doi: 10.5114/ceji.2015.52839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng KY, Zhang ZX, Guo AJ, Bi CW, Zhu KY, Xu SL, Zhan JY, Lau DT, Dong TT, Choi RC, Tsim KW. Salidroside stimulates the accumulation of HIF-1α protein resulted in the induction of EPO expression: a signaling via blocking the degradation pathway in kidney and liver cells. Eur J Pharmacol. 2012;679:34–9. doi: 10.1016/j.ejphar.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Liu A, Hou R, Zhang J, Jia X, Jiang W, Chen J. Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. Eur J Pharmacol. 2009;607:6–14. doi: 10.1016/j.ejphar.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 117.Wu T, Zhou H, Jin Z, Bi S, Yang X, Yi D, Liu W. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur J Pharmacol. 2009;613:93–9. doi: 10.1016/j.ejphar.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 118.Zheng KY, Guo AJ, Bi CW, Zhu KY, Chan GK, Fu Q, Xu SL, Zhan JY, Lau DT, Dong TT, Choi RC, Tsim KW. The extract of Rhodiolae Crenulatae Radix et Rhizoma induces the accumulation of HIF-1α via blocking the degradation pathway in cultured kidney fibroblasts. Planta Med. 2011;77:894–9. doi: 10.1055/s-0030-1250627. [DOI] [PubMed] [Google Scholar]
- 119.Zheng KY, Zhang ZX, Guo AJ, Bi CW, Zhu KY, Xu SL, Zhan JY, Lau DT, Dong TT, Choi RC, Tsim KW. Salidroside stimulates the accumulation of HIF-1α protein resulted in the induction of EPO expression: a signaling via blocking the degradation pathway in kidney and liver cells. Eur J Pharmacol. 2012;679:34–9. doi: 10.1016/j.ejphar.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 120.Li L, Wang H, Zhao X. Effects of Rhodiola on production, health and gut development of broilers reared at high altitude in Tibet. Sci Rep. 2014;4:7166. doi: 10.1038/srep07166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu ZW, Chen X, Jin XH, Meng XY, Zhou X, Fan FX, Mao SY, Wang Y, Zhang WC, Shan NN, Li YM, Xu RC. SILAC-based proteomic analysis reveals that salidroside antagonizes cobalt chloride-induced hypoxic effects by restoring the tricarboxylic acid cycle in cardiomyocytes. J Proteomics. 2016;130:211–20. doi: 10.1016/j.jprot.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 122.Chen M, Cai H, Yu C, Wu P, Fu Y, Xu X, Fan R, Xu C, Chen Y, Wang L, Huang X. Salidroside exerts protective effects against chronic hypoxia-induced pulmonary arterial hypertension via AMPKα1-dependent pathways. Am J Transl Res. 2016;8:12–27. [PMC free article] [PubMed] [Google Scholar]
- 123.Hsu SW, Chang TC, Wu YK, Lin KT, Shi LS, Lee SY. Rhodiola crenulata extract counteracts the effect of hypobaric hypoxia in rat heart via redirection of the nitric oxide and arginase 1 pathway. BMC Complement Altern Med. 2017;17:29. doi: 10.1186/s12906-016-1524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rabinowitz MH. Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: tricking the body into mounting orchestrated survival and repair responses. J Med Chem. 2013;56:9369–402. doi: 10.1021/jm400386j. [DOI] [PubMed] [Google Scholar]
- 125**.Zhang J, Kasim V, Xie YD, Huang C, Sisjayawan J, Dwi Ariyanti A, Yan XS, Wu XY, Liu CP, Yang L, Miyagishi M, Wu SR. Inhibition of PHD3 by salidroside promotes neovascularization through cell-cell communications mediated by muscle-secreted angiogenic factors. Sci Rep. 2017;7:43935. doi: 10.1038/srep43935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu L, Yuan J, Zhang S. Rejuvenating activity of salidroside (SDS): dietary intake of SDS enhances the immune response of aged rats. Age (Dordr) 2013;35:637–46. doi: 10.1007/s11357-012-9394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guan S, He J, Guo W, Wei J, Lu J, Deng X. Adjuvant effects of salidroside from Rhodiola rosea L. on the immune responses to ovalbumin in mice. Immunopharmacol Immunotoxicol. 2011;33:738–43. doi: 10.3109/08923973.2011.567988. [DOI] [PubMed] [Google Scholar]
- 128**.Zhao X, Lu Y, Tao Y, Huang Y, Wang D, Hu Y, Liu J, Wu Y, Yu Y, Liu C. Salidroside liposome formulation enhances the activity of dendritic cells and immune responses. Int Immunopharmacol. 2013;17:1134–40. doi: 10.1016/j.intimp.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 129.Peng H, Dong R, Wang S, Zhang Z, Luo M, Bai C, Zhao Q, Li J, Chen L, Xiong H. A pH-responsive nano-carrier with mesoporous silica nanoparticles cores and poly(acrylic acid) shell-layers: fabrication, characterization and properties for controlled release of salidroside. Int J Pharm. 2013;446:153–9. doi: 10.1016/j.ijpharm.2013.01.071. [DOI] [PubMed] [Google Scholar]
- 130.Diwaker D, Mishra KP, Ganju L, Singh SB. Rhodiola inhibits dengue virus multiplication by inducing innate immune response genes RIG-I, MDA5 and ISG in human monocytes. Arch Virol. 2014;159:1975–86. doi: 10.1007/s00705-014-2028-0. [DOI] [PubMed] [Google Scholar]
- 131.Liu MW, Su MX, Zhang W, Zhang LM, Wang YH, Qian CY. Rhodiola rosea suppresses thymus T-lymphocyte apoptosis by downregulating tumor necrosis factor-α-induced protein 8-like-2 in septic rats. Int J Mol Med. 2015;36:386–98. doi: 10.3892/ijmm.2015.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu X, Li P, Zhang P, Chu M, Liu H, Chen X, Ge Q. Differential effects of Rhodiola rosea on regulatory T cell differentiation and interferon-γ production in vitro and in vivo. Mol Med Rep. 2016;14:529–36. doi: 10.3892/mmr.2016.5278. [DOI] [PubMed] [Google Scholar]
- 133.Marchev AS, Dimitrova P, Koycheva IK, Georgiev MI. Altered expression of TRAIL on mouse T cells via ERK phosphorylation by Rhodiola rosea L. and its marker compounds. Food Chem Toxicol. 2017 doi: 10.1016/j.fct.2017.02.009. pii: S0278–6915(17)30055–8. [DOI] [PubMed] [Google Scholar]
- 134.Song B, Huang G, Xiong Y, Liu J, Xu L, Wang Z, Li G, Lu J, Guan S. Inhibitory effects of salidroside on nitric oxide and prostaglandin E2 production in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Med Food. 2013;16:997–1003. doi: 10.1089/jmf.2012.2473. [DOI] [PubMed] [Google Scholar]
- 135.Li D, Fu Y, Zhang W, Su G, Liu B, Guo M, Li F, Liang D, Liu Z, Zhang X, Cao Y, Zhang N, Yang Z. Salidroside attenuates inflammatory responses by suppressing nuclear factor-κB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm Res. 2013;62:9–15. doi: 10.1007/s00011-012-0545-4. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Xu CF, Liu YJ, Mao YF, Lv Z, Li SY, Zhu XY, Jiang L. Salidroside Attenuates Ventilation Induced Lung Injury via SIRT1-Dependent Inhibition of NLRP3 Inflammasome. Cell Physiol Biochem. 2017;42:34–43. doi: 10.1159/000477112. [DOI] [PubMed] [Google Scholar]
- 137.Qi Z, Qi S, Ling L, Lv J, Feng Z. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265–71. doi: 10.1016/j.intimp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 138.Chang YW, Yao HT, Hsieh SH, Lu TJ, Yeh TK. Quantitative determination of salidroside in rat plasma by on-line solid-phase extraction integrated with high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:164–9. doi: 10.1016/j.jchromb.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 139.Han F, Li YT, Mao XJ, Zhang XS, Guan J, Song AH, Yin R. Metabolic profile of salidroside in rats using high-performance liquid chromatography combined with Fourier transform ion cyclotron resonance mass spectrometry. Anal Bioanal Chem. 2016;408:1975–81. doi: 10.1007/s00216-015-9080-9. [DOI] [PubMed] [Google Scholar]
- 140.Mao Y, Zhang X, Zhang X, Lu G. Development of an HPLC method for the determination of salidroside in beagle dog plasma after administration of salidroside injection: application to a pharmacokinetics study. J Sep Sci. 2007;30:3218–22. doi: 10.1002/jssc.200700273. [DOI] [PubMed] [Google Scholar]
- 141.Yu S, Liu L, Wen T, Liu Y, Wang D, He Y, Liang Y, Liu X, Xie L, Wang G, Wei W. Development and validation of a liquid chromatographic/electrospray ionization mass spectrometric method for the determination of salidroside in rat plasma: application to the pharmacokinetics study. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861:10–5. doi: 10.1016/j.jchromb.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 142**.Guo N, Zhu M, Han X, Sui D, Wang Y, Yang Q. The metabolism of salidroside to its aglycone p-tyrosol in rats following the administration of salidroside. PLoS One. 2014;9:e103648. doi: 10.1371/journal.pone.0103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y, Li L, Lin L, Liu J, Zhang Z, Xu D, Xiang F. Pharmacokinetics, tissue distribution, and excretion of salidroside in rats. Planta Med. 2013;79:1429–33. doi: 10.1055/s-0033-1350807. [DOI] [PubMed] [Google Scholar]
- 144*.Wang M, Luo L, Yao L, Wang C, Jiang K, Liu X, Xu M, Shen N, Guo S, Sun C, Yang Y. Salidroside improves glucose homeostasis in obese mice by repressing inflammation in white adipose tissues and improving leptin sensitivity in hypothalamus. Sci Rep. 2016;6:25399. doi: 10.1038/srep25399. [DOI] [PMC free article] [PubMed] [Google Scholar]