Abstract
Prior research has demonstrated the link between maternal depression during pregnancy (i.e., prenatal depression) and increased neurodevelopmental dysregulation in offspring. However, little is known about the roles of key hypothalamic–pituitary axis regulatory genes in the placenta modulating this association. This study will examine whether placental gene expression levels of 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2), glucocorticoid receptor (NR3C1), and mineralocorticoid receptor (NR3C2) can help elucidate the underlying mechanisms linking prenatal depression to infant temperament, particularly in infants with high negativity and low emotion regulation. Stored placenta tissues (N = 153) were used to quantify messenger ribonucleic acid levels of HSD11B2, NR3C1, and NR3C2. Assessments of prenatal depression and infant temperament at 6 months of age were ascertained via maternal report. Results found that prenatal depression was associated with increased Negative Affectivity (p < .05) after controlling for postnatal depression and psychosocial characteristics. Furthermore, the association between prenatal depression and Negative Affectivity was moderated by gene expression levels of HSD11B2, NR3C1, and NR3C2 such that greater gene expression significantly lessened the association between prenatal depression and Negative Affectivity. Our findings suggest that individual differences in placental gene expression may be used as an early marker of susceptibility or resilience to prenatal adversity.
Depression is a major stress-related disorder for women, with 10–25% of women experiencing depression during pregnancy (Field, Diego, & Hernandez-Reif, 2006; Marcus, Flynn, Blow, & Barry, 2003; Stowe, Hostetter, & Newport, 2005; de Tychey et al., 2005). Prenatal depression affects fetal activity and growth (Dieter et al., 2001), and infants of mothers who are depressed during pregnancy have been shown to exhibit increased levels of fear, stress, irritability, and reactivity (Davis, Glynn, Waffarn, & Sandman, 2011; Davis et al., 2004, 2007; Diego et al., 2004; Field, 2011; Gaynes et al., 2005; Zuckerman, Bauchner, Parker, & Cabral, 1990). Prior studies have also found that prenatal depression predicts developmental delays (Deave, Heron, Evans, & Emond, 2008), emotional and behavioral problems (Korhonen, Luoma, Salmelin, & Tamminen, 2012; Luoma et al., 2001, 2004), changes in cortical thickness (Sandman, Buss, Head, & Davis, 2015) in childhood and adolescence, and stress-related disease in adulthood (Barker, 2002; Kajantie, 2006).
Decades of interdisciplinary research have explored the underlying biological mechanisms that connect maternal depression, or exposure to prenatal stress, to offspring developmental outcomes. Much of this research has focused on glucocorticoid stress hormones such as cortisol as a possible component of the underlying mechanism. Highly stressed pregnant women may secrete a greater amount of cortisol compared to nonstressed pregnant women (Chrousos, 1992; Glover, O’Connor, & O’Donnell, 2010), although the evidence for this is mixed. As cortisol passes through the placenta to the fetus, increased levels have been strongly associated with depression in the general population (Knorr, Vinberg, Kessing, & Wetterslev, 2010; Pariante & Lightman, 2008) and in pregnant mothers (Field, Diego, Hernandez-Reif, et al., 2004; Field et al., 2006). The pathway lies in fetal overexposure to glucocorticoids that impact development of the central nervous system and the hypothalamic–pituitary (HPA) axis (Sandman, 2015; Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999), which may set the child on a suboptimal neurodevelopmental trajectory (Davis et al., 2004).
The placenta plays a vital role in regulating the amount of glucocorticoids in circulation in the fetal environment (Moisiadis & Matthews, 2014; Seckl, 1998; Seckl & Holmes, 2007; Seckl & Meaney, 2004). The placental enzyme, 11β-HSD2, converts active cortisol to inactive cortisone (Benediktsson, Calder, Edwards, & Seckl, 1997). This enzyme is encoded by the HSD11B2 gene. Higher 11β-HSD2 levels in the placenta create a barrier to the transfer of excessive glucocorticoids, thus protecting the fetus, while a relative deficiency of 11β-HSD2 allows greater passage of maternal glucocorticoids to the fetus. Greater placental HSD11B2 DNA methylation has been associated with poor infant outcomes, such as lower birthweight, lower quality of movement, and lower muscle tone (Conradt, Lester, Appleton, Armstrong, & Marsit, 2013; Marsit, Maccani, Padbury, & Lester, 2012). DNA methylation is most frequently studied as a proxy measure of epigenetic regulation and is known to be associated with a reduction in gene expression (Marsit et al., 2012). However, one previous work by Räikkönen et al. (2015) found an inverse association where greater placental HSD11B2 expression was correlated with infant dysregulated behavior, as measured by crying, feeding, spitting, bowel movement, sleeping, and predictability.
Among other important regulators of glucocorticoids in the placenta are the glucocorticoid receptors (GR; encoded by NR3C1) and mineralocorticoid receptors (MR; encoded by NR3C2), which modulate the actions of glucocorticoids on gene transcription (Conradt et al., 2015; Reynolds et al., 2015; Seckl, 1998; Seckl & Holmes, 2007; Seckl & Meaney, 2004). The activation of GR and MR inhibits HPA axis activity, which releases more cortisol through negative feedback inhibition in brain tissues such as the hippocampus (Matthews, 2002). GR deficiency in the placenta and insufficient placental glucocorticoid response may lead to impaired GR functions and thereby possibly contribute to the development of infant dysregulated behavior (Conradt et al., 2015; Paquette et al., 2015). Increased placental NR3C1 methylation has been associated with decreased gene expression (Bromer, Marsit, Armstrong, Padbury, & Lester, 2013), poorer attention, greater cortisol stress reactivity, higher arousal, and decreased self-regulation in infants (Bromer et al., 2013; Conradt et al., 2015; Paquette et al., 2015). In contrast, some studies found that increased placental NR3C1 expression, or decreased NR3C1 methylation, was associated with adverse infant neurobehavioral outcomes, such as lower quality of movement, more lethargy, poorer attention, and decreased self-regulation (Räikkönen et al., 2015; Stroud et al., 2016). Finally, no association between placental MR (NR3C2) and infant neurobehavioral development has been noted. Similar to GR, MR is responsive to glucocorticoids and plays a significant role in glucocorticoid regulation. Prior research found that prenatal depression was associated with altered placental NR3C2 gene expression (Reynolds et al., 2015), which suggests the involvement of MR in the molecular mechanisms that underlie the programming effects of prenatal depression in offspring development.
In one recent study, Conradt et al. (2013) examined whether placental HSD11B2 and NR3C1 methylation would interact with prenatal depression and anxiety in predicting infant neurobehavior between 37 and 41 weeks of age. They found that prenatal depression and anxiety alone did not predict infant neurobehavior, with the exception that prenatal depression was associated with lower muscle tone. However, when they interacted with DNA methylation they found that in the context of prenatal depression alone, greater NR3C1 methylation on the CpG island (specifically in the region of CpG2) was associated with decreased infant self-regulation, lower muscle tone, and more lethargy. In addition, only in the context of prenatal anxiety, greater HSD11B2 methylation on the CpG island (in the region of CpG4) conferred risk for lower muscle tone.
There are some limitations in Conradt et al.’s study. First, possible confounding factors (e.g., postnatal depression) have not been clearly specified. Second, placental DNA methylation of HSD11B2 and NR3C1 genes was conducted as markers for epigenetic vulnerabilities and the roles of the expression of those genes in the placenta were not evaluated. The relationship between DNA methylation and gene expression is not straightforward because gene expression is regulated by genetics and other epigenetic processes, such as histone modification and noncoding RNA (Jaenisch & Bird, 2003; Pastinen et al., 2004), and all of those functions collectively lead to the altered levels of gene expression (see Maccani & Marsit, 2009 for a review). Consequently, to gain a more accurate evaluation of placental gene functioning, gene expression data (mRNA expression) must be provided.
Although there is evidence supporting the role of placental gene functions in regulating the relationship between prenatal depression and infant neurobehavior, no study has examined early temperament, which has been viewed as a precursor for subsequent mental health disorders (De Pauw & Mervielde, 2010; Hellemans, Sliwowska, Verma, & Weinberg, 2010). In the current study, we used gene expression data from placental tissue and hypothesized that prenatal maternal depression would be associated with infant temperament at 6 months, especially in areas related to negative affectivity and regulation. We predicted that prenatal depression would interact with the three key genes (HSD11B2, NR3C1, and NR3C2) involved in placental glucocorticoid metabolism or transfer to predict infant temperament. In particular, we hypothesized that the effect of prenatal depression on negative affectivity and dysregulation would be significantly stronger among infants with lower placental HSD11B2, NR3C1, and NR3C2 expression and that greater expression would “protect” the infant from the deleterious effects of prenatal maternal depression, as evidenced by a lower association between maternal depression and infant temperament. As prior research suggested that both prenatal and postnatal maternal depression affect infant temperament (Davis et al., 2004), we also controlled for postnatal maternal depression.
METHOD
Participants
Data in this study came from a subsample of 153 mother–child dyads, whose placenta tissues were stored as a part of an ongoing prospective study of child development (see a detailed description PMID: 26418562). Participants were recruited at the prenatal obstetrics and gynecological clinics at Mount Sinai Hospital and New York Presbyterian Queens, NY, followed from their 2nd trimester through delivery, and then prospectively after birth. Exclusion criteria included HIV infection, maternal psychosis, maternal age <15 years, life-threatening maternal medical complications, and congenital/ chromosomal abnormalities in the fetus. The participants (N = 153) in the current study and those active participants of the parent study without placenta tissue (N = 173) were not significantly different across gestational age (p = .411), birthweight (p = .168), child gender (p = .813), maternal age (p = .995), marital status (p = .385), maternal education (p = .265), and race (p = .086).
Preterm infants born before 34 weeks’ gestation were also excluded because of their risks for severe health and developmental problems (Crowther, Crosby, & Henderson-Smart, 2010; Loftin et al., 2010; Vohr, 2013). Infants were born with a mean gestational age of 39.1 (SD = 1.84) weeks (range 35.0–42.2 weeks) (Table 1 shows the demographic characteristics of the sample used in the current study).
TABLE 1.
Demographic Characteristics (N = 153)
| Gestational age (weeks) | Mean (SD) | 39.05 (1.84) |
| Birthweight (g) | <2,500, N (%) | 7 (4.58) |
| Mean (SD) | 3,307.25 (482.94) | |
| Child’s sex | Boy, N (%) | 86 (56.2) |
| Girl, N (%) | 67 (43.8) | |
| Marital status | Married, N (%) | 55 (35.9) |
| Common law, N (%) | 12 (7.8) | |
| Single, N (%) | 84 (54.9) | |
| Divorced/separate/widowed, N (%) | 2 (1.4) | |
| On welfare | Yes, N (%) | 117 (76.5) |
| No, N (%) | 36 (23.5) | |
| Mother’s education | Primary/some high school, N (%) | 27 (17.6) |
| High school degree/GED, N (%) | 34 (22.2) | |
| Some college, N (%) | 39 (25.5) | |
| Associate’s degree, N (%) | 19 (12.4) | |
| Bachelor’s degree, N (%) | 19 (12.4) | |
| Graduate-level degree, N (%) | 15 (9.8) | |
| Mother’s race | White, N (%) | 17 (11.1) |
| Black, N (%) | 38 (24.8) | |
| Hispanic, N (%) | 80 (52.3) | |
| Asian, N (%) | 11 (7.2) | |
| Others, N (%) | 7 (4.6) | |
| Maternal age | Mean (SD) | 27.53 (5.8) |
| Use of antidepressant | Yes, N (%) | 3 (2.0) |
| No, N (%) | 150 (98) | |
| Negative affectivity | Mean (SD) | 3.23 (0.89) |
| Regulation | Mean (SD) | 5.42 (0.66) |
| HSD11B2 | Mean (SD) | 10.30 (1.76) |
| NR3C1 | Mean (SD) | 10.82 (0.45) |
| NR3C2 | Mean (SD) | 7.06 (0.68) |
| Prenatal depression | Mean (SD) | 6.97 (5.27) |
| Postnatal depression | Mean (SD) | 5.75 (5.51) |
Note. SD, standard deviations.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All mothers gave written informed consent before any assessment or data collection. All procedures involving human subjects in this study were approved by the Institutional Review Boards at the City University of New York, New York Presbyterian/ Queens, and the Icahn School of Medicine at Mount Sinai.
Measures
Maternal depression
The Edinburgh Postnatal Depression Scale (EPDS; Cox, Holden, & Sagovsky, 1987) was used to measure mother’s depressive symptomatology during pregnancy and the postpartum period. The EPDS is a validated self-report questionnaire consisting of 10 question items. For each item, mothers reported how they felt in the past 7 days on a 4-point Likert scale from 0 to 3, based on levels of severity. Some items were reverse coded, and an aggregate sum score was generated. The EPDS is well validated and has acceptable reliability ranging from .79 to .86 (Kheirabadi, Maracy, Akbaripour, & Masaeli, 2012; Mazhari & Nakhaee, 2007; Montazeri, Torkan, & Omidvari, 2007; Small, Lumley, Yelland, & Brown, 2007), satisfactory sensitivity (78%), and specificity (75%) (Kheirabadi et al., 2012). EPDS has been used as a valid and reliable measure for depression during the prenatal and postnatal periods (Bolten et al., 2013; Luoma et al., 2001; Oberlander et al., 2008). Mothers completed the EPDS during their 2nd trimester and at 6 months postpartum. Forty-two (27%) mothers met criteria for clinical depression (≥10) during the prenatal period, and 28 (18%) met criteria during the postpartum period. In this study, the internal reliability (Cronbach’s alpha) of prenatal and postnatal EPDS was .78 and .85, respectively.
Infant temperament
The Short Form of the Infant Behavior Questionnaire-Revised (IBQ-R; Garstein & Rothbart, 2003; Putnam, Helbig, Gartstein, Rothbart, & Leerkes, 2014) was used to measure infants’ temperament at approximately 6 months. The IBQ-R is a parent report questionnaire, which is typically completed by the mother. It consists of 91 items about the frequency of their infant/child’s specific behaviors. The 14 subscales include activity level, cuddliness, fear, sadness, high-intensity pleasure, low-intensity pleasure, approach, smiling and laughter, falling reactivity, duration of orienting, perceptual sensitivity, distress to limitations, vocal reactivity, and soothability. The IBQ-R has been found to form three hierarchical factors: Negative Affectivity, Regulatory Capacity/Orienting (Regulation), and Positive Emotionality/Surgency (Bayly & Gartstein, 2013; Gartstein, Bell, & Calkins, 2014). As prior evidence indicates that prenatal mood state is particularly associated with negative affectivity (Blair, Glynn, Sandman, & Davis, 2011; Pluess et al., 2011) and dysregulation (Babineau et al., 2015; Gutteling et al., 2005), we focused on the Negative Affectivity and Regulation factors, but not Positive Emotionality/Surgency. Negative Affectivity includes falling reactivity, fear, distress to limitations, and sadness subscales, with an internal reliability of .76. Regulation consists of low-intensity pleasure, cuddliness, duration of orienting, and soothability subscales, with an internal reliability of .82 (Gartstein & Rothbart, 2003).
Placenta sampling and gene expression profiling
Biopsies, free of maternal decidua, were collected from each placenta quadrant midway between the cord insertion and the placenta rim within an hour of delivery to maintain the optimal integrity of DNA and RNA (Diplas et al., 2009; Lambertini et al., 2008). Tissue was washed in cold sterile water, blotted in sterile gauze, snapfrozen in a liquid nitrogen tank for 24 hours, and stored in an ultra-freezer at −80°C until use. Frozen placenta tissues from each biopsy were ground into powder in a −80°C cooled TissueLyser II (Qiagen, Valencia, CA, USA) and mixed in equal amounts to represent the whole placenta. DNA and RNA were isolated from powder in Maxwell 16 automated DNA/RNA extraction system (Promega, Madison, WI, USA).
Placental gene expression profiling was carried out using the NanoString™ technology with the nCounter Analysis System (Seattle, WA, USA). Briefly, 100 ng of RNA was incubated in the presence of reporter and capture probes overnight at 65°C. Following hybridization, unbound probes were removed, and the purified complexes were aligned and immobilized on imaging cartridges using an nCounter Prep Station II. Cartridges were then sealed and scanned in an nCounter Digital Analyzer for code count detection. The nanoString Norm package (Waggott et al., 2012) was used to normalize nCounter data. Code count data were first normalized against the geometric mean of spike-in controls and against the geometric mean of the housekeeping genes, GAPDH, RPL19, and RPLP0. The limit of detection (LOD) for each sample was set at two standard deviations above the mean of the included negative control probes. Expression below background threshold was set to the value of the LOD divided by the square root of 2 to maintain sample variability.
Missing data and outliers
Infant’s birthweight and maternal postnatal depression variables were missing from two subjects. Little’s MCAR tests indicated that data were missing completely at random (p = .372). No outliers (>3 SDs from the mean) and violation of normality were detected.
Statistical analysis
First, the unadjusted associations between the study variables were examined using Pearson correlation. Gender differences were examined by a series of independent sample t-tests. Using the PROCESS macro for SPSS (Hayes, 2013; Preacher & Hayes, 2004), which provides the model coefficient estimations using ordinary least squares regression, we tested the moderating effect of gene expression on the association between prenatal depression and infant temperament at 6 months. We computed the bootstrapped bias-corrected 95% confidence intervals by taking 10,000 bootstrapped samples (Hayes, 2013), with each continuous variable centered at its mean. Through this analysis, we were able to test the main effects of prenatal depression, gene expression, and their interaction effect, in predicting temperament (Negative Affectivity and Regulation). All interactions were further examined using the simple slope analysis procedure as implemented in the PROCESS macro (Aiken & West, 1991; Hayes, 2012). The interaction effect was probed to determine whether the simple slope of infant temperament on prenatal depression was statistically significant for higher (i.e., +1 SD) and lower (i.e., −1 SD) values of gene expression.
Covariates and cofounders
Possible infant (gender, gestational age at birth, and birthweight) and maternal characteristics (maternal education and welfare status) were further adjusted in the model. Prenatal maternal depression has been found to influence fetal growth in terms of gestational age and birthweight (Diego et al., 2004; Dieter et al., 2001; Field, Diego, Dieter, et al., 2004), and there are individual differences in infant behaviors with respect to psychosocial factors. Partially adjusted moderation analyses were controlled only for postnatal depression, while fully adjusted moderation analyses were controlled for postnatal depression and all infant and maternal characteristics listed above.
RESULTS
Descriptive statistics
The correlation metrics and descriptive statistics are presented in Table 2. As seen, Negative Affectivity and Regulation were negatively correlated (r = −.28, p < .001). Prenatal and postnatal depression were both positively correlated with Negative Affectivity, but negatively correlated with Regulation. There were moderate correlations among the expression level of the three selected genes (HSD11B2, NR3C1, and NR3C2). Gene expression was associated with neither prenatal nor postnatal depression.
TABLE 2.
Unadjusted Associations Between Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Negative affectivity | 1 | ||||||
| 2. Regulation | −.28** | 1 | |||||
| 3. HSD11B2 | −.14 | .13 | 1 | ||||
| 4. NR3C1 | .05 | −.03 | .19** | 1 | |||
| 5. NR3C2 | −.08 | −.11 | .48** | .50** | 1 | ||
| 6. Prenatal depression | .32** | −.20* | .04 | −.03 | .00 | 1 | |
| 7. Postnatal depression | .40** | −.21* | −.01 | −.02 | .05 | .50** | 1 |
Note. Negative Affectivity and Regulation were measured by the Infant Behavior Questionnaire-Revised. Prenatal and postnatal depression was measured by the Edinburg Postnatal Depression Scale.
p < .05,
p < .01.
Boys exhibited lower scores than girls on Negative Affectivity (boys, mean = 3.09, SD = 0.88; girls, mean = 3.41, SD = 0.89, p = .031). Gender difference was not observed in Regulation, prenatal and postnatal depression, or gene expression measures.
Moderation effects of gene expression on temperament
Negative affectivity
HSD11B2
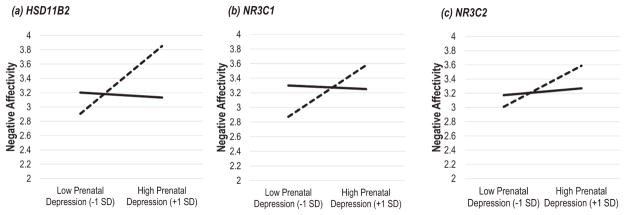
There was a significant main effect for prenatal depression on Negative Affectivity (p values = .005 and .004 in partially and fully adjusted analyses, respectively), whereas the main effect of HSD11B2 on Negative Affectivity was not significant (see Table 3). There was a significant interaction between maternal depression and HSD11B2 expression on Negative Affectivity (p < .001). Simple slope analysis showed that prenatal depression was only positively associated with Negative Affectivity at the levels of low HSD11B2 expression (p < .001), but at the levels of high expression (see Figure 1a).
TABLE 3.
Regression and Follow-Up Analyses: Predicting Negative Affectivity
| Model 1—Postnatal depression adjusted
|
Model 2—Fully adjusted
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression model | β | t | p | 95% CI | Regression model | β | t | p | 95% CI |
| Postnatal depression | .30 | 3.63 | <.001 | .022, .075 | Postnatal depression | .31 | 3.69 | <.001 | .024, .078 |
| Child gender | .18 | 2.59 | .011 | .078, .580 | |||||
| Gestational age | −.07 | −0.95 | .345 | −.131, .046 | |||||
| Birthweight | −.08 | −0.98 | .331 | −.0004, .0001 | |||||
| Maternal education | −.08 | −0.80 | .427 | −.165, .070 | |||||
| Welfare status | −.17 | −1.61 | .110 | −.822, .084 | |||||
| HSD11B2 | −.11 | −1.50 | .137 | −.126, .017 | HSD11B2 | −.12 | −1.65 | .101 | −.132, .012 |
| Prenatal depression | .24 | 2.89 | .005 | .013, .070 | Prenatal depression | .25 | 2.91 | .004 | .013, .070 |
| HSD11B2 × prenatal depression | −.29 | −3.67 | <.001 | −.042, −.013 | HSD11B2 × prenatal depression | −.28 | −3.65 | <.001 | −.042, −.013 |
| Simple slope analyses for interaction | Simple slope analyses for interaction | ||||||||
| Low HSD11B2 (−1 SD) | .53 | 4.14 | <.001 | .047, .133 | Low HSD11B2 (−1 SD) | .53 | 4.15 | <.001 | .047, .133 |
| High HSD11B2 (+1 SD) | −.04 | −0.41 | .684 | −.041, .027 | High HSD11B2 (+1 SD) | −.04 | −0.39 | .698 | −.04, .027 |
| Regression model | β | t | p | 95% CI | Regression model | β | t | p | 95% CI |
| Postnatal depression | .31 | 3.60 | <.001 | .023, .078 | Postnatal depression | .30 | 3.51 | <.001 | .022, .078 |
| Child gender | .20 | 2.64 | .009 | .089, .627 | |||||
| Gestational age | −.03 | −0.34 | .733 | −.110, .077 | |||||
| Birthweight | −.07 | −0.89 | .375 | −.0004, .0002 | |||||
| Maternal education | −.06 | −0.51 | .608 | −.155, .091 | |||||
| Welfare status | −.20 | −1.78 | .078 | −.893, .046 | |||||
| NR3C1 | .04 | 0.50 | .621 | −.220, .367 | NR3C1 | .03 | 0.38 | .705 | −.238, .351 |
| Prenatal depression | .18 | 2.12 | .036 | .002, .059 | Prenatal depression | .18 | 2.16 | .032 | .003, .060 |
| NR3C1 × prenatal depression | −.19 | −2.06 | .041 | −.139, −.003 | NR3C1 × prenatal depression | −.21 | −2.27 | .025 | −.149, −.010 |
| Simple slope analyses for interaction | Simple slope analyses for interaction | ||||||||
| Low NR3C1 (−1 SD) | .37 | 2.79 | .006 | .018, .107 | Low NR3C1 (−1 SD) | .40 | 2.95 | .004 | .022, .112 |
| High NR3C1 (+1 SD) | −.01 | −0.06 | .954 | −.041, .038 | High NR3C1 (+1 SD) | −.03 | −0.23 | .817 | −.044, .035 |
| Regression model | β | t | p | 95% CI | Regression model | β | t | p | 95% CI |
| Postnatal depression | .32 | 3.71 | <.001 | .024, .080 | Postnatal depression | .32 | 3.63 | <.001 | .024, .080 |
| Child gender | .19 | 2.56 | .012 | .077, .600 | |||||
| Gestational age | −.06 | −0.72 | .474 | −.125, .059 | |||||
| Birthweight | −.07 | −0.83 | .410 | −.0004, .0002 | |||||
| Maternal education | −.06 | −0.51 | .613 | −.154, .091 | |||||
| Welfare status | −.19 | −1.71 | .089 | −.878, .064 | |||||
| NR3C2 | −.07 | −.86 | .392 | −.279, .110 | NR3C2 | −.04 | −0.58 | .565 | −.250, .137 |
| Prenatal depression | .19 | 2.14 | .034 | .002, .060 | Prenatal depression | .19 | 2.19 | .030 | .003, .061 |
| NR3C2 × prenatal depression | −.12 | −1.81 | .072 | −.064, .003 | NR3C2 × prenatal depression | −.13 | −1.98 | .049 | −.067, −.0001 |
| Simple slope analyses for interaction | Simple slope analyses for interaction | ||||||||
| Low NR3C2 (−1 SD) | .31 | 2.59 | .011 | .012, .092 | Low NR3C2 (−1 SD) | .32 | 2.72 | .007 | .015, .095 |
| High NR3C2 (+1 SD) | .06 | 0.63 | .533 | −.023, .044 | High NR3C2 (+1 SD) | .05 | 0.54 | .589 | −.024, .043 |
Note. At Model 1, adjusted for postnatal depression.
At Model 2, adjusted for postnatal depression, child gender, gestational age, birthweight, maternal education, and welfare status.
Figure 1.
Gene expression moderation of prenatal depression on Negative Affectivity. Solid line represents high gene expression (+1 SD), and dashed line represents low gene expression (−1 SD). Prenatal maternal depression is linked to higher levels of Negative Affectivity only among infants with the low levels of placental gene expression (p values < .001, =.004, and .007 for HSD11B2, NR3C1, and NR3C2, respectively), but not high levels of gene expression (p values = .698, .817, and .589 for HSD11B2, NR3C1, and NR3C2, respectively). Figures are based on the fully adjusted analyses.
NR3C1
There was a significant main effect for prenatal depression on Negative Affectivity (p values = .036 and .032 in partially and fully adjusted analyses, respectively), whereas the main effect of NR3C1 on Negative Affectivity was not significant. There was a significant interaction between maternal depression and NR3C1 expression on Negative Affectivity (p values = .041 and .025 in partially and fully adjusted analyses, respectively). Simple slope analysis showed that prenatal depression was only positively associated with Negative Affectivity at the levels of low NR3C1 expression (p values = .006 and .004 in partially and fully adjusted analyses, respectively), but not at the levels of high expression (see Figure 1b).
NR3C2
There was a significant main effect of prenatal depression on Negative Affectivity (p values = .034 and .030 in partially and fully adjusted analyses, respectively), whereas the main effect of NR3C2 on Negative Affectivity was not significant. The interaction between maternal depression and NR3C2 on Negative Affectivity was marginally significant or significant (p values = .072 and .049 in partially and fully adjusted analyses, respectively). Simple slope analysis showed that prenatal depression was only positively associated with Negative Affectivity at the levels of low NR3C2 expression (p values = .011 and .007 in partially and fully adjusted analyses, respectively), but not at the levels of high expression (Figure 1c).
Regulation
Neither the main effects of prenatal depression and gene expression nor their interaction were significantly related to Regulation (see Table 4).
TABLE 4.
Regression and Follow-Up Analyses: Predicting Regulation
| Model 1—Postnatal depression adjusted
|
Model 2—Fully adjusted
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression model | β | t | p | 95% CI | Regression model | β | t | p | 95% CI |
| Postnatal depression | −.15 | −1.63 | .106 | −.041, .004 | Postnatal depression | −.14 | −1.45 | .148 | −.040, .006 |
| Child gender | .03 | 0.37 | .715 | −.176, .255 | |||||
| Gestational age | .05 | 0.63 | .532 | −.052, .100 | |||||
| Birthweight | −.05 | −0.52 | .606 | −.0003, .0002 | |||||
| Maternal education | −.01 | −0.07 | .946 | −.104, .098 | |||||
| Welfare status | .13 | 1.04 | .298 | −.183, .594 | |||||
| HSD11B2 | .13 | 1.68 | .095 | −.009, .110 | HSD11B2 | .11 | 1.33 | .186 | −.020, .103 |
| Prenatal depression | −.16 | −1.68 | .094 | −.044, .004 | Prenatal depression | −.14 | −1.42 | .157 | −.042, .007 |
| HSD11B2 × prenatal depression | .04 | 0.42 | .675 | −.010, .015 | HSD11B2 × prenatal depression | .02 | 0.18 | .859 | −.012, .014 |
| Simple slope analyses for interaction | Simple slope analyses for interaction | ||||||||
| Low HSD11B2 (−1 SD) | −.19 | −1.37 | .173 | −.060, .011 | Low HSD11B2 (−1 SD) | −.15 | −1.05 | .297 | −.056, .017 |
| High HSD11B2 (+1 SD) | −.12 | −1.10 | .275 | −.043, .012 | High HSD11B2 (+SD) | −.12 | −1.06 | .291 | −.044, .013 |
| Regression model | β | t | p | 95% CI | Regression model | β | t | p | 95% CI |
| Postnatal depression | −.16 | −1.72 | .088 | −.042, .003 | Postnatal depression | −.14 | −1.48 | .141 | −.041, .006 |
| Child gender | .04 | 0.44 | .658 | −.170, .269 | |||||
| Gestational age | .04 | 0.45 | .657 | −.060, .095 | |||||
| Birthweight | −.05 | −0.51 | .611 | −.0003, .0002 | |||||
| Maternal education | −.02 | −0.17 | .866 | −.111, .093 | |||||
| Welfare status | .13 | 1.10 | .274 | −.174, .607 | |||||
| NR3C1 | −.02 | −0.31 | .760 | −.273, .200 | NR3C1 | −.03 | −0.37 | .713 | −.290, .199 |
| Prenatal depression | −.14 | −1.53 | .127 | −.041, .005 | Prenatal depression | −.12 | −1.32 | .189 | −.040, .008 |
| NR3C1 × prenatal depression | .04 | 0.40 | .689 | −.044, .066 | NR3C1 × prenatal depression | .02 | 0.19 | .847 | −.052, .063 |
| Simple slope analyses for interaction | Simple slope analyses for interaction | ||||||||
| Low NR3C1 (−1 SD) | −.18 | −1.27 | .207 | −.059, .013 | Low NR3C1 (−1 SD) | −.14 | −0.97 | .333 | −.056, .019 |
| High NR3C1 (+1 SD) | −.10 | −0.81 | .422 | −.045, .019 | High NR3C1 (+1 SD) | −.10 | −0.80 | .424 | −.046, .020 |
| Regression model | β | t | p | 95% CI | Regression model | β | t | p | 95% CI |
| Postnatal depression | −.15 | −1.66 | .099 | −.041, .004 | Postnatal depression | −.13 | −1.40 | .164 | −.040, .007 |
| Child gender | .04 | 0.45 | .653 | −.167, .265 | |||||
| Gestational age | .05 | 0.57 | .571 | −.054, .098 | |||||
| Birthweight | −.05 | −0.56 | .573 | −.0003, .0002 | |||||
| Maternal education | −.03 | −0.24 | .809 | −.113, .088 | |||||
| Welfare status | .14 | 1.12 | .263 | −.167, .608 | |||||
| NR3C2 | −.09 | −1.14 | .258 | −.246, .066 | NR3C2 | −.10 | −1.24 | .218 | −.259, .060 |
| Prenatal depression | −.13 | −1.43 | .154 | −.040, .006 | Prenatal depression | −.11 | −1.20 | .231 | −.038, .009 |
| NR3C2 × prenatal depression | −.02 | −0.25 | .804 | −.030, .023 | NR3C2 × prenatal depression | −.03 | −0.45 | .655 | −.034, .021 |
| Simple slope analyses for interaction | Simple slope analyses for interaction | ||||||||
| Low NR3C2 (−1 SD) | −.12 | −0.90 | .369 | −.047, .017 | Low NR3C2 (−1 SD) | −.08 | −0.62 | .539 | −.043, .023 |
| High NR3C2 (+1 SD) | −.15 | −1.41 | .161 | −.046, .008 | High NR3C2 (+1 SD) | −.15 | −1.35 | .181 | −.046, .009 |
Note. At Model 1, adjusted for postnatal depression.
At Model 2, adjusted for postnatal depression, child gender, gestational age, birthweight, maternal education, and welfare status.
DISCUSSION
The current study has two main findings: (1) Prenatal depression was associated with increased Negative Affectivity at 6 months of age and (2) the association between prenatal depression and Negative Affectivity was moderated by the levels of HSD11B2, NR3C1, and NR3C2 gene expression, in which prenatal depression was linked to increased Negative Affectivity only at the lower HSD11B2, NR3C1, and NR3C2 gene expression levels. Our finding is consistent with previous studies that showed fetal exposure to maternal depression was conducive to programming effects, with downstream implications for infant temperament development. It also extends prior research by showing initial evidence that the impact of maternal depression may be alleviated by the level of gene expression in the placenta tissues.
Depression is the leading cause of disability worldwide (WHO, 2017) and is a significant public health concern. It has been widely speculated that children of mothers with higher prenatal depression are more likely to exhibit cognitive, behavioral, and emotional problems (Field, 2011) as a result of exposure to elevated maternal cortisol in the intrauterine environment. Our results show that greater maternal depression is independently associated with greater Negative Affectivity even after postnatal depression and other psychosocial characteristics were controlled for. This finding is consistent with prior research, which showed that prenatal maternal stress (e.g., depression, anxiety) predicted increased emotionality in offspring independent of postnatal maternal mood state (Davis et al., 2004, 2007; Huot, Brennan, Stowe, Plotsky, & Walker, 2004; O’Connor, Heron, Golding, Beveridge, & Glover, 2002).
We attempted to extend our understanding of whether the adverse effect of maternal depression during pregnancy on their offspring’s temperament could be moderated by placental gene function. Beyond the effects of prenatal depression, postnatal depression, and background characteristics, placental HSD11B2, NR3C1, and NR3C2 expression moderated the impact of exposure to prenatal depression. Greater prenatal depression and lower placental gene expression together appeared to confer risk for infants’ negative temperament, whereas higher placental gene expression buffers the infant from the effect of prenatal depression. Lower expression of HSD11B2, NR3C1, and NR3C2, the key genes that metabolize and regulate glucocorticoids in the placenta, may contribute to levels of glucocorticoids exposure that may harm the fetus (Conradt et al., 2013; Marsit et al., 2012; Moisiadis & Matthews, 2014; Paquette et al., 2015). Specifically, lower placental HSD11B2 expression may lead to a reduction in 11β-HSD2, thereby failing to create an effective barrier between mother and fetus by converting cortisol into cortisone, while lower NR3C1 and NR3C2 expression may result in decreased levels of GR and MR that may constrain glucocorticoid signaling in utero. While prior research has linked increased maternal cortisol to poor self-regulation and temperament problems among affected offspring (Buss et al., 2012; Davis et al., 2007; de Weerth, van Hees, & Buitelaar, 2003), it is also possible that greater expression of HSD11B2, NR3C1, and NR3C2 may protect against the impact of elevated levels of stress-induced glucocorticoids produced by the mother during pregnancy. Consequently, some infants may be less vulnerable to temperamental problems despite mother’s prenatal depression.
Interestingly, neither prenatal depression and gene expression nor their interaction were related to Regulation. Negative Affectivity and Regulation represent different constructs of temperament (Bayly & Gartstein, 2013; Gartstein et al., 2014) as measured by the IBQ-R Negative Affectivity scale, which measures infants’ response to distress, fear, crying, and sad mood. The development of these temperament traits may be more likely to be influenced by the dysregulated HPA axis functioning in offspring. The fetal HPA axis is developed and demonstrates enhanced activity during late gestation (Challis et al., 2001). The alternation in the development of the fetal HPA axis is subject to changes in fetal glucocorticoid exposure, which could be influenced by the changes in the placental HSD11B2, NR3C1, and NR3C2 gene expression levels. In contrast, the IBQ-R Regulation scale measures infant temperament traits including pleasure related to low stimulus intensity, expression of enjoyment, duration of attention, and the ability to become calm when soothed by the caregiver. The development of emotion regulation in younger children is a complex phenomenon and is likely influenced by other neurobiological systems, such as the brain stem, limbic, and cortical systems (Geva & Feldman, 2008). Our findings suggest that infant’s negative emotionality may be selectively affected by prenatal depression and further confirm that lower placental HSD11B2, NR3C1, and NR3C2 levels are more likely to promote increased impaired emotionality but not problems with emotion regulation in infancy. However, the underlying mechanisms behind the differences between the two dimensions of temperament still need to be explored.
Prior literature suggests that gene expression itself might be susceptible to adverse environmental influences, particularly in the prenatal period, although we did not find direct associations between maternal depression and gene expression. Our findings indicate several inconsistences in the literature. Prenatal depression has been associated with increased NR3C1 and NR3C2 expression in the placenta (Räikkönen et al., 2015; Reynolds et al., 2015), but greater NR3C1 methylation (i.e., lower expression) in cord blood (Conradt et al., 2013; Oberlander et al., 2008). No previous research has found a significant correlation between maternal depression and placental HSD11B2 expression (O’Donnell et al., 2012; Ponder et al., 2011; Räikkönen et al., 2015; Reynolds et al., 2015), although lower expression has been associated with increased prenatal anxiety (O’Donnell et al., 2012).
It is worth noting that our results appear to be contrary to those of Räikkönen et al. (2015), where higher placental gene expression of HSD11B2 and NR3C1 (but not NR3C2) was associated with more difficulties in regulatory behaviors (i.e., crying, sleeping, feeding, spitting, bowel movement, sleeping, and predictability) in newborn infants around 15 days, and higher placental NR3C1 gene expression partially mediated the association between prenatal depression and infant regulatory behaviors. Differences might stem from the fact that we measured temperament at 6 months using the IBQ-R, whereas Räikkönen et al. measured regulatory behaviors at 15 days using the Neonatal Perception Inventory. They suspected that greater expression of NR3C1 might lead to placental glucocorticoid oversensitivity, resulting in dysregulated infant behaviors. The investigation of placental gene expression of HSD11B2, NR3C1, NR3C2 in relation to infant temperament has not been well explored. While the differences might be a result of the methodological differences between the two studies, future replication is necessary to increase understanding of the relationship between glucocorticoid metabolism in the placenta and emerging temperament in early childhood.
There are some limitations to the current study. First, both the measures of maternal prenatal depression and infant temperament relied on mothers’ report. Future studies will benefit from assessments of infant temperament by multiple informants or via observation (Boyd, Zayas, & McKee, 2006; Edhborg, Lundh, Seimyr, & Widström, 2001; Pauli-Pott, Mertesacker, & Beckmann, 2004). Similarly, generalization of the findings for clinical-level depression may be limited as we relied on self-report depression symptomatology. The application of a more structured psychiatric interview (DSM-5) of depressive symptoms is warranted. Second, our sample was limited by the availability of placenta tissues. While key demographic characteristics between the infants with and without placenta tissues did not differ significantly, we acknowledge that this may have limited external validity. Furthermore, maternal depression was assessed only once, in mid-gestation. It is possible that mothers’ depression fluctuated during pregnancy and its interaction with placental gene function would also be differentially associated with infant’s temperament. Third, our study utilized a candidate gene approach and only examined three genes, while a genomewide analysis may offer new and more comprehensive insights. Fourth, given that the sample consisted of more families of low SES and minority status (Black and Hispanic), results may not generalize to families with higher economic resources or to other racial/ethnic groups (Blackmore et al., 1993; Lu & Halfon, 2003). Fifth, a very small proportion of our sample (2% or n = 3) used antidepressants during pregnancy. As there is a slight possibility that the association between prenatal depression and infant neurodevelopment may be influenced by antidepressant exposure (Casper et al., 2003; Weikum et al., 2013), the analyses were rerun again by excluding these three cases. Although the results showed no change, we have to remain cautious. Finally, placenta samples collected in the current study were obtained from pregnancies free of major obstetric complications, which may constrain the variances in both gene expression levels and infants’ outcomes in an unknown fashion. Despite these limitations, the current study demonstrated that a greater level of maternal depression during pregnancy is associated with increased Negative Affectivity in infants. Furthermore, the negative effect of maternal depression on Negative Affectivity is present only among infants with lower placental HSD11B2, NR3C1, and NR3C2 expression.
These findings support the concept that placental genes play a crucial role in regulating infant growth during early development. Understanding the effects of the expression of human placental genes can help uncover the role of prenatal depression in influencing infant development, thus contributing to preventive interventions for prenatal maternal depression to mitigate its negative effects on offspring development. Moving forward, a priority for researchers and clinicians alike should be working toward translating these findings into interventions to detect and prevent later psychopathology among high-risk infants. Our findings support the view that preventing early negative/ difficult temperament (e.g., greater negative affectivity) and future suboptimal neurobehavioral development characterized by internalizing and externalizing behaviors (De Pauw & Mervielde, 2010; Hellemans et al., 2010) should involve interventions that begin during pregnancy. This information can aid in identifying offspring of depressed mothers. However, the clinical implications (i.e., incorporating gene expression in multidimensional risk indices) are still premature. Placental gene expression cannot be assessed when pregnancy continues, so studies must be undertaken using peripheral tissues. The circulating placental RNA (cpRNA) released from the placenta into the maternal bloodstream during pregnancy has been related to the gene expression levels in the placenta. As cpRNA may reflect changes in the placenta, it has been used as a noninvasive approach to examine placental functions for prenatal diagnosis (such as pre-eclampsia, preterm birth) when pregnancy continues (Tsui et al., 2004; Whitehead, Walker, & Tong, 2016). Although the degree of correlations in gene expression across different tissues (maternal blood versus placenta) remains in question, these studies suggest potential for the discovery of placental mRNA markers in the cpRNA that can be used for monitoring placental functions, particularly in modulating glucocorticoid circulation in utero. Finally, decreasing negative emotionality and/or increasing emotion regulation could lead to more positive socioemotional development in the child’s life (Denham, Wyatt, Bassett, Echeverria, & Knox, 2009; Sanson, Hemphill, & Smart, 2004).
Acknowledgments
We thank all the parents and children who consented to participate in this study. We also thank current and former research staff and assistants at Queens College, City University of New York for their contributions to this study. Gene expression assays were conducted in Chen’s laboratory at Icahn School of Medicine at Mount Sinai. We thank Yula Ma, MD and Jia Chen, Sc.D. for gene expression assays and analysis.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests with respect to this report.
Contributor Information
Wei Zhang, Queens College, CUNY.
Jackie Finik, Queens College, CUNY and Icahn School of Medicine at Mount Sinai and Graduate School of Public Health and Health Policy, CUNY.
Kathryn Dana, Queens College, CUNY and The Graduate Center, CUNY.
Vivette Glover, Imperial College London.
Jacob Ham, Icahn School of Medicine at Mount Sinai.
Yoko Nomura, Queens College, CUNY and Icahn School of Medicine at Mount Sinai and The Graduate Center, CUNY.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications Inc; 1991. [Google Scholar]
- Babineau V, Green CG, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, … Wazana A. Prenatal depression and 5-HTTLPR interact to predict dysregulation from 3 to 36 months—A differential susceptibility model. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2015;56(1):21–29. doi: 10.1111/jcpp.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Fetal programming of coronary heart disease. Trends in Endocrinology & Metabolism. 2002;13:364–368. doi: 10.1016/S1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Bayly B, Gartstein M. Mother’s and father’s reports on their child’s temperament: Does gender matter? Infant Behavior and Development. 2013;36(1):171–175. doi: 10.1016/j.infbeh.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clinical Endocrinology. 1997;46(2):161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Blackmore CA, Ferré CD, Rowley DL, Hogue CJ, Gaiter J, Atrash H. Is race a risk factor or a risk marker for preterm delivery? Ethnicity & Disease. 1993;3:372–377. [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14:644–651. doi: 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolten M, Nast I, Skrundz M, Stadler C, Hellhammer DH, Meinlschmidt G. Prenatal programming of emotion regulation: Neonatal reactivity as a differential susceptibility factor moderating the outcome of prenatal cortisol levels. Journal of Psychosomatic Research. 2013;75(4):351–357. doi: 10.1016/j.jpsychores.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Boyd RC, Zayas LH, McKee MD. Mother-infant interaction, life events and prenatal and postpartum depressive symptoms among urban minority women in primary care. Maternal and Child Health Journal. 2006;10(2):139–148. doi: 10.1007/s10995-005-0042-2. [DOI] [PubMed] [Google Scholar]
- Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Developmental Psychobiology. 2013;55(7):673–683. doi: 10.1002/dev.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. Journal of Pediatrics. 2003;142(4):402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Sloboda D, Matthews SG, Holloway A, Alfaidy N, Patel FA, … Newnham J. The fetal placental hypothalamic–pituitary–adrenal (HPA) axis, parturition and post natal health. Molecular and Cellular Endocrinology. 2001;185(1–2):135–144. doi: 10.1016/S0303-7207(01)00624-4. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The concepts of stress and stress system disorders. JAMA. 1992;267:1244. doi: 10.1001/jama.1992.03480090092034. [DOI] [PubMed] [Google Scholar]
- Conradt E, Fei M, LaGasse L, Tronick E, Guerin D, Gorman D, … Lester BM. Prenatal predictors of infant self-regulation: The contributions of placental DNA methylation of NR3C1 and neuroendocrine activity. Frontiers in Behavioral Neuroscience. 2015;9:130. doi: 10.3389/fnbeh.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8:1321–1329. doi: 10.4161/epi.26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Crosby DD, Henderson-Smart DJ. Vitamin K prior to preterm birth for preventing neonatal periventricular haemorrhage. Cochrane Database of Systematic Reviews. 2010;(1):CD000229. doi: 10.1002/14651858.cd000164. (Online). [DOI] [PMC free article] [PubMed]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52(2):119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6(3):319–331. doi: 10.1207/s15327078in0603_1. [DOI] [Google Scholar]
- Deave T, Heron J, Evans J, Emond A. The impact of maternal depression in pregnancy on early child development. BJOG: An International Journal of Obstetrics and Gynaecology. 2008;115:1043–1051. doi: 10.1111/j.1471-0528.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Denham SA, Wyatt TM, Bassett HH, Echeverria D, Knox SS. Assessing social-emotional development in children from a longitudinal perspective. Journal of Epidemiology and Community Health. 2009 Dec;63(Suppl 1):i37–i52. doi: 10.1136/jech.2007.070797. 2008. [DOI] [PubMed] [Google Scholar]
- De Pauw SSW, Mervielde I. Temperament, personality and developmental psychopathology: A review based on the conceptual dimensions underlying childhood traits. Child Psychiatry & Human Development. 2010;41:313–329. doi: 10.1007/s10578-009-0171-8. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004;67(1):63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- Dieter JN, Field T, Hernandez-Reif M, Jones NA, Lecanuet JP, Salman FA, Redzepi M. Maternal depression and increased fetal activity. Journal of Obstetrics and Gynaecology: The Journal of the Institute of Obstetrics and Gynaecology. 2001;21:468–473. doi: 10.1080/01443610120072009. [DOI] [PubMed] [Google Scholar]
- Diplas AI, Lambertini L, Lee MJ, Sperling R, Lee YL, Wetmur JG, Chen J. Differential expression of imprinted genes in normal and IUGR human placentas. Epigenetics. 2009;4(4):235–240. doi: 10.4161/epi.9019. [DOI] [PubMed] [Google Scholar]
- Edhborg M, Lundh W, Seimyr L, Widström AM. The long-term impact of postnatal depressed mood on mother-child interaction: A preliminary study. Journal of Reproductive and Infant Psychology. 2001;19(1):61–71. doi: 10.1080/02646830123255. [DOI] [Google Scholar]
- Field T. Prenatal depression effects on early development: A review. Infant Behavior and Development. 2011;34(1):1–14. doi: 10.1016/j.infbeh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, … Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27:216–229. doi: 10.1016/j.infbeh.2003.09.010. [DOI] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: A review. Infant Behavior and Development. 2006;29(3):445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, … Gonzalez-Garcia A. Prenatal maternal biochemistry predicts neonatal biochemistry. The International Journal of Neuroscience. 2004;114:933–945. doi: 10.1080/00207450490461305. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Bell MA, Calkins SD. EEG asymmetry at 10 months of age: Are temperament trait predictors different for boys and girls? Developmental Psychobiology. 2014;56:1327–1340. doi: 10.1002/dev.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development. 2003;26(1):64–86. doi: 10.1016/S0163-6383(02)00169-8. [DOI] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, … Miller WC. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evidence Report/ Technology Assessment. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva R, Feldman R. A neurobiological model for the effects of early brainstem functioning on the development of behavior and emotion regulation in infants: Implications for prenatal and perinatal risk. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49:1031–1041. doi: 10.1111/j.1469-7610.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, O’Donnell K. Prenatal stress and the programming of the HPA axis. Neuroscience & Biobehavioral Reviews. 2010;35(1):17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, De Weerth C, Willemsen-Swinkels SHN, Huizink AC, Mulder EJH, Visser GHA, … Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. European Child and Adolescent Psychiatry. 2005;14(1):41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper] 2012 Retrieved from http://www.afhayes.com/public/process2012.pdf.
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: The Guilford Press; 2013. [Google Scholar]
- Hellemans KGC, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neuroscience & Biobehavioral Reviews. 2010;34(6):791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot RL, Brennan PA, Stowe ZN, Plotsky PM, Walker EF. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Annals of the New York Academy of Sciences. 2004;1032(1):234–236. doi: 10.1196/annals.1314.028. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33(3s):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Kajantie E. Fetal origins of stress-related adult disease. Annals of the New York Academy of Sciences. 2006;1083(1):11–27. doi: 10.1196/annals.1367.026. [DOI] [PubMed] [Google Scholar]
- Kheirabadi GR, Maracy MR, Akbaripour S, Masaeli N. Psychometric properties and diagnostic accuracy of the Edinburgh postnatal depression scale in a sample of Iranian women. Iranian Journal of Medical Sciences. 2012;37(1):32–38. [PMC free article] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35:1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Korhonen M, Luoma I, Salmelin R, Tamminen T. A longitudinal study of maternal prenatal, postnatal and concurrent depressive symptoms and adolescent well-being. Journal of Affective Disorders. 2012;136(3):680–692. doi: 10.1016/j.jad.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Lambertini L, Diplas AI, Lee MJ, Sperling R, Chen J, Wetmur JG. A sensitive functional assay reveals frequent loss of genomic imprinting in human placenta. Epigenetics. 2008;3(5):261–269. doi: 10.4161/epi.3.5.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftin RW, Habli M, Snyder CC, Cormier CM, Lewis DF, DeFranco EA. Late preterm birth. Reviews in Obstetrics and Gynecology. 2010;3(1):10–19. doi: 10.3909/riog0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal. 2003;7(1):13–30. doi: 10.1023/A:1022537516969. [DOI] [PubMed] [Google Scholar]
- Luoma I, Kaukonen A, Antymaa M, Puura K, Tamminen T, Salmelin R. A longitudinal study of maternal depressive symptoms, negative expectations and perceptions of child problems. Child Psychiatry and Human Development. 2004;35(1):37–53. doi: 10.1023/b:chud.0000039319.96151.63. [DOI] [PubMed] [Google Scholar]
- Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, Almqvist F. Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:1367–1374. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Maccani MA, Marsit CJ. Epigenetics in the placenta. American Journal of Reproductive Immunology. 2009;62(2):78–89. doi: 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women’s Health. 2003;12(4):373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One. 2012;7(3):e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends in Endocrinology and Metabolism. 2002;13:373–380. doi: 10.1016/S1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- Mazhari S, Nakhaee N. Validation of the Edinburgh Postnatal Depression Scale in an Iranian sample. Archives of Women’s Mental Health. 2007;10:293–297. doi: 10.1007/s00737-007-0204-x. [DOI] [PubMed] [Google Scholar]
- Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 2: Mechanisms. Nature Reviews Endocrinology. 2014;10:403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- Montazeri A, Torkan B, Omidvari S. The Edinburgh Postnatal Depression Scale (EPDS): Translation and validation study of the Iranian version. BMC Psychiatry. 2007;7(1):11. doi: 10.1186/1471-244X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. The British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 2012;37:818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Paquette AG, Lester BM, Lesseur C, Armstrong DA, Guerin DJ, Appleton AA, Marsit CJ. Placental epigenetic patterning of glucocorticoid response genes is associated with infant neurodevelopment. Epigenomics. 2015;7(5):767–779. doi: 10.2217/epi.15.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends in Neurosciences. 2008 Sep;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pastinen T, Sladek R, Gurd S, Sammak A, Ge B, Lepage P, … Hudson TJ. A survey of genetic and epigenetic variation affecting human gene expression. Physiological Genomics. 2004;16(2) doi: 10.1152/physiolgenomics.00163.2003. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U, Mertesacker B, Beckmann D. Predicting the development of infant emotionality from maternal characteristics. Development and Psychopathology. 2004;16(1):19–42. doi: 10.1017/S0954579404044396. [DOI] [PubMed] [Google Scholar]
- Pluess M, Velders FP, Belsky J, Van IJzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VWV, … Tiemeier H. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biological Psychiatry. 2011;69:520–525. doi: 10.1016/j.biopsych.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ponder KL, Salisbury A, McGonnigal B, Laliberte A, Lester B, Padbury JF. Maternal depression and anxiety are associated with altered gene expression in the human placenta without modification by antidepressant use: Implications for fetal programming. Developmental Psychobiology. 2011;53:711–723. doi: 10.1002/dev.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society Inc. 2004;36(4):717–731. doi: 10.3758/BF03206553. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E. Development and assessment of short and very short forms of the Infant Behavior Questionnaire-Revised. Journal of Personality Assessment. 2014;96(4):445–458. doi: 10.1080/00223891.2013.841171. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Pesonen AK, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, … Reynolds RM. Maternal depressive symptoms during pregnancy, placental expression of genes regulating glucocorticoid and serotonin function and infant regulatory behaviors. Psychological Medicine. 2015;45:3217–3226. doi: 10.1017/S003329171500121X. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Pesonen AK, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, … Räikkönen K. Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity. Psychological Medicine. 2015;45:2023–2030. doi: 10.1017/S003329171400316X. [DOI] [PubMed] [Google Scholar]
- Sandman CA. Fetal exposure to placental corticotropin-releasing hormone (pCRH) programs developmental trajectories. Peptides. 2015;72:145–153. doi: 10.1016/j.peptides.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, Davis EP. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry. 2015;77(4):324–334. doi: 10.1016/j.biopsych.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology. 1999;34(3):163–173. doi: 10.1002/(SICI)1098-2302(199904)34:3<163:AID-DEV1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sanson A, Hemphill SA, Smart D. Connections between temperament and social development: A review. Social Development. 2004;13(1):142–170. doi: 10.1046/j.1467-9507.2004.00261.x. [DOI] [Google Scholar]
- Seckl JR. Physiologic programming of the fetus. Clinics in Perinatology. 1998;25(4):939–962. vii. [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: Glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nature Clinical Practice Endocrinology & Metabolism. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Annals of the New York Academy of Sciences. 2004;1032(1):63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Small R, Lumley J, Yelland J, Brown S. The performance of the Edinburgh Postnatal Depression Scale in English speaking and non-English speaking populations in Australia. Social Psychiatry and Psychiatric Epidemiology. 2007;42(1):70–78. doi: 10.1007/s00127-006-0134-3. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. American Journal of Obstetrics and Gynecology. 2005;192(2):522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Salisbury AL, Phipps MG, Huestis MA, Niaura R, … Lester BM. Epigenetic regulation of placental NR3C1: Mechanism underlying prenatal programming of infant neurobehavior by maternal smoking? Child Development. 2016;87(1):49–60. doi: 10.1111/cdev.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui NBY, Chim SSC, Chiu RWK, Lau TK, Ng EKO, Leung TN, … Lo YMD. Systematic micro-array based identification of placental mRNA in maternal plasma: Towards noninvasive prenatal gene expression profiling. Journal of Medical Genetics. 2004;41:461–467. doi: 10.1136/jmg.2003.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tychey C, Spitz E, Briançon S, Lighezzolo J, Girvan F, Rosati A, … Vincent S. Pre- and postnatal depression and coping: A comparative approach. Journal of Affective Disorders. 2005;85(3):323–326. doi: 10.1016/j.jad.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Vohr B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clinics in Perinatology. 2013;40(4):739–751. doi: 10.1016/j.clp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Waggott D, Chu K, Yin S, Wouters BG, Liu FF, Boutros PC. NanoStringNorm: An extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics. 2012;28:1546–1548. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development. 2003;74(2):139–151. doi: 10.1016/S0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Weikum WMW, Brain U, Chau CMY, Grunau RE, Boyce WT, Diamond A, Oberlander TF. Prenatal serotonin reuptake inhibitor (SRI) antidepressant exposure and serotonin transporter promoter genotype (SLC6A4) influence executive functions at 6 years of age. Frontiers in Cellular Neuroscience. 2013 Oct;7:180. doi: 10.3389/fncel.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead CL, Walker SP, Tong S. Measuring circulating placental RNAs to non-invasively assess the placental transcriptome and to predict pregnancy complications. Prenatal Diagnosis. 2016;36:997–1008. doi: 10.1002/pd.4934. [DOI] [PubMed] [Google Scholar]
- WHO. WHO | Depression. 2017 Retrieved from http://www.who.int/mediacentre/factsheets/fs369/en/
- Zuckerman B, Bauchner H, Parker S, Cabral H. Maternal depressive symptoms during pregnancy, and newborn irritability. Journal of Developmental & Behavioral Pediatrics. 1990;11(4):190–194. doi: 10.1097/00004703-199008000-00006. [DOI] [PubMed] [Google Scholar]