Abstract
Substituted alkenes are pivotal structural motifs found in pharmaceuticals and agrochemicals. Although numerous methods have been developed to construct substituted alkenes, a generally efficient, mild, catalytic platform for the conversion of alkynes to this highly functionalized scaffold via successive C–C bond forming steps remains in high demand. Here we describe an intermolecular, regio- and syn-stereoselective alkylarylation of terminal alkynes with tertiary alkyl oxalates via photoredox-Ni dual catalysis. This catalytic protocol, synergistically combining Ir/Ni-catalyzed alkyne difunctionalization with photoinduced alkene isomerization, affords trisubstituted alkenes with excellent efficiency and syn-stereoselectivity. The mild conditions tolerate many functional groups, allowing for a broad scope with respect to terminal alkynes, aryl bromides, and alkyl oxalates.
Converting alkynes into alkenes with high stereoselectivity via two consecutive C-C bond forming steps is a desirable process, yet very challenging. Here, the authors describe a dual photoredox-nickel catalytic system for the regio- and syn-selective alkylarylation of terminal alkynes with alkyl oxalates and aryl bromides.
Introduction
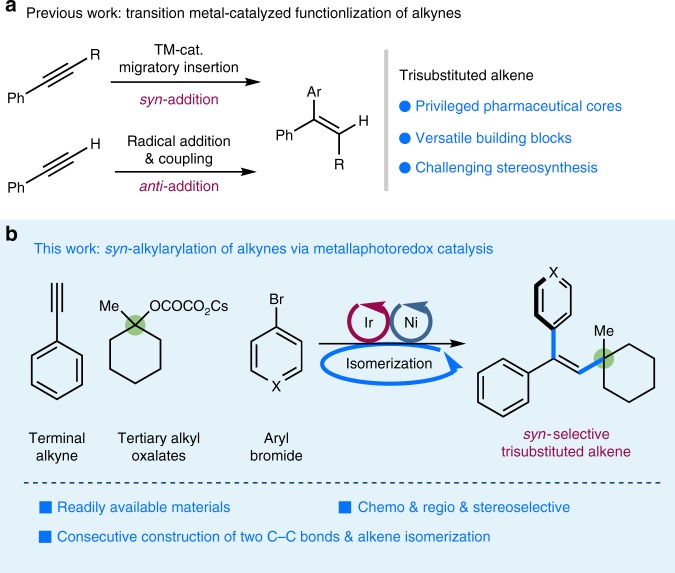
Substituted alkenes are pivotal structural motifs found in pharmaceuticals, agrochemicals, and biologically active natural products1–3, as well as versatile synthetic building blocks in organic synthesis4,5. Consequently, the development of general protocols for the regio- and stereoselective synthesis of alkenes, particularly employing readily available and abundant precursors, is a long-standing goal of chemical synthesis6,7. Transition metal-catalyzed direct functionalization of alkynes is a powerful method to access substituted alkenes with stereoselectivity control8–21. Generally, two distinct strategies have been utilized: one is a catalytic generation of syn-substituted alkenyl metal species, via migratory insertion controlled by the stereoelectronic effect, followed by protonation or coupling to deliver trisubstituted alkenes with syn-stereoselectivity8–28; the other is transition metal-catalyzed radical addition/coupling reaction of alkynes, typically favoring anti-addition which is dominated by steric factors of vinyl radicals in the coupling step (Fig. 1a)29–37. Although numerous methods have been developed toward addressing the challenge of regio- and stereo-selectively forming trisubstituted alkenes, a generally efficient, mild, catalytic platform for the conversion of alkynes to this highly functionalized scaffold via successive C–C bond forming steps would be highly demanding and represent a valuable advance in synthetic methodology.
Fig. 1.
syn-Alkylarylation of terminal alkynes via the combination of photoredox and nickel catalysis. a Alkyne functionalizations via transition metal catalysis. b syn-Alkylarylation of alkynes via metallaphotoredox catalysis
Over the last decade, visible light photocatalysis have emerged as a powerful platform in organic synthesis by activating organic molecules through either single-electron transfer or energy transfer38–43. Particularly, the ability of the photoredox catalyst to modulate the oxidation state of organometallic species has enabled the efficient construction of challenging C–C bonds44–49. Recently, this solar-energy-driven catalytic technology has been utilized to facilitate contra-thermodynamic E → Z isomerization of olefins through an energy-transfer manifold, enabling the facile synthesis of Z-olefins50–56. We recently questioned whether metallaphotoredox catalysis could serve as an alternative platform to access trisubstituted alkenes with high control over stereoselectivity57–59. Specifically, a catalytic protocol including three sequential events, (i) alkyl radical addition to the C≡C bond29, (ii) cross-coupling of the resulting alkenyl radical with nickel complex, and (iii) photochemical E → Z isomerization of olefins, would deliver the stereodefined trisubstituted alkenes. We envisioned that the unique metallaphotoredox manifold would enable the generation of alkyl radicals from readily available feedstocks60 such as alcohols61, and more importantly could be leveraged to enrich the stereoselectivity of alkyne addition reactions. Herein, we demonstrate the first example of alkylarylation of terminal alkynes employing simple tertiary alcohol derivatives and aryl halides through the synergistic merger of photoredox and nickel catalysis62,63, furnishing a wide array of trisubstituted alkenes with syn-stereoselectivity under mild conditions (Fig. 1b). This photoredox protocol provides complementary reactivity and stereoselectivity to a previous nickel system with alkyl halides as radical precursors, which affords the trisubstituted alkenes with anti-stereoselectivity at elevated temperature (80–120 °C)34.
Results
Design plan
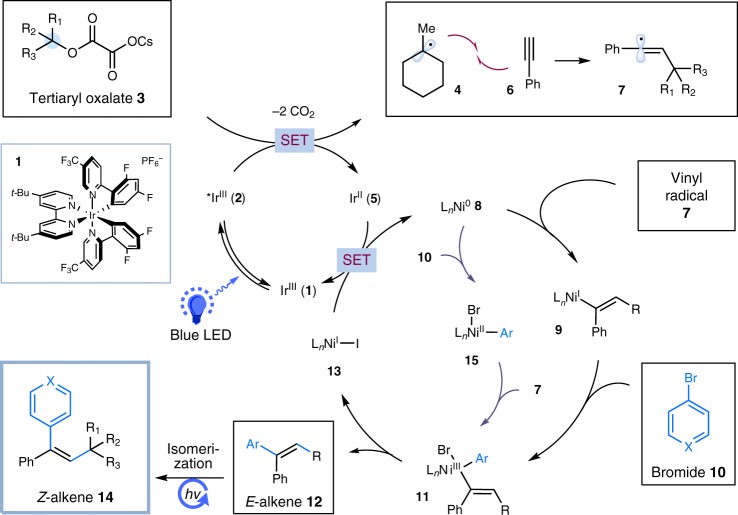
Inspired by Overman and MacMillan’s oxalate half-ester chemistry64, we chose tertiary alkyl oxalates as the alkyl precursors for our proposed metallaphotoredox alkyne chemistry. Tertiary oxalate salts are bench stable, and can be easily prepared from corresponding, abundant tertiary alcohols. As depicted in Fig. 2, we envisioned that a single-electron oxidation of tertiary alkyl oxalate 3 (E1/2 = + 1.28 V vs SCE in CH3CN for tert-BuOCOCO2Cs)64 by photoexcited Ir[dF(CF3)ppy]2(dtbbpy)(PF6) 1 (E1/2[Ir*III/IrII = + 1.21 V vs SCE in CH3CN]65 should generate alkyl radical 4 upon loss of two molecules of CO2 as well as reduced Ir(II) species 5. Alkyl radical 4 is expected to undergo regioselective addition to terminal alkyne 6 to produce linearized alkenyl radical 7 due to resonance stabilization effect29. An anti-addition of the high-energy alkenyl radial 7 and Ni(0) 8 is expected to deliver the (E)-alkenyl-Ni(I) species 966. Subsequent oxidative addition of (E)-alkenyl-Ni(I) 9 with aryl bromide 10 would afford (E)-alkenyl-Ni(III) complex 1134,67, which undergoes a facile reductive elimination to produce substituted alkene 12 with concomitant generation of Ni(I) complex 13. Single-electron transfer between Ir(II) 5 {E1/2[IrIII/IrII = −1.37 V vs SCE in CH3CN]}65 and Ni(I) 13 (E1/2[NiII/Ni0 = −1.2 V vs SCE in DMF]68 would regenerate ground-state Ir(III) 1 and Ni(0) to close the two catalytic cycles. Given the polarity of 12, at this juncture, we hypothesized that a E → Z isomerization of 12 would be possible through a photoinduced energy transfer manifold50–56, delivering the desired alkene 14 with syn-stereoselectivity. Alternatively, another catalytic pathway involving oxidative addition of Ni(0) with aryl bromide 1062,63,69, followed by trapping of the nucleophilic vinyl radical 7 by aryl-Ni(II) 15 to furnish the key Ni(III) intermediate 11, is also plausible.
Fig. 2.
Proposed mechanism. Two possible reaction pathways proposed on the basis of previous literature
Optimization study
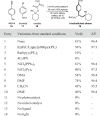
We examined the feasibility of this proposed metallaphotoredox alkyne protocol by employing 4-tert-butylphenylacetylene 16 and 4-bromobenzaldehyde 17 as standard substrates. As shown in Table 1, upon irradiation by a 90 W blue LED of a solution of alkyne 16, bromide 17, and cesium oxalate 18 derived from 1-methyl-1-cyclohexanol in the presence of catalytic amounts of Ir[dF(CF3)ppy]2(dtbbpy)PF6 1, NiCl2•glyme, 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbbpy) in DMSO, 81% yield of the desired trisubstituted alkene product 19 was obtained with excellent chemo-, regio-, and syn-selectivity (Z/E = 96:4) (entry 1). The structurally similar photocatalyst Ir[dF(CF3)ppy]2(phen)PF6 also promoted this transformation with moderate efficiency and excellent syn-selectivity (entry 2). Switching to other commonly employed photocatalysts, such as Ru(bpy)3(PF6)2 and 4CzlPN (2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile), resulted in a dramatic decrease in efficiency (entries 3–4). The reaction proceeded with moderate to good efficiency in the presence of NiCl2(PPh3)2 or precatalyst NiCl2(Py)4 (entries 5–6). The choice of solvent demonstrated a dramatic effect on the reaction efficiency, with DMSO proving to be optimal (entries 7–10). Interestingly, stereoselectivity of the alkene product appeared to be solvent independent (entries 7–10). Finally, control experiments demonstrated that light, photocatalyst, nickel catalyst, and ligand are all essential for the desired transformation to proceed (entries 11–14) (for additional control experiments, see Supplementary Tables 1, 2).
Table 1.
Optimization of reaction conditions.a
|
aReaction conditions: photocatalyst (3 mol%), NiCl2•glyme (20 mol%), dtbbpy (20 mol%), alkyne (0.1 mmol), oxalate (1.5 equiv.), aryl bromide (2.0 equiv.), DMSO [0.05 M], 90 W blue LED, 36 °C, 18 h. Yields determined by 1H NMR with an internal standard, and the ratio of the two isomers was determined by 1H NMR analysis of the crude reaction mixture. dtbbpy = 4,4′-di-tert-butyl-2,2′-dipyridyl; 4CzlPN = 2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile
Substrate scope
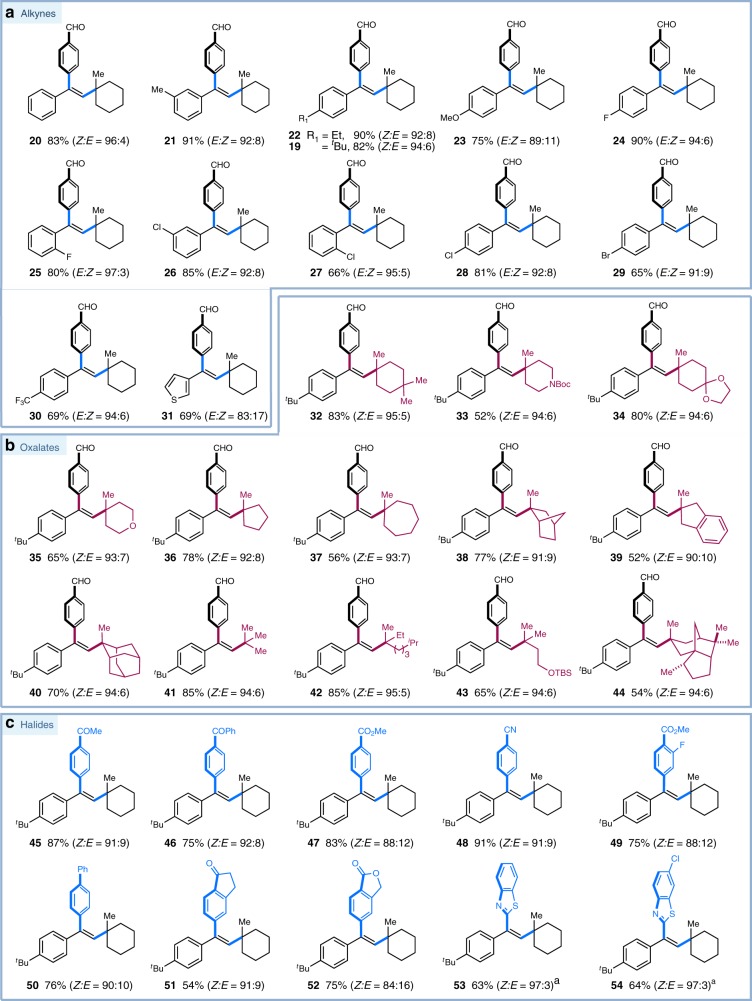
With optimal conditions in hand, we explored the generality of this metallaphotoredox protocol with respect to the alkyne fragment. As depicted in Fig. 3a, terminal arylalkynes bearing electron-neutral, -donating, and -withdrawing substituents proceeded smoothly under the optimal conditions, furnishing the corresponding trisubstituted alkenes with high to excellent yields and stereoselectivity (products 19−30, 65−90% yields, >95:5 syn-selectivity). Notably, halides (F, Cl, Br) on the aryl ring of alkynes remained untouched, offering valuable handles for further manipulations (product 24−29, 65−90% yields, >91:9 syn-selectivity). Moreover, ortho substituents on the aryl ring had little effect to the reaction efficiency and selectivity (products 25 and 27, 80% and 66% yield, >95:5 syn-selectivity, respectively). A slight erosion of yield and selectivity was observed when 3-ethynylthiophene was subjected to this multicomponent system (products 31, 69% yield, 83:17 syn-selectivity). However, internal alkynes are not compatible in this reaction protocol, a result we attribute to increased steric hindrance.
Fig. 3.
Substrate scope. a Scope of alkynes. b Scope of cesium oxalates. c Scope of aryl halides. Reaction conditions: Ir-1 (3 mol%), NiCl2•glyme (20 mol%), dtbbpy (20 mol%), alkyne (0.1 mmol), oxalate (1.5 equiv.), bromide (2.0 equiv.), DMSO [0.05 M], 90 W blue LED, 36 °C, 18 h. All cited yields are isolated yields. The ratios of the two isomers were determined by 1H NMR analysis of the crude reaction mixtures. aHeteroaryl chloride was employed. dtbbpy = 4,4′-di-tert-butyl-2,2′-dipyridyl
Next, we evaluated the scope of cesium tertiary alkyl oxalates in this protocol. As shown in Fig. 3b, a variety of tertiary cesium oxalates, readily prepared from the corresponding tertiary alcohols, can be successfully employed with high levels of efficiency and stereoselectivity. Cyclic oxalates, derived from cyclohexanols, cyclopentanols, and cycloheptanols, underwent the desired addition/coupling smoothly, yielding the (Z)-selective trisubstituted alkenes with high efficiency (products 32−40 and 44, 52−83% yields, Z/E up to 95:5). Heterocycles, in the form of tetrahydropyran and piperidine, were also viable substrates (products 33 and 35, 52% and 65% yields, Z/E > 93:7). A number of polycyclic oxalates could be effectively employed without loss in yield and selectivity (products 34, 38−40, and 44, 49−84% yields, Z/E up to 94:6). Moreover, this photoredox protocol could further be applied to acyclic tertiary oxalates, furnishing the desired alkenes in good yields and excellent stereoselectivity favoring syn-addition (products 41−43, 65−85% yields, Z/E > 94:6). Notably, natural-product-derived substrates, such as cedrol oxalate, proved successful, indicating the potential utility of this mild protocol with complex molecules (products 44, 54% yield, Z/E = 94:6).
Finally, we turned our attention to the scope of aryl bromides that can participate in this catalytic protocol. As revealed in Fig. 3c, a variety of electron-deficient aryl bromides can be readily employed with high efficiency and moderate-to-high stereoselectivity (products 45−54, 54−91% yields, Z/E up to 97:3). Many valuable functional groups, including ketones, esters, nitrile, and lactones were found to be well tolerated under the mild conditions (products 45−52, 54−91% yields, Z/E up to 92:8). Gratifyingly, benzothiazole-derived heteroaromatic chlorides could be efficiently employed in this synergistic protocol without any loss in stereoselectivity, albeit with a slight decrease in yields (products 53 and 54, 63% and 64% yields, Z/E = 97:3, respectively). At this stage in our studies on this metallaphotoredox protocol, the scope of aryl halides is currently limited to electron-poor and electron-neutral system, in which a conjugated substituent at the para position is crucial to achieve excellent syn-stereoselectivity control (see Supplementary Figs. 13, 14).
To highlight the synthetic utility of this metallaphotoredox difunctionalization manifold, a gram-scale reaction of alkyne 16 was performed. The reaction proceeded smoothly, affording the desired alkylarylation product 19 in 65% yield with excellent stereoselectivity (Z/E > 98:2) (Fig. 4).
Fig. 4.
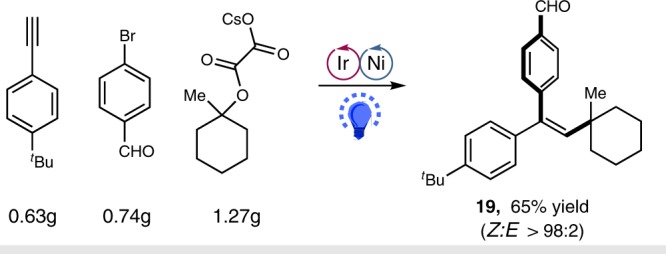
Reaction on large scale. Reaction condition: Ir-1 (3 mol%), NiCl2•glyme (20 mol%), dtbbpy (20 mol%), alkyne (4 mmol), oxalate (1.5 equiv.), bromide (2.0 equiv.), DMSO [0.05 M], 90 W blue LED, 36 °C, 18 h. Isolated yield. The ratio of the two isomers were determined by 1H NMR analysis of the crude reaction mixture. dtbbpy = 4,4′-di-tert-butyl-2,2′-dipyridyl
Mechanistic studies
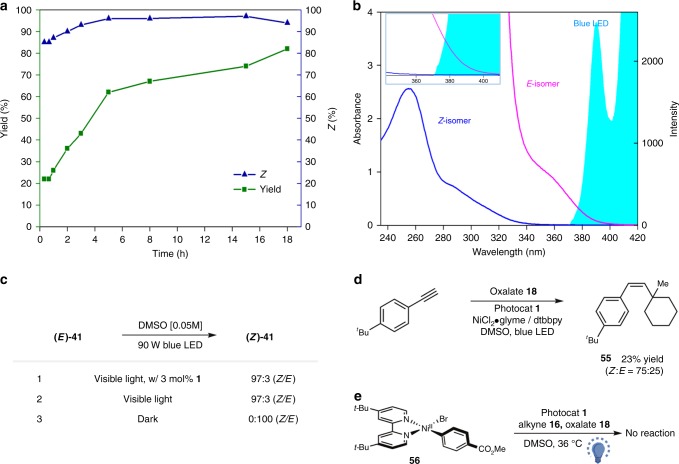
To probe the reaction pathway of this metallaphotoredox three-component coupling protocol, we conducted a series of preliminary mechanistic experiments. Time course studies showed that yields steadily increased overtime, while high stereoselectivity was observed at the early stage, suggesting that stereoselectivity of this transformation might be catalyst-independent (Fig. 5a). To probe the stereo-enrichment process, (E)-alkene 41 was prepared and subjected to the photoinduced system (Fig. 5c). In the presence or absence of photocatalyst 1, (E)-41 underwent the isomerization to yield (Z)-41 with excellent efficiency, indicating that photocatalyst does not actually engage in the isomerization event (Fig. 5c). As expected, no isomerization of 41 was observed in the absence of visible light (Fig. 5c). On the basis of these results, we expected that trisubstituted alkene itself might act as a photosensitizer for this photochemical isomerization. This hypothesis was further confirmed by UV–Vis absorption spectrometry of (E)-41, which exhibited considerable overlap with the blue LED spectrum (Fig. 5b). Particularly, the (E)-isomer showed strong bathochromic shift relative to the (Z)-isomer (Fig. 5b). Predictably, deconjugation of the π-system in the (Z)-isomer product secures high levels of stereocontrol in this contra-thermodynamic, photoinduced E → Z isomerization (see Supplementary Fig. 3 for density functional theory (DFT)-based conformational analysis). Furthermore, reaction of oxalate 18 with alkyne 16 in the absence of aryl bromide afforded the alkene product 55 in 23% yield (Fig. 5d), providing supporting for the addition of alkyl radical to alkyne step shown in Fig. 2. Nevertheless, only a trace amount of tert-alkyl-aryl coupling product was observed in the reaction of oxalate 18 with aryl bromide 17 (see Supplementary Fig. 10). These results suggest that, under these metallaphotoredox conditions, tertiary alkyl radical would be more prone to undergo radical addition to alkyne, as opposed to capture by nickel species, probably due to steric hindrance70. Finally, we have prepared Ni(II) oxidative addition complex69,71 56 to evaluate an alternative pathway involving radical capture by aryl-Ni(II) species 15 (Fig. 2). Irradiation with a 90 W blue LED of a solution of isolated aryl-NiII-Br 56, alkyne 16, and oxalate 18 in the presence of stoichiometric amount of photocatalyst 1 led to no formation of the desired trisubstituted alkene product (Fig. 5e). The major byproduct was biaryl, which could be formed via homo-coupling from disproportionation of Ni(II) complex 56 (see Supplementary Fig. 12 and Supplementary Table 4). Therefore, we expected that a catalytic Ni0/I/III pathway, proceeding via capture of alkenyl radical by Ni(0), followed by oxidative addition and then reductive elimination, could be operative in this metallaphotoredox manifold (Fig. 2).
Fig. 5.
Mechanistic studies. a Time course studies; b UV–Vis absorption spectrometry; c Isomerization experiments; d Reaction of alkyne with oxalate; e Stoichiometric reaction of isolated Ni(II) complex. dtbbpy = 4,4′-di-tert-butyl-2,2′-dipyridyl
Discussion
In conclusion, we have developed a generic protocol for the intermolecular, regioselective, syn-alkylarylation of terminal alkynes with tertiary alkyl oxalates through a synergistic merger of photoredox and nickel catalysis. A one-pot, three-step sequence, involving radical addition, transition-metal-based coupling, and alkene isomerization, proceeds with high efficiency under the light-induced mild conditions. This manifold forges two vicinal C–C bonds, yielding a variety of trisubstituted alkenes with excellent regioselectivity and syn-stereoselectivity. We expect that the operational simplicity and generality of this methodology and readily availability of the starting materials will allow it to enjoy extensive application in the area of organic chemistry.
Methods
General procedure for the syn-selective alkylarylataion reaction
To a flame dried 8 mL reaction vial was charged with Ir[dF(CF3)ppy]2(dtbbpy)(PF6) (0.003 mmol, 3 mol%), NiCl2•DME (0.02 mmol, 20 mol%), 4,4′-di-tert-butyl-2,2′-dipyridyl (0.02 mmol, 20 mol%), aryl bromide (0.2 mmol, 2.0 equiv.), and cesium alkyl oxalate (0.15 mmol, 1.5 equiv.). The vial was capped. After evacuated and backfilled nitrogen three times, DMSO [0.05 M] was added via a syringe, followed by the addition of terminal alkyne (0.1 mmol, 1.0 equiv.). The reaction mixture was irritated with a 90 W blue LED, with cooling from a fan (36 °C). After 18 h, the reaction was quenched with H2O, extracted with ethyl acetate. The combined organic layers were dried with MgSO4, filtered, and concentrated in vacuo. The crude material was purified by flash chromatography to afford the products. See Supplementary Methods for further experimental details.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
We thank the National Natural Science Foundation of China (21702029), and the “Thousand Plan” Youth Program and the Shanghai Sailing Program (17YF1400100) for financial support. We thank Prof. David MacMillan (Princeton University) for discussions, and Prof. Chao Zheng (Shanghai Institute of Organic Chemistry, CAS) for DFT calculations.
Author contributions
L.C. conceived and designed the project. L.C. and L.G. designed the experiments. L.G., S.F., S.Z. and H.L. performed the experiments and analyzed the data. L.C. prepared the manuscript.
Data availability
The authors declare that all the data supporting the findings of this work are available within the article and its Supplementary Information files, or from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-06904-9.
References
- 1.Stewart J, Charest MP, Herr F. A pharmacological investigation of potential antidepressants of the amitriptyline-type. J. Med. Chem. 1963;6:338–339. doi: 10.1021/jm00339a032. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. J. Med. Chem. 2003;46:1081–1111. doi: 10.1021/jm020450x. [DOI] [PubMed] [Google Scholar]
- 3.Cipriani A, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trost, B. M. & Fleming, I. Comprehensive Organic Synthesis (Pergamon Press, 1991).
- 5.Itami K, Yoshida Ji. Multisubstituted olefins: platform synthesis and applications to materials science and pharmaceutical chemistry. Bull. Chem. Soc. Jpn. 2006;79:811–824. doi: 10.1246/bcsj.79.811. [DOI] [Google Scholar]
- 6.Flynn AB, Ogilvie WW. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 2007;107:4698–4745. doi: 10.1021/cr050051k. [DOI] [PubMed] [Google Scholar]
- 7.Wang, J. Stereoselective Alkene Synthesis (Springer, 2012).
- 8.Fallis AG, Forgione P. Metal mediated carbometallation of alkynes and alkenes containing adjacent heteroatoms. Tetrahedron. 2001;57:5899–5913. doi: 10.1016/S0040-4020(01)00422-7. [DOI] [Google Scholar]
- 9.Alonso F, Beletskaya IP, Yus M. Transition-metal-catalyzed addition of heteroatom−hydrogen bonds to alkynes. Chem. Rev. 2004;104:3079–3160. doi: 10.1021/cr0201068. [DOI] [PubMed] [Google Scholar]
- 10.Moslin, R. M., Miller-Moslin, K. & Jamison, T. F. Regioselectivity and enantioselectivity in nickel-catalysed reductive coupling reactions of alkynes. Chem. Commun. 4441–4449 (2007). [DOI] [PMC free article] [PubMed]
- 11.Marek, I., Chinkov, N. & Banon-Tenne, D. Carbometallation Reactions (Wiley-VCH Verlag GmbH, 2008).
- 12.Chinchilla R, Nájera C. Chemicals from alkynes with palladium catalysts. Chem. Rev. 2014;114:1783–1826. doi: 10.1021/cr400133p. [DOI] [PubMed] [Google Scholar]
- 13.Greenhalgh Mark D, Jones Alison S, Thomas Stephen P. Iron-catalysed hydrofunctionalisation of alkenes and alkynes. ChemCatChem. 2014;7:190–222. doi: 10.1002/cctc.201402693. [DOI] [Google Scholar]
- 14.Sam B, Breit B, Krische Michael J. Paraformaldehyde and mMethanol as C1 feedstocks in metal-catalyzed C-C cCouplings of p-unsaturated reactants: beyond hydroformylation. Angew. Chem. Int. Ed. 2014;54:3267–3274. doi: 10.1002/anie.201407888. [DOI] [PubMed] [Google Scholar]
- 15.Jackson EP, et al. Mechanistic basis for regioselection and regiodivergence in nickel-catalyzed reductive couplings. Acc. Chem. Res. 2015;48:1736–1745. doi: 10.1021/acs.accounts.5b00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida H. Borylation of alkynes under base/coinage metal catalysis: some recent developments. ACS Catal. 2016;6:1799–1811. doi: 10.1021/acscatal.5b02973. [DOI] [Google Scholar]
- 17.Ghosh A, Johnson KF, Vickerman KL, Walker JA, Stanley LM. Recent advances in transition metal-catalysed hydroacylation of alkenes and alkynes. Org. Chem. Front. 2016;3:639–644. doi: 10.1039/C6QO00023A. [DOI] [Google Scholar]
- 18.Boyarskiy VP, Ryabukhin DS, Bokach NA, Vasilyev AV. Alkenylation of arenes and heteroarenes with alkynes. Chem. Rev. 2016;116:5894–5986. doi: 10.1021/acs.chemrev.5b00514. [DOI] [PubMed] [Google Scholar]
- 19.Suess AM, Lalic G. Copper-catalyzed hydrofunctionalization of alkynes. Synlett. 2016;27:1165–1174. doi: 10.1055/s-0035-1561357. [DOI] [Google Scholar]
- 20.Jordan AJ, Lalic G, Sadighi JP. Coinage metal hydrides: synthesis, characterization, and reactivity. Chem. Rev. 2016;116:8318–8372. doi: 10.1021/acs.chemrev.6b00366. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Zi W. Transition-metal catalyzed enantioselective hydrofunctionalization of alkynes. Tetrahedron Lett. 2018;59:2205–2213. doi: 10.1016/j.tetlet.2018.04.057. [DOI] [Google Scholar]
- 22.Herath A, Montgomery J. Highly chemoselective and stereoselective synthesis of Z-eEnol sSilanes. J. Am. Chem. Soc. 2008;130:8132–8133. doi: 10.1021/ja802844v. [DOI] [PubMed] [Google Scholar]
- 23.Shi SL, Buchwald SL. Copper-catalysed selective hydroamination reactions of alkynes. Nat. Chem. 2014;7:38. doi: 10.1038/nchem.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue F, Zhao J, Hor TSA, Hayashi T. Nickel-catalyzed three-component domino reactions of aryl Grignard reagents, alkynes, and aryl halides producing tetrasubstituted alkenes. J. Am. Chem. Soc. 2015;137:3189–3192. doi: 10.1021/ja513166w. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Nakajima M, Serrano E, Martin R. Alkyl bromides as mild hydride sources in Ni-catalyzed hydroamidation of alkynes with isocyanates. J. Am. Chem. Soc. 2016;138:15531–15534. doi: 10.1021/jacs.6b10351. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Derosa J, Engle KM. Palladium(II)-catalyzed regioselective syn-hydroarylation of disubstituted alkynes using a removable directing group. J. Am. Chem. Soc. 2016;138:13076–13081. doi: 10.1021/jacs.6b08818. [DOI] [PubMed] [Google Scholar]
- 27.Kortman GD, Hull KL. Copper-catalyzed hydroarylation of internal alkynes: highly regio- and diastereoselective synthesis of 1,1-diaryl, trisubstituted olefins. ACS Catal. 2017;7:6220–6224. doi: 10.1021/acscatal.7b01847. [DOI] [Google Scholar]
- 28.Clarke C, Incerti-Pradillos CA, Lam HW. Enantioselective nickel-catalyzed anti-carbometallative cyclizations of alkynyl electrophiles enabled by reversible alkenylnickel E/Z iIsomerization. J. Am. Chem. Soc. 2016;138:8068–8071. doi: 10.1021/jacs.6b04206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wille U. Radical cascades initiated by intermolecular radical addition to alkynes and related triple bond systems. Chem. Rev. 2013;113:813–853. doi: 10.1021/cr100359d. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, García-Domínguez A, Nevado C. Pd-catalyzed stereoselective carboperfluoroalkylation of alkynes. J. Am. Chem. Soc. 2015;137:11610–11613. doi: 10.1021/jacs.5b07432. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Zhu N, Chen P, Ye J, Liu G. Copper-catalyzed trifluoromethylazidation of alkynes: efficient access to CF3-substituted azirines and aziridines. Angew. Chem. Int. Ed. 2015;54:9356–9360. doi: 10.1002/anie.201503412. [DOI] [PubMed] [Google Scholar]
- 32.Xu T, Hu X. Copper-catalyzed 1,2-addition of α-carbonyl iodides to alkynes. Angew. Chem. Int. Ed. 2015;54:1307–1311. doi: 10.1002/anie.201410279. [DOI] [PubMed] [Google Scholar]
- 33.He YT, Li LH, Wang Q, Wu W, Liang YM. Synthesis of β-difluoroalkylated acrylonitriles in the presence of copper powder. Org. Lett. 2016;18:5158–5161. doi: 10.1021/acs.orglett.6b02627. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, García-Domínguez A, Nevado C. Nickel-catalyzed stereoselective dicarbofunctionalization of alkynes. Angew. Chem. Int. Ed. 2016;55:6938–6941. doi: 10.1002/anie.201601296. [DOI] [PubMed] [Google Scholar]
- 35.Domański S, Chaładaj W. A broadly applicable method for Pd-catalyzed carboperfluoro-alkylation of terminal and internal alkynes: a convenient route to tri- and tetrasubstituted olefins. ACS Catal. 2016;6:3452–3456. doi: 10.1021/acscatal.6b00777. [DOI] [Google Scholar]
- 36.Garcia-Dominguez A, Muller S, Nevado C. Nickel-catalyzed intermolecular carbosulfonylation of alkynes via sulfonyl radicals. Angew. Chem. Int. Ed. 2017;56:9949–9952. doi: 10.1002/anie.201704862. [DOI] [PubMed] [Google Scholar]
- 37.García-Domínguez A, Li Z, Nevado C. Nickel-catalyzed reductive dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 2017;139:6835–6838. doi: 10.1021/jacs.7b03195. [DOI] [PubMed] [Google Scholar]
- 38.Stephenson, C. R. J., Yoon, T. P. & MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry (Wiley-VCH, 2018).
- 39.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JR, Hu XQ, Lu LQ, Xiao WJ. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016;45:2044–2056. doi: 10.1039/C5CS00655D. [DOI] [PubMed] [Google Scholar]
- 41.Douglas JJ, Sevrin MJ, Stephenson CRJ. Visible light photocatalysis: applications and new disconnections in the synthesis of pharmaceutical agents. Org. Process Res. Dev. 2016;20:1134–1147. doi: 10.1021/acs.oprd.6b00125. [DOI] [Google Scholar]
- 42.Romero NA, Nicewicz DA. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 43.Marzo L, Pagire SK, Reiser O, Konig B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 2018;57:10034–10072. doi: 10.1002/anie.201709766. [DOI] [PubMed] [Google Scholar]
- 44.Tellis JC, et al. Single-electron transmetalation via photoredox/nickel dual catalysis: unlocking a new paradigm for sp3–sp2 cross-coupling. Acc. Chem. Res. 2016;49:1429–1439. doi: 10.1021/acs.accounts.6b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopkinson MN, Tlahuext-Aca A, Glorius F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res. 2016;49:2261–2272. doi: 10.1021/acs.accounts.6b00351. [DOI] [PubMed] [Google Scholar]
- 46.Gui YY, Sun L, Lu ZP, Yu DG. Photoredox sheds new light on nickel catalysis: from carbon-carbon to carbon-heteroatom bond formation. Org. Chem. Front. 2016;3:522–526. doi: 10.1039/C5QO00437C. [DOI] [Google Scholar]
- 47.Fabry DC, Rueping M. Merging visible light photoredox catalysis with metal catalyzed C–H aActivations: on the role of oxygen and superoxide ions as oxidants. Acc. Chem. Res. 2016;49:1969–1979. doi: 10.1021/acs.accounts.6b00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavalcanti LN, Molander GA. Photoredox catalysis in nickel-catalyzed cross-coupling. Top. Curr. Chem. 2016;374:39. doi: 10.1007/s41061-016-0037-z. [DOI] [PubMed] [Google Scholar]
- 49.Twilton J, et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017;1:0052. doi: 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- 50.Singh K, Staig SJ, Weaver JD. Facile sSynthesis of Z-alkenes via uphill catalysis. J. Am. Chem. Soc. 2014;136:5275–5278. doi: 10.1021/ja5019749. [DOI] [PubMed] [Google Scholar]
- 51.Metternich JB, Gilmour R. A bio-inspired, catalytic E→Z isomerization of activated olefins. J. Am. Chem. Soc. 2015;137:11254–11257. doi: 10.1021/jacs.5b07136. [DOI] [PubMed] [Google Scholar]
- 52.Metternich JB, Gilmour R. One photocatalyst, n activation modes strategy for cascade catalysis: emulating coumarin biosynthesis with (−)-rRiboflavin. J. Am. Chem. Soc. 2016;138:1040–1045. doi: 10.1021/jacs.5b12081. [DOI] [PubMed] [Google Scholar]
- 53.Molloy JJ, Metternich JB, Daniliuc CG, Watson AJB, Gilmour R. Contra-thermodynamic, photocatalyticThermodynamic, Photocatalytic E-->Z isomerization of styrenyl boron species: vectors to facilitate exploration of two-dimensional chemical space. Angew. Chem. Int. Ed. 2018;57:3168–3172. doi: 10.1002/anie.201800286. [DOI] [PubMed] [Google Scholar]
- 54.Faßbender SI, Metternich JB, Gilmour R. Spatiotemporal control of pre-existing alkene geometry: a bio-inspired route to 4-trifluoromethyl-2H-chromenes. Org. Lett. 2018;20:724–727. doi: 10.1021/acs.orglett.7b03859. [DOI] [PubMed] [Google Scholar]
- 55.Metternich Jan B, et al. Covalent iImmobilization of (−)-riboflavin on polymer functionalized silica particles: application in the photocatalytic E → Z isomerization of polarized alkenes. Chem. Eur. J. 2018;24:4228–4233. doi: 10.1002/chem.201800231. [DOI] [PubMed] [Google Scholar]
- 56.Pearson CM, Snaddon TN. Alkene photo-isomerization inspired by vision. ACS Cent. Sci. 2017;3:922–924. doi: 10.1021/acscentsci.7b00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng HP, Fan XZ, Chen ZH, Xu QH, Wu J. Photoinduced nickel-catalyzed chemo- and regioselective hydroalkylation of internal alkynes with ether and amide α-hHetero C(sp3)–H bBonds. J. Am. Chem. Soc. 2017;139:13579–13584. doi: 10.1021/jacs.7b08158. [DOI] [PubMed] [Google Scholar]
- 58.Hou J, et al. Visible-light-driven alkyne hydro-/carbocarboxylation using CO2 via iridium/cobalt dual catalysis for divergent heterocycle synthesis. J. Am. Chem. Soc. 2018;140:5257–5263. doi: 10.1021/jacs.8b01561. [DOI] [PubMed] [Google Scholar]
- 59.Till NA, Smith RT, MacMillan DWC. Decarboxylative hydroalkylation of alkynes. J. Am. Chem. Soc. 2018;140:5701–5705. doi: 10.1021/jacs.8b02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsui JK, Lang SB, Heitz DR, Molander GA. Photoredox-mediated routes to radicals: the value of catalytic radical generation in synthetic methods development. ACS Catal. 2017;7:2563–2575. doi: 10.1021/acscatal.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, MacMillan DWC. Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3–sp2 cross-coupling of oxalates with aryl halides. J. Am. Chem. Soc. 2016;138:13862–13865. doi: 10.1021/jacs.6b09533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tellis JC, Primer DN, Molander GA. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science. 2014;345:433–436. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuo Z, et al. Merging photoredox with nickel catalysis: CCoupling of a-carboxyl sp3-carbons with aryl halides. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nawrat CC, Jamison CR, Slutskyy Y, MacMillan DWC, Overman LE. Oxalates as activating groups for alcohols in visible light photoredox catalysis: formation of quaternary centers by redox-neutral fragment coupling. J. Am. Chem. Soc. 2015;137:11270–11273. doi: 10.1021/jacs.5b07678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowry MS, et al. SSingle-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III)C complex. Chem. Mater. 2005;17:5712–5719. doi: 10.1021/cm051312+. [DOI] [Google Scholar]
- 66.Cheung CW, Zhurkin FE, Hu X. Z-selective olefin synthesis via iron-catalyzed reductive coupling of alkyl halides with terminal arylalkynes. J. Am. Chem. Soc. 2015;137:4932–4935. doi: 10.1021/jacs.5b01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, Ren Q, Zhang X, Gong H. Preparation of vinyl arenes by nickel-catalyzed reductive coupling of aryl halides with vinyl bromides. Angew. Chem. Int. Ed. 2016;55:15544–15548. doi: 10.1002/anie.201607959. [DOI] [PubMed] [Google Scholar]
- 68.Durandetti M, Devaud M, Perichon J. New J. Chem. 1996;20:659–667. [Google Scholar]
- 69.Heitz DR, Tellis JC, Molander GA. Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc. 2016;138:12715–12718. doi: 10.1021/jacs.6b04789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Primer DN, Molander GA. Enabling the cross-coupling of tertiary organoboron nucleophiles through radical-mediated alkyl transfer. J. Am. Chem. Soc. 2017;139:9847–9850. doi: 10.1021/jacs.7b06288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun SZ, Martin R. Nickel-catalyzed umpolung arylation of ambiphilic a-bromoalkyl boronic esters. Angew. Chem. Int. Ed. 2018;57:3622–3625. doi: 10.1002/anie.201712428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that all the data supporting the findings of this work are available within the article and its Supplementary Information files, or from the corresponding author upon request.