Abstract
Short term changes in exposure to outdoor fine particulate matter (PM2.5) concentrations are associated with an increased risk of mortality. However, less is known about how oxidant gases may modify the acute health effects of PM2.5. Our objective was to investigate whether associations between acute exposure to PM2.5 and mortality were modified by the oxidant gases O3 and NO2 using their redox-weighted average (Ox). We conducted a multi-city case-crossover study in 24 cities across Canada between 1998–2011 including 1,179,491 nonaccidental mortality events. Interquartile increases in lag-0 and 3-day mean PM2.5 and Ox concentrations were each associated with small increases in nonaccidental and cardiovascular mortality. In stratified analyses, associations between PM2.5 and nonaccidental and cardiovascular mortality tended to be greatest in the highest tertile of Ox with a significant interaction observed between lag 0 PM2.5 and 3-day mean Ox (interaction p-value = 0.04). There was no evidence of effect modification by Ox in the relationship between PM2.5 and respiratory mortality. Overall, the relationship between short-term changes in outdoor PM2.5 and nonaccidental mortality may be greater when oxidant gas concentrations are also elevated. In some regions, reductions in oxidant gas concentrations may also reduce the acute health impacts of PM2.5.
Introduction
Short-term increases in outdoor fine particulate air pollution (PM2.5) are known to be associated with increased mortality1–3. Other pollutants including nitrogen dioxide (NO2) and ozone (O3) have also been associated with daily mortality events4,5, but it is not clear how these oxidant gases may modify the acute health effects of PM2.5. This is an important public health issue as populations are simultaneously exposed to both PM2.5 and oxidant gases (e.g. O3 and NO2). Moreover, understanding interactions between these pollutants may help to inform preventative measures aimed at reducing the public health impacts of outdoor air pollution.
A recent study conducted in London, England found that O3, NO2, and their combined oxidant capacity (Ox) were each associated with daily mortality with the strongest associations observed for Ox6. However, this study did not specifically evaluate how Ox may modify the acute health effects of PM2.5. Recently, we reported that the strength of associations between long-term exposures to outdoor PM2.5 and nonaccidental, cardiovascular, and respiratory mortality were greater in regions with higher Ox concentrations7. Biological mechanisms explaining this observation may include the fact that oxidant gases are known to deplete anti-oxidants in the lung lining fluid8 and increase the permeability of the lung epithelium9–12. Alternatively, photochemical aging of PM2.5 may increase particle toxicity13,14 and this process may be accelerated in regions with higher oxidant gas concentrations. In either case, this evidence suggests that PM2.5 may be more harmful on days with increased concentrations of oxidant gases.
In this study, we examined how Ox may modify the acute health effects of PM2.5 using a multi-city case-crossover study of non-accidental, cardiovascular, and respiratory mortality.
Results
Descriptive statistics are provided in Tables 1 and 2. In total, 1,179,491 nonaccidental mortality events occurred, including 401,719 cases of cardiovascular mortality and 105,980 cases of respiratory mortality. Nonaccidental mortality cases tended to be younger than cases of cardiovascular or respiratory deaths and both genders were present in approximately equal proportions. Table 2 shows the distribution of ambient air pollutants and weather variables in 24 cities across Canada during the study period. Mean daily concentrations were 8.84 µg/m3 for PM2.5, 16.48 ppb for NO2, 20.87 ppb for O3 and 19.38 for Ox. The average daily mean temperature was 7.36 °C, varying from −39.7 to 31.51 °C (interquartile range of 15.3 °C). Table S1 shows Pearson correlation coefficients among the air pollutants and weather variables. PM2.5 was moderately correlated with NO2 and weakly correlated with the other pollutants and weather variables.
Table 1.
Distribution of the number of deaths for nonaccidental, cardiovascular, and respiratory mortality in 24 cities across Canada (1998–2011).
| Outcome | Number of deaths | % Male | Mean Age (years) |
|---|---|---|---|
| Nonaccidental deaths | 1,179,491 | 49.7 | 75.0 |
| All cardiovascular deaths | 401,719 | 49.5 | 79.0 |
| All respiratory deaths | 105,980 | 50.5 | 80.1 |
Table 2.
Daily concentrations of ambient air pollutants and weather variables in 24 cities across Canada (1998–2011).
| Air Pollutants & weather variables | Mean (SD) | Median | IQR | Range |
|---|---|---|---|---|
| PM2.5 (µg/m3) | 8.84 (6.50) | 7.06 | 6.63 | <1-98.15 |
| NO2 (ppb) | 16.48 (8.33) | 15.36 | 10.91 | <1-68.44 |
| O3 (ppb) | 20.87 (9.99) | 20.12 | 13.61 | <1-89.78 |
| Ox (ppb) | 19.38 (6.25) | 18.73 | 8.13 | <1-62.40 |
| Temperature (°C) | 7.36 (10.62) | 8.00 | 15.3 | −39.7–31.51 |
| Relative Humidity (%) | 72.22 (12.85) | 72.96 | 17.41 | 16.04–100 |
IQR, interquartile range; Ox, redox-weighted oxidant capacity of NO2 and O3.
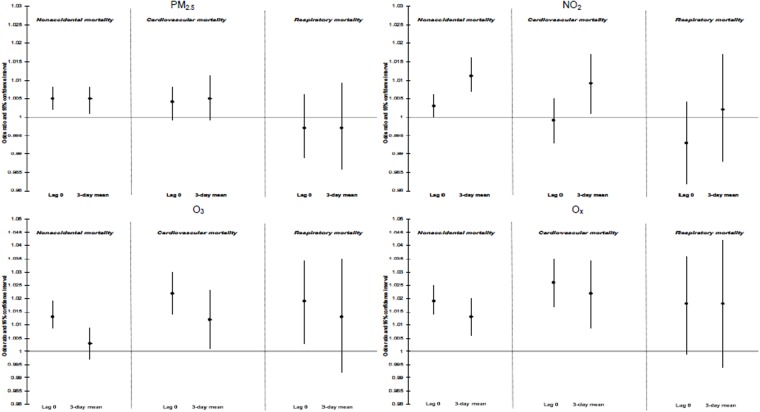
Figure 1 and Table S2 show associations between ambient air pollutants and nonaccidental, cardiovascular and respiratory mortality during the time period of 1998 to 2011. For PM2.5, lag-0 and 3-day mean concentrations were each associated with small increases in nonaccidental and cardiovascular mortality. Short term changes in lag-0 and 3-day mean Ox concentrations were positively associated with all three mortality outcomes, but 95% confidence intervals for respiratory mortality included the null. In general, risk estimates for Ox tended to be slightly larger than for O3 or NO2 individually with the exception of lag-0 respiratory mortality which was similar for O3 and Ox.
Figure 1.
Odds ratios (ORs)1 and 95% CIs for nonaccidental, cardiovascular, and respiratory mortality associated with acute exposure to ambient air pollutants in 24 cities across Canada (1998–2011). ORs reflect a 6.63 µg/m3 change in PM2.5, a 10.91 ppb change in NO2, a 13.61 ppb change in O3, and a 8.13 ppb change in Ox. All models are adjusted for 3-day mean ambient temperature (cubic splines) and relative humidity and daily counts of hospitalization for influenza (in respiratory mortality models only).
In single pollutant models, the strongest association was between lag-0 Ox and cardiovascular mortality (OR = 1.026; 95% CI: 1.017, 1.035 per 10.91 ppb). As sensitivity analyses, we examined two-pollutant models including linear terms for both PM2.5 and Ox. In these models, Ox remained positively associated with nonaccidental (OR = 1.011, 95% CI: 1.004, 1.018) and cardiovascular mortality (OR = 1.020, 95% CI: 1.008, 1.033) whereas risk estimates for PM2.5 decreased slightly (nonaccidental: OR = 1.005, 95% CI: 1.001, 1.008; cardiovascular: OR = 1.005, 95% CI: 0.999, 1.011) (Tables S3 and S4).
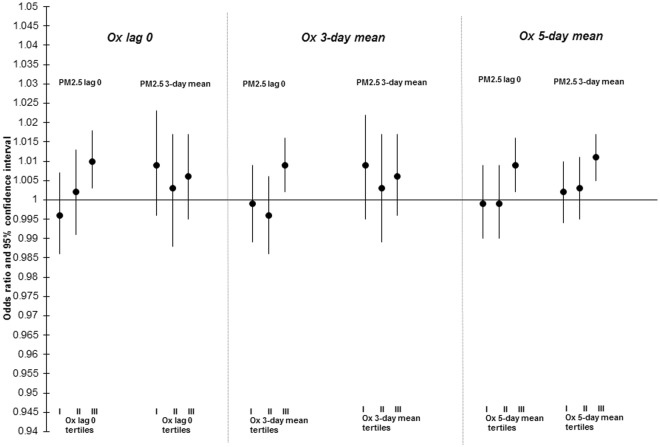
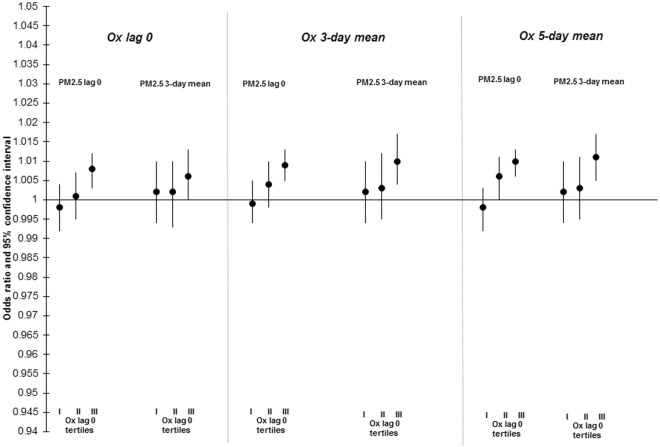
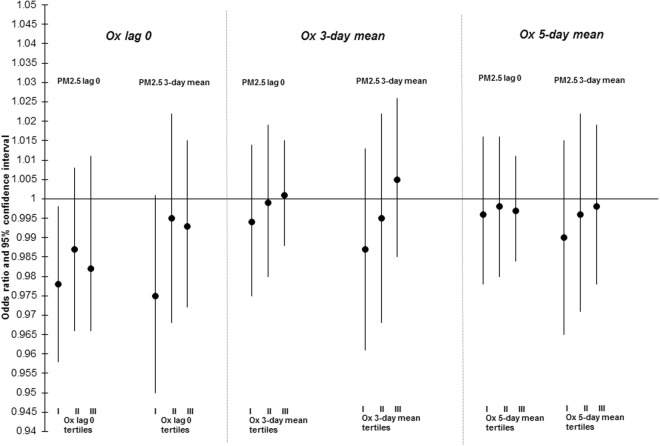
The results of stratified analyses examining the relationship between PM2.5 and mortality across tertiles of Ox are presented in Figs 2–4 and Table S5. For lag-0 PM2.5, increased risks of nonaccidental (interaction p-value = 0.04) and cardiovascular mortality (interaction p-value = 0.19) were limited to the highest tertiles of Ox. This trend was less clear for 3-day PM2.5 concentrations and evidence of effect modification by Ox was not observed for the relationship between PM2.5 and respiratory mortality. In sensitivity analyses, PM2.5-mortality associations were not modified by NO2 or O3 individually (data not shown). As well, findings when restricted to the warm season only were similar to the whole year analyses (data not shown).
Figure 3.
Odds ratios (ORs)1 and 95% CIs for associations between lag 0 and 3-day mean PM2.5 and cardiovascular mortality across tertiles (I, II, III) of same day, 3-day mean, and 5-day mean Ox in 24 cities across Canada (1998–2011). ORs reflect a 6.63 µg/m3 change in PM2.5. All models are adjusted for 3-day mean ambient temperature (cubic splines) and relative humidity.
Figure 2.
Odds ratios (ORs)1 and 95% CIs for associations between lag 0 and 3-day mean PM2.5 and nonaccidental mortality across tertiles (I, II, III) of same day, 3-day mean, and 5-day mean Ox in 24 cities across Canada (1998–2011). ORs reflect a 6.63 µg/m3 change in PM2.5. All models are adjusted for 3-day mean ambient temperature (cubic splines) and relative humidity.
Figure 4.
Odds ratios (ORs)1 and 95% CIs for associations between lag 0 and 3-day mean PM2.5 and respiratory mortality across tertiles (I, II, III) of same day, 3-day mean, and 5-day mean Ox in 24 cities across Canada (1998–2011). ORs reflect a 6.63 µg/m3 change in PM2.5. All models are adjusted for 3-day mean ambient temperature (cubic splines) and relative humidity and daily counts of hospitalization for influenza.
Discussion
In this study, we examined how oxidant gases may modify associations between short-term changes in outdoor PM2.5 concentrations and nonaccidental, cardiovascular, and respiratory mortality. As in previous studies, we found that short-term changes in ambient air pollution concentrations were associated with small increased risks of mortality, predominantly nonaccidental and cardiovascular mortality. For PM2.5 specifically, we noted that same day exposures were only associated with nonaccidental and cardiovascular mortality during periods with the highest Ox concentrations (i.e. above 21.38 ppb). Moreover, we found that short term changes in Ox were more strongly associated with nonaccidental and cardiovascular mortality than PM2.5 in mutually adjusted models.
One previous study conducted in London, England reported an association between daily variations in Ox and mortality6, but to our knowledge this is the first study to evaluate how oxidant gases may modify the relationship between short-term changes in outdoor PM2.5 and mortality. However, we recently reported that Ox levels modified the relationship between short-term changes in ambient PM2.5 and the risk of myocardial infarction and our results for cardiovascular mortality are consistent with this finding15. In addition, we previously reported that oxidant gases modified the relationship between long-term PM2.5 exposures and mortality with stronger associations observed in areas with higher Ox concentrations7. Collectively, these findings suggest that oxidant gases may modify both the acute and chronic health effects of PM2.5 exposures with larger risk estimates observed for chronic health impacts.
While our study could not directly evaluate how Ox concentrations may modify PM2.5 health impacts existing evidence suggests that such a relationship is biologically plausible. For example, one possibility is that oxidant gases deplete anti-oxidants in the lung lining fluid which in turn may lower our natural defense against reactive oxygen species generated in response to PM2.58,16. Moreover, some findings suggest that the lung epithelium barrier is more permeable following ozone exposures and this may facilitate the absorption of particles and/or inflammatory mediators from the lungs directly into the systemic circulation9–12. On the other hand, increased Ox concentrations may influence the toxicity of particles themselves as photochemical aging has been shown to increase particle toxicity13,14.
While this study had a number of important strengths including a large number of mortality cases from multiple cities across Canada it is important to recognize several limitations. First, as in all epidemiological studies, exposure measurement error likely impacted our results as mean daily air pollution concentrations were assigned to case and control periods using fixed-site monitors at the city level. This error was likely most important for NO2 exposures as within-city spatial variations are greater for NO2 than for O3 or PM2.5 and fixed-site measurements may not adequately represent spatial differences in NO2 exposures over large geographic areas. However, assuming that measurement errors are non-differential between case and control periods, this would usually result in an underestimation of risk estimates and is not a likely explanation of increased PM2.5 mortality association in upper tertiles of Ox. Satellite based air pollutants modeling have been proved as an effective method to accurately capture the spatial variability of ambient air pollution17–19. However, in this study, we could not obtain daily satellite-based air pollutant concentrations, as these are mainly available on a long-term basis across Canada20. In addition, we relied on data from 1998 to 2011 and thus more recent years are excluded from our analyses.
In summary, our results suggest that oxidant gases may act to strengthen associations between same day PM2.5 exposures and nonaccidental and cardiovascular mortality. While these risks remain small, they suggest that the health benefits of reductions in Ox concentrations may be larger than expected as such reductions may also decrease the health impacts of PM2.5 even if mass concentrations remain unchanged.
Methods
Study population
A time-stratified case crossover study design21 was used to estimate associations between short-term changes in outdoor air pollution concentrations and the risk of non-accidental (ICD-10: A00 to R99), cardiovascular (ICD-10: I10–99), and respiratory mortality (ICD-10: J00-J99). Mortality data were obtained for the years 1998 to 2011 from the Canadian Mortality Database maintained by Statistics Canada. All subjects who died and were residents of the corresponding cities under investigation were eligible to be included in the analyses. The following 24 cities across Canada were included: Abbotsford, Calgary, Edmonton, Halifax, Hamilton, Kingston, Kitchener, London, Montreal, Oakville, Oshawa, Ottawa, Regina, Saint John (New Brunswick), Sarnia, Saskatoon, Sault Ste Marie, St-John’s (New Found Land and Labrador), Thunder Bay, Toronto, Vancouver, Victoria, Windsor, Winnipeg.
Daily Air Pollution Data
Daily average concentrations of ambient PM2.5, NO2 and O3 were obtained from fixed-site monitoring stations operated by the National Air Pollution Surveillance (NAPS) network maintained by Environment Canada. Daily mean temperature and relative humidity data were also collected from weather stations in the corresponding cities. If daily air pollution concentrations were available for multiple monitors in a single city, daily concentrations were averaged over all available monitors. We calculated the combined oxidant capacity (Ox) of O3 and NO2 for each day in each city using a weighted average with weights equivalent to their respective redox potentials (i.e. Ox = [(1.07 × NO2) + (2.075 × O3)]/3.145)22. Exposures were assigned to case and control periods based on the monitoring station located in each subjects’ city of residence.
Statistical analysis
Conditional logistic regression models were used to estimate the association between short-term changes in ambient air pollutant concentrations and the risk of mortality21. All models pooled cases across cities using a random intercept at the city level to account for potential within-city correlations. We developed models for the whole year and separately for the warm season (April–September) in order to specifically capture the portion of the year with elevated O3 concentrations. All ambient air pollutants (i.e. PM2.5, NO2, O3, and Ox, as defined above) were evaluated in single pollutant models and all odds ratios (and 95% confidence intervals (CI)) reflect interquartile range (IQR) changes in pollutant concentrations. We used lag-0 IQR values for all statistical analyses since interquartile ranges were similar across exposure lag periods (within 1 µg/m3 for PM2.5 and within 1 ppb for NO2 and O3).
We evaluated two different exposure periods for ambient air pollutants: lag-0 (the same day as the mortality event or the control period) and 3-day mean concentrations (including the day of the mortality even or the control period). As sensitivity analyses we also examined the time periods lag-1 (the day prior to the event or the control period) and lag-2 (two days prior to the event or the control period); the magnitudes of these associations were similar to or less than values for the main analyses and are not discussed further. Since the case-crossover design compares cases to themselves at different points in time it adjusts for factors that do not vary within individuals over short time-periods (e.g. age, smoking status, body mass index). In this study, the case period consisted of the day of the mortality event and control periods were selected on the same day of the week in the same month and year as the case period. This time-stratified approach to referent selection has been shown to result in unbiased conditional logistic regression estimates in case-crossover studies23. All models were adjusted for 3-day mean ambient temperature with a quadratic B-spline with three internal knots placed at the 10th, 75th, and 90th percentiles of location-specific temperature distributions and 3-day mean relative humidity24.
To evaluate effect modification by Ox in the relationship between acute exposure to PM2.5 and mortality we conducted stratified analyses across tertiles of Ox (<16.41 ppb, 16.41–<21.38 ppb, ≥21.38 ppb) based on the distribution of Ox across all cities. We evaluated the statistical significance of effect modification by including a cross-product interaction term between PM2.5 and the categorical variable for tertiles of Ox. Wald’s method was used to assess the presence of interaction on the multiplicative scale. Effect modification was considered statistically significant if the p-value for the interaction term was less than 0.05.
As sensitivity analyses, we investigated two-pollutant models to evaluate the extent to which PM2.5-mortality associations may be confounded by Ox. We also evaluated effect modification of PM2.5-mortality associations across tertiles of NO2 and O3. Finally, we investigated effect modification of Ox in the warm season only. All statistical analyses were conducted with R software (version 3.2.4) using the packages dlnm and lme4.
Institutional Approvals
The use of the data in this study was approved by the Statistics Canada Policy Committee after consultation with the Statistics Canada Confidentiality and Legislation Committee, Data Access and Control Services Division, and the Federal Privacy Commissioner. This approval is equivalent to that of standard research ethics boards.
Electronic supplementary material
Acknowledgements
The authors thank Dr. Dave Stieb and Mr. Phil Blagden for reviewing a previous version of the manuscript.
Author Contributions
Dr. Lavigne was the lead author of the manuscript and conducted all statistical analyses. Drs Weichenthal and Burnett contributed to writing portions of the manuscript and interpretation of findings.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34599-x.
References
- 1.Krall JR, Anderson GB, Dominici F, Bell ML, Peng RD. Short-term exposure to particulate matter constituents and mortality in a national study of U.S. urban communities. Environ. Health Perspect. 2013;121:1148–1153. doi: 10.1289/ehp.1206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Q, et al. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA. 2017;318:2446–2456. doi: 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achilleos S, et al. Acute effects of fine particulate matter constituents on mortality: A systematic review and meta-regression analysis. Environ. Int. 2017;109:89–100. doi: 10.1016/j.envint.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills IC, Atkinson RW, Kang S, Walton H, Anderson HR. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open. 2015;5:e006946-2014-006946. doi: 10.1136/bmjopen-2014-006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams ML, Atkinson RW, Anderson HR, Kelly FJ. Associations between daily mortality in London and combined oxidant capacity, ozone and nitrogen dioxide. Air. Qual. Atmos. Health. 2014;7:407–414. doi: 10.1007/s11869-014-0249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weichenthal S, Pinault LL, Burnett RT. Impact of Oxidant Gases on the Relationship between Outdoor Fine Particulate Air Pollution and Nonaccidental, Cardiovascular, and Respiratory Mortality. Sci. Rep. 2017;7:16401-017-16770-y. doi: 10.1038/s41598-017-16770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakey PS, et al. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016;6:32916. doi: 10.1038/srep32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomberg A, et al. Clara cell protein as a biomarker for ozone-induced lung injury in humans. Eur. Respir. J. 2003;22:883–888. doi: 10.1183/09031936.03.00048203. [DOI] [PubMed] [Google Scholar]
- 10.Broeckaert F, et al. Serum clara cell protein: a sensitive biomarker of increased lung epithelium permeability caused by ambient ozone. Environ. Health Perspect. 2000;108:533–537. doi: 10.1289/ehp.00108533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciencewicki Jonathan, Trivedi Shweta, Kleeberger Steven R. Oxidants and the pathogenesis of lung diseases. Journal of Allergy and Clinical Immunology. 2008;122(3):456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rattanavaraha W, et al. The reactive oxidant potential of different types of aged atmospheric particles: An outdoor chamber study. Atmospheric Environment. 2011;45:3848–3855. doi: 10.1016/j.atmosenv.2011.04.002. [DOI] [Google Scholar]
- 14.Saffari A, Daher N, Shafer MM, Schauer JJ, Sioutas C. Global perspective on the oxidative potential of airborne particulate matter: a synthesis of research findings. Environ. Sci. Technol. 2014;48:7576–7583. doi: 10.1021/es500937x. [DOI] [PubMed] [Google Scholar]
- 15.Weichenthal S, Lavigne E, Evans G, Pollitt K, Burnett RT. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study. Environ. Health. 2016;15:46-016-0129-9. doi: 10.1186/s12940-016-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crobeddu B, Aragao-Santiago L, Bui LC, Boland S, Baeza Squiban A. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017;230:125–133. doi: 10.1016/j.envpol.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 17.Fang X, Zou B, Liu X, Sternberg T, Zhai L. Satellite-based ground PM2.5 estimation using timely structure adaptive modeling. Remote Sensing of Environment. 2016;186:152–163. doi: 10.1016/j.rse.2016.08.027. [DOI] [Google Scholar]
- 18.Zou B, Zheng Z, Wan N, Qiu Y, Wilson JG. An optimized spatial proximity model for fine particulate matter air pollution exposure assessment in areas of sparse monitoring. Int. J. Geogr. Inf. Sci. 2016;30:727–747. doi: 10.1080/13658816.2015.1095921. [DOI] [Google Scholar]
- 19.Zou B, et al. High-Resolution Satellite Mapping of Fine Particulates Based on Geographically Weighted Regression. IEEE Geoscience and Remote Sensing Letters. 2016;13:495–499. doi: 10.1109/LGRS.2016.2520480. [DOI] [Google Scholar]
- 20.Crouse DL, et al. Ambient PM2.5, O(3), and NO(2) Exposures and Associations with Mortality over 16 Years of Follow-Up in the Canadian Census Health and Environment Cohort (CanCHEC) Environ. Health Perspect. 2015;123:1180–1186. doi: 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am. J. Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 22.Bratsch, S.V. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. https://www.nist.gov/sites/default/files/documents/srd/jpcrd355.pdf (1988).
- 23.Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005;16:717–726. doi: 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- 24. Gasparrini, A. et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.