Abstract
Bacillus velezensis ZY-1-1 was isolated from the larval gut of the lignocellulose-rich diet-fed scarab beetle, Holotrichia parallela, and confirmed to possess extremely high xylanase (48153.8 ± 412.1 U/L) and relatively moderate cellulase activity (610.1 ± 8.2 U/L). Notably, these xylanase and cellulase activities were enhanced by xylan (1.4 and 5.8-fold, respectively) and cellulose (1.1 and 3.5-fold, respectively), which indicated the hemicellulosic/cellulosic substrate-inducible lignocellulolytic activities of this strain. The complete genome of B. velezensis ZY-1-1 comprises of 3,899,251 bp in a circular chromosome with a G + C content of 46.6%. Among the predicted 3688 protein-coding genes, 24 genes are involved in the degradation of lignocellulose and other polysaccharides, including 8, 7 and 2 critical genes for the degradation of xylan, cellulose and lignin, respectively. This genome-based analysis will facilitate our understanding of the mechanism underlying the biodegradation of lignocellulose and the biotechnological application of this novel lignocellulolytic bacteria or related enzymes.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1490-x) contains supplementary material, which is available to authorized users.
Keywords: Bacillus velezensis, Complete genome, Xylanase, Cellulase, Substrate induction
Genome reports
The lignocellulosic biomass of plants is the most abundantly available raw material that is used for producing renewable biofuels and other high-value chemicals. However, these lignocellulosic materials are primarily composed of cellulose and hemicellulose strands, which are stably held together by lignin. Currently, lignocellulose degradation, the first key step of lignocellulosic biomass application, is still a great challenge (Glaser 2015; Mansour et al. 2016). In fact, there are many specific environments and niches (including the gut of insects living on lignocellulose-rich diets) that possess lignocellulolytic abilities, which have been partially attributed to the microbes and considered valuable treasures for screening lignocellulolytic microbes and related enzymes (Hongoh 2010; Sheng et al. 2012; de Gonzalo et al. 2016). This strategy of enzymatic hydrolysis of lignocellulose based on microbes and microbial sourced genes has received remarkable attention both in the industry and the academic communities worldwide.
In this study, we isolated a new lignocellulose-degrading bacterium, strain ZY-1-1, from the larval gut of the scarab beetle, Holotrichia parallela (coleoptera: scarabaeidae), which is fed on lignocellulose-rich diets (Zhang and Jackson 2008). Strain ZY-1-1 showed high extracellular lignocellulolytic activities, including hemicellulosic/cellulosic substrate-inducible, extremely high xylanase and relatively moderate cellulase activities. First, using Congo red staining method (Teather and Wood 1982), we found that strain ZY-1-1 grew well and formed a gradually increasing clear zone along with the strain’s growth on a basic agar plate with xylan (for xylanase screening) or carboxymethylcellulose (CMC, for cellulase screening). Strain ZY-1-1, growing on the basic agar plate with xylan, formed larger clear zones and higher diameter ratios of clear zone to bacterial colony (highest ratio value of 9.70 ± 1.35 vs. 6.31 ± 0.47), compared to the growth on the basic agar plate with CMC (Fig. 1a–d). Then, the extracellular lignocellulolytic activities of strain ZY-1-1 were determined by the dinitrosalisylic acid (DNS) spectrophotometric method (Dutta et al. 2014). Strain ZY-1-1 was cultured in liquid Luria broth (LB) for 23 h, and the xylanase and cellulase activities of the supernatant were determined to be 48153.8 ± 412.1 and 610.1 ± 8.2 U/L, respectively (Fig. 1e). To evaluate the carbohydrate substrate-inducing effect on the lignocellulolytic activities, strain ZY-1-1 was cultured in the liquid basic medium with or without carbohydrate substrates (basic medium, basic + xylan medium and basic + CMC medium). The xylanase and cellulase activities were highest just before the early-stationary phase (22–23 h) during the growth, and then fluctuated in the stationary phase (Fig. 1f–h). After 23 h culturing, the xylanase activity of strain ZY-1-1 in the supernatant of the basic + xylan medium (35892.3 ± 234.3 U/L) was 1.3- and 1.4-fold higher than that of the basic + CMC medium (27988.2 ± 524.8 U/L) and the basic medium (26119.5 ± 111.1 U/L), respectively. Meanwhile, after culturing for 23 h, the cellulase activities in the supernatant of the basic + xylan medium, the basic + CMC medium and the basic medium were 2104.9 ± 65.7, 1274.0 ± 23.2 and 365.8.5 ± 0.5 U/L, respectively, which indicated a significant xylan and CMC-induced effect on cellulase activity of strain ZY-1-1 (5.8- and 3.5-fold, respectively) (Fig. 1i). Taken together, the strain ZY-1-1 exhibited a significant hemicellulosic/cellulosic substrate-induced, and novel pattern of lignocellulolytic activities, which indicated much higher xylanase activity than cellulose activity. Our results are quite different from previous reports of other lignocellulolytic Bacillus strains, such as Bacillus sp. 275, Bacillus sp. R2 and B. velezensis 157, which indicated much higher cellulase activities than xylanase activities (Khelil et al. 2016; Gong et al. 2017; Chen et al. 2018). This implies that the strain ZY-1-1 possess a varied regulation mechanism for novel genes encoding lignocellulolytic enzymes, that deserves further investigation.
Fig. 1.
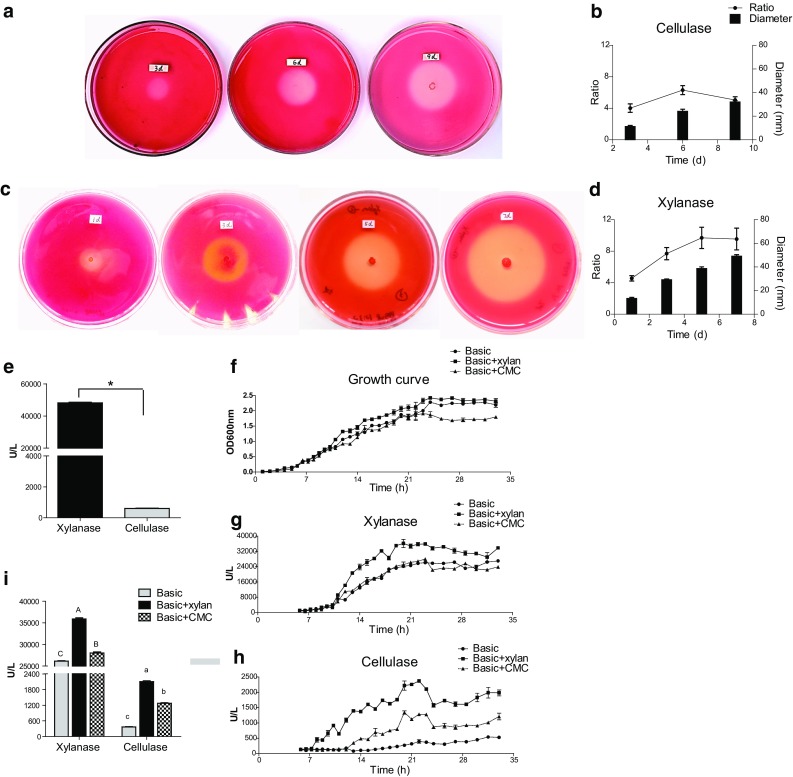
Lignocellulolytic activities of Bacillus velezensis ZY-1-1. a, b Degradation of carboxymethylcellulose (CMC) during the cultivation of Bacillus ZY-1-1 for 3 days, 6 days and 9 days on the basic agar plate with CMC was determined by the Congo red staining (Teather and Wood 1982) (a); meanwhile, the clear zone diameter and the diameter ratio of clear zone to bacterial colony (b) were calculated. c, d The degradation of xylan during cultivation of Bacillus ZY-1-1 for 1 day, 3 days, 5 days and 7 days on the basic agar plate with xylan was determined by the Congo red staining (c); meanwhile, the clear zone diameter and the diameter ratio of clear zone to bacterial colony (d) were calculated. e The xylanase and cellulase activities after cultivation in liquid Luria broth (LB) for 23 h; “Asterisk” indicates a significant difference between xylanase and cellulase activities (P < 0.05, t test). f–h The growth curve (f) and the dynamics of xylanase (g) and cellulase (h) activities of B. velezensis ZY-1-1 during the cultivation in the liquid basic, basic + xylan and basic + CMC medium were determined by the method described in the Supplementary Methods. The optimal temperature and pH for the enzymatic reaction were confirmed as 50 °C and pH 5.0 for xylanase, and 50 °C and pH 4.0 for cellulase (Fig. S1). (i) The xylanase or cellulase activities of B. velezensis ZY-1-1 were compared between the culture supernatants of the liquid basic, basic + xylan and basic + CMC medium after 23 h cultivation. The different letters indicate significant differences in the activity (P < 0.05, Tukey’s test following ANOVA analysis). The values are means ± SE. The repetition numbers (n) were 3 for (b) and (d), and 4 for (e–i). All medium formulas and statistical analyses mentioned above were described in the Supplementary Methods
Then, the genome of strain ZY-1-1 was sequenced using two sequencing techniques, including the PacBio RSII system (MenloPark, CA, USA) as the third-generation sequencing technology and Illumina HiSeq (151-bp paired-end) as the second-generation sequencing technology. The reads from the former system were assembled by Canu software (Koren et al. 2017) and the latter by A5-miseq (Tatusova et al. 2016). Then, to form the complete genome sequence, the assembled contigs from both sequencing systems were combined and rectified using pilon software (Walker et al. 2014). The complete genome sequence was then generated by combining the data followed by rectification using pilon software (Walker et al. 2014). The complete genome of strain ZY-1-1 consisted of one 3,899,251 bp chromosome with 3688 protein-coding genes, 87 tRNA genes and 27 rRNA genes, and an average G + C content of 46.57% (Table 1 and Fig. S2).
Table 1.
Genome features of B. velezensis ZY-1-1
| Features | Chromosome |
|---|---|
| Genome size (bp) | 3,899,251 |
| G + C content (%) | 46.6 |
| Total genes | 3919 |
| Protein-coding genes (CDS) | 3688 |
| 5 s rRNA | 9 |
| 16 s rRNA | 9 |
| 23 s rRNA | 9 |
| tRNA | 87 |
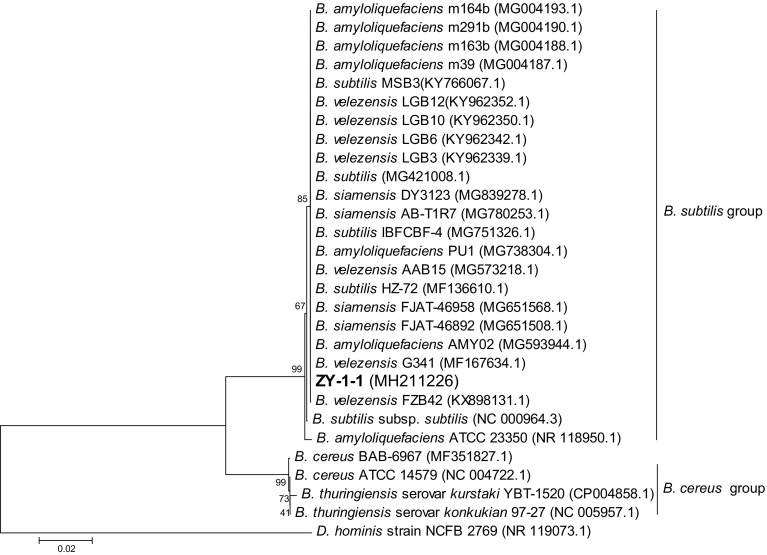
Afterward, strain ZY-1-1 was classified into B. velezensis, which was a re-classified Bacillus species including conspecific B. velezensis, B. methylotrophicus and B. amyloliquefaciens subsp. Plantarum (Dunlap et al. 2016; Fan et al. 2017), according to its morphological characteristic, 16S rRNA and complete genome sequences. Strain ZY-1-1 held the typical morphological characteristics of the Bacillus species with rod-shaped vegetative cells and endospores (Fig. S1). Based on the 16S rRNA gene phylogenetic analysis result, it was then affiliated with the B. subtilis group (Fig. 2). To identify the species information, the whole genome sequence of strain ZY-1-1 was analyzed by genome BLAST, and six strains (including B. velezensis JJ-D34, B. velezensis M75, B. velezensis CAU B946, B. velezensis NJN-6, B. amyloliquefaciens Y14 and B. amyloliquefaciens LM2303) were found to be extremely similar with strain ZY-1-1 (all identities = 99%), and all six strains belonged to the B. velezensis (Table 2). Correspondingly, high average nucleotide identity (ANI) values (> 97.5%) and a similar G + C content were observed when the genome sequence of strain ZY-1-1 was compared with 19 strains of B. velezensis, while all ANI values were below 94.5% when compared with strains of other Bacillus species (Table 2). Based on the above results, the strain ZY-1-1 was named B. velezensis ZY-1-1.
Fig. 2.
Phylogenetic tree based on the partial 16S rRNA sequence of B. velezensis ZY-1-1 and other homologous Bacillus strains. The tree was constructed using the neighbor-joining method by MEGA7 (Kumar et al. 2016). The GenBank accession numbers of all the sequences are indicated in parentheses. The bar represents 0.02 substitutions per site. Dermabacter hominis (D. hominis) strain NCFB 2769 was used as the outgroup
Table 2.
Average nucleotide identity (ANI) analysis of B. velezensis ZY-1-1
| Strain name | Accession number | Assembly level | G + C% | OrthoANIu value (%)a |
|---|---|---|---|---|
| Operational group B. amyloliquefaciensb | ||||
| B. velezensis (B. velezensis /B. methylotrophicus/B. amyloliquefaciens ssp. Plantarum)c | ||||
| B. velezensis KCTC 13012T | GCA_001267695.1 | Scaffold | 46.3 | 97.8 |
| B. velezensis JJ-D34d | CP011346.1 | Complete | 46.4 | 99.5 |
| B. velezensis M75d | CP016395.1 | Complete | 46.4 | 99.4 |
| B. velezensis AS43.3 | NC_019842.1 | Complete | 46.6 | 97.7 |
| B. velezensis CAU B946d | NC_016784.1 | Complete | 46.5 | 99.4 |
| B. velezensis NAU-B3 | NC_022530.1 | Complete | 46.0 | 97.7 |
| B. velezensis NJN-6d | NZ_CP007165.1 | Complete | 46.6 | 99.5 |
| B. velezensis SQR9 | NZ_CP006890.1 | Complete | 46.1 | 97.7 |
| B. velezensis TrigoCor1448 | NZ_CP007244.1 | Complete | 46.5 | 97.7 |
| B. amyloliquefaciens ssp. plantarum FZB42T | NC_009725.1 | Complete | 46.5 | 97.7 |
| B. amyloliquefaciens ssp. plantarum CAU B946 | HE617159.1 | Complete | 46.4 | 99.4 |
| B. amyloliquefaciens CC178 | NC_022653.1 | Complete | 46.5 | 97.7 |
| B. amyloliquefaciens IT-45 | NC_020272.1 | Complete | 46.6 | 99.5 |
| B. amyloliquefaciens KHG19 | NZ_CP007242.1 | Complete | 46.6 | 97.7 |
| B. amyloliquefaciens Y14d | CP017953.1 | Complete | 46.4 | 99.5 |
| B. amyloliquefaciens LM2303d | CP018152.1 | Complete | 46.4 | 99.5 |
| B. amyloliquefaciens LFB112 | NC_023073.1 | Complete | 46.7 | 99.4 |
| B. amyloliquefaciens UMAF6639 | NZ_CP006058.1 | Complete | 46.3 | 97.6 |
| B. methylotrophicus KACC 13105T | GCA_000960265.2 | Contig | 46.4 | 97.8 |
| B. amyloliquefaciens | ||||
| B. amyloliquefaciens DSM 7T | NC_014551.1 | Complete | 46.1 | 94.0 |
| B. amyloliquefaciens TA208 | NC_017188.1 | Complete | 45.8 | 93.9 |
| B. amyloliquefaciens XH7 | NC_017191.1 | Complete | 45.8 | 94.0 |
| B. siamensis | ||||
| B. siamensis KCTC 13613T | AJVF00000000 | Contig | 46.3 | 94.3 |
| B. siamensis XY18 | LAGT01000000 | Contig | 46.3 | 94.3 |
| B. siamensis 7551 | NPCI01000000 | Contig | 46.4 | 94.3 |
| Other Bacillus species | ||||
| B. vallismortis | ||||
| B. vallismortis DV1-F-3 T | AFSH01000000 | Scaffold | 43.8 | 76.8 |
| B. vallismortis TD3 | NXEM01000000 | Scaffold | 43.9 | 77.1 |
| B. vallismortis B4144_201601 | LQYR01000000 | Scaffold | 43.0 | 77.1 |
| B. subtilis | ||||
| B. subtilis subsp. subtilis 168 | NC_000964.3 | Complete | 43.5 | 77.2 |
| B. cereus | ||||
| B. cereus ATCC 14579T | NC_004722.1 | Complete | 35.3 | 68.1 |
| B. thuringiensis | ||||
| B. thuringiensis serovar konkukian 97 − 27 | NC_005957.1 | Complete | 34.9 | 68.3 |
aThe ANI values were calculated using OrthoANIu, an improved algorithm and software for calculating ANI (Yoon et al. 2017), through online tools of EZBioCloud (https://www.ezbiocloud.net/tools/ani)
bOperational group B. amyloliquefaciens is composed of B. velezensis, B. amyloliquefaciens and B. siamensis (Dunlap et al. 2016; Fan et al. 2017)
c B. velezensis is a re-classified Bacillus species including conspecific B. velezensis, B. methylotrophicus and B. amyloliquefaciens subsp. Plantarum (Dunlap et al. 2016)
dThis strain was extremely similar (identity = 99%) to B. velezensis ZY-1-1 based on genome BLAST analysis
The B. velezensis ZY-1-1 genes were annotated using the NCBI Prokaryotic Genomes Annotation Pipeline (PGAP) (Tatusova et al. 2016). Then, the protein-coding genes were further annotated and were classified into 22, 74, and 43 functional classes based on Cluster of Orthologous Groups (COG), Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG), respectively. In addition, 243, 126, and 232 carbohydrate metabolism-related genes were classified into the classes of “Carbohydrate transport and metabolism”, “Carbohydrate metabolic process” and “Carbohydrate metabolism” through the COG, GO and KEGG analyses, respectively (Table S1). The detailed classification information for the protein-coding genes could be found in Fig. S4–S6. Based on HMMER (version 3.0) software, which is based on the Carbohydrate active enzymes (CAZy) database, 125 genes were predicted into CAZy family, including 40 glycoside hydrolases (GHs) genes, 34 glycosyl transferases (GTs) genes, 2 polysaccharide lyases (PLs) genes, 31 carbohydrate esterases (CEs) genes and 6 auxiliary activities (AAs) genes. Moreover, 33, 22 and 3 genes were predicted for antibiotic resistance, antibiotic target and antibiotic biosynthesis, respectively, through the BLAST analysis against Comprehensive Antibiotic Resistance Database (CARD) (McArthur et al. 2013).
To identify the genes related to the lignocellulosic degradation in B. velezensis ZY-1-1, the genes encoding known lignicellulolytic enzymes, which were confirmed in other bacterial species, were selected as the query sequences, and a BLASTp search of the B. velezensis ZY-1-1 genome was carried out (Table 3, S2). For xylan degradation, the genes encoding endo-1,4-β-xylanase (AVX15758.1), glucuronoxylanase (AVX17235.1) and 1,4-β-xylosidase (AVX17306.1) to hydrolyze the main chain of xylan were annotated, which cooperated with arabinosidase (AVX16491.1, AVX16510.1), arabinoxylan arabinofuranohydrolase (AVX17234.1), β-mannanase (AVX15568.1), and acetylxylan esterase (AVX18602.1) to hydrolyze the branched chain of xylan or other hemicelluloses (Sheng et al. 2014). For cellulose degradation, we observed the endo-1,4-β-glucanase (AVX17239.1) and endo-β-1,3-1,4 glucanase (AVX15545.1) for endo-form hydrolysis of (1-4)-beta-d-glucosidic linkages, and β-glucosidase (AVX18716.1, AVX15571.1, AVX15585.1, AVX16352.1, AVX17112.1) for hydrolysis of cellobiose or cellooligosaccharides (Wilson 2011). Meanwhile, for lignin degradation, the genes encoding laccase (AVX18327.1) and deferrochelatase (AVX15607.1) were also found. Furthermore, we observed some other glycosidases, including endo-1,5-α-L-arabinanase (AVX16483.1), galactanase (AVX17829.1), 6-phospho-β-galactosidase (AVX17823.1), oligo-1,6-glucosidase (AVX16273.1, AVX18210.1, AVX18633.1) and 6-phospho-α-d-glucosidase (AVX18175.1). These findings imply that this lignocellulolytic strain may have the potential ability to utilize other polysaccharides, such as arabinan, starch and galactoside.
Table 3.
Annotated genes encoding lignocellulose-degrading enzymes in B. velezensis ZY-1-1
| Gene of B. velezensis ZY-1-1 | Reference gene | BLASTp identity (%) | BLASTp coverage (%) | |||
|---|---|---|---|---|---|---|
| Annotation | Accession no. (NCBI) | Accession no. (UniProtKB) | Species | Annotated Gene | Reference Gene | |
| Hemicellulose-related | ||||||
| Endo-1,4-β-xylanase | AVX15758.1 | P18429 | B. subtilis 168 | 95.00 | 100.00 | 100.00 |
| Glucuronoxylanase | AVX17235.1 | Q45070 | B. subtilis 168 | 90.00 | 100.00 | 100.00 |
| 1,4-β-Xylosidase | AVX17306.1 | P94489 | B. subtilis 168 | 94.90 | 100.00 | 88.37 |
| Arabinosidase | AVX16491.1 | P94531 | B. subtilis 168 | 87.10 | 99.40 | 99.20 |
| Arabinosidase | AVX16510.1 | P94552 | B. subtilis 168 | 80.73 | 99.40 | 99.60 |
| Arabinoxylan arabinofuranohydrolase | AVX17234.1 | Q45071 | B. subtilis 168 | 91.56 | 100.00 | 87.72 |
| β-Mannanase | AVX15568.1 | O05512 | B. subtilis 168 | 74.31 | 100.00 | 100.00 |
| Acetylxylan esterase | AVX18602.1 | P94388 | B. subtilis 168 | 83.96 | 100.00 | 100.00 |
| Endo-1,5-α-l-arabinanase | AVX16483.1 | Q93HT9 | Geobacillus thermodenitrificans | 51.00 | 89.41 | 94.25 |
| Galactanase | AVX17829.1 | Q65CX5 | B. licheniformis ATCC 14580 | 30.48 | 79.19 | 70.52 |
| 6-Phospho-β-galactosidase | AVX17823.1 | C7N8L9 | Leptotrichia buccalis ATCC 14201 | 61.00 | 99.36 | 99.36 |
| Cellulose-related | ||||||
| Endo-1,4-β-glucanase | AVX17239.1 | P07983 | B. subtilis DLG | 96.79 | 100.00 | 100.00 |
| β-Glucanase/Endo-β-1,3 − 1,4 glucanase | AVX15545.1 | P07980 | B. amyloliquefaciens | 92.47 | 98.35 | 100.00 |
| β-Glucosidase | AVX18716.1 | Q7WUL3 | Cellulomonas fimi | 29.12 | 65.78 | 68.44 |
| 6-Phospho-β-glucosidase | AVX15571.1 | O05508 | B. subtilis 168 | 81.96 | 98.71 | 98.92 |
| 6-Phospho-β-glucosidase | AVX15585.1 | P46320 | B. subtilis 168 | 94.57 | 100.00 | 100.00 |
| 6-Phospho-β-glucosidase | AVX16352.1 | P46320 | B. subtilis 168 | 29.50 | 99.31 | 97.51 |
| Aryl-phospho-β-d-glucosidase | AVX17112.1 | P42973 | B. subtilis 168 | 88.66 | 99.17 | 99.37 |
| Oligo-1,6-glucosidase | AVX16273.1 | P29093 | Bacillus sp. F5 | 47.56 | 90.40 | 99.61 |
| Oligo-1,6-glucosidase | AVX18210.1 | O06994 | B. subtilis 168 | 50.27 | 98.04 | 99.11 |
| Oligo-1,6-glucosidase | AVX18633.1 | O06994 | B. subtilis 168 | 49.19 | 98.57 | 98.75 |
| 6-Phospho-α-D-glucosidase | AVX18175.1 | P54716 | B. subtilis 168 | 92.43 | 100.00 | 100.00 |
| Lignin-related | ||||||
| Laccase | AVX18327.1 | D4GPK6 | Haloferax volcanii ATCC 29605 | 38.92 | 98.83 | 85.32 |
| Deferrochelatase | AVX15607.1 | Q8XAS4 | Escherichia coli O157:H7 | 39.04 | 84.93 | 86.76 |
The references which confirmed the specific enzymatic activities of the reference genes are listed in Table S2
In conclusion, B. velezensis ZY-1-1 displayed tremendous xylanolytic activity and relatively moderate cellulolytic activity. Both activities were significantly induced by hemicellulosic/cellulosic substrates. Based on the complete genome information, the lignocellulose degradation-related genes were annotated using a BLAST analysis by comparing them to reference genes with confirmed lignicellulolytic activities. This genome-based analysis facilitated the identification of novel functional genes and provided an insight into the regulation mechanism underlying the degradation of lignocellulose in bacteria, especially the genus Bacillus. These results shed light into the bacteria-sourced mechanism of lignocellulolytic degradation and enhanced the application potential of B. velezensis ZY-1-1 for the biomass energy industry.
Accession numbers
The genome sequence of B. velezensis ZY-1-1 was deposited into the GenBank under the accession number CP027061. The strain is available from the China Center for Type Culture Collection (CCTCC) with the deposition number M2018180.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by National Natural Science Foundation of China (31501634 and 30671404) and National Key R&D Program of China (2017YFD0202000).
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Chen L, Gu W, Xu H, Yang G, Shan X, Chen G, Wang CF, Qian AD. Complete genome sequence of Bacillus velezensis 157 isolated from Eucommia ulmoides with pathogenic bacteria inhibiting and lignocellulolytic enzymes production by SSF. 3 Biotech. 2018;8(2):114. doi: 10.1007/s13205-018-1326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalo G, Colpa DI, Habib MH, Fraaije MW. Bacterial enzymes involved in lignin degradation. J Biotechnol. 2016;236:110–119. doi: 10.1016/j.jbiotec.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Dunlap CA, Kim S, Kwon S, Rooney AP. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int J Syst Evol Micr. 2016;66(3):1212–1217. doi: 10.1099/ijsem.0.000858. [DOI] [PubMed] [Google Scholar]
- Dutta N, Mukhopadhyay A, Dasgupta AK, Chakrabarti K. Improved production of reducing sugars from rice husk and rice straw using bacterial cellulase and xylanase activated with hydroxyapatite nanoparticles. Bioresour Technol. 2014;153:269–277. doi: 10.1016/j.biortech.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Fan B, Blom J, Klenk HP, Borriss R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “Operational group B. amyloliquefaciens” within the B. subtilis species complex. Front Microbiol. 2017;8:22. doi: 10.3389/fmicb.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. Enzyme-based lignocellulose hydrolyzation—Sauter mean diameter of raw materials as a basis for cellulase performance characterization and yield prediction. J Biotechnol. 2015;214:9–16. doi: 10.1016/j.jbiotec.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Gong G, Kim S, Lee SM, Woo HM, Park TH, Um Y. Complete genome sequence of Bacillus sp. 275, producing extracellular cellulolytic, xylanolytic and ligninolytic enzymes. J Biotechnol. 2017;254:59–62. doi: 10.1016/j.jbiotec.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Hongoh Y. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci Biotechnol Biochem. 2010;74(6):1145–1151. doi: 10.1271/bbb.100094. [DOI] [PubMed] [Google Scholar]
- Khelil O, Choubane S, Cheba BA. Polyphenols content of spent coffee grounds subjected to physico-chemical pretreatments influences lignocellulolytic enzymes production by Bacillus sp. R2. Bioresour Technol. 2016;211:769–773. doi: 10.1016/j.biortech.2016.03.112. [DOI] [PubMed] [Google Scholar]
- Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27(5):722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AA, Da CA, Arnaud T, Lu-Chau TA, Fdz-Polanco M, Moreira MT, Cacho RJ. Review of lignocellulolytic enzyme activity analyses and scale-down to microplate-based assays. Talanta. 2016;150:629–637. doi: 10.1016/j.talanta.2015.12.073. [DOI] [PubMed] [Google Scholar]
- McArthur AG, Waglechner N, Nizam F, et al. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother. 2013;57(7):3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng P, Huang S, Wang Q, Wang A, Zhang H. Isolation, screening, and optimization of the fermentation conditions of highly cellulolytic bacteria from the hindgut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae) Appl Biochem Biotechnol. 2012;167(2):270–284. doi: 10.1007/s12010-012-9670-3. [DOI] [PubMed] [Google Scholar]
- Sheng P, Xu J, Saccone G, Li K, Zhang H. Discovery and characterization of endo-xylanase and beta-xylosidase from a highly xylanolytic bacterium in the hindgut of Holotrichia parallela larvae. J Mol Catal B-Enzym. 2014;105:33–40. doi: 10.1016/j.molcatb.2014.03.019. [DOI] [Google Scholar]
- Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44(14):6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather RM, Wood PJ. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. Plos One. 2014;9(11):e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DB. Microbial diversity of cellulose hydrolysis. Curr Opin Microbiol. 2011;14(3):259–263. doi: 10.1016/j.mib.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Ha SM, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. 2017;110(10):1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jackson TA. Autochthonous bacterial flora indicated by PCR-DGGE of 16S rRNA gene fragments from the alimentary tract of Costelytra zealandica (Coleoptera: Scarabaeidae) J Appl Microbiol. 2008;105(5):1277–1285. doi: 10.1111/j.1365-2672.2008.03867.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.