Abstract
The viscoelastic properties of four novel, low molecular weight hyaluronic acid derivatives were investigated and compared to the parent hyaluronic acid compound. Briefly, all derivatives were synthesized by first deacetylating the parent hyaluronic acid. One sample was left as such, while two others were reacytelated. The final compound, of particular interest for its anti-inflammatory properties, was butyrylated. The compounds were dissolved in phosphate buffer solution (PBS) and studied at a concentration of 5 mg/mL. Shear thinning behaviour was observed for all compounds, however, derivative samples had a lower viscosity than the parent compound at high shear rates. Viscoelastic properties were also observed to decrease as a result of the derivative preparation method. It is believed that these changes are primarily caused by a decrease in hyaluronic acid molecular weight. By increasing the concentration of the anti-inflammatory compound, it may be possible to modulate the viscoelastic properties to more closely resemble those of commercial viscosupplements. As a result, an anti-inflammatory derivative of hyaluronic acid may potentially improve upon existing viscosupplements used to treat patients who are susceptible to flare up.
Keywords: Shear thinning, Viscoelastic, Viscosupplement, Anti-inflammatory, Osteoarthritis
Introduction
Synovial fluid is a naturally produced lubricant consisting of proteins, lipids and hyaluronic acid [1]. Healthy synovial fluid protects human joints by lubricating the articulating surfaces and absorbing shock. Hyaluronic acid is the primary component of synovial fluid, and consequently is a major contributor to rheological properties that make it an effective lubricant [2–4].
With the onset of joint disease including osteoarthritis and rheumatoid arthritis the composition of synovial fluid is observed to change [5]. This change manifests as a decrease in hyaluronic acid concentration and molecular weight, and an increase in protein concentration [5, 6]. The concentration of white blood cells in synovial fluid is also observed to increase as osteoarthritis progresses [1]. With the progression of osteoarthritis, lubricating and shock absorption properties of synovial fluid degrade and can result in cartilage wear and in severe cases, bone on bone contact [7, 8].
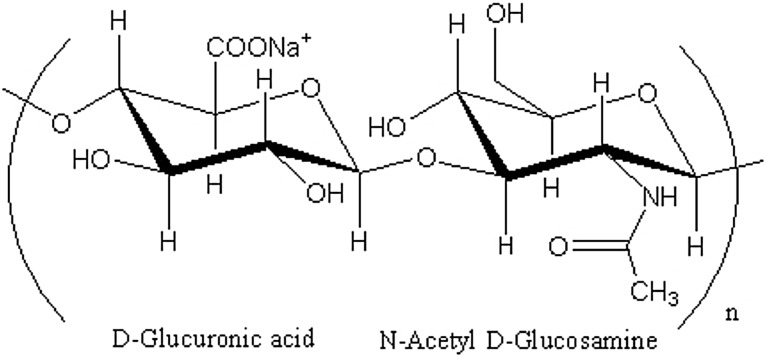
Viscosupplementation has been developed as a treatment for osteoarthritis in weight bearing joints. Viscosupplementation involves injecting a bio-compatible fluid that is shear thinning and viscoelastic directly to the intra-articular joint cavity in order to improve joint lubrication and shock absorption. Viscosupplements typically consist of hyaluronic acid that has been synthesized to minimize friction and maximize shock absorption within the joint [4, 9]. A diagram of the hyaluronic acid molecule is presented in Figure 1.
Fig. 1.
Schematic illustration of hyaluronic acid molecule [23]
Among existing viscosupplements it is common to manipulate molecular weight, degree of crosslinking and preparation methods of hyaluronic acid to overcome common treatment issues such as injectibility, compromise between gel and liquid behaviour, and prolonging treatment effect [10–12].
Recent research has focused on controlling viscosupplement viscoelastic properties by adding hyaluronic acid nano-particles [13] while other research has attempted to emulated healthy synovial fluid with aurophilically crosslinked hyaluronic acid [14]. Addition of mannitol to the viscosupplement has been investigated as a possible method to prevent breakdown of hyaluronic acid polymer chains, thereby increasing the duration of treatment effect [15].
While parent (intact) high molecular weight hyaluronic acid is protective in joints, low molecular weight hyaluronic acid has been shown to be pro-inflammatory in a number of systems [16–18]. The hyaluronic acid derivatives in this study were developed with the purpose of investigating pro-inflammatory response of low molecular weight hyaluronic acid samples and to synthesize a hyaluronic acid derivative with anti-inflammatory properties [16]. Reacylation with butyryic anhydride resulted in partially substituted low molecular weight hyaluronic acid which had anti-inflammatory properties. This was not the case with other N-acylations [16].
The objectives of this study are to investigate the rheological properties of these low molecular weight hyaluronic acid derivatives with the purpose of better understanding the effects of the chemical preparation on the final solution rheology and to evaluate the suitability of the anti-inflammatory derivative as a potential treatment for mild to moderate osteoarthritis.
Materials and methods
Hyaluronic acid derivatives
Parent (intact) hyaluronic acid derived from streptococcus equi along with four, low molecular weight derivatives were studied. The samples were prepared according to the procedure described by Babsola et al. [16] at Queen’s University, Kingston, ON and were shipped to the University of British Columbia, Vancouver, BC in dehydrated form. The hyaluronic acid derivatives, preparation method and sample number are presented in Table 1. Briefly, the derivative samples were formed by first partially deacetylating one lot the parent compound, which was then divided into lots for generating the individual compounds [16]. This approach permits the generation of compounds with equimolar amounts of N-acyl substituents. One sample was left as such, while the two other samples were reacetylated. The final sample was butyrylated and was found to have anti-inflammatory properties, in assays of pro-inflammatory cytokine production by a cultured line of macrophages of human origin [16]. The % butyrylation of BHA (in Table 1) was 22.7 ± 3.8 [16]. The individual compounds were characterized by agarose gel electrophoresis, 1H NMR and mass spectrometry and were previously reported [16]. The hydrolysis procedures used resulted in a reduction of the molecular mass of the parent hyaluronic acid to 30–214 kDa, by gel electrophoresis [16].
Table 1.
Hyaluronic acid derivatives and the corresponding sample number
| Compound | Preparation | Sample |
|---|---|---|
| Hyaluronic acid (parent compound) | – | Sample 1 |
| DHA | Deacetylated hyaluronic acid | Sample 2 |
| AHA-1 | Reacetylated DHAa | Sample 3 |
| AHA-2 | Reactylated DHAb | Sample 4 |
| BHA | Butyrylated DHAa | Sample 5 |
aDeacetylated by hydrazinolysis
bDeacetylated by NaOH
It should be noted that the deacetylation step in the formulation of all derivatives in this study causes bond cleavage, resulting in a decrease in molecular weight from parent to derivative compounds [16].
In preparation for rheological testing each compound was diluted to a concentration of 5 mg/mL in phosphate buffer solution (PBS) 7.2 pH at room temperature and was subsequently mixed for 3 hours using a magnetic stirrer. Rheological tests were conducted immediately following mixing.
Methods
All measurements were conducted using a Malvern Kinexus Ultra rheometer with a 50 mm, 1 degree cone and plate geometry. The rheometer was calibrated with Newtonian standard oil prior to conducting all tests. A sample volume of 0.58 mL was required for loading the rheometer with the given geometry. All tests were conducted at 37o C. To reduce sample evaporation, a base plate with a water trap was used. The sample was then covered with aluminium and plastic covers respectively.
Three replicate runs were completed for each test. The final results presented are the average of all three runs completed.
Shear rheology
All samples were pre-sheared at 0.1 Pa for 1 minute followed by a zero shear rest period of 2 minutes to remove any loading effects and shear history. Shear viscosity was then evaluated over the shear range of 0.1 to 1000 s−1. It is important to note that this shear range does not describe the entire spectrum of shear rates observed in knee joints, however viscosity at shear rates outside this range cannot be measured accurately with the given apparatus due to low sample viscosity, sample inertia, and inherent limitations in conventional rheometry [5]. This shear range does describe the range of shears typically observed during walking and the transition to running [11].
The shear viscosity data for all samples was fitted with the Carreau–Yassuda model. The Carreau–Yassuda model has previously been used to model both synovial fluid and viscosupplement viscosity, and describes a zero shear plateau, a shear thinning region and an infinite shear plate [19, 20].The Carreau–Yassuda model is given in Equation 1.
| 1 |
where η is the sample viscosity, η ∞ is the viscosity at high shear, η 0 is the viscosity at zero shear, 1/λ is the critical shear rate at which shear thinning behaviour is observed, a is the Yassuda exponent and is proportional to the extent over which shear thinning behaviour is observed, and n is the power law index, related to the degree of shear thinning [19].
Estimates for η 0 and η ∞ are determined experimentally while the remaining parameter estimates for the Carreau–Yassuda were obtained using the curve fitting tool cftool in MATLAB.
Viscoelastic properties
Prior to commencing oscillatory measurements all samples were pre-sheared at 0.1 Pa for 1 minute followed by a zero shear rest period for 2 minutes to remove any shear history from the shear measurements.
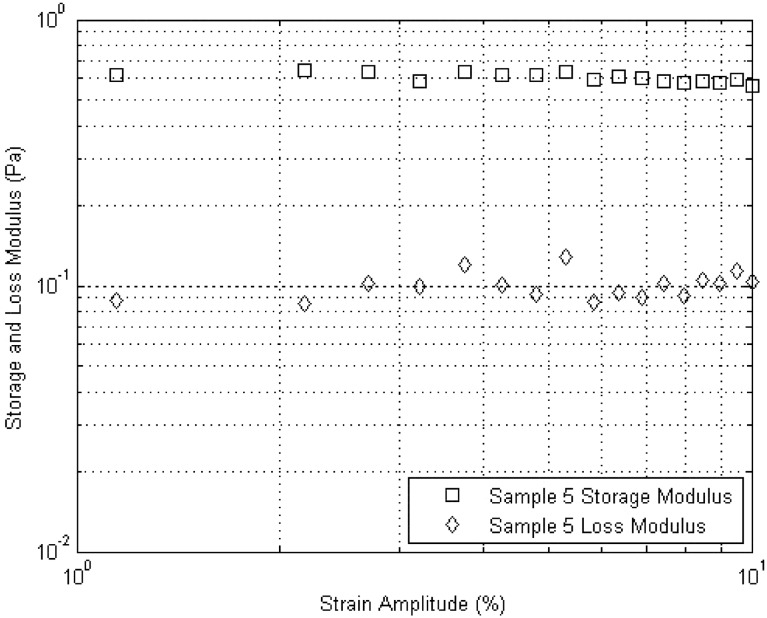
Oscillatory flow was used to investigate the viscoelastic properties of the hyaluronic acid derivatives. Strain amplitude sweep measurements were first conducted to identify the linear viscoelastic region for each sample. 5% strain was determined to fall within the linear viscoelastic region for all compounds investigated and was therefore used for all frequency sweep measurements. A typical amplitude sweep curve is shown in Figure 2.
Fig. 2.
Amplitude sweep for Sample 5. The linear viscoelastic region is clearly shown up to 10% strain
Following strain amplitude sweep measurements a pre-shear at 0.1 Pa for 1 minute and a zero shear rest period of 2 minutes was used to remove any history effects prior to commencing frequency sweep measurements.
Frequency sweeps were completed over an oscillation frequency range of 1 to 100 Hz in order to obtain estimates for the storage and loss moduli (G′ and G″). In general, the data was accurate over the range of 1 to 10 Hz. For the lower viscosity samples accurate measurements could only be obtained over a smaller range of frequencies as was the case for Samples 4 and 5.
Averages from three replicate runs were used to plot the data. Where applicable, the averaged data sets were used to determine the crossover frequency.
Results
Shear rheology
The shear viscosity is plotted as a function of shear rate for all samples in Figure 3. The solid lines represent the Carreau–Yassuda model fits.
Fig. 3.
Shear viscosity as a function of shear rate. A Newtonian plateau is observed at low shear rates for Sample 1 with shear thinning behaviour at higher shear rates. Samples 2 through 5 were not observed to demonstrate a zero-shear plateau in the investigated range. Solid lines represent the Carreau–Yassuda fit
Non-Newtonian shear thinning behaviour is observed for all samples, however Sample 1 (parent hyaluronic acid) is observed to exhibit distinct behaviour from the four derivative samples. At low shear rates, Sample 1 is found to exhibit a Newtonian plateau while at higher shear rates exhibits moderate shear thinning behaviour.
The four derivative samples exhibited no Newtonian plateau over the investigated shear range.
The Newtonian plateau at high shear rates was similar for all samples. Parameter estimates for the Carreau–Yassuda model are presented in Table 2.
Table 2.
Carreau–Yassuda model parameters for each sample
| a | n | λ | η 0 | η ∞ | |
|---|---|---|---|---|---|
| Sample 1 | 1.02 | 0.330 | 0.03 | 0.08 | 0.003 |
| Sample 2 | 60 | 0.015 | 110.050 | 32.00 | 0.002 |
| Sample 3 | 50.56 | 0.046 | 100.044 | 9.04 | 0.002 |
| Sample 4 | 60.6 | 0.057 | 120.001 | 0.50 | 0.003 |
| Sample 5 | 60.075 | 0.060 | 100.257 | 0.908 | 0.002 |
All parameters were found to be significant at the 95% confidence level
All parameters were found to be significant at the 95% confidence level suggesting the models are a good fit for the data over the observed shear rates.
Viscoelastic properties
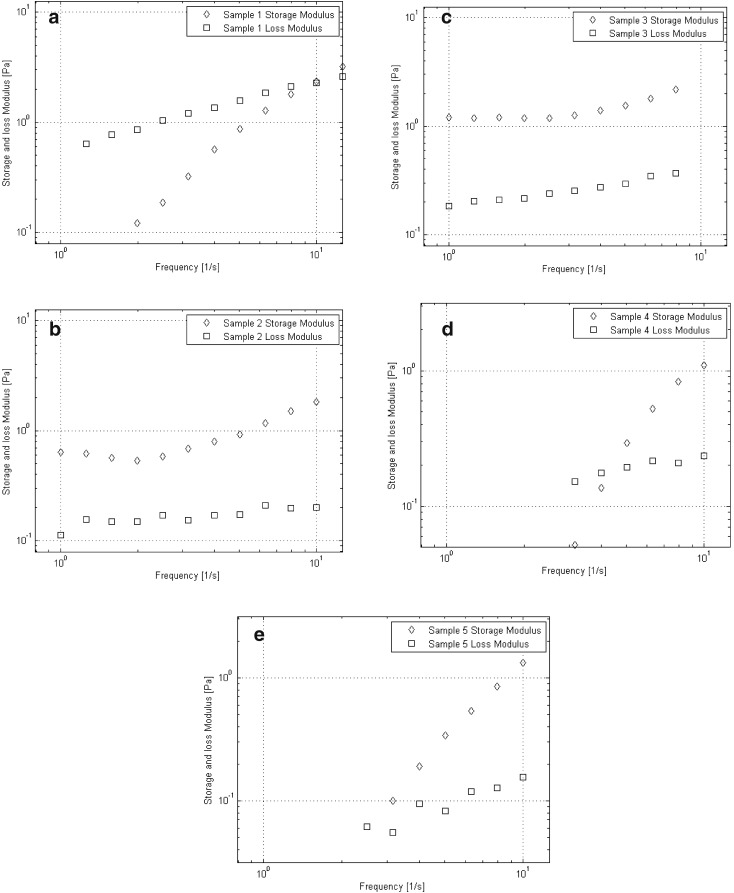
The dynamic moduli for hyaluronic acid and the four derivatives are plotted as functions of oscillation frequency in Figure 4a–e.
Fig. 4.
a Storage and loss moduli as functions of oscillation frequency for Sample 1. A crossover of storage and loss moduli is observed at approximately 10 Hz. b Storage and loss moduli are plotted as functions of oscillation frequency for Sample 2. Gel like behaviour is observed over the observed frequency range. c Storage and loss moduli as functions of oscillation frequency for Sample 3. Gel like behaviour is observed over the observed frequency range. d Storage and loss moduli as functions of oscillation frequency for Sample 4. A crossover frequency of approximately 4.2 Hz is observed. e Storage and loss moduli as functions of oscillation frequency for Sample 5. A crossover frequency of approximately 2.8 Hz is observed
As expected, Sample 1 exhibited clear viscoelastic behaviour, with a crossover frequency at approximately 10 Hz. Samples 2 and 3 both exhibited purely gel behaviour in the investigated range of frequencies while Sample 4 exhibited a crossover frequency at approximately 4.2 Hz. Sample 5 exhibited a crossover frequency at approximately 2.8 Hz. A summary of the viscoelastic behaviour of all samples along with those for three commercial viscosupplements is provided in Table 3 [20]. Note that dynamic moduli are commonly reported at 2.5 Hz as it roughly corresponds to the frequency of running [21].
Table 3.
Crossover frequency, dynamic moduli at crossover and dynamic moduli at 2.5 Hz
| f c (Hz) | G c (Pa) | G′ 2.5 | G″2.5 | Solution behaviour | |
|---|---|---|---|---|---|
| Sample 1 | 10.0 | 2.3 | 0.19 | 1.04 | Viscoelastic |
| Sample 2 | – | – | 0.58 | 0.17 | Gel |
| Sample 3 | – | – | 1.18 | 0.24 | Gel |
| Sample 4 | 4.5 | 0.20 | 0.129 | 0.15 | Viscoelastic |
| Sample 5 | 2.8 | 0.06 | 0.013 | 0.063 | Viscoelastic |
| Orthovisc® | 0.398 | – | 111.2 | 61.48 | Viscoelastic |
| Suplasyn® | – | – | 3.36 | 10.78 | Viscous |
| Synvisc® | – | – | 118.1 | 22.46 | Gel |
Discussion
Shear rheology
As observed in Figure 3, The disappearance of the Newtonian plateau at low shear rates in the derivative samples is also attributed to the decrease in molecular weight as the larger, parent molecule is hypothesized to undergo a greater degree of molecular entanglement thereby requiring a larger shear rate to cause shear thinning behaviour. As shown in Table 2, the infinite shear viscosity of all derivative samples are nearly identical, suggesting the processing steps involved in each compound formulation have minimal effect on the shear viscosity at high shear rates. It is therefore hypothesized that at high shear rates, the viscosity is primarily a function of the hyaluronic acid molecular weight and that N-acetyl and N-butyryl have minimal impact on the high shear viscosity.
The shear viscosity of the hyaluronic acid derivatives was found to be significantly lower than those reported for commercial viscosupplements [5, 11, 20]. This difference is primarily attributed to the low molecular weight of the hyaluronic acid derivatives and the relatively low concentration at which the samples were studied [5, 11]. The molecular weight and concentration of several commercial viscosupplements are presented for comparison to values for the samples in this study in Table 4.
Table 4.
The molecular weight and concentration of hyaluronic acid derivatives and several commercial viscosupplements are compared [11, 16, 24–26]
| Viscosupplement | Molecular weight (kDa) | Concentration (mg/mL) |
|---|---|---|
| HA derivsa | 30–214 | 5 |
| Hyalgan® | 500–700 | 10 |
| Hyalubrix® | 1500 | 15 |
| Durolane® | 1000 | 20 |
| Synvisc® | 6000–7000 | 8 |
| Suplasyn® | 730 | 20 |
| Orthovisc® | 1000–2900 | 15 |
aHyaluronic acid derivatives in the current study
Viscoelastic properties
The deacetylation process appears to have reduced the viscoelastic properties of the derivative samples as Sample 1 was found to have greater dynamic moduli than Samples 2 through 5 over the investigated range of frequencies. Samples 2 and 3 were found to exhibit purely gel-like behaviour in the range of studied frequencies while the remaining samples displayed a cross-over frequency and viscoelastic behaviour. This may indicate that deacetylation via hydrazinolysis causes gel behaviour while deacetylation by NaOH does not. This may also suggest that the butyrylation process causes a return towards viscoelasticity. This may suggest that moieties such as N-acetyl and butyryl groups have an effect on the viscoelastic behaviour of the hyaluronic acid derivatives. Sample 5 was observed to exhibit the smallest dynamic moduli, suggesting that butyrylation may further reduce sample viscoelasticity beyond the initial reduction caused by deacetylation.
As was observed with the shear viscosity, the oscillatory behaviour of the investigated samples was found to be significantly less viscoelastic than commercially available viscosupplements and other hyaluronic acid solutions [10, 11, 15, 20]. This result is attributed to the low molecular weight and concentration of hyaluronic acid in the derivative samples studied here.
Similarly to shear viscosity measurements, the viscoelastic measurements demonstrate that sample rheology is influenced by method of deacetylation and butyrylation. This shows that rheology of hyaluronic acid solutions is not only dependent on average molecular weight, concentration, and degree of cross-linking.
Suitability of anti-inflammatory hyaluronic acid as a viscosupplement
Sample 5 (butyrylated, anti-inflammatory) was found to exhibit shear thinning behaviour and viscoelastic behaviour, traits present in both healthy synovial fluid and commercial viscosupplements [5, 11, 20]. This is a promising result as prior studies have shown that viscosity and viscoelasticity can be modulated by adjusting the solution concentration and degree of cross-linking [5, 10]. Therefore, even though at the investigated concentration of 5 mg/mL the viscosity and viscoelastic properties were significantly lower than those for three commercial viscosupplements, it is expected that Sample 5 could be modified to enhance the viscosity and viscoelastic properties to fall within the range for a typical viscosupplement [5, 10, 12, 13].
In addition to potentially suitable viscous and viscoelastic properties, Sample 5 also possesses anti-inflammatory properties which may reduce the incidence of flare up [16]. The effect of flare up in patients with knee osteoarthritis has been observed to significantly affect synovial fluid properties such as hyaluronic acid molecular weight, concentration, and protein concentration [22].
Incidents of flare up may be detrimental long-term to joint health by reducing the viscosity and viscoelasticity as a result of a decrease in concentration and molecular weight of hyaluronic acid [5, 22]. It is therefore expected that a viscosupplement that adequately lubricates and protects an osteoarthritic knee while also providing anti-inflammatory properties would be advantageous in the treatment of mild to moderate osteoarthritis.
Conclusions
The shear viscosity of four low molecular weight hyaluronic acid derivative samples was investigated. All samples were found to exhibit shear thinning behaviour which was successfully fitted with the Carreau–Yassuda model.
The oscillatory behaviour of the samples was also investigated. Viscoelastic behaviour was observed for all samples. Samples 2 and 3 were found to exhibit purely gel behaviour over the investigated range. This result suggests that the method of deacetylation and the butyrylation step may affect the viscoelastic behaviour.
Variation in rheology across derivative compounds demonstrates that the rheology of hyaluronic acid solutions is not only dependent on polymer concentration, average molecular weight and degree of cross-linking but may also be affected by method of deacetylation and butyrylation.
The viscosity and viscoelasticity of all derivative samples were found to be lower than values reported elsewhere for commercially available viscosupplements. It is believed that this result is primarily caused by a decrease in hyaluronic acid molecular weight and concentration. It is expected that by adjusting the hyaluronic acid concentration and degree of cross linking, the viscosity and viscoelasticity of the investigated samples could be modulated to more closely resemble properties of commercial viscosupplements.
By adjusting the concentration and degree of cross-linking, it is believed that Sample 5 could be used as an effective viscosupplement. The effect of flare up in cases of knee osteoarthritis has been proven to be harmful to both short term and long term joint health, and it may therefore be beneficial to treat mild to moderate cases of knee osteoarthritis with an anti-inflammatory viscosupplement.
Acknowledgements
Support from the Canada Foundation for Innovation grant for rheometer purchase is acknowledged. Financial support from the Natural Sciences and Engineering Research Council of Canada, Discovery Grant, is gratefully acknowledged.
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflict of interest in relation to the work in this article.
References
- 1.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Goudoulas TB, Kastrinakis EG, Nychas SG, Papazoglou LG, Kazakos GM, Kosmas PV. Rheological study of synovial fluid obtained from dogs: healthy, pathological, and post-surgery, after spontaneous rupture of cranial cruciate ligament. Ann Biomed Eng. 2010;38(1):57–65. doi: 10.1007/s10439-009-9832-9. [DOI] [PubMed] [Google Scholar]
- 3.Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci. 2007;104(15):6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wobig M, Bach G, Beks P, Dickhut A, Runzheimer J, Schwieger G, Vetter G, Balazs E. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan g-f 20 and a lower-molecular-weight hyaluronan. Clin Ther. 1999;21(9):1549–1562. doi: 10.1016/S0149-2918(00)80010-7. [DOI] [PubMed] [Google Scholar]
- 5.Fam H, Kontopoulou M, Bryant JT. Effect of concentration and molecular weight on the rheology of hyaluronic acid/bovine calf serum solutions. Biorheology. 2009;46(1):31–43. doi: 10.3233/BIR-2009-0521. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthr Rheum. 2012;64(12):3963–3971. doi: 10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 7.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin N Am. 2013;39(1):1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56(3):882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 9.Mensitieri M, Ambrosio L, Iannace S, Nicolais L, Perbellini A. Viscoelastic evaluation of different knee osteoarthritis therapies. J Mater Sci Mater Med. 1995;6(3):130–137. doi: 10.1007/BF00120288. [DOI] [Google Scholar]
- 10.Choi SC, Ae Yoo M, Lee SY, Lee HJ, Son DH, Jung J, Noh I, Kim CW. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J Biomed Mater Res A. 2015;103(9):3072–3080. doi: 10.1002/jbm.a.35437. [DOI] [PubMed] [Google Scholar]
- 11.Finelli I, Chiessi E, Galesso D, Renier D, Paradossi G. A new viscosupplement based on partially hydrophobic hyaluronic acid: a comparative study. Biorheology. 2011;48(5–6):263–275. doi: 10.3233/BIR-2011-0596. [DOI] [PubMed] [Google Scholar]
- 12.Shimojo AAM, Pires AMB, Lichy R, Rodrigues AA, Santana MHA. The crosslinking degree controls the mechanical, rheological, and swelling properties of hyaluronic acid microparticles. J Biomed Mater Res A. 2015;103(2):730–737. doi: 10.1002/jbm.a.35225. [DOI] [PubMed] [Google Scholar]
- 13.Fakhari A, Phan Q, Thakkar SV, Middaugh CR, Berkland C. Hyaluronic acid nanoparticles titrate the viscoelastic properties of viscosupplements. Langmuir. 2013;29(17):5123–5131. doi: 10.1021/la304575x. [DOI] [PubMed] [Google Scholar]
- 14.Casuso P, Perez-San Vicente A, Iribar H, Gutierrez-Rivera A, Izeta A, Loinaz I, Cabanero G, Grande H-J, Odriozola I, Dupin D. Aurophilically cross-linked “dynamic” hydrogels mimicking healthy synovial fluid properties. Chem Commun. 2014;50:15199–15201. doi: 10.1039/C4CC05735J. [DOI] [PubMed] [Google Scholar]
- 15.Conrozier T, Mathieu P, Rinaudo M. Mannitol preserves the viscoelastic properties of hyaluronic acid in an in vitro model of oxidative stress. Rheumatol Ther. 2014;1(1):45–54. doi: 10.1007/s40744-014-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babasola O, Rees-Milton KJ, Bebe S, Wang J, Anastassiades TP. Chemically modified n-acylated hyaluronan fragments modulate proinflammatory cytokine production by stimulated human macrophages. J Biol Chem. 2014;289(36):24779–24791. doi: 10.1074/jbc.M113.515783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inammation in mouse chondrocytes. Biochimie. 2010;92(2):204–215. doi: 10.1016/j.biochi.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Small hyaluronan oligosaccharides induce inammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol. 2010;80(4):480–490. doi: 10.1016/j.bcp.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Mezger TG (2006) “The Rheology handbook”. Vincentz Network, Hannover
- 20.Bhuanantanondh P, Grecov D, Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. J Med Biol Eng. 2012;32(1):12–16. doi: 10.5405/jmbe.834. [DOI] [Google Scholar]
- 21.Mazzucco D, McKinley G, Scott RD, Spector M. Rheology of joint fluid in total knee arthroplasty patients. J Orthop Res. 2002;20(6):1157–1163. doi: 10.1016/S0736-0266(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 22.Conrozier T, Balblanc J-C, Piperno M, Rinaudo M. Differences in the osteoarthritic synovial fluid composition and rheology between patients with or without are-up. A pilot study. Osteoarthr Cartil. 2012;20:S244. doi: 10.1016/j.joca.2012.02.404. [DOI] [PubMed] [Google Scholar]
- 23.Maleki A, Kjoniksen AL, Nystrom B. Anomalous viscosity behavior in aqueous solutions of hyaluronic acid. Polym Bull. 2007;59(2):217–226. doi: 10.1007/s00289-007-0760-2. [DOI] [Google Scholar]
- 24.Aggarwal A, Sempowski IP. Hyaluronic acid injections for knee osteoarthritis. Systematic review of the literature. Can Fam Phys. 2004;50(2):249–256. [PMC free article] [PubMed] [Google Scholar]
- 25.Bhuanantanondh P. Rheology of synovial fluid with and without viscosupplements in patients with osteoarthritis: a pilot study. Master’s thesis, University of British Columbia; 2009.
- 26.Neustadt D, Caldwell J, Bell M, Wade J, Gimbel J. Clinical effects of intraarticular injection of high molecular weight hyaluronan (orthovisc) in osteoarthritis of the knee: a randomized, controlled, multicenter trial. J Rheumatol. 2005;32(10):1928–1936. [PubMed] [Google Scholar]