Abstract
Conventional biomedical imaging modalities in wide clinical use, such as ultrasound imaging, X-ray computed tomography, magnetic resonance imaging, and positron emission tomography, can provide morphological, anatomical, and functional information about biological tissues. However, single mode imaging in conventional medicine provides only limited information for definitive diagnoses. Thus, combinational diagnosis using multiple imaging modalities has become increasingly important. Recently, photoacoustic imaging (PAI) has gained significant attention, and several PAI prototypes have been used in clinical trials. At the same time, PAI has been tested in combination with conventional imaging modalities. For all these imaging modalities, various contrast-enhancing agents have been developed for various purposes. In this review article, we will focus on recent progress in developing dual mode contrast agents for PAI in combination with other conventional imaging modalities.
Keywords: Photoacoustic imaging, Dual-mode imaging, Biomedical imaging
Introduction
Conventional biomedical imaging techniques, such as ultrasound imaging (USI), fluorescence imaging (FLI), X-ray computed tomography (X-ray CT), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), and positron emission tomography (PET), typically provide morphological and functional information about biological tissues. However, single imaging modalities have inherent weaknesses, such as shallow tissue penetration depth, poor detection sensitivity, low spatial resolution, harmful ionizing radiation, slow imaging speed, inaccuracy, and/or high cost. For example, FLI can provide excellent spatial resolution and sensitivity in shallow tissues but cannot penetrate in deep tissues. USI can provide good spatial resolution at low cost, but is limited by low contrast. X-ray CT can achieve a high penetration depth but is limited by low sensitivity and the need for ionizing radiation. MRI can also achieve a high penetration depth and good spatial resolution, but is limited by low sensitivity, slow imaging speed, and high cost. PET has high sensitivity, but is limited by poor spatial resolution and by the need for ionizing radiation. Therefore, single modality imaging, while invaluable, is an imperfect tool for acquiring overall structural and functional information. To resolve this problem, multimodality imaging has been widely pursued for acquiring complementary information [1–6].
Photoacoustic imaging (PAI) is a hybrid imaging technique that takes advantage of both optical and acoustic properties [7]. PAI is based on the photoacoustic (PA) effect, discovered by Alexander Graham Bell, which converts light to sound [8]. When a short-pulsed light (typically nanoseconds in pulse duration) illuminates biological tissue, a PA wave is generated from thermoelastic expansion of the optically absorbing target, and the propagated PA waves are detected by an ultrasound (US) transducer. PAI has various advantages, such as high spatial resolution in deep tissue, strong optical contrast, and the use of non-ionizing radiation. In addition, PAI can use both intrinsic and extrinsic optical absorbers. Intrinsic optical absorbers include melanin, lipids, water, oxy- and deoxyhemoglobin. Extrinsic optical absorbers include various organic and inorganic chemicals with strong optical absorption, such as organic dyes (e.g., indocyanine green and methylene blue), gold nanomaterials, and polymeric nanoparticles [9–16]. More importantly, PAI can easily operate in tandem with other imaging techniques, such as FLI, USI, X-ray CT, MRI, and PET [17–21].
In the past decade, fast progress in nanotechnology has driven rapid advancements in molecular imaging. For molecular imaging, the above mentioned imaging modalities must use imaging contrast agents with a specific targeting ligand. In this way, the molecular imaging capabilities of these modalities can provide enhanced imaging contrast for earlier diagnosis of disease, track the disease process, and help evaluate treatment [22]. Typically, FLI, USI, X-ray CT, MRI, and PET respectively use fluorophores, microbubbles, high density molecules, paramagnetic agents, and radioisotopes as contrast agents. Because some of these imaging probes exhibit strong optical absorption, they are inherently excellent contrast agents for PAI as well. In this review, we will report on recent attempts to develop dual- or multi-mode contrast agents for PAI and other imaging modalities.
Molecular dual-mode imaging
Contrast agents for dual-mode PAI and FLI
In biomedical imaging, FLI has great advantages as a noninvasive and inexpensive imaging modality [23–26]. FL imaging also offers high sensitivity, down to a single molecule, especially in superficial detection. However, optical blurring and bleaching rapidly decrease the in vivo spatial resolution rapidly with penetration depth. Because both PAI and FLI use light as an excitation source, they can be easily integrated into a single system. To simultaneously perform FLI and PAI, exogenous contrast agents which produce PA and FL signals are needed [27–30]. When the input laser illuminates a target area, the molecules absorb the light energy. If the light is then converted into other wavelengths of light, it produces FL light; if the light is converted into heat energy, it produces PA waves. Single agents which provide both phenomena enable dual model imaging by PAI and FLI.
In general, indocyanine green (ICG) has been widely used in PAI and FLI for in vivo experiments. ICG has been used for diagnosing medical parameters such as cardiac output, hepatic function, and liver blood flow, applications already approved by the Food and Drug Administration (FDA). It can be used for sentinel lymph node (SNL) mapping and imaging of breast and bladder tumors [31, 32]. ICG primarily absorbs the light between 600 and 900 nm and emits fluorescence between 750 and 950 nm. Because the FL quantum yield of ICG is approximately 11% in water, ICG can be used as a contrast agent for both modalities [33].
In addition to ICG, there are several other dual mode contrast agents for PAI and FLI. Liu et al. [34] developed fluorescent dye-loaded mesoporous silica nanoparticles (MSNPs), which are NIR dye (Cy754, emission wavelength of 795 nm) doped MSNPs (Cy754-MSNPs) that have an absorption peak at 754 nm [34]. Figure 1a, b show PA and FL images of 4T1 tumor models with Cy754-MNSPs used for SLN mapping. After injection of Cy754-MSNPs into both control and 4T1 model mice, fluorescent signals from the lymph node were detected only in the 4T1 model (Fig. 1a, right). These signals were acquired using an IVIS imaging system. PA signals from the SLN were also obtained by the PAI system. Figure 1b shows PA images before and after injection of Cy754-MSNPs (100 μL, 4 mg/mL) into the mouse’s foot. Although the SLN was invisible before injection (Fig. 1b, top), strong PA signals from the SLN were easily detected from accumulated Cy754-MSNPs after injection (Fig. 1b, bottom).
Fig. 1.
PA and FL imaging with a dual mode contrast agent. a FL and b PA images of a SLN with Cy754-MSNPs. c FL and d PA images of a SLN with red-emissive C-dots. Figure reproduced with permission from [34, 35]
Fluorescent carbon dots (C-dots) have been also used for dual mode FLI and PAI. Ge et al. [35] developed C-dots with florescence emission peaked at 650 nm as a dual mode contrast agent with high water solubility, low toxicity, high biocompatibility, and broad absorption wavelength range. Figure 1c, d show in vivo FL and PA images of HeLa tumor-bearing nude mice. After intravenous (i.v.) injection of C-dots, C-dots were accumulated in the tumor site (Fig. 1c, right), but no accumulation was with normal tissue (Fig. 1c, left). A PA image of the tumor was also easily obtained after injection of C-dots (Fig. 1d, bottom). The C-dots plentifully accumulated in the tumor, and provided clear FL and PA images in living mice.
These results demonstrate that the combination of PAI and FLI can image deeply, with high spatial resolution and sensitivity. Extrapolating from these results, the possible application of dual-mode PAI and FLI with combination of single contrast agents could be a vital tool for preclinical applications.
Contrast agents for photoacoustic microscopy (PAM) and fluorescence microscopy (FLM)
Planar FLI has great advantages in biomedical imaging as a non-invasive and inexpensive imaging tool. But this technology has low resolution and can provide only 2D images. Confocal and multiphoton fluorescence microscopy (FLM) are free from these limitations. In confocal fluorescence microscopy (CFM), a pinhole is added at the confocal plane of the lens to increase the optical resolution and image contrast and eliminate out-of-focus light. More importantly, the pinhole enables the reconstruction of three-dimensional structures. Multiphoton microscopy (MPM) is made possible by focusing high peak power energy within a volume of one picoliter at the high NA objective lens focal spot to create multiphoton excitation. The multiphoton excitation rapidly decreases outside the focal spot, and thus MPM can perform high-resolution FLI without using a pinhole. MPM also has the advantage of less photobleaching than other FLM techniques, and can image deeper tissues than CFM because its longer wavelength illumination [36, 37].
Confocal, multiphoton, and other FL microscopy technologies are based on emission of fluorescence light from fluorescent materials. As mentioned earlier, energy decay following light exciation can be both radiative and non-radiative. Thus, CFM, MPM, and/or PAM can be implemented together with the use of single contrast agents. An integrated CFM and PAM system was developed and tested in live animals [38]. Using 570 and 593 nm illumination, the PAM image showed the vascular networks and maps of hemoglobin oxygen saturation. CFM was imaged to the lymphatic system by measuring FL intensity following the injection of 20% rhodamine B isothiocyanate(RITC)-dextran fluorescent dye. Microvasculares are well visible in the PAM image at 570 nm (Fig. 2a) while lymphatic systems were identified in a CFM image after the injection of 20% RITC-dextran (Fig. 2b). The overlaid CFM and PAM images are shown in Fig. 2c, and an optical microscopy image is shown in Fig. 2d. In addition, an MPM and PAM integrated system using fluorescent beads and carbon particles was developed and studied [39]. Second harmonic generation imaging (SHG), third harmonic generation imaging (THG), MPM, and PAM were integrated into a single modality (Fig. 2e–i) [40].
Fig. 2.
Top row combined functional vascular and lymphatic images of a mouse ear in vivo using an integrated PAM and CFM system: a PAM, b CFM, c PAM and CFM merged, and d optical microscope image. Lower rows agent-free imaging of a mouse ear ex-vivo using the multimodal imaging system. e Photoacoustic mesoscopy, f MPM, g PAM, h Seond harmonic generation(SHG), i Third harmonic generation.(THG). Figures reproduced with permission from [38, 40]
Contrast agents for dual-mode PAI and USI
The main advantage of PAI is that it can be easily implemented with conventional USI because both technologies can share the acoustic detection mechanism [41]. Thus, the implementation is rather simple compared to that of MRI/CT, MRI/PET, PET/CT, and other combinations. The combined PAI/USI system can be portable and cost effective. In additon, real-time imaging and image display are feasible. This combinational implementation is probably the best current candidate for clinical translation and commercialization. Recently, combined US & PAT technology has demonstrated its clinical usefulness in various research efforts regarding breast cancer diagnosis [31, 42–45].
Ultrasound contrast agents contains very strong acoustic scatterers in the form of internal gas. When microbubbles circulate in the blood stream, US is strongly scattered by their high acoustic impedance. As a result, microbubbles can be used as both passive and active targeting agents in USI, and can be used in clinical applications [46]. For combined PAI and USI, optical absorbers can be combined with conventional microbubbles. In recent years, one study has reported on porphyrin shell microbubbles formed by porphyrin-lipid shell encapsulating a fluorinated gas [47]. In another recent study, a dual modality contrast agent was developed in the form of ink-encapsulated micro- or nano-bubbles [48]. When optical dyes are incooperated with microbubbles, the toxicity of the specific optical dye can be an issue, and the use of clinically acceptable dye is important. As an example, methylene blue microbubbles formed by combining a standard ultrasound contrast agent with a standard PA dye have been reported [49].
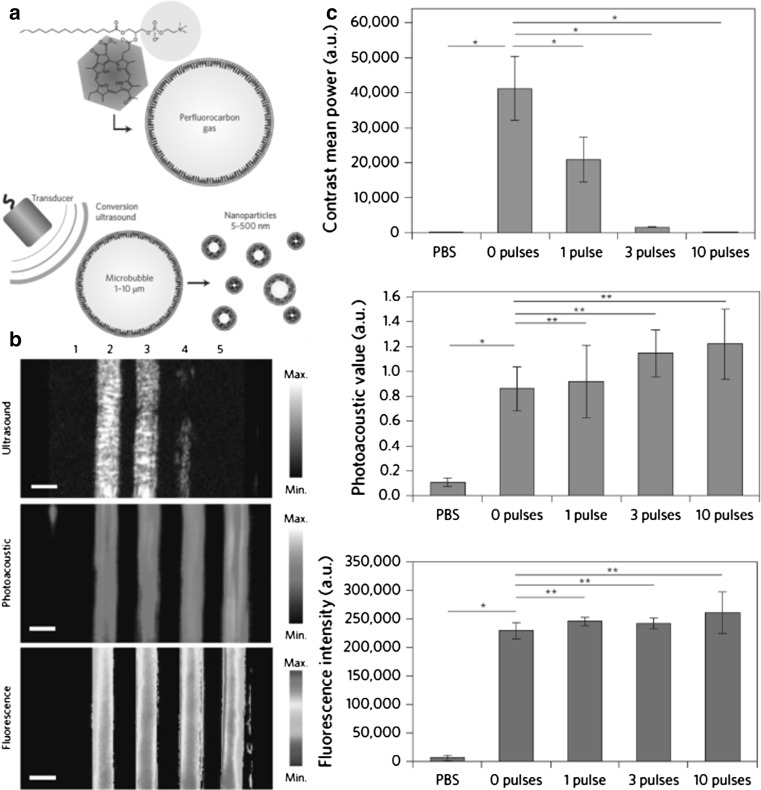
A recent report by Huynh et al. [50] shows the conversion process of nanoparticles from microbubbles in multimodal imaging. Low-frequency ultrasound was used to burst the microbubbles and to convert form smaller nanoparticles, and then USI, PAI, and FLI were performed. Figure 3a is a schematic diagram of porphyrin microbubbles (pMBs) and their transformation from microbubbles to nanobubbles. Figure 3b, c show multimodal images of pMBs and porphyrin nanoparticles (pNPs) by ultrasound application. Figure 4c shows quantified US, PA, and FL signals with various US pulses.
Fig. 3.
a Schematic diagram of conversion from porphyrin microbubbles (pMBs) to porphyrin nanoparticles (pNPs). b, c Multimodal images of pMBs and resulting pNPs after ultrasound application. b Ultrasound, photoacostic, fluorescence images was pMBs and pNPs embedded in an acrylamide gel phantom. Samples: (1) phosphate buffered saline (PBS), (2) pMBs without any ultrasound application (zero pulses), and after applying (3) one pulse, (4) three pulses, and (5) ten pulses. Scale bars 2 mm. c Quantified signals from Fig. 3b. Figure reproduced with permission from [50]
Fig. 4.
a PA 2008 tumor image before injection and b 6 h post-injection of GNRs. c Raman mapping of the tumored area. d Raman spectrum of tumor area of panel e and reference signals. e In vivo photoacoustic images of mouse post injection of the nanoconstruct. f SERS spectra of the targeted tumor. Figure reproduced with permission from [53, 55]
Contrast agents for PAI and Raman spectroscopy
Surface enhanced Raman spectroscopy (SERS) is an emerging bio-sensing optical technique which uses localized surface plasmon resonance from nanoscale metal materials. Recently, SERS has gained wide pre-clinical use due to its sensitive chemical specificity and selective detection ability, and its ability to be multiplexed [51, 52]. Dual-mode Raman spectroscopy and PAI is quite attractive for biomedical imaging, and these two technologies can offset their own drawbacks. Raman spectroscopy is not able to penetrate deep below the skin surface due to optical diffusion in biological tissues. Furthermore, PAI is necessary to achieve high chemically-specific and sensitive images with high spatial resolution in deep tissue. These disadvantages can be overcome by integrating the two modalities. In general, the combination of SERS and PAI provides dual-mode images with metal nanoparticles (NPs) as an exogenous contrast agents.
Gold nanorods (AuNRs), gold nanoparticles (AuNPs), and carbon nanotubes have been effectively used in dual-mode imaging with PAI and SERS [53–55]. These gold nanostructures exhibit strong optical absorption due to localized surface plasmon resonance, so PA signals from them are very strong. Moreover, they generate enhanced Raman signals close to the metal surface, so they provide sufficient signals for Raman imaging. As shown in Fig. 4, AuNRs were used as an exogenous agent for dual mode imaging, with an absorption peak at 756 nm [5]. When these agents are functionalized with specific Raman molecules, they have the same optical absorption, but have different and unique Raman spectra. These Raman signalling dyes have been used in dual-mode analysis. In vivo PA and Raman images of ovarian cancer (OvCA) cells in living mice were acquired after i.v injection of AuNRs. The multimodal images were obtained by separate PA and Raman imaging systems. PA images pre- and post-injection of AuNRs are shown in Fig. 4a, b, where the PA imaging was performed with 756 nm excitation. Figure 4c shows Raman mapping of the tumor and its background. The Raman spectrum of the brighter part matches with the reference spectrum of the AuNRs (Fig. 4d). The other parts of the Raman mapping do not match with the reference spectrum, so the brighter part represents the tumor and clearly shows its boundaries. AuNPs were used in the same manner [7]. The experimental AuNPs have an absorption peak at 532 nm. After injection of AuNPs with Cy7-lip reporter molecules, PAI produced a wide field image of the targeted tumor (Fig. 4e). For real-time spectroscopic photoacoustic tomographic imaging, the PA signals were acquired at six wavelengths: 680, 700, 750, 800, 850, and 900 nm. Then SERS chemically identified the molecules by their unique Raman spectra (Fig. 4f). As a possible clinical application, SERS could be used for image-guided variable tumor surgery because of the image for resection margin identification can distinguish between tumor and normal tissue. By combining PAI and SERS, dual-mode imaging overcomes the poor penetration depth of SERS and the limited chemical sensitivity of PA. This dual mode application might be widely used in pre and intraoperative imaging for accurately targeting tumors.
Contrast agents for PAI and SPECT/CT
X-ray CT is a computerized medical imaging system that provides cross sectional tomographic images by combining X-ray images from different angles [56–58]. CT provides accurate deep imaging with high resolution and 3D visualization of biological structures; however, radiation exposure and the relatively low sensitivity of soft tissue are weaknesses of this medical imaging system [59–61]. Integrating PAI and CT can provide accurate and intuitive diagnostic images. PA imaging provides high resolution soft tissue structural images of, for example, blood vessels, the spleen, kidney, liver, and cecum, and it is also able to image functional information e.g., hemoglobin oxygen saturation. CT provides 3D whole body structural information based on X-ray absorption. Moreover, to enhance the soft tissue contrast of CT and selectively visualize the high contrast of cancerous tissue with PA imaging, contrast agents can be used. For CT, these include small quantity of iodinated compounds for vessel imaging and barium sulfate for digestive system imaging; for PA, they include organic dyes, nanoparticles, and reporter genes.
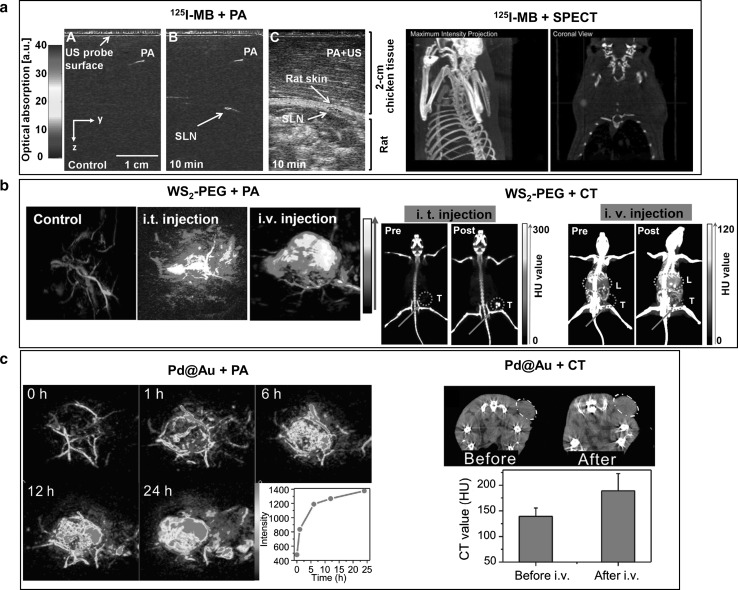
In recent research, 125I sodium iodide radiolabeled methylene blue (MB), has been used for sentinel lymph node mapping with PAI, and for its verification with single-photon emission computed tomography (SPECT) [18]. Additionally, two dimensional nanomaterials, specifically PEGylated WS2 nanosheets and Pd@Au nanoplates, have been studied as PA and CT dual mode imaging contrast agents and photothermal therapy agents [62, 63]. Still other studies explored the use of TaOx@PPy, composed of X-ray absorbing tantalum sub-oxides encapsulated in UV-vis-NIR absorbing polypyrrole, and Au@PB, composed of light absorbing Prussian blue coated gold nanoparticles that absorb X-rays [64, 65]. These materials, which are composed of high X-ray attenuation materials that are radiolabeled, encapsulated, or coated with highly light absorbing materials, greatly enhanced PA and CT image contrast, and they clearly visualized tumors by accumulating in the tumor region. With 125I labeled MB, sentinel lymph nodes were clearly detected by PAI. The combination of PAI and USI provided functional and high resolution anatomical information. Before 125I labeled MB injection, significant PA signals were not detected under the skin; however, after injection, sentinel lymph nodes were clearly mapped in PA images and in US and PA combined images (Fig. 5a, left). In the SPECT image, sentinel lymph nodes were clearly visualized after 125I labeled MB injection (Fig. 5a, right). Polyethylene glycol coated WS2 nanosheets (WS2-PEG) showed high X-ray attenuation, thanks to their high atomic number material, as well as high optical absorbance in the NIR region, providing both CT and PAI tissue contrast in vivo. In CT images, the tumor region was clearly detected following a WS2-PEG intratumoral injection, and the CT signal was dramatically enhanced, by approximately six times in Hounsfield units (HU) (Fig. 5b, right). After i.v. injection, contrast in the tumor and liver regions was enhanced, which indicated that WS2-PEG accumulated in the liver via the reticuloendothelial system. In PA images at an optical wavelength of 700 nm, only blood vessels around the tumor were visualible before WS2-PEG injection (Fig. 5b, left). After intratumoral injection, the PA signal was enhanced by approximately five times, and by three times after i.v. injection of WS2-PEG. In the PAI images after i.v. injection, the PA signal distribution was more uniform than after intratumoral injection at the tumor region, and the tumor boundary was clearly visualized (Fig. 5b, left). To acquire the CT and PA images, the passive dual mode contrast agent avoided the need to sacrifice the mouse. Moreover, the combination of CT and PA dual mode imaging and the contrast agent provided complementary information, e.g., whole body imaging without a limitation of penetration depth limit, high spatial resolution imaging, and images of the tumor microstructure. Another two dimensional structure nanomaterial, core shell Pd@Au nanoplates, showed highly stable high X-ray attenuation and high optical absorbance in the NIR region. In PAI with an optical wavelength of 700 nm, the PA signal increased gradually in the tumor region after i.v. injection of Pd@Au, and the tumor boundary was clearly visualized after accumulation of Pd@Au in the tumor (Fig. 5c, left). Moreover, 24 hours after an i.v. injection of the dual-mode contrast agent Pd@Au, the CT value signifiantly increased, which indicated considerable tumor accumulation of Pd@Au (Fig. 5c, right). Combined CT and PA dual mode imaging with Pd@Au visualized contrast agent accumulation in vivo, providing whole body anatomical information and high spatial resolution.
Fig. 5.
PA and SPECT/CT imaging with dual mode contrast agents. a PA and SPECT imaging with 125I labeled MB. b PA and CT imaging with WS2-PEG. c PA and CT imaging with Pd@Au. Figure reproduced with permission from [18, 62, 63]
Contrast agents for PAI and MRI
Magnetic resonance imaging (MRI) is a widely using medical imaging system that provides non-invasive anatomical and functional images with great penetration depth [66, 67]. MRI is based on magnetic fields generated from spinning hydrogen atoms and protons’ energy relaxation in water, lipids, and other molecules [68]. Compared to X-ray CT, MRI does not use ionizing radiation and provides better soft tissue contrast. However, its low contrast resolution, low sensitivity, slow imaging speed, and unsuitablity for an intraoperative environment are considered weaknesses. However, combining MRI and PAI can provide complementary information to improve diagnostic accuracy and supply functional information and high resolution 3D images of soft tissue boundaries.
To enhance the image contrast of both MRI and PAI, recent studies have explored nanomaterials which show high optical absorbance and a magnetic response, e.g., magnetic nanoparticles, superparamagnetic iron oxide, gadolinium chelates, and reporter genes. On the atomic scale, thin polyacrylic-acid-functionalized Co9Se8 (PAA-Co9Se8) nanosheets have been studied as a PAI and MRI dual mode contrast agent which shows strong optical absorbance in the NIR region. PAI was performed with a pulsed laser wavelength of 808 nm and T2-weighted MR signal enhancement [69]. In the PA image before applying the contrast agent, only blood vessels near the tumor region were visualized (Fig. 6a, left). After PAA-Co9Se8 injection, the PA signal near the tumor was dramatically enhanced, and the PA image clearly showed the tumor region (Fig. 6a, left). Moreover, in the T2-weighted MR images, a darkening effect in the tumor region was obviously detected after injection of PAA-Co9Se8 (Fig. 6a, right).
Fig. 6.
PA and MR imaging with dual mode contrast agenta. a PA and MR imaging with PAA-Co9Se8 nanosheets. The dotted circle indicates the tumor region. b PA and MR imaging with MoS2/Fe3O4 nanoparticles. c PA and MR imaging with . Left PA images; right MR images. Figures reproduced with permission from [69–71]
Another PA and MRI combined dual mode contrast agent that combines MoS2 and Fe3O4 nanoparticles (MoS2/Fe3O4) has been researched as a magnetic targeted contrast agent [70]. MoS2 showed high light to heat convergence efficiency, and Fe3O4 showed external magnet targeting at the desired tumor location. After an MoS2/Fe3O4 i.v. injection, a T2-weighted MR image showed contrast enhancement at three time points (0.5, 6.0, and 24.0 h) at the targeted tumor region (Fig. 6b, right). Additionally, the PA signal at the tumor region increased 6 h after MoS2/Fe3O4 injection with magnet targeting (Fig. 6b, left top). However, when an external magnetic was not imposed on tumor region (Fig. 6b, left bottom), the PA signal was relatively low compared to magnetic targeting, which indicated that external magnet targeting provided efficient accumulation of MoS2/Fe3O4 in the tumor region. With the MoS2/Fe3O4dual mode imaging contrast, the MR sensitivity and PA signal penetration depth were enhanced, and MoS2/Fe3O4 provided magnetic targeting at the tumor.
In still other work, a single component material, copper sulfide nanocrystal (), was used as a PA/MR dual-mode imaging contrast agent [71]. Thanks to the high concentration of free carriers in , which is a kind of p-type semiconductor, showed high optical absorbance at wavelengths around 1000 nm in the NIR region, with a peak around 1160 nm. Additionally, unpaired electrons of enabled a magnetic response which enhanced the MRI sensitivity. Compared to a PBS injected model, the injected model’s T1-weighted MR signal was enhanced by 15.5% after i.v. injection, and by 51.4% after intratumoral injection, and the MR images showed a brightened region at the tumor location (Fig. 6c, right). For PAI, 1064 nm laser pulses were used, and USI provided anatomical information. After injection, an approximately 170 times stronger PA signal was detected, and the penetration depth was increased up to 1.5 cm at 5 seconds after injection (Fig. 6c, left). The PA signal decreased as time elapsed; however, the PA signal still remained strong compared to before injection.
The combination of PA and MRI provided three dimensional volumetric soft tissue anatomical information, tumor boundary detection, and the microstructure of blood vessels, the tumor, and the biological system. Additionally, dual mode imaging with one contrast agent that enhanced both MR and PA contrast provided a synergetic effect, e.g., dual mode sensitivity enhancement, deeper penetration depth, and contrast enhancement. Dual mode imaging with one contrast agent offers strong advantages for tumor detection, therapy monitoring, and other preclinical and clinical research.
Contrast agents for PAI and PET
Positron emission tomography (PET) is a nuclear imaging tool used for both preclinical and clinical applications in areas such as pharmacokinetics, disease monitoring, and neuroimaging [13, 72–74]. PET imaging provides a noninvasive, highly sensitive, and quantitative record of a radiotracer’s targeting efficiency in organs and tissue. However, PET is an ionizing imaging technique, using exogenous contrast agents labeled with radionuclides that emit positrons. Moreover, PET also has several other disadvantages, such as a poor spatial resolution, low imaging speed, and high operation cost. Still, combining PAI and PET can provide useful information about anatomical structures, the distribution of contrast agents, and quantitative tracking in tumors of contrast agents labeled with radioisotopes.
Recent research has explored dual mode contrast agents that synthsize various nanomaterials that can be used as exogenous contrast agents for PA imaging with radioisotopes such as 64Cu, 13N, 68Ga, 18F, 72As and 89Zr [75]. Organic nanoformulated naphthalocianine (nanonap), which has tunable and very high optical absorption in the near infrared region, has been used as a contrast agent for PA gastrointestinal (GI) tract imaging in mouse [76]. Figure 7a shows PA MAP images of the GI tracts of small animals after oral administration of nanonaps. The movement of nanonaps in the small intestine was confirmed in the time-resolved PA MAP images. The depth information from the skin to the small intestine was encoded using pseudo-color (Fig. 7b). In whole body GI tract PET, the images show the movement of the 64Cu labeled nanonaps (Fig. 7c). After oral gavage, 64Cu labeled nanonaps were observed in stomach and intestine at 0.5 h and 3h, respectively.
Fig. 7.
a PA MIP images of ZnBNc nanonaps in the mouse GI tract. Red arrows show the transit of nanonaps. b Depth-encoded PA image of nanonaps in the intestine c PET image of ONc nanonap labeled with 64Cu after gavage. d PA MIP images of 4T1 tumor in a control mouse and after i.v. injection with MoS2-IO-(d)PEG. e PET images after iv injection with 64Cu-MoS2-IO-(d)PEG. Blue circles show the 4T1 tumor region. f 2D (left) and 3D (right) PA and US images at variable time points of the U87MF tumor region after i.v. injection with rGO-AuNRVe. g PET images of a U87MF tumor on mouse after i.v. injection with 64Cu-rGO-AuNRVe. Figures reproduced with permission from [76, 80, 81]
MoS2 nanosheets, which have good physiological stability, biocompatibility, and a high absorption coefficient in NIR wavelengths, have been tested in a similar manner [77]. 4T1 tumor bearing mice were intravenously injected with MoS2-IO-(d)PEG (double PEGylated MoS2-iron oxide nanoparticles) nanosheets ([MoS2] = 0.68 mg/mL−1, 0.2mL), and then PA images were acquired at various time points (Fig. 7d). The PA signal was stronger after the i.v. injection of MoS2-IO-(d)PEG than before the injection. For PET, 64Cu was labeled with MoS2-IO-(d)PEG in a chleator-free condition. The 64Cu-MoS2-IO-(d)PEG was intravenously injected into a 4T1 mouse tumor (Fig 7e). The positron signal from 64Cu at the tumor site was clearly detected at 3 h post-injection.
Reduced graphene oxide (rGO)-loaded small plasmonic gold nanorod vesicles (rGO-AuNRVe) were explored as a PET and PAI contrast agent. Further, reduced rGO nanoparticles with a large surface area have been reported for photothermal therapy and drug delivery for chemotherapy [78, 79]. To demonstrate the feasibility of rGO-AuNRVe as a PA contrast agent, it was intravenously injected into U87MG tumor-bearing mice. Subsequently, 2D and 3D PA images of the tumor region were acquired at various time points after injection (Fig 7f). The PA signal was then overlaid with the US image. The PA signal of the tumor region increased after injection and reached a maximum at 24 h after injection. To research in vivo bio-distribution of rGO-AuNRVe using PET, 64Cu was combined with rGO-AuNRVe in a chelator free method and i.v. injected into U87MG tumor-bearing mice (Fig 7g). After the i.v. injection, the PET signal from rGO-AuNRVe in the tumor region increased by up to 9.7% ID/g at 24 h.
Conclusion
Existing medical imaging modalities in clinics can supply morphological, molecular, and functional information about biological tissues. However, it is difficult to definitely diagnose the diseases’ status. Therefore, a multimodal imaging approach becomes crucial. Recently, PAI has become a popular preclinical imaging tool and several clinical trials using this modality have been performed. Thus, PAI can be a good candidate for the multimodal imaging approach. To increase detection sensitivities and specificities, a variety of contrast agents have been developed thanks to recent explosive studies on nanomedicine. In this review article, we have discussed about the dual mode contrast boosters for PAI with other imaging modalities. In spite of thrive on nanomedicine in research, more commercially and clinically available agents need to be developed. The key issue should lay on the safeties of these compounds in clinical environments.
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI15C1817). Additional funding was received from the China-ROK (NRF-2013K1A3A1A20046921) and from the NRF Pioneer Research Center Program (NRF-2014M3C1A3017229 and NRF-2015M3C1A3056409), and from the Marine Biotechnology Program (20150220) funded by the Ministry of Oceans and Fisheries, Korea.
References
- 1.Townsend DW. Dual-modality imaging: combining anatomy and function. J Nucl Med. 2008;49:938–955. doi: 10.2967/jnumed.108.051276. [DOI] [PubMed] [Google Scholar]
- 2.Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–365. [PubMed] [Google Scholar]
- 3.Schillaci O. Hybrid SPECT/CT: a new era for SPECT imaging? Eur J Nucl Med Molec Imaging. 2005;32:521–524. doi: 10.1007/s00259-005-1760-9. [DOI] [PubMed] [Google Scholar]
- 4.Gaemperli O, Schepis T, Valenta I, Husmann L, Scheffel H, Duerst V, et al. Cardiac image fusion from stand-alone SPECT and CT: clinical experience. J Nucl Med. 2007;48:696–703. doi: 10.2967/jnumed.106.037606. [DOI] [PubMed] [Google Scholar]
- 5.Bagci U, Udupa JK, Mendhiratta N, Foster B, Xu Z, Yao J, et al. Joint segmentation of anatomical and functional images: applications in quantification of lesions from PET, PET-CT, MRI-PET, and MRI-PET-CT images. Med Image Anal. 2013;17:929–945. doi: 10.1016/j.media.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park H, Wood D, Hussain H, Meyer CR, Shah RB, Johnson TD, et al. Introducing parametric fusion PET/MRI of primary prostate cancer. J Nucl Med. 2012;53:546–551. doi: 10.2967/jnumed.111.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C, Favazza C, Wang LV. In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chem Rev. 2010;110:2756–2782. doi: 10.1021/cr900266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell AG. The photophone. Sci Am. 1880;10:3921–3923. [Google Scholar]
- 9.Song J, Kim J, Hwang S, Jeon M, Jeong S, Kim C, et al. “Smart” gold nanoparticles for photoacoustic imaging: an imaging contrast agent responsive to the cancer microenvironment and signal amplification via pH-induced aggregation. Chem Commun. 2016;52:8287–8290. doi: 10.1039/C6CC03100E. [DOI] [PubMed] [Google Scholar]
- 10.Lee M-Y, Lee C, Jung HS, Jeon M, Kim KS, Yun SH, Kim C, Hahn SK. Biodegradable photonic melanoidin for theranostic applications. ACS nano. 2015;10(1):822–31. [DOI] [PubMed]
- 11.Kim J, Park S, Lee C, Kim JY, Kim C. Organic nanostructures for photoacoustic imaging. ChemNanoMat. 2015;2:156–166.
- 12.Roy I, Shetty D, Hota R, Baek K, Kim J, Kim C, et al. A multifunctional subphthalocyanine nanosphere for targeting, labeling, and killing of antibiotic-resistant bacteria. Angew Chem. 2015;127:15367–15370. doi: 10.1002/ange.201507140. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Kim J, Zhang Y, Jeon M, Liu C, Song L, et al. Dual-color photoacoustic lymph node imaging using nanoformulated naphthalocyanines. Biomaterials. 2015;73:142–148. doi: 10.1016/j.biomaterials.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Jeon M, Song W, Huynh E, Kim J, Kim J, Helfield BL, et al. Methylene blue microbubbles as a model dual-modality contrast agent for ultrasound and activatable photoacoustic imaging. J Biomed Opt. 2014;19:016005. doi: 10.1117/1.JBO.19.1.016005. [DOI] [PubMed] [Google Scholar]
- 15.Koo J, Jeon M, Oh Y, Kang HW, Kim J, Kim C, et al. In vivo non-ionizing photoacoustic mapping of sentinel lymph nodes and bladders with ICG-enhanced carbon nanotubes. Phys Med Biol. 2012;57:7853. doi: 10.1088/0031-9155/57/23/7853. [DOI] [PubMed] [Google Scholar]
- 16.Lee C, won WK, Beack S, Lee D, Park Y, Kim H, et al. Biodegradable nitrogen-doped carbon nanodots for non-invasive photoacoustic imaging and photothermal therapy. [DOI] [PMC free article] [PubMed]
- 17.Jeon M, Kim C. Multimodal photoacoustic tomography. IEEE Trans Multimed. 2013;15:975–982. doi: 10.1109/TMM.2013.2244203. [DOI] [Google Scholar]
- 18.Akers WJ, Edwards WB, Kim C, Xu B, Erpelding TN, Wang LV, et al. Multimodal sentinel lymph node mapping with single-photon emission computed tomography (SPECT)/computed tomography (CT) and photoacoustic tomography. Transl Res. 2012;159:175–181. doi: 10.1016/j.trsl.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S, Lee C, Kim J, Kim C. Acoustic resolution photoacoustic microscopy. Biomed Eng Lett. 2014;4:213–222. doi: 10.1007/s13534-014-0153-z. [DOI] [Google Scholar]
- 20.Kim J, Park S, Jung Y, Chang S, Park J, Zhang Y, et al. Programmable real-time clinical photoacoustic and ultrasound imaging system. Sci Rep. 2016;6:35137. doi: 10.1038/srep35137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee D, Lee C, Kim S, Zhou Q, Kim J, Kim C. In vivo near infrared virtual intraoperative surgical photoacoustic optical coherence tomography. Sci Rep. 2016;6:35176. doi: 10.1038/srep35176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 23.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 24.Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 25.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 26.Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol. 2007;18:17–25. doi: 10.1016/j.copbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Akers WJ, Kim C, Berezin M, Guo K, Fuhrhop R, Lanza GM, et al. Noninvasive photoacoustic and fluorescence sentinel lymph node identification using dye-loaded perfluorocarbon nanoparticles. ACS Nano. 2010;5:173–182. doi: 10.1021/nn102274q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda A, Bu J, Chen J, Zheng G, DaCosta RS. Dual in vivo photoacoustic and fluorescence imaging of HER2 expression in breast tumors for diagnosis, margin assessment, and surgical guidance. Mol Imag. 2014;13:1–9. doi: 10.2310/7290.2014.00043. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Zhao Y-X, Qiao Z-Y, Mayerhöffer U, Spenst P, Li X-J, et al. Nano-confined squaraine dye assemblies: new photoacoustic and near-infrared fluorescence dual-modular imaging probes in vivo. Bioconj Chem. 2014;25:2021–2029. doi: 10.1021/bc5003983. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Maslov K, Kim C, Hu S, Wang LV. Integrated photoacoustic and fluorescence confocal microscopy. IEEE Trans Biomed Eng. 2010;57:2576–2578. doi: 10.1109/TBME.2010.2059026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim C, Song KH, Gao F, Wang LV. Sentinel lymph nodes and lymphatic vessels: noninvasive dual-modality in vivo mapping by using indocyanine green in rats—volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging 1. Radiology. 2010;255:442–450. doi: 10.1148/radiol.10090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Wang H, Song L. A novel folate-receptor targeted indocyanine green nanoprobe for in vivo photoacoustic/fluorescence dual-modality imaging of breast carcinoma. In: Optics in the Life Sciences, Vancouver, 2015/04/12. OSA Technical Digest (online). Optical Society of America, p OW3D.2. 2015. doi:10.1364/OMP.2015.OW3D.2.
- 33.Park S, Kim J, Jeon M, Song J, Kim C. In vivo photoacoustic and fluorescence cystography using clinically relevant dual modal indocyanine green. Sensors. 2014;14:19660–19668. doi: 10.3390/s141019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Rong P, Yu L, Zhang X, Yang C, Guo F, et al. Dual-modality noninvasive mapping of sentinel lymph node by photoacoustic and near-infrared fluorescent imaging using dye-loaded mesoporous silica nanoparticles. Molec Pharm. 2015;12:3119–3128. doi: 10.1021/mp500698b. [DOI] [PubMed] [Google Scholar]
- 35.Ge J, Jia Q, Liu W, Guo L, Liu Q, Lan M, et al. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv Mater. 2015;27:4169–4177. doi: 10.1002/adma.201500323. [DOI] [PubMed] [Google Scholar]
- 36.Kobat D, Horton NG, Xu C. In vivo two-photon microscopy to 1.6-mm depth in mouse cortex. J Biomed Opt. 2011;16:106014. doi: 10.1117/1.3646209. [DOI] [PubMed] [Google Scholar]
- 37.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Maslov K, Kim C, Hu S, Wang LV. Integrated photoacoustic and fluorescence confocal microscopy. Biomed Eng IEEE Trans. 2010;57:2576–2578. doi: 10.1109/TBME.2010.2059026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sela G, Razansky D, Shoham S. Multimodal optoacoustic and multiphoton fluorescence microscopy. In: SPIE BiOS, 2013. International Society for Optics and Photonics, pp. 85812H.
- 40.Soliman D, Tserevelakis GJ, Omar M, Ntziachristos V. Combining microscopy with mesoscopy using optical and optoacoustic label-free modes. Sci Rep. 2015;5:12902. doi: 10.1038/srep12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Lee D, Jung U, Kim C. Photoacoustic imaging platforms for multimodal imaging. Ultrasonography. 2015;34:88–97. doi: 10.14366/usg.14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim C, Erpelding TN, Jankovic L, Wang LV. Performance benchmarks of an array-based hand-held photoacoustic probe adapted from a clinical ultrasound system for non-invasive sentinel lymph node imaging. Philos Trans A Math Phys Eng Sci. 2011;369:4644–4650. doi: 10.1098/rsta.2010.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song L, Kim C, Maslov K, Shung KK, Wang LV. High-speed dynamic 3D photoacoustic imaging of sentinel lymph node in a murine model using an ultrasound array. Med Phys. 2009;36:3724. doi: 10.1118/1.3168598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erpelding TN, Kim C, Pramanik M, Jankovic L, Maslov K, Guo Z, et al. Sentinel lymph nodes in the rat: noninvasive photoacoustic and US imaging with a clinical US system 1. Radiology. 2010;256:102–110. doi: 10.1148/radiol.10091772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erpelding TN, Garcia-Uribe A, Krumholz A, Ke H, Maslov K, Appleton C, Margenthaler J, Wang LV. A dual-modality photoacoustic and ultrasound imaging system for noninvasive sentinel lymph node detection: preliminary clinical results. In: SPIE BiOS. International Society for Optics and Photonics, 2014. pp 894359.
- 46.Wen Q, Wan S, Liu Z, Xu S, Wang H, Yang B. Ultrasound contrast agents and ultrasound molecular imaging. J Nanosci Nanotechnol. 2014;14:190–209. doi: 10.1166/jnn.2014.9114. [DOI] [PubMed] [Google Scholar]
- 47.Huynh E, Lovell JF, Helfield BL, Jeon M, Kim C, Goertz DE, et al. Porphyrin shell microbubbles with intrinsic ultrasound and photoacoustic properties. J Am Chem Soc. 2012;134:16464–16467. doi: 10.1021/ja305988f. [DOI] [PubMed] [Google Scholar]
- 48.Kim C, Qin R, Xu JS, Wang LV, Xu R. Multifunctional microbubbles and nanobubbles for photoacoustic and ultrasound imaging. J Biomed Opt. 2010;15:010510. doi: 10.1117/1.3302808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeon M, Song W, Huynh E, Kim J, Kim J, Helfield BL, et al. Methylene blue microbubbles as a model dual-modality contrast agent for ultrasound and activatable photoacoustic imaging. J Biomed Opt. 2014;19:16005. doi: 10.1117/1.JBO.19.1.016005. [DOI] [PubMed] [Google Scholar]
- 50.Huynh E, Leung BY, Helfield BL, Shakiba M, Gandier JA, Jin CS, et al. In situ conversion of porphyrin microbubbles to nanoparticles for multimodality imaging. Nat Nanotechnol. 2015;10:325–332. doi: 10.1038/nnano.2015.25. [DOI] [PubMed] [Google Scholar]
- 51.Bergholt MS, Zheng W, Huang Z. Development of a multiplexing fingerprint and high wavenumber Raman spectroscopy technique for real-time in vivo tissue Raman measurements at endoscopy. J Biomed Opt. 2013;18:030502. doi: 10.1117/1.JBO.18.3.030502. [DOI] [PubMed] [Google Scholar]
- 52.Kang JW, So PT, Dasari RR, Lim D-K. High resolution live cell raman imaging using subcellular organelle-targeting SERS-sensitive gold nanoparticles with highly narrow intra-nanogap. Nano Lett. 2015;15:1766–1772. doi: 10.1021/nl504444w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice. ACS Nano. 2012;6:10366–10377. doi: 10.1021/nn304347g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee D-E, Koo H, Sun I-C, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41:2656–2672. doi: 10.1039/C2CS15261D. [DOI] [PubMed] [Google Scholar]
- 55.Dinish U, Song Z, Ho CJH, Balasundaram G, Attia ABE, Lu X, et al. Single molecule with dual function on nanogold: biofunctionalized construct for in vivo photoacoustic imaging and SERS biosensing. Adv Funct Mater. 2015;25:2316–2325. doi: 10.1002/adfm.201404341. [DOI] [Google Scholar]
- 56.Hsieh J. Computed tomography: principles, design, artifacts, and recent advances. Bellingham, WA: SPIE, 2009.
- 57.Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps) J Am Coll Cardiol. 2014;63:1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 58.Suomalainen A, Vehmas T, Kortesniemi M, Robinson S, Peltola J. Accuracy of linear measurements using dental cone beam and conventional multislice computed tomography. Dentomaxillofac Radiol. 2008;37(1):10–17. [DOI] [PubMed]
- 59.Cavalcanti M, Rocha S, Vannier M. Craniofacial measurements based on 3D-CT volume rendering: implications for clinical applications. Dentomaxillofac Radiol 2004;33(3):170–6. [DOI] [PubMed]
- 60.Oberoi S, Knueppel S. Three-dimensional assessment of impacted canines and root resorption using cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:260–267. doi: 10.1016/j.tripleo.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 61.Yitschaky O, Redlich M, Abed Y, Faerman M, Casap N, Hiller N. Comparison of common hard tissue cephalometric measurements between computed tomography 3D reconstruction and conventional 2D cephalometric images. Angle Orthod. 2011;81:11–16. doi: 10.2319/031710-157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Tang S, Guo Z, Wang X, Mo S, Huang X, et al. Core–Shell Pd@ Au nanoplates as theranostic agents for in-vivo photoacoustic imaging, CT imaging, and photothermal therapy. Adv Mater. 2014;26:8210–8216. doi: 10.1002/adma.201404013. [DOI] [PubMed] [Google Scholar]
- 63.Cheng L, Liu J, Gu X, Gong H, Shi X, Liu T, et al. PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv Mater. 2014;26:1886–1893. doi: 10.1002/adma.201304497. [DOI] [PubMed] [Google Scholar]
- 64.Jin Y, Li Y, Ma X, Zha Z, Shi L, Tian J, et al. Encapsulating tantalum oxide into polypyrrole nanoparticles for X-ray CT/photoacoustic bimodal imaging-guided photothermal ablation of cancer. Biomaterials. 2014;35:5795–5804. doi: 10.1016/j.biomaterials.2014.03.086. [DOI] [PubMed] [Google Scholar]
- 65.Jing L, Liang X, Deng Z, Feng S, Li X, Huang M, et al. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials. 2014;35:5814–5821. doi: 10.1016/j.biomaterials.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 66.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30:2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatr 2005;162(9):1605–13. [DOI] [PMC free article] [PubMed]
- 68.Brown RW, Cheng Y-CN, Haacke EM, Thompson MR, Venkatesan R. Magnetic resonance imaging: physical principles and sequence design. London: Wiley; 2014. [Google Scholar]
- 69.Song XR, Wang X, Yu SX, Cao J, Li SH, Li J, Liu G, Yang HH, Chen X. Co9Se8 nanoplates as a new theranostic platform for photoacoustic/magnetic resonance dual-modal-imaging-guided chemo-photothermal combination therapy. Adv Mater. 2015;27(21):3285–91. [DOI] [PMC free article] [PubMed]
- 70.Yu J, Yin W, Zheng X, Tian G, Zhang X, Bao T, Dong X, Wang Z, Gu Z, Ma X. Smart MoS2/Fe3O4 nanotheranostic for magnetically targeted photothermal therapy guided by magnetic resonance/photoacoustic imaging. Theranostics 2015;5(9):931. [DOI] [PMC free article] [PubMed]
- 71.Mou J, Liu C, Li P, Chen Y, Xu H, Wei C, et al. A facile synthesis of versatile Cu2−xS nanoprobe for enhanced MRI and infrared thermal/photoacoustic multimodal imaging. Biomaterials. 2015;57:12–21. doi: 10.1016/j.biomaterials.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Gourni E, Mansi R, Jamous M, Waser B, Smerling C, Burian A, et al. N-terminal modifications improve the receptor affinity and pharmacokinetics of radiolabeled peptidic gastrin-releasing peptide receptor antagonists: examples of 68Ga-and 64Cu-labeled peptides for PET imaging. J Nucl Med. 2014;55:1719–1725. doi: 10.2967/jnumed.114.141242. [DOI] [PubMed] [Google Scholar]
- 73.Kim E, Howes OD, Kim B-H, Jeong JM, Lee JS, Jang I-J, et al. Predicting brain occupancy from plasma levels using PET: superiority of combining pharmacokinetics with pharmacodynamics while modeling the relationship. J Cereb Blood Flow Metabol. 2012;32:759–768. doi: 10.1038/jcbfm.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luster M, Karges W, Zeich K, Pauls S, Verburg FA, Dralle H, et al. Clinical value of 18F-fluorodihydroxyphenylalanine positron emission tomography/computed tomography (18F-DOPA PET/CT) for detecting pheochromocytoma. Eur J Nucl Med Mol Imag. 2010;37:484–493. doi: 10.1007/s00259-009-1294-7. [DOI] [PubMed] [Google Scholar]
- 75.Sun X, Cai W, Chen X. Positron emission tomography imaging using radiolabeled inorganic nanomaterials. Acc Chem Res. 2015;48:286–294. doi: 10.1021/ar500362y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y, et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol. 2014;9:631–638. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chou SS, De M, Kim J, Byun S, Dykstra C, Yu J, et al. Ligand conjugation of chemically exfoliated MoS2. J Am Chem Soc. 2013;135:4584–4587. doi: 10.1021/ja310929s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W, Guo ZY, Huang DQ, Liu ZM, Guo X, Zhong HQ. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32:8555–8561. doi: 10.1016/j.biomaterials.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 79.Kim H, Namgung R, Singha K, Oh I-K, Kim WJ. Graphene oxide–polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug Chem. 2011;22:2558–2567. doi: 10.1021/bc200397j. [DOI] [PubMed] [Google Scholar]
- 80.Liu T, Shi S, Liang C, Shen S, Cheng L, Wang C, et al. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano. 2015;9:950–960. doi: 10.1021/nn506757x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song J, Yang X, Jacobson O, Lin L, Huang P, Niu G, et al. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano. 2015;9:9199–9209. doi: 10.1021/acsnano.5b03804. [DOI] [PMC free article] [PubMed] [Google Scholar]