Dear Editor,
Several recent clinical studies have indicated that dietary supplementation with branched-chain amino acids (BCAA), particularly with leucine, is an effective anti-atrophic therapy (Bauer et al., 2015; Tsien et al., 2015; English et al., 2016). In animal models, BCAA can prevent denervation (Ribeiro et al., 2015), hindlimb suspension (Maki et al., 2012; Jang et al., 2015) or dexamethasone-induced (Yamamoto et al., 2010) muscle atrophy. General control nonderepressible 2 kinase (GCN2) is a well-known amino-acid sensor. Under conditions of amino-acid deprivation, the increased level of uncharged transfer RNA (tRNA) activates GCN2 through binding to the histadyl-tRNA synthetase-like domain (Wek et al., 1995). Upon activation, GCN2 phosphorylates eukaryotic initiation factor 2 alpha at Ser51, which leads to translational arrest and restoration of amino acid homeostasis (Wek et al., 1995; Sood et al., 2000). As amino acids are potent modulators of protein turnover in skeletal muscle, we proposed that GCN2 may affect denervation-induced muscle atrophy, but the detail mechanism remains unclear.
To investigate the impact of GCN2 on the development of muscle atrophy, we performed sciatic denervation procedure on hindlimb muscles in wild-type (WT) and Gcn2−/− mice. On day 7 after denervation, WT tibial anterior (TA) muscle mass decreased to 70.9% ± 1.8% of the contralateral level, while GCN2-deficient TA muscle mass remained at 83.1% ± 1.6% of its contralateral level (Fig. 1A). GCN2 deficiency also significantly attenuated the muscle mass loss in atrophied gastrocnemius (GAS) and extensor digitorum longus (EDL) muscles. Similar results were observed on day 14 after denervation (Fig. S1). Wheat germ agglutinin (WGA) staining of muscle cryosections demonstrated that GCN2-deficient TA muscles had a better preservation of myofiber size in response to denervation (Fig. 1B). After denervation, myofiber size distribution calculated from WT TA muscles showed a leftward shift from its contralateral conditions. However, such shift was delayed in atrophied GCN2-deficient TA muscles (Fig. 1C). To further verify whether activation of GCN2 contributes to muscle atrophy, we overexpressed GCN2 in flexor digitorum brevis (FDB) muscles using in vivo electroporation. The transfection efficiency was confirmed by Western blot (Fig. S2). After denervation for ten days, the diameter of WT FDB myofiber decreased from 37.2 ± 0.9 μm to 24.2 ± 0.7 μm, while the diameter of Gcn2−/− FDB myofiber was maintained at 32.3 ± 0.7 μm. Overexpression of GCN2 resulted in a further reduction in the diameter of FDB myofibers in both WT and Gcn2−/− mice. However, the reduction was more dramatically in Gcn2−/− FDB muscle and the significant difference in the diameter of atrophied FDB myofiber between WT and Gcn2−/− mice was diminished after transfected with the GCN2 plasmid (Fig. 1D).
Figure 1.

Effect of GCN2 on denervation-induced atrophy. Sciatic denervation was performed on 2- to 3-month-old WT and Gcn2−/− mice. (A) Seven days after denervation, the percentage of denervated to contralateral skeletal muscle mass was determined. ctrl, contralateral; denerv, denervated. (B) Cryosections from contralateral and denervated TA muscle of WT and Gcn2−/− mice were stained with wheat germ agglutinin (WGA). Scale bars = 100 μm. The average myofiber cross-sectional area (CSA) was measured by Image J, and data are expressed as the percentage of denervated to contralateral fiber CSA of WT and Gcn2−/− mice. (C) TA myofiber size distribution showed a reduced leftward shift in GCN2-deficient TA muscles compared to WT muscles. (D) Flexor digitorum brevis (FDB) muscle fibers were transfected with the pIRS2-EGFP or pIRS2-EGFP-GCN2 plasmid. On day 10 after denervation, representative images were taken and FDB myofiber diameters were measured. 4 mice were used for each group and 20–40 myofiber diameters were measured for each mouse. Scale Bar = 50 μm. *P < 0.05 compared to WT or control mice
Emerging evidence suggests that the protein degradation in muscle atrophy is mediated by FoxO3a, an important regulator of Atrogin-1, MuRF-1 and LC3 (Sandri et al., 2004; Zhao et al., 2007; Guo et al., 2016). Upon atrophy stimuli, FoxO3a shuffled into the myofiber nucleus, which leads to transcriptional activation (Sandri et al., 2004). Through Western blotting and immunostaining, we observed that the phosphorylation level of FoxO3a at Ser207 were increased in TA muscles of WT mice after denervation, and FoxO3 was mainly located in the nucleus. However, the denervation-induced phosphorylation and nuclear accumulation of FoxO3a were significantly less in GCN2-deficient muscles compared with that in WT muscles (Fig. 2A–B). We also observed significant increases in protein expression of MuRF-1 and Atrogin-1, as well as the ratio of conversion of LC3 into the activated forms (LC3-II/I) in WT atrophic TA muscles. However, increases in E3 ubiquitin ligases expression and activation of autophagy were statistically less remarkable in atrophic Gcn2−/− muscles than in WT (Fig. S3).
Figure 2.
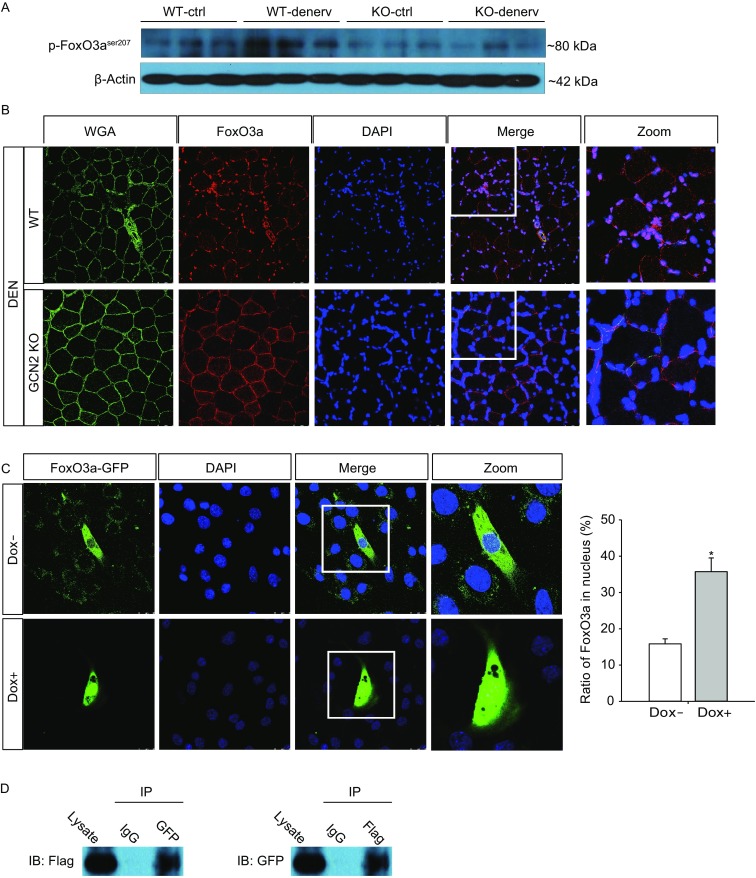
Effect of GCN2 on FoxO3a activation and nuclear translocation. (A) TA muscles were collected from WT and GCN2−/− mice seven days after denervation, and lysates were examined by Western blotting. The immunoblot band intensities were quantified by densitometry and the normalized values with respect to corresponding loading controls (β-actin) are plotted as bar graphs in panels. (B) Representative immunostaining using WGA and FoxO3a antibody on TA muscle sections obtained on denervation day 7. (C) C2C12 cells were stably transfected with doxycycline (Dox)-controlled Flag-tagged mouse GCN2 (mGCN2-C2C12), cultured for 24 h with (Dox+) or without (Dox−) 1 μg/mL Dox, and analyzed for expression of Flag-tagged GCN2 by Western blotting. (D) mGCN2-C2C12 cells were transfected with pEGFP-C2-FoxO3a plasmid, cultured for 48 h with (Dox+) or without (Dox−) 1 μg/mL Dox. Representative fluorescent images were taken and the ratio of FoxO3a in the nucleus was quantitatively analyzed. (D) mGCN2-C2C12 cells were transfected with pEGFP-C2-FoxO3a plasmid and grown for 48 h with 1 μg/mL Dox. Anti-GFP and anti-Flag antibodies were used to immunoprecipitate (IP) GFP-FoxO3a and Flag-GCN2 from whole-cell extracts, respectively, followed by Western blotting analyses using anti-Flag (left) and anti-GFP (right) antibodies to detect Flag-GCN2 and GFP-FoxO3a, respectively. Figures are chosen as the representative of three independent experiments. *P < 0.05
To investigate whether GCN2 activation directly causes FoxO3a nuclear translocation in muscle atrophy, we generated a stable C2C12 cell line (mGCN2-C2C12) with doxycycline (Dox)-controlled expression of flag-tagged mouse GCN2 (Fig. 2C), and found that GCN2 overexpression induced by Dox (Dox+) significantly increased the ratio of FoxO3a in the nucleus (Fig. 2D). Furthermore, co-immunoprecipitation experiments using lysates from FoxO3a plasmid-transfected mGCN2-C2C12 cells with Dox demonstrated that GCN2-Flag and FoxO3a-EGFP were specifically co-precipitated with anti-GFP and anti-Flag antibodies, respectively (Fig. 2E), demonstrating that GCN2 and FoxO3a can physically interact with each other in cells. Using the differentiated mGCN2-C2C12 cells, we also found that overexpression of GCN2 exacerbated dexamethasone-induced upregulation of Atrogin-1 and LC3-II in differentiated C2C12 cells (Fig. S4).
It has been demonstrated dietary deprivation of essential amino acids, which activates GCN2 via increasing uncharged tRNA levels (Wek et al., 1995), caused diffuse atrophy in the rectus femoris muscles (Kamata et al., 2014). In contrast, leucine or other BCAA supplementation, which attenuates GCN2 activity (Wek et al., 1995), has been regarded as a potential pharmaconutrient for the treatment of numerous muscle wasting conditions (Bauer et al., 2015; Tsien et al., 2015; English et al., 2016). In agreement with those findings, we demonstrated that GCN2 deletion attenuates, whereas GCN2 overexpression exacerbates denervation-induced muscle atrophy. Furthermore, the detrimental effect of GCN2 in denervation-induced atrophy was related to FoxO3a activation, which upregulates genes involved in both the ubiquitin-proteasome pathway and autophagy in muscle atrophy (Sandri et al., 2004; Bertaggia et al., 2012; Wei et al., 2013; Guo et al., 2016). Thus, reducing GCN2 activity may be a potential therapeutic approach for the clinical treatment of muscle atrophy.
FOOTNOTES
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81470520, 91643206, 91743104, 31371430 to HW and 31300976 to BW) and Chinese Academy of Sciences (KJRH2015-005 and Hundred Talents Program).
Yuting Guo, Huiwen Wang, Yinglong Tang, Yue Wang, Mengqi Zhang, Zhiguang Yang, Eric Nyirimigabo, Bin Wei, Zhongbing Lu, and Guangju Ji declare that they have no conflicts of interest. All institutional and national guidelines for the care and use of laboratory animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Contributor Information
Zhongbing Lu, Email: luzhongbing@ucas.ac.cn.
Guangju Ji, Email: gj28@ibp.ac.cn.
References
- Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo ME, Mets T, Seal C, Wijers SL, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Bertaggia E, Coletto L, Sandri M. Posttranslational modifications control FoxO3 activity during denervation. Am J Physiol Cell Physiol. 2012;302:C587–C596. doi: 10.1152/ajpcell.00142.2011. [DOI] [PubMed] [Google Scholar]
- English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103:465–473. doi: 10.3945/ajcn.115.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Meng J, Tang Y, Wang T, Wei B, Feng R, Gong B, Wang H, Ji G, Lu Z. AMP-activated kinase alpha2 deficiency protects mice from denervation-induced skeletal muscle atrophy. Arch Biochem Biophys. 2016;600:56–60. doi: 10.1016/j.abb.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Jang J, Yun HY, Park J, Lim K. Protective effect of branched chain amino acids on hindlimb suspension-induced muscle atrophy in growing rats. J Exerc Nutr Biochem. 2015;19:183–189. doi: 10.5717/jenb.2015.15062704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata S, Yamamoto J, Kamijo K, Ochiai T, Morita T, Yoshitomi Y, Hagiya Y, Kubota M, Ohkubo R, Kawaguchi M, et al. Dietary deprivation of each essential amino acid induces differential systemic adaptive responses in mice. Mol Nutr Food Res. 2014;58:1309–1321. doi: 10.1002/mnfr.201300758. [DOI] [PubMed] [Google Scholar]
- Maki T, Yamamoto D, Nakanishi S, Iida K, Iguchi G, Takahashi Y, Kaji H, Chihara K, Okimura Y. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutr Res. 2012;32:676–683. doi: 10.1016/j.nutres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro CB, Christofoletti DC, Pezolato VA, de Cassia Marqueti Durigan R, Prestes J, Tibana RA, Pereira EC, de Sousa Neto IV, Durigan JL, da Silva CA. Leucine minimizes denervation-induced skeletal muscle atrophy of rats through akt/mtor signaling pathways. Front Physiol. 2015;6:73. doi: 10.3389/fphys.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics. 2000;154:787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ, Engelen MP, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Dui W, Liu D, Xing Y, Yuan Z, Ji G. MST1, a key player, in enhancing fast skeletal muscle atrophy. BMC Biol. 2013;11:12. doi: 10.1186/1741-7007-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol. 1995;15:4497–4506. doi: 10.1128/MCB.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D, Maki T, Herningtyas EH, Ikeshita N, Shibahara H, Sugiyama Y, Nakanishi S, Iida K, Iguchi G, Takahashi Y, et al. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve. 2010;41:819–827. doi: 10.1002/mus.21621. [DOI] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


