Abstract
Naphthalocyanines (Ncs) are a family of aromatic small molecule with large near infrared extinction coefficients, making them appealing contrast agent candidates for photoacoustic imaging (PAI). Depending on the substitutions on the Nc periphery or metal center, different spectrally-resolved absorption peak wavelengths are possible, which can enable photoacoustic contrast multiplexing. Owing to their generally poor aqueous solubility, approaches have been developed to modify Ncs or formulate them as biocompatible contrast agents for PAI. Due to their inherent capacity for metal ion chelation, Ncs hold potential for complementary multimodal contrast imaging techniques such as 64Cu positron emission tomography. In this research perspective, we highlight some recent reports involving the use of Ncs in PAI.
Keywords: Photoacoustic, Naphthalocyanines, Multimodal imaging, Contrast agents
Introduction
Photoacoustic imaging (PAI) has emerged as a promising, noninvasive optical imaging modality [1–3]. PAI involves conversion of non-ionizing laser pulses into heat within tissue, resulting in thermoelastic expansion and creation of ultrasonic (US) pressure waves which are recorded with conventional US transducers. The signal obtained by the transducer is processed to obtain the photoacoustic image. PAI is also referred to as thermoacoustic or optoacoustic imaging. PAI can make use of endogenous contrast originating from blood absorption which makes it useful for vasculature imaging [4]. Another added advantage of PAI is its high contrast imaging capability with improved spatial resolution when imaging in deep tissue [5]. Since other near-infrared optical imaging lacks the capability to image deeper tissue due to low contrast imaging stemming from light scattering, PAI has enabling imaging capability in deep tissues.
PAI has been explored in numerous imaging applications including prostate cancer detection [6] and tumor imaging [7], Crohn’s disease detection [8], detection of macrophages in atherosclerotic plaques [9], drug delivery [10], and brain function monitoring [11], to name a few. Much effort has been on early detection of diseases and real time imaging [12, 13]. PAI can be a convenient method for real time imaging since it is non-invasive. Moreover, near infrared (NIR) radiation, unlike other nuclear imaging methods, is non-ionizing method which is a safer option for imaging. With these advantages, together with good portability and low cost of production and operation, photoacoustic imaging is in the process of being translated to clinic.
PAI has been evaluated for breast imaging. X-ray mammogram is the most common method of imaging the breast. Although X-ray imaging is able to identify tumors, in cases of young subjects, these might not be detected due to dense breast tissue. Some tumors display rapid growth that might not be able detect due to the longer intervals between two imaging tests owing to the harmful effects of x-ray imaging [14]. PAI could be an appealing screening technique since it has real-time monitoring capability without ionizing radiation. A mammoscope developed based on a PAI system demonstrated feasibility [15].
Conventional optical imaging approaches suffer from low contrast and poor spatial resolution in deep tissues whereas PAI overcomes those disadvantages with certain endogenous biological chromophores. In particular, hemoglobin in blood has been widely exploited as a useful PAI contrast agent in this way [16]. However, the spatial resolution of the image depends on oxygen saturation level as well as concentration of hemoglobin in the region and despite its high concentration, hemoglobin possesses a low NIR extinction coefficient. Thus, for higher contrast and/or deeper tissue PAI, exogenous contrast agents may be required for adequate image formation. Depth dependent information provided by PAI might not be able to provide a complete imaging picture. Therefore, multimodal imaging has commonly been explored for PAI resulting in the use of complementary imaging methods like ultrasound (US) imaging [17, 18], optical coherence tomography [19], fluorescence imaging [20], or fluorescence confocal microscopy [21] resulting in enhanced diagnostic abilities. PAT is amenable to be combined with other modalities since it is safe, portable and has low operation cost [22].
Contrast agents
Most PAI imaging agents are designed to absorb light in the NIR region, as tissue penetration of light of this wavelength exhibits reduced absorption and scattering in tissues. A large variety of nanomaterials have been explored as exogenous PAI contrast agents [23–25]. These include inorganic materials (gold nanomaterials [26, 27], carbon nanotubes [7], graphene oxide [28], core–shell [29], and gold nanocages [30]) and organic materials (porphysomes [31] ], albumin-NIR dye assemblies [32], and semi-conducting polymers [33]). Gold nanomaterials are probably one of the well-studied PAI contrast agent [26, 27]. Gold nanoparticles are appealing due to a large and highly tunable surface plasmon resonance effect to produce NIR contrast. Clinical translation of gold nanoparticles has seen some progress, but like any new contrast agent faces barriers to achieve a reproducible and acceptable level of biodegradability and cytotoxicity [34, 35].
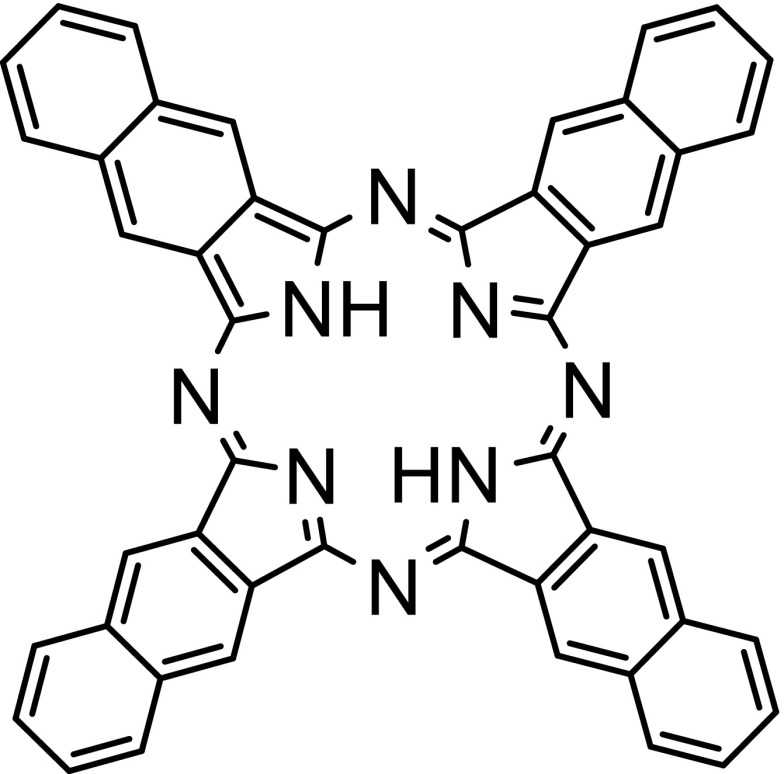
Porphyrins and related compounds have found numerous applications in theranostics and in photomedicine [36, 37]. The related naphthalocyanines (Ncs) and phthalocyanines (Pcs) are naphtho- and benzo- substituted porphyrazines, respectively. The structure of the basic Nc framework is shown in Fig. 1. Ncs are organic chromophores that possess high NIR extinction coefficients and capability for diverse metal chelation in the macrocycle center [38]. Pcs and Ncs have also been used for numerous theranostic applications [39]. Ncs show strong absorption in NIR-I region with their typical absorption peak between 700 and 900 nm. It is possible to vary the substituents of Pcs and Ncs to achieve absorption in the NIR-II window beyond 1000 nm for deeper PAI (through over 10 cm chicken breast tissue) [18]. Ncs have also been used for phototherapeutic applications including photodynamic and photothermal therapy [40, 41].
Fig. 1.
Chemical structure of naphthalocyanine
Although Ncs display high extinction coefficients, they are typically insoluble in water, so they must be solubilized prior to use in PAI. The delivery of Ncs to their intended site usually involves the use of a nanocarrier [40, 42, 43]. Alternatively, Ncs have been structurally modified to increase their solubility. In addition to their low solubility in aqueous solutions, they tend to form aggregates owing to stacking and strong van-der-Waals forces. To decrease inter-molecular interactions and to therefore increase the solubility, axial substitution methods or chelation of metals has been employed [44]. One of the first metal chelated Pc was synthesized unintentionally [45]. In 1928, when researchers were synthesizing phthalimide with phthalic anhydride and ammonia as starting material, iron from the container reacted with the starting material resulting in iron-chelated Pc. Later the product was isolated and characterized as iron-chelated Pc. Other metals such as zinc, copper, aluminium have also been chelated, imparting slightly shifted absorbance peaks. Different metal-chelated Ncs have been shown to have different contrast properties for PAI, with nickel Nc being found to be particularly promising [46]. Metalloporphyrin nanoparticles have been used in many theranostic biomedical applications that could also suit metal Ncs [47]. One of the main reason for Ncs to be used as contrast agent for PAI is their extremely high extinction coefficients which are typically up to 2.8 × 105 M−1 cm−1. [48] Nc chelation with metals such as silicon can provide for very high extinction coefficients (~ 4 × 105 M−1 cm−1) at a peak absorption wavelength 810 nm [40].
Nanocarriers
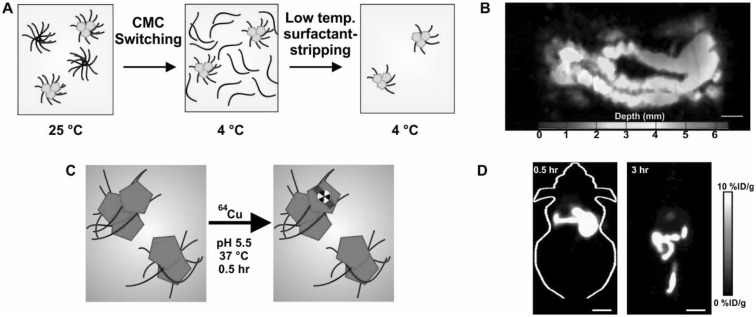
Several nanocarriers like micelles and liposomes have capacity for delivering hydrophobic cargos like Ncs [49, 50]. Pluronic F127 (PF127) micelles were able to solubilize Ncs and then concentrate them [42]. PF127 forms micelles in water at room temperature, based on its critical micelle concentration (CMC). However, due to the temperature-sensitive CMC properties, they dissociate and form unimers at 4 °C. After dispersing Ncs in PF127 micelles at room temperature, the solution was subjected to low temperature and membrane-based removal of free and loose PF127 while providing full retention of the dye in highly concentrated Nc surfactant-stripped micelles (Fig. 2a). Subsequent work demonstrated that Pluronic type triblock copolymer surfactants, but not several other types of surfactants, are required to generate high NIR absorption with the surfactant-stripping approach [51]. These surfactant-stripped Ncs have been used in characterization of PAI imaging systems [52]. The same approach was extended to other dyes such as pheophytin [53]. Multiple Pcs and Ncs could be formulated in surfactant-stripped micelles in this way, generating colloidal suspensions with very high NIR absorption. Surfactant-stripped Ncs did not alter their absorbance peak wavelength even at a observed, calculated NIR absorption values of 1000. On the other hand, small molecule PAI contrast agents like indocyanine green and methylene blue show noticeable peak shifts when the concentration is high. There was no obvious toxicity originating from surfactant-stripped 5,9,14,18,23,27,32,36-octabutoxy-2,3-naphthalocyanine (ONc) or zinc-2,11,20,29-tetra-tert-butyl-2,3-naphthalocyanine (ZnBNc) and these passed safely through the intestine following oral gavage. After ZnBNc surfactant-stripped Ncs were gavaged, PAI results revealed a clear PA signal from the intestine (Fig. 2b). Real-time functional imaging of peristalsis was possible with this approach.
Fig. 2.
Surfactant-stripped Ncs for multimodal imaging. a Formulation of highly concentrated Nc encapsulated micelles using a surfactant-stripping method. b Non-invasive photoacoustic image of a mouse intestine following oral administration of surfactant-stripped Nc (zinc-2,11,20,29-tetra-tert-butyl-2,3-naphthalocyanine; ZnBNc). c Seamless labeling of surfactant-stripped Nc (5,9,14,18,23,27,32,36-Octabutoxy-2,3-naphthalocyanine; ONc) by simple incubation with 64Cu. Whole body positron emission tomography of mice 0.5 and 3 h after oral administration of 64Cu Ncs.
Used with permission from refs. [42, 53]. Copyright 2016, Wiley and 2014 Springer Nature
Mulitmodal imaging is increasingly being used to address limitations of individual imaging modalities [54]. For PAI, even though imaging is possible much deeper than other optical techniques, the maximum imaging depth in tissue is still limited. Thus, combining contrast PAI with another imaging technique, such as positron emission tomograph (PET) in which there are no depth limitations, could be complementary. To demonstrate multimodal imaging capabilities of the surfactant-stripped Ncs, 64Cu was chelated to ONc seamlessly by simple incubation (Fig. 2c). 64Cu chelated surfactant-stripped Ncs enabled PET whole body imaging of the intestine (Fig. 2d).
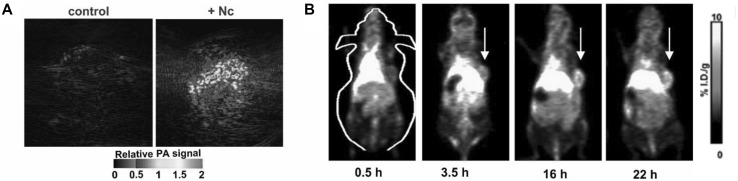
Ncs have potential for tumor imaging. In another study involving surfactant-stripped Ncs, the same surfactant-stripped ONc micelles were intravenously injected into mouse bearing 4T1 tumor [41]. Photoacoustic imaging of tumor showed the region of tumor to have a very high PA signal whereas control mouse (with no ONc injection) had no signal (Fig. 3a). Again, 64Cu was seamlessly labeled by simple incubation of surfactant-stripped ONc micelles with the radioisotope, resulting in a labeling yield of 83.2%. With intravenous injection of 64Cu labeled surfactant-stripped Ncs, whole body imaging of mice was carried out at several timepoints. As shown in Fig. 3b, from 3.5 h, the tumor was increasingly visible, demonstrating multimodal PAI and PET imaging of tumor.
Fig. 3.
Tumor photoacoustic imaging and PET imaging. a Photoacoustic tumor imaging of a 4T1 tumor in a mouse 24 h following intravenous injection of surfactant-stripped Nc. b PET imaging of 4T1 tumor at several timepoints following intravenous injection of the same surfactant-stripped Nc micelles, labeled with 64Cu
Used with permission from ref [41]. Copyright 2017, Royal Society of Chemistry
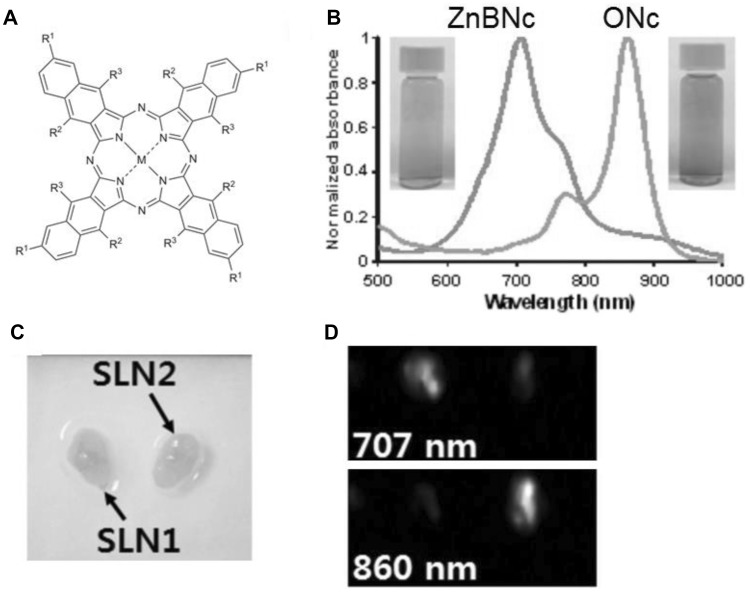
Multi-wavelength multiplexing is a capability of PAI and other optical imaging techniques that is difficult for other imaging modalities (e.g. ultrasound, PET, MRI) to achieve. PAI multiplexing holds potential for molecular imaging [26]. Substitution of various peripheral functional groups and metal chelation can lead to varied optical properties of Ncs. Another study that was amongst the first to report dual color channel photoacoustic lymph node imaging made use of two Nc variants: ONc and ZnBNc [43]. The structures are shown in Fig. 4a. This study involved the use of surfactant stripped Ncs with two distinct wavelengths, 707 nm (ZnBnc) and 860 nm (ONc), for lymph node imaging. The spectra of these Ncs is shown in Fig. 4b. Lymph node mapping can be helpful in identifying the regions for surgical procedure or in identifying the potential site of cancer spread to lymph nodes noninvasive imaging. Surfactant-stripped Ncs were injected into the forepaws of mice individually and the lymph nodes were imaged. SLNs injected with surfactant-stripped ZnBNc were clearly visible at 707 and lymph nodes injected with surfactant-stripped ONc showed strong signal when imaged at 860 nm. To confirm simultaneous dual imaging capability of the system, both the dyes were injected in separate lymphatic system and the nodes were imaged ex vivo at 707 and 860 nm (Fig. 4c, d). The images show spectrally resolved imaging of the correct type of Nc in the separate lymph nodes.
Fig. 4.
Two color PAI of sentinel lymph nodes with surfactant stripped Ncs. a Chemical structure of Ncs. 707 nm ZnBNc has: M = Zn; R1 = t−Bu; R2 = H. 860 nm ONc has: M = 2H; R1 = H; R2 = O−(CH2)3CH3. b Normalized absorbance spectra of ZnBNc (green) and ONc (orange) following surfactant-stripping. c white-light image of excised lymph nodes following injection at different sites. d PAI of lymph nodes with 707 and 860 nm light. The left lymph node was the draining site for ZnBNc micelles and the right lymph node was the draining site for ONc lymph nodes.
Used with permission from Ref. [43]. Copyright 2015, Elsevier. (Color figure online)
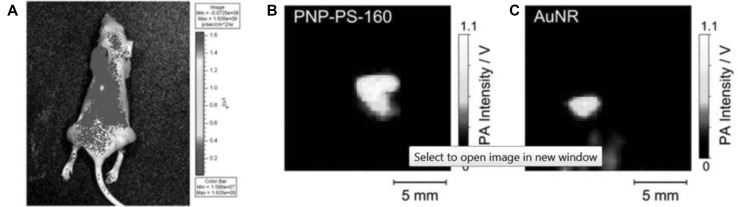
Silicon naphthalocyanines (SiNc) have also been studied for photoacoustic applications. One such study prepared a solution of SiNc by coating it with 10% Cremophor EL and 1% Propanediol [55]. An emulsion containing SiNc was injected into tumor-bearing mice for PAI. After 15 min, when the tumor was imaged, the signal in the tumor region was strong whereas the signal from blood was declining indicating the movement of SiNc to tumor. In a similar study utilizing SiNc as a PAI agent, SiNc was encapsulated in polymeric nanoparticles [20]. In vivo tests of SiNc encapsulated in polymeric nanoparticle revealed a five-fold higher PA signal in comparison with gold nanorods (Fig. 5). Therefore, modified Ncs with minimal toxicity can serve as strong PAI imaging contrast agents.
Fig. 5.
a In vivo fluorescence image of a mouse injected with polymeric nanoparticles containing SiNc. Photoacoustic image of mouse injected with b Polymeric nanoparticle encapsulated with SiNc. c Gold nanorods.
Used with permission from Ref. [20]. Copyright 2015, Royal Society of Chemistry
Structure modification
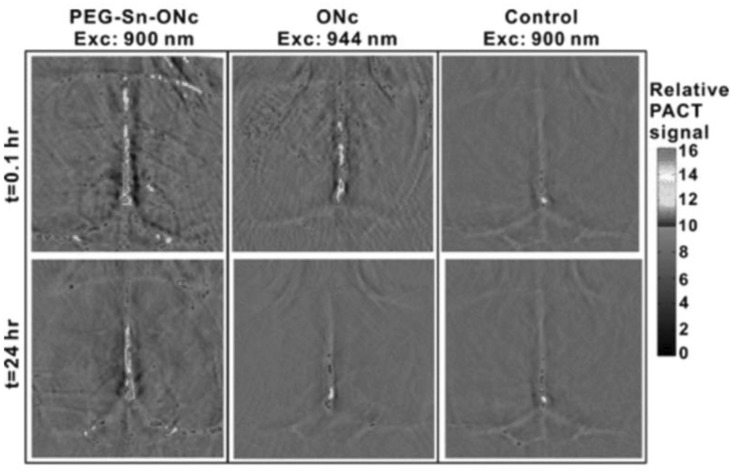
Another strategy that has been applied to increase the solubility of Ncs is to covalently modify the structure. This can confer improved water solubility to the dye, although this approach has been more commonly reported for Pc dyes [56, 57]. Metal chelation can also impact solubility. Therefore, modified Ncs have been synthesized with 70 distinct elements to the macrocycle for variety of applications [58]. One such example is chelation of tin (Sn) to ONc [44]. Sn–ONc was subsequently modified at the axial metal position with conjugation of polyethylene glycol to further enhance its solubility. ONc was reacted with SnCl2 to chelate tin to the central macrocycle of ONc. Sn–ONc displayed red-shifted spectra with a 70 nm shift (to 930 nm). However the solubility of metal-chelated ONc remained limited, implying that metal chelation had no effect on the solubility of ONc. To improve the solubility, axial PEGylation of Sn–ONc was done which improved the solubility in methanol drastically. Moreover, in vivo tests showed that PEGylated Sn–ONc circulated for longer time compared to ONc. Owing to the prolonged circulation time, PEGylated Sn–ONc was able to produce a strong PA signal even 24 h after intravenous injection (Fig. 6). Thus, PEG not only improved the solubility but also assisted in prolonged circulation for PAI.
Fig. 6.
Non-invasive photoacoustic imaging of blood vessels in the mouse brain imaged at 0.1 and 24 h following intravenous injection of axially PEGylated Sn–ONc.
Used with permission from Ref. [44]. Copyright 2016, American Chemical Society
Conclusion
PAI holds potential to play a role in diagnosis and treatment monitoring of cancer and other diseases. Given the high extinction coefficient of Ncs they can serve as useful PAI contrast agents to immediately impart high contrast for deep tissue imaging. Ncs usually need to be formulated with nanocarriers for intravenous administration. With appropriate targeting moieties, these probably could be delivered to regions of interest for molecular imaging. Many Ncs have inherent capacity for use in multimodal imaging owing to simplicity of seamless chelation with 64Cu. Ncs have been demonstrated for multi-color imaging which exhibits their versatile capabilities to serve as contrast agent. Further improvements in modification of Ncs with improved physical properties are desired. In depth toxicity characterization of Ncs is warranted. Just as PAI itself needs to firmly establish itself as a useful imaging modality, more work is needed to take advantage of the unique chemical and optical properties of Ncs to establish them as enabling contrast agents for PAI.
Acknowledgements
This work was supported by the National Institutes of Health (R01EB017270 and DP5OD017898).
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Wang LV, Hu S. Photoacoustic tomography. In vivo imaging from organelles to organs. Science. 2012;335(6075):1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang LV, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nat Methods. 2016;13:627. doi: 10.1038/nmeth.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao J, Wang LV. Photoacoustic tomography: fundamentals, advances and prospects. Contrast Media Mol Image. 2011;6(5):332–345. doi: 10.1002/cmmi.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu S, Wang LV. Photoacoustic imaging and characterization of the microvasculature. J. Biomed. Opt. 2010;15(1):011101–011101-15. doi: 10.1117/1.3281673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu M, Wang LV. Photoacoustic imaging in biomedicine. Rev Sci Instr. 2006;77(4):041101. doi: 10.1063/1.2195024. [DOI] [Google Scholar]
- 6.Agarwal A, et al. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J Appl Phys. 2007;102(6):064701. doi: 10.1063/1.2777127. [DOI] [Google Scholar]
- 7.De La Zerda A, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3(9):557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knieling F, et al. Multispectral optoacoustic tomography for assessment of crohn’s disease activity. New Engl J Med. 2017;376(13):1292–1294. doi: 10.1056/NEJMc1612455. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, et al. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2008;9(6):2212–2217. doi: 10.1021/nl801852e. [DOI] [PubMed] [Google Scholar]
- 10.Yao X, et al. pH-responsive metallo-supramolecular nanogel for synergistic chemo-photodynamic therapy. Acta Biomater. 2015;25:162–171. doi: 10.1016/j.actbio.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Yao J, et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat Methods. 2015;12:407. doi: 10.1038/nmeth.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lao Y, et al. Noninvasive photoacoustic imaging of the developing vasculature during early tumor growth. Phys Med Biol. 2008;53(15):4203. doi: 10.1088/0031-9155/53/15/013. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, et al. Photoacoustic imaging of early inflammatory response using gold nanorods. Appl Phys Lett. 2007;90(22):223901. doi: 10.1063/1.2743752. [DOI] [Google Scholar]
- 14.Lashof JC, et al. Mammography and beyond: developing technologies for the early detection of breast cancer. Washington, DC: Institute of Medicine; 2000. [PubMed] [Google Scholar]
- 15.Piras D, et al. Photoacoustic imaging of the breast using the twente photoacoustic mammoscope: present status and future perspectives. IEEE J Sel Top Quantum Electron. 2010;16(4):730–739. doi: 10.1109/JSTQE.2009.2034870. [DOI] [Google Scholar]
- 16.Hoelen C, et al. Three-dimensional photoacoustic imaging of blood vessels in tissue. Opt Lett. 1998;23(8):648–650. doi: 10.1364/OL.23.000648. [DOI] [PubMed] [Google Scholar]
- 17.Kim C, et al. Deeply penetrating in vivo photoacoustic imaging using a clinical ultrasound array system. Biomed Opt Express. 2010;1(1):278–284. doi: 10.1364/BOE.1.000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, et al. A phosphorus phthalocyanine formulation with intense absorbance at 1000 nm for deep optical imaging. Theranostics. 2016;6(5):688. doi: 10.7150/thno.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang EZ, et al. Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging. Biomed Opt Express. 2011;2(8):2202–2215. doi: 10.1364/BOE.2.002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki H, et al. Near-infrared absorbing polymer nano-particle as a sensitive contrast agent for photo-acoustic imaging. Nanoscale. 2015;7(1):337–343. doi: 10.1039/C4NR04724A. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, et al. In vivo integrated photoacoustic and confocal microscopy of hemoglobin oxygen saturation and oxygen partial pressure. Opt Lett. 2011;36(7):1029–1031. doi: 10.1364/OL.36.001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon M, Kim C. Multimodal photoacoustic tomography. IEEE Trans Multimed. 2013;15(5):975–982. doi: 10.1109/TMM.2013.2244203. [DOI] [Google Scholar]
- 23.Kim C, Favazza C, Wang LV. In Vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths. Chem Rev. 2010;110(5):2756–2782. doi: 10.1021/cr900266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, Lovell JF. Advanced functional nanomaterials for theranostics. Adv Func Mater. 2017;27(2):1603524. doi: 10.1002/adfm.201603524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaster JE, Jokerst JV. What is new in nanoparticle-based photoacoustic imaging? WIRES Nanomed Nanobiotechnol. 2017;9(1):e1404. doi: 10.1002/wnan.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallidi S, et al. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009;9(8):2825–2831. doi: 10.1021/nl802929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y-S, et al. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt Express. 2010;18(9):8867–8878. doi: 10.1364/OE.18.008867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng Z, et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials. 2013;34(21):5236–5243. doi: 10.1016/j.biomaterials.2013.03.090. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, et al. Core-shell Pd@ Au nanoplates as theranostic agents for in-vivo photoacoustic imaging, CT imaging, and photothermal therapy. Adv Mater. 2014;26(48):8210–8216. doi: 10.1002/adma.201404013. [DOI] [PubMed] [Google Scholar]
- 30.Kim C, et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano. 2010;4(8):4559–4564. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovell JF, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;10:324. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, et al. Albumin-NIR dye self-assembled nanoparticles for photoacoustic pH imaging and pH-responsive photothermal therapy effective for large tumors. Biomaterials. 2016;98:23–30. doi: 10.1016/j.biomaterials.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, et al. Broadband absorbing semiconducting polymer nanoparticles for photoacoustic imaging in second near-infrared window. Nano Lett. 2017;17(8):4964–4969. doi: 10.1021/acs.nanolett.7b02106. [DOI] [PubMed] [Google Scholar]
- 34.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 35.Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41(12):1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, et al. Emerging applications of porphyrins in photomedicine. Front Phys. 2015;3:23. doi: 10.3389/fphy.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Lovell JF. Porphyrins as theranostic agents from prehistoric to modern times. Theranostics. 2012;2(9):905–915. doi: 10.7150/thno.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali H, van Lier JE. Metal complexes as photo- and radiosensitizers. Chem Rev. 1999;99(9):2379–2450. doi: 10.1021/cr980439y. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Lovell JF. Recent applications of phthalocyanines and naphthalocyanines for imaging and therapy. WIRES Nanomed. Nanobiotechnol, 2017;9(1):e1420. [DOI] [PMC free article] [PubMed]
- 40.Song L, et al. Naphthalocyanine-reconstituted LDL nanoparticles for in vivo cancer imaging and treatment. Int J Nanomed. 2007;2(4):767. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, et al. Surfactant-stripped naphthalocyanines for multimodal tumor theranostics with upconversion guidance cream. Nanoscale. 2017;9(10):3391–3398. doi: 10.1039/C6NR09321C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol. 2014;9(8):631–638. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C, et al. Dual-color photoacoustic lymph node imaging using nanoformulated naphthalocyanines. Biomaterials. 2015;73:142–148. doi: 10.1016/j.biomaterials.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 44.Huang H, et al. Axial PEGylation of tin octabutoxy naphthalocyanine extends blood circulation for photoacoustic vascular imaging. Bioconj Chem. 2016;27(7):1574–1578. doi: 10.1021/acs.bioconjchem.6b00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory P. Industrial applications of phthalocyanines. J Porphyr Phthalocyanines 2000;4(4):432–7.
- 46.Duffy MJ, et al. Towards optimized naphthalocyanines as sonochromes for photoacoustic imaging in vivo. Photoacoustics. 2018 doi: 10.1016/j.pacs.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao S, Rajendiran V, Lovell JF. Metalloporphyrin nanoparticles: coordinating diverse theranostic functions. Coord Chem Rev. 2017 doi: 10.1016/j.ccr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busetti A, et al. Photothermal sensitization of amelanotic melanoma cells by Ni (II)-octabutoxy-naphthalocyanine. J Photochem Photobiol B. 1999;53(1):103–109. doi: 10.1016/S1011-1344(99)00132-3. [DOI] [PubMed] [Google Scholar]
- 49.Luo D, Carter KA, Lovell JF. Nanomedical engineering: shaping future nanomedicines. WIRES Nanomed. Nanobiotechnol. 2015;7(2):169–188. doi: 10.1002/wnan.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2012;64:302–315. doi: 10.1016/j.addr.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, et al. Therapeutic surfactant-stripped frozen micelles. Nat Commun. 2016;7:11649. doi: 10.1038/ncomms11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, et al. programmable real-time clinical photoacoustic and ultrasound imaging system. Sci Rep. 2016;6:35137. doi: 10.1038/srep35137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, et al. Surfactant-stripped frozen pheophytin micelles for multimodal gut imaging. Adv Mater. 2016;28(38):8524–8530. doi: 10.1002/adma.201602373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rieffel J, Chitgupi U, Lovell JF. Recent advances in higher-order, multimodal, biomedical imaging agents. Small. 2015;11(35):4445–4461. doi: 10.1002/smll.201500735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bézière N, Ntziachristos V. Optoacoustic Imaging of Naphthalocyanine: potential for contrast enhancement and therapy monitoring. J Nucl Med. 2015;56(2):323–328. doi: 10.2967/jnumed.114.147157. [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro AO, et al. [1,2,3,4-Tetrakis(α/β-d-galactopyranos-6-yl)phthalocyaninato]zinc(II): a water-soluble phthalocyanine. Tetrahedron Lett. 2006;47(52):9177–9180. doi: 10.1016/j.tetlet.2006.10.155. [DOI] [Google Scholar]
- 57.De Filippis MP, et al. Synthesis of a new water-soluble octa-cationic phthalocyanine derivative for PDT. Tetrahedron Lett. 2000;41(47):9143–9147. doi: 10.1016/S0040-4039(00)01638-5. [DOI] [Google Scholar]
- 58.Chen Y, et al. Axially modified gallium phthalocyanines and naphthalocyanines for optical limiting. Chem Soc Rev. 2005;34(6):517–529. doi: 10.1039/b416368k. [DOI] [PubMed] [Google Scholar]