Abstract
Premature birth is a leading cause of infant mortality which is often attributed to irregular breathing and apnea of prematurity. A common treatment for apnea is caffeine to stimulate the brain’s respiratory center. However, caffeine’s long term effect on infant development is not fully comprehended. We hypothesized that noninvasive localized body stimulation regularizes breathing pattern. We investigated the impact of electrical or mechanical stimulation on breathing in mice. After the mice were ventilated for 28 s to induce apnea, mice were taken off the ventilator while receiving mechanical, electrical, or no stimulation in a randomized order. Both stimuli targeted the diaphragm area through a custom-built belt with vibrating motors or adhesive electrodes. After each apnea cycle, the time to take the first breath (T) was recorded. The electrical stimulation given at 4.5, 8.3, 16.7 V (pulse rate = 3 Hz, pulse width = 120 μs) showed no reduction in T. Electrical stimulation at pulse rates of 10 or 20 Hz (16.7 V, pulse width 260 μs) showed a detrimental effect increasing T by ~ 7% compared to control values (p = 0.005, p = 0.038 respectively). High and medium intensity mechanical stimulations significantly reduced T by 11.74 (p < 10−13) and by 17.08% (p < 10−8), respectively. Further reducing the amplitude of vibrations did not affect T. When the probe was attached to the ankles, only the high intensity vibrations resulted in a decrease in T (p < 10−13). Mechanical vibrations, applied at various intensities and locations, could be used to treat irregular breathing and apnea in infants.
Keywords: Apnea of prematurity, Breathing regulation, Noninvasive, Mechanical vibrations, Electrical stimulation
Introduction
Apnea of prematurity (AOP) affects more than half of all infants born at a gestational age of 37 weeks or less [1]. During AOP, infants stop breathing with occasional inter-breath intervals (IBIs) as long as 70 s without proper gas exchange [2]. Such a long period is mainly attributed to the underdeveloped brain respiratory center and weak chemoreceptor function which may cause bradycardia or hypoxia [1, 3]. Currently, the preferred treatment for AOP is caffeine, which is used as a respiratory stimulant [1, 3, 4]. In addition to treating AOP, it has been shown that caffeine reduces bronchopulmonary dysplasia, failure to extubate, and the time on mechanical ventilators [5, 6]. However, caffeine was also shown to impair cerebral and intestinal blood flow velocity [7], and if it is administered improperly, such as overdosing, it might have detrimental effects on preterm infants [8, 9]. Furthermore, Carnielli et al. [10] showed that caffeine treatment increases energy expenditure and suggested that it might retard infant growth.
While the majority of the short-term treatment effects have mainly favored the reduction of symptoms and survival of preterm infants, the long term effects of caffeine are still open for speculations and require additional studies. Several long term studies up to early childhood revealed no side effects of caffeine. Some of these studies were concerned with sleep duration and sleep apnea (5–12 years) [11]. Other studies considered death, cerebral palsy, and cognitive delay (18–21 months) [12]. Schmidt et al. [13] found, that despite the short term benefits of caffeine, a 5-year follow-up did not show any improvement in survival rate without disability between very low birth weight infants treated with caffeine and those with placebo. Thus, further investigations are required to clarify caffeine’s long term effects on neurodevelopmental outcomes as well as optimal dosage, and time for treatment initiation and discontinuation [14, 15].
The remaining controversy of the invasive caffeine treatment warrants the development of noninvasive methods and without chemical stimulants. Mechanical stimulation has already been extensively explored and proven successful in the clinic by many [16–22]. These mechanical vibrators employed different settings (frequency and amplitude) and stimulated different body regions (e.g., feet, thorax, and whole body). Based on these results, we aimed to further study and compare various stimulus types (mechanical vs. electrical), settings, and locations. Specifically, we hypothesized that (1) mechanical or electrical vibrations applied at different locations on the body surface and with different settings can be used to stimulate the respiratory center of the brain in order to reduce the length of apnea and (2) that reduction is dependent on the properties of the stimulation.
Materials and methods
Stimulation devices
For electrical stimulations, two self-adhesive replacement gel pads with electronic pulse stimulators (ChoiceMMed America Co., PA) were cut into smaller pieces (1–2 mm in dimensions), connected to the TENS Unit 3000 (Roscoe Medical, Compass Health Brand, OH) and placed over the abdomen (Fig. 1). For mechanical stimulations, a custom-designed vibrating belt was placed over the gel pads and abdomen as well (Fig. 1). Two miniature vibrating belts were separately also applied to the ankles. The belts were made with Velcro so that they could be strapped around the abdomen or the ankle and contained small rotating motors at 13,000 rpm (no-load condition) which resulted in vibrations of constant intensity (RadioShack, 3V DC Micro-Vibration Motor, Catalog #: 2730107). The single belt applied at the abdomen had two vibrating motors, while the two belts at the ankle each had one motor. The complete protocol included eight different stimulation settings. The three mechanical stimulations had different intensities (high, medium, and low) achieved by connecting the motors to varying resistive loads (51, 220, and 470 Ω, respectively). The loads were selected based on commercially available resistors which produced small to large tolerable sensations and vibrations from the bracelet on the investigators’ palm. These stimulations were applied to the abdomen or the ankles. The four square-wave electrical stimulations, applied to the abdomen, were conducted at different voltages (4.5, 8.3, 16.7 V), pulse rates (3, 10, 20 Hz), and pulse widths (120, 260 μs) using the TENS unit. Similar to mechanical sensations, many combinations of the three electrical settings were tested, and based on their resulting sensation on the investigators’ palm, those combinations were selected that contributed to a range of tolerable small to large intensity vibrations. The strongest sensation was the one with the highest voltage, frequency, and pulse widths.
Fig. 1.
Cartoon of a mouse with a vibrating bracelet around abdomen and ankle (dark gray)
Mouse ventilation
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Boston University. Mice (n = 8, C57BL/6, body weight: 26.6 ± 2.7 g, Charles River) were weighed and 10% Nembutal® (Pentobarbital) was administered intraperitoneally (80 mg/kg). The experiments lasted for several hours, and to maintain a steady anesthesia, additional dosages during the experiment were administered (10 mg/kg). Following the initial dose, tracheostomy was performed and the mice were put on a rodent mechanical ventilator (flexiVent, Scireq Inc., Montreal). The ventilator was set to deliver a tidal volume of 8 ml/kg at a rate between 180 and 240 breaths per minute superimposed on a positive end-expiratory pressure (PEEP) of 3 cmH2O. In order to recruit the lung and acclimate the mouse to the ventilation, mice were given a short stabilization period starting with a deep inhalation (3 s pressure ramp from 3 to 30 cmH2O airway pressure followed by a 3 s pressure hold at 30 cmH2O), followed by a 1 min default ventilation, and ending with another deep inhalation.
After stabilization, mice were given a ventilation cycle (Fig. 2a) which included the default ventilation pattern followed by a deep inhalation. Apnea was stimulated by slight over-ventilation due to the deep inhalation. Consequently, the Hering–Breuer inflation reflex was triggered and mice tended to delay regaining spontaneous breathing. Prior to each experiment, a subset of the total stimulations (Table 1) were chosen and were written on individual pieces of paper. An extra piece of paper for no stimulation (control) was also added. These paper slips were folded and placed in a box. Prior to the end of every ventilation cycle, a paper slip was randomly selected to decide whether a stimulation with a given set of properties followed the cycle or no stimulation (control) was given. The selected stimulation type was then excluded from the remaining choices. Once all paper slips were withdrawn from the box, they were returned and shuffled, and the process was repeated guaranteeing the same number of repetitions of every stimulation settings within approximately the same time period. Spontaneous breathing was monitored through a voltage signal measured by a pressure transducer (World Precision Instruments (WPI), 07B PNEU05) attached to a side tap of the tracheotomy tube. The voltage signal from the transducer was amplified (WPI, Model TBM4-F), digitized (WPI, DataTrax), and displayed on a computer (WPI, Quad 16-EFA-400). After allowing several spontaneous breaths, the cycle was repeated. Once the experiment was complete, the voltage time series was converted to gauge pressure.
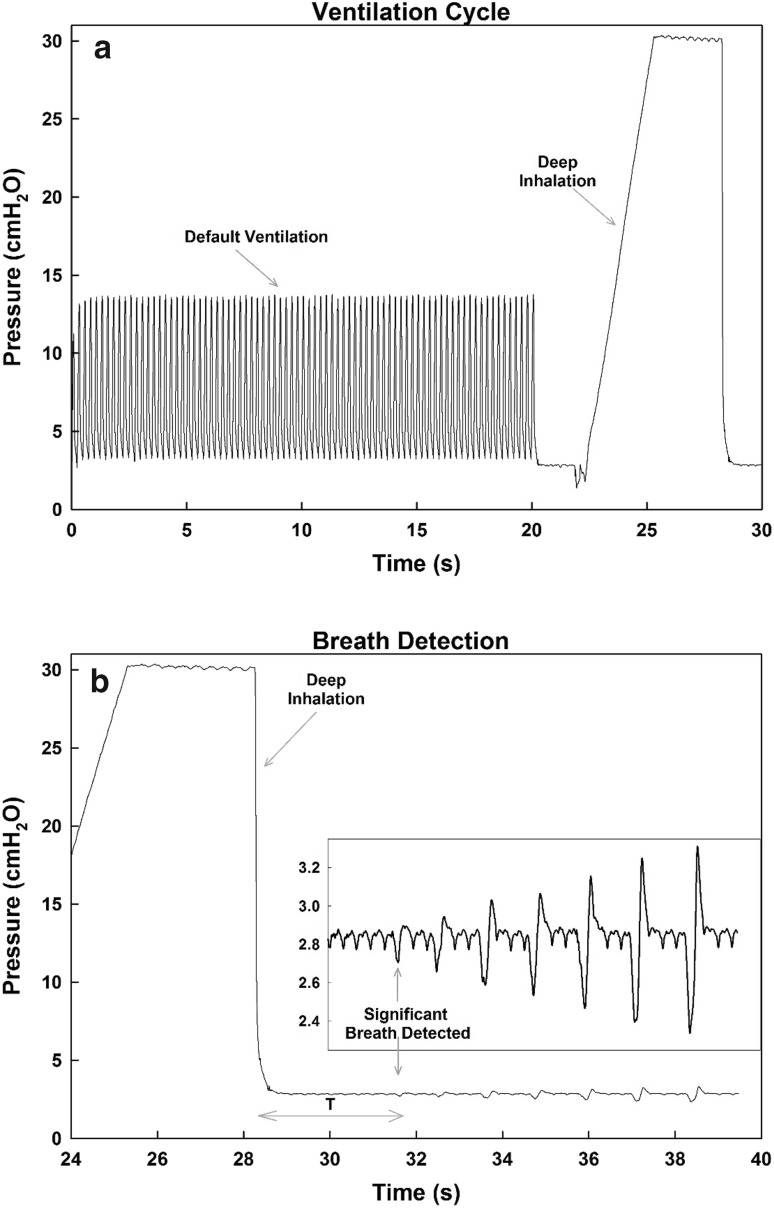
Fig. 2.
Ventilation cycle and breath detection. a A ventilation cycle consists of a 20-s default period of controlled ventilation (8 ml/kg) followed by a 6-s deep inhalation before stopping ventilation. b The time to take a breath T is determined by the time interval between the end of the deep inhalation and the first breath that has an apex exceeding the threshold defined as 0.128 cmH2O + mean + standard deviation of the values immediately following the end of the ventilation cycle without breathing
Table 1.
Results from all stimulation conditions
| Stimulation | Specifications | Stimulation site | p value | No. of mice | No. of paired trials | % Change in T |
|---|---|---|---|---|---|---|
| Mechanical | High intensity | Abdomen | < 10−13 | 6* | 216 | − 11.74 |
| Mechanical | Medium intensity | Abdomen | < 10−8 | 4** | 84 | − 17.08 |
| Mechanical | Low intensity | Abdomen | NS | 1 | 11 | − 11.11 |
| Electrical | V = 16.7, F = 3, PW = 120 | Abdomen | NS | 1 | 51 | − 2.39 |
| Electrical | V = 8.3, F = 3, PW = 120 | Abdomen | NS | 1 | 20 | − 8.22 |
| Electrical | V = 4.5, F = 3, PW = 120 | Abdomen | NS | 1 | 20 | 0.75 |
| Electrical | V = 16.7, F = 10, PW = 260 | Abdomen | 0.0049 | 1 | 40 | 7.78 |
| Electrical | V = 16.7, F = 20, PW = 260 | Abdomen | 0.0378 | 1 | 40 | 6.69 |
| Mechanical | High intensity | Ankle | < 10−13 | 4** | 151 | − 29.44 |
| Mechanical | Medium intensity | Ankle | NS | 2 | 86 | − 4.54 |
| Mechanical | Low intensity | Ankle | NS | 1 | 56 | − 2.6 |
Sign rank test results (p values) indicate whether the change in T is significant compared to no stimulation (control). A negative % change in T indicates shorter apnea length whereas a positive % change in T indicates a longer and potentially detrimental apnea length
V voltage (V), F frequency (Hz), PW pulse width (μs), NS not significant
*Significant for all individuals
**3/4 mice showed individual significance
Data analysis
Using the collected data, the time (T) for the mouse to start spontaneous breathing on its own after every ventilation cycle was measured (Fig. 2b). In order to be considered a valid breath from which T was measured, the peak pressure during a breathing attempt had to exceed a pressure threshold. To determine the threshold, the mean and standard deviation of the pressure measurements directly following the ventilation cycle (when the ventilator was off and the mouse was not breathing) were calculated. Then the threshold was set to be the sum of the mean, standard deviation and a constant (0.128 cmH2O). Sign rank tests were used to assess the effects of different stimulation settings on T (Table 1). For the significant scenarios, further statistical tests were carried out between the different groups (control vs. stimulation) and within each group separately. However, T was first normalized (Tn) with the median of the control case for each mouse. This was done in order to reduce the effects of inter-individual mouse breathing patterns as well as the effect of anesthesia across the long experimental hours because occasionally mice showed no spontaneous breathing. The Brown–Forsythe test was used to test for equal variances and the Kolmogorov–Smirnov test was used to compare probability distribution functions (pdf). Data analysis was performed using SigmaPlot (Systat Software Inc., CA) and MATLAB R2015a (MathWorks, CA). Statistical significance was accepted at the level of 0.05. Figures were generated using SigmaPlot.
Results
Table 1 summarizes the comparison of the eight different stimulation experiments to the no stimulation (control) case. High and medium intensity mechanical stimulations at the abdomen produced mean T values that were 11.7 (p < 10−13) and 17.1% (p < 10−8) lower than control, respectively. However, low intensity mechanical stimulation had no impact on T. When the stimulation was applied to the ankles, only the high intensity mechanical stimulation reduced T significantly by 29% (p < 10−13). On the other hand, in one mouse, repeated electrical stimulation using a voltage of 16.7 V at two frequencies of 10 and 20 Hz significantly increased mean T by 7.8 (p = 0.005) and 6.7% (p = 0.038), respectively. No other electrical stimulations yielded any significant result. Due to the detrimental effect of the electrical stimulations, no further trials were tested on other mice.
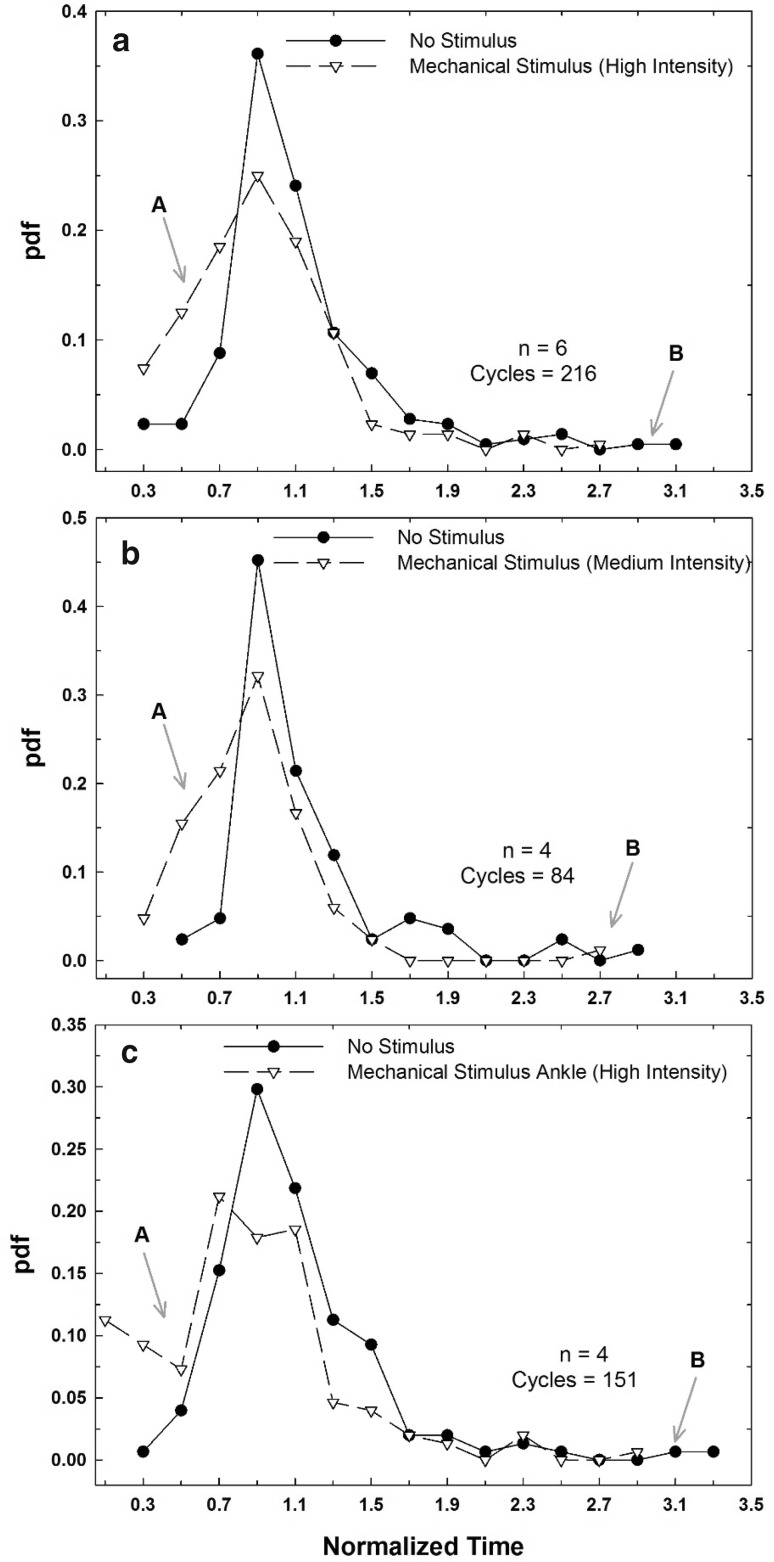
Table 2 summarizes the normalized Tn values for three stimulation conditions which gave statistically significantly different results than the controls. Table 3 summarizes the results of the Brown–Forsythe and the Kolmogorov–Smirnov tests between controls and stimulations. Only the high intensity ankle stimulation (Tn = 0.809 ± 0.486) had a minor but statistically significant increase (p = 0.021) in variance compared to its control (1 ± 0.436). Furthermore, the variance for the ankle was significantly higher than for the abdomen with the medium intensity (p = 0.005) and high intensity (p = 0.027) stimulations (not shown in Table 3). However, there was no difference between any control sets. Figure 3 plots the probability density functions for these mechanical stimulations. As indicated in the figure by arrows “A” and “B”, the distributions of T for each stimulation are shifted to the left and had a shorter tail. According to the Kolmogorov–Smirnov test, all three stimulations were highly significantly different from control (p: 7.58 × 10−8, 2.68 × 10−6 and 2.66 × 10−8 for the high intensity abdomen, medium intensity abdomen, and high intensity ankle, respectively). Furthermore, the pdf of the high intensity ankle stimulation was also different from that of the high intensity abdomen (p = 0.027).
Table 2.
Tn values for the significant stimulations
| Stimulation type | Tn (no stimulation) | Tn (stimulation) |
|---|---|---|
| High intensity abdomen | 1 (0.414) | 0.896 (0.391) |
| Medium intensity abdomen | 1 (0.410) | 0.846 (0.348) |
| High intensity ankle | 1 (0.436) | 0.809 (0.486) |
T was normalized with the value of the control before statistical analysis
Tn data reported as median (standard deviation)
Table 3.
Summary of the statistics carried out on Tn for the stimulations that were significantly different from control in Table 1
| Stimulation type | p value variance test | p value pdf test |
|---|---|---|
| High intensity abdomen | 0.516 | 7.58 × 10−8 |
| Medium intensity abdomen | 0.843 | 2.69 × 10−6 |
| High intensity ankle | 0.021 | 2.96 × 10−7 |
Using Brown–Forsythe test, the variance of the high intensity ankle stimulation was significantly higher than control. Using Kolmogorov–Smirnov tests, all stimulations were different from their respective controls. There was no difference in any of these tests for pairwise control comparisons (p values not shown)
Fig. 3.
Probability density distribution functions for the median normalized T values (Tn) for the three stimulation types in Table 2. a High intensity abdomen mechanical stimulation and control (n = 6, 216 ventilation cycles per group). b Medium intensity abdomen mechanical stimulation and control (n = 4, 84 ventilation cycles per group). c High intensity ankle mechanical stimulation and control (n = 4, 151 ventilation cycles per group). For all stimulations, it was observed that the distribution of Tn for the mechanical stimulus had a higher density on the left of the graph representing more frequent lower Tn values (see arrow with “A”). The graph of the control had a longer tail to the right (see arrow with “B”), showing a wider distribution and the presence of larger Tn values
Discussion
Our study has several implications. First, we confirmed that noninvasive mechanical stimulation can shorten T in individual mice as well as in the population. In contrast, electrical stimulation has no effects or may have a negative impact on T (Table 1). Second, the results suggest that the effect of the stimulus is intensity as well as location dependent (Tables 1, 2). Furthermore, there could be an interaction between these two factors since high intensity at the ankle was significantly different from high intensity at the abdomen with regard to median and variance (smaller median but larger variance), but it was not different from the medium intensity stimulation at the abdomen. Finally, since the high intensity stimulation at the ankle and the medium intensity stimulation at the abdomen were both significantly different compared to control in the population but not in all individuals (compared to high intensity at the abdomen), the threshold above which improvements are seen could be subject specific. The fact that there was no difference in controls across stimulation types in all the statistical tests further validates that the observed reductions in Tn are due to the stimulations as well as indicates that the normalization procedure removed the effects of the anesthesia, subject variation, and long experimental time. The general observation was that the pdf of Tn (Fig. 3) showed a leftward and upward shift due to stimulation. While the exact threshold of an apnea is not unilaterally agreed upon, it is assumed to range from several to tens of seconds [16, 23–25]. Regardless of the exact threshold, the simple shift of the pdf supports the need for further investigating and optimizing the impact of small amplitude mechanical vibrations on T applied at various positions for the treatment of AOP.
In order to consider possible implications of mechanical stimulation on preterm infants, we can apply the observed effect of mechanical stimulation on T to the inter-breath interval (IBI) distributions reported by Frey et al. [2]. In their study, they showed that the distribution p of IBIs exhibits a long tail that can be modeled as a power law: where α is the exponent of the distribution and is related to breathing stability. In a longitudinal data set, α increased from 2.62 ± 0.4 at post-natal age of 9.3 weeks to 3.22 ± 0.4 at a post-natal age of 12.6 weeks (p < 0.002) [2]. If we assume that mechanical stimulation reduces IBI in babies in a similar manner as it reduces T in mice by a factor , then we can write IBIs = gIBIc, where subscripts c and s represent control and stimulation respectively. It is easy to show that the distribution of IBIs will also be a power law: . Substituting IBIc = IBIs/g into , we obtain . The risk that an IBI exceeds a given value X is the area under the distribution from X to infinity. The ratio of the risk for control to the risk for stimulated breathing is then simply . Our data suggest that for the 11.7 (lowest) and 29% (highest) reduction in T, the values of g are 0.883 and 0.71, respectively. Taking α = 2.6 for premature infants, the corresponding estimated reductions in risk due to stimulation are ~ 0.82 and 0.58, respectively. These numbers in turn suggest 18–42% lower risks for long apneas in the presence of mechanical stimulations. Despite the several assumptions in this analysis, these predictions warrant experimental testing.
The primary result of this study is also consistent with several previous studies. A 2003 study done by Pichardo et al. [17] found that stimulation given as vibrations at the thorax of infants is an effective noninvasive treatment for AOP. In their clinical trial, the stimulation was a 3 s, 10 V, 250 Hz square wave pulse administered manually by a nurse when AOP was observed. In 2009, Bloch-Salisbury et al. [16] designed a vibrating mattress capable of delivering small stochastics displacements. These displacements helped to stabilize breathing by reducing IBIs, durations of oxygen desaturation, and apnea. Their stimulation was a band-limited white noise between 30 and 60 Hz arguing that subthreshold mechanical noise is able to stabilize a nonlinear system. Stimulation was triggered on and off sequentially for 10 min intervals. Though their study measured different parameters, they reported a 50% reduction in IBI (< 5 s) whereas in our study we found a 17.1–29% reduction in T with a predicted 18–42% reduction in risk. However, unlike our study, they also observed a 40% reduction in the variance of IBI due to the stimulation. The lower reduction in IBI in our study could be due to the fact that our mice were mature and the nervous system was naturally more responsive. Additionally, the lack of a reduction in variance might be explained by the differences in the signal type (monotonic vs. stochastic) and the location of the applied vibrations (direct exposure of abdomen or ankle vs. whole body vibration) Nevertheless, our study suggests that a simple monotonic input and local stimulation (abdomen or ankle) can lead to improvement in AOP and potentially regularize breathing.
It is also noteworthy that the mechanism which resulted in a reduction in IBI, subthreshold stochastic resonance [16], must be different from the mechanism of the reduced T in the current study because our vibrations contained monotonous excitation and likely above the tactile threshold. While the actual mechanism is not known, it was proposed that tactile sensory stimulation most likely generates an excitatory but nonspecific neuronal activity in the brainstem respiratory center and hence stimulate respiratory activity [26]. This is a very attractive scenario because the stimulation of the excitatory glutamatergic system could counterbalance the enhanced sensitivity of the inhibitory neurotransmitters (e.g., gamma-aminobutyric acid, adenosine, or serotonin), which is another feature of the premature infant’s respiratory control system [4]. Additionally, we speculate that the stimulation of somatosensory afferents by vibrotactile stimuli could promote the stability of breathing in neonates by specific mechanosensitive responses. Indeed Lin et al. [27] found that sensory nerve terminals respond to mechanical stimuli that is related to cell architecture, particularly to the microtubule and actin microfilament network organization.
Our study is not without limitations. The most important limitation is that the mice in our study were adult with fully developed nervous system. Thus, compared to preterm mice, the respiratory system of adult mice might be much more responsive to stimulation which could explain the positive local effects using a monotonous signal. Nevertheless, the Hartford 2003 study [17] applied a single frequency square wave signal and still observed an effect of stimulation. Further trials should be performed on preterm animals or preterm infants to determine the effect the stimulus has on the underdeveloped nervous system. The findings of this study and the idea of a small locally vibrating device may also find applications in relieving sleep apnea or snoring in the elderly. Indeed, it has been shown that vibrating insoles helped regularize walking of elderly humans through subthreshold stimulation of the nervous system [28]. Thus, it is possible that a vibrating device could have similar effects on the brain respiratory center.
Acknowledgements
The authors thank Rana Akleh for the mouse cartoon in Fig. 1
Conflict of interest
All authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Contributor Information
Samer Bou Jawde, Phone: 617 961 2710, Email: sbujawde@bu.edu.
Béla Suki, Email: bsuki@bu.edu.
References
- 1.Zhao J, Gonzalez F, Mu D. Apnea of prematurity: from cause to treatment. Eur J Pediatr. 2011;170:1097–1105. doi: 10.1007/s00431-011-1409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey U, Silverman M, Barabási AL, Suki B. Irregularities and power law distributions in the breathing pattern in preterm and term infants. J Appl Physiol (Bethesda, MD) 1985;1998(85):789–797. doi: 10.1152/jappl.1998.85.3.789. [DOI] [PubMed] [Google Scholar]
- 3.Eichenwald EC. Newborn C on FA. Apnea of prematurity. Pediatrics. 2015 [Google Scholar]
- 4.Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5(Suppl A):S377–S382. doi: 10.1016/S1526-0542(04)90067-X. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 6.Steer P, Flenady V, Shearman A, Charles B, Gray PH, Henderson-Smart D, et al. High dose caffeine citrate for extubation of preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2004;89:F499–F503. doi: 10.1136/adc.2002.023432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoecker C, Nelle M, Poeschl J, Beedgen B, Linderkamp O. Caffeine impairs cerebral and intestinal blood flow velocity in preterm infants. Pediatrics. 2002;109:784–787. doi: 10.1542/peds.109.5.784. [DOI] [PubMed] [Google Scholar]
- 8.Ergenekon E, Dalgiç N, Aksoy E, Koç E, Atalay Y. Caffeine intoxication in a premature neonate. Paediatr Anaesth. 2001;11:737–739. doi: 10.1046/j.1460-9592.2001.00753.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson BJ, Gunn TR, Holford NH, Johnson R. Caffeine overdose in a premature infant: clinical course and pharmacokinetics. Anaesth Intensive Care. 1999;27:307–311. doi: 10.1177/0310057X9902700316. [DOI] [PubMed] [Google Scholar]
- 10.Carnielli VP, Verlato G, Benini F, Rossi K, Cavedagni M, Filippone M, et al. Metabolic and respiratory effects of theophylline in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2000;83:F39–F43. doi: 10.1136/fn.83.1.F39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus CL, Meltzer LJ, Roberts RS, Traylor J, Dix J, D’ilario J, et al. Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am J Respir Crit Care Med. 2014;190:791–799. doi: 10.1164/rccm.201406-1092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 14.Nobile S, Carnielli VP. Caffeine for preterm infants: current indications and uncertainties. Acta Bio-Medica Atenei Parm. 2015;86(Suppl 1):32–35. [PubMed] [Google Scholar]
- 15.Poets CF, Khan SR. Former preterm infants, caffeine was good for you, but now beware of snoring! Am J Respir Crit Care Med. 2014;190:720–721. doi: 10.1164/rccm.201409-1588ED. [DOI] [PubMed] [Google Scholar]
- 16.Bloch-Salisbury E, Indic P, Bednarek F, Paydarfar D. Stabilizing immature breathing patterns of preterm infants using stochastic mechanosensory stimulation. J Appl Physiol (Bethesda, MD) 1985;2009(107):1017–1027. doi: 10.1152/japplphysiol.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichardo R, Adam JS, Rosow E, Bronzino J, Eisenfeld L. Vibrotactile stimulation system to treat apnea of prematurity. Biomed Instrum Technol. 2003;37:34–40. doi: 10.2345/0899-8205(2003)37[34:VSSTTA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Smith VC, Kelty-Stephen D, Qureshi Ahmad M, Mao W, Cakert K, Osborne J, et al. Stochastic resonance effects on apnea, bradycardia, and oxygenation: a randomized controlled trial. Pediatrics. 2015;136:e1561–e1568. doi: 10.1542/peds.2015-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo VC, Honorato da Silva S, Freitas de Amorim M, Nohama P. Instrumentation for the detection and interruption of apnea episodes for premature newborn. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2014;2014:2127–2130. doi: 10.1109/EMBC.2014.6944037. [DOI] [PubMed] [Google Scholar]
- 20.Lovell JR, Eisenfeld L, Rosow E, Adam J, Bronzino JD. The design, development and application of a virtual instrument system to assess vibrotactile stimulation to interrupt neonatal apnea. Proc. 19th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 1997;3:1150–1153. [Google Scholar]
- 21.Svenningsen NW, Wittström C, Hellström-Westas L. OSCILLO-oscillating air mattress in neonatal care of very preterm babies. Technol Health Care Off J Eur Soc Eng Med. 1995;3:43–46. [PubMed] [Google Scholar]
- 22.Korner AF, Guilleminault C, den Hoed JV, Baldwin RB. Reduction of sleep apnea and bradycardia in preterm infants on oscillating water beds: a controlled polygraphic study. Pediatrics. 1978;61:528–533. [PubMed] [Google Scholar]
- 23.Williamson JR, Bliss DW, Browne DW, Indic P, Bloch-Salisbury E, Paydarfar D. Using physiological signals to predict apnea in preterm infants. 2011 Conf. Rec. Forty Fifth Asilomar Conf. Signals Syst. Comput. ASILOMAR, 2011, p. 1098–102. 10.1109/acssc.2011.6190183.
- 24.Hoppenbrouwers T, Hodgman JE, Harper RM, Hofmann E, Sterman MB, McGinty DJ. Polygraphic studies of normal infants during the first six months of life: III. Incidence of apnea and periodic breathing. Pediatrics. 1977;60:418–425. [PubMed] [Google Scholar]
- 25.Morton SU, Smith VC. Treatment options for apnoea of prematurity. Arch Dis Child Fetal Neonatal Ed. 2016;101:F352–F356. doi: 10.1136/archdischild-2015-310228. [DOI] [PubMed] [Google Scholar]
- 26.Gaugler C, Marlier L, Messer J. Sensory stimulations for the treatment of idiopathic apneas of prematurity. Arch Pediatr Organe Off Soc Francaise Pediatr. 2007;14:485–489. doi: 10.1016/j.arcped.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y-W, Cheng C-M, LeDuc PR, Chen C-C. Understanding sensory nerve mechanotransduction through localized elastomeric matrix control. PLoS ONE. 2009;4:e4293. doi: 10.1371/journal.pone.0004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. Lancet Lond Engl. 2003;362:1123–1124. doi: 10.1016/S0140-6736(03)14470-4. [DOI] [PubMed] [Google Scholar]