Abstract
Plant growth-promoting rhizobacteria (PGPR) have been reported to influence plant growth, yield, and nutrient uptake by an array of mechanisms. Uncovering the behavioral dynamics of PGPR is one of the most important issues necessary for understanding their functional performances. In this study, strain NJAU-Z9 which was found to possess complex functions and efficient rhizospheric colonization ability was selected from plenty of bacterial strains isolated randomly from the pepper rhizosphere soil and identified as Bacillus velezensis. Repeated seedling nursing tests performed absolute growth-promoting advantage for the novel isolated strain. After that, primers for the quantitative detection were designed based on its whole genome sequence (WGS), and a real-time PCR method was utilized to explore strategies for monitoring the strain in natural soil and in the pepper rhizosphere. Results showed based on the whole genome, two primers were identified as NJAU-Z9-specific quantitative PCR primers. Two seasonal pot experiments demonstrated that strain NJAU-Z9 effectively colonized the rhizosphere measured by the novel abundance detecting strategy, improved plant growth, and showed a positive correlation between bacterial number and biomass. This study offers a strategy based on a real-time PCR method for directly monitoring B. velezensis strain NJAU-Z9 in the soil and the rhizosphere and provides a reference for the quantitative study of other PGPR strains based on WGSs.
Electronic supplementary material
The online version of this article (10.1007/s00284-018-1563-4) contains supplementary material, which is available to authorized users.
Introduction
Plant growth-promoting rhizobacteria (PGPR) use one or more different mechanisms to promote plant growth and health directly or indirectly [1]. Among PGPR, Bacillus spp. possess excellent performance with many advantages, including wide distribution, easy isolation and culturing, and formation of oval endospores [2] Many representatives of this genus perform functions such as the solubilization of nutrients, nitrogen fixation, and the production of growth regulators and are related to the growth promotion efficiency observed in inoculated plants [3]. In the past dozen years, in order to have beneficial effects on a target plant, numerous studies have introduced PGPR agents in large number by seed or seed piece inoculation and soil amendment [4, 5]. Survival of these inoculated strains in a new environment, however, does play a critical role in function performance, since effective colonization is necessary for the successful stimulation of plant growth or the soil microbial ecosystem by the target strain [6]. Thus, one of the most important issues in the field is to uncover the behavioral dynamics of the inoculants in the soil.
Early studies focused on culturing strategies to monitor functional microbes [7]; however, single cell and culture conditions do not adequately replicate the natural environment [8]. Moreover, species/strain-specific selective media are seldom available; the procedure is extremely time consuming; and the results are not immediate. In fact, agricultural soils are rich in a wide variety of native microorganisms, and most species in many environments have never been described [9, 10]. Contemporarily, based on DNA analyses, a variety of molecular methods are employed to identify the abundance of functional microbes as well as different genes that could be involved in soil processes [11, 12]. Among these, real-time quantitative PCR (qPCR) is widely used not only for the detection and quantification of 16S rRNA genes [13] but also for identifying functional genes involved in rhizosphere-related processes due to its high specificity, sensitivity, and speed [14]. Although these reports showed that qPCR is a valuable technique for quantitatively monitoring populations of unlabeled bacteria in greenhouse experiments, utilization of strain primers leads to robust detection for target microbes. In addition, the application of strain primers is more difficult in field experiments where closely related indigenous bacteria may interfere with amplification and quantification.
Since a type strain CBMB205T (= KACC 13015T), recognized as separate from other members of the genus Bacillus, was isolated from rice rhizosphere soil (Korea, in 2010), novel species named Bacillus velezensis was suggested [15]. Actually, only minor differences of the genomes between this strain and B. velezensis NRRL B-41580T, B. oryzicola KACC 18228, and Bacillus amyloliquefaciens subsp. plantarum FZB42T based on comparative genomic analysis were observed, and B. methylotrophicus, B. amyloliquefaciens subsp. plantarum, and B. oryzicola were later proposed to be heterotypic synonyms of B. velezensis [15–20]. However, regardless of the taxonomy of B. velezensis, these strains were commonly isolated as plant pathogen antagonists and developed as biological control and plant growth promotion agents [21, 22]. Nevertheless, few studies have focused on the quantification of the B. velezensis group.
In this study, a promising phytostimulatory plant growth-promoting bacterium (PGPR), NJAU-Z9, isolated from pepper rhizosphere was identified as B. velezensis, and it showed a significant promoting effect on pepper growth by pre-colonization at the seedling stage. The whole genome was sequenced by Single-Molecule, Real-Time (SMRT) technology, and the unique and efficient quantitative primers were designed based on genome fragments to explore strategies for monitoring the functional species using qPCR (Fig. S1) and subsequently to verify the correlation between the number of strain NJAU-Z9 in the rhizosphere and plant growth promotion (Fig. S2). It is hoped that the results will provide technical assistance to microbial ecology researchers of the species in the future and will be a reference for the quantitative study of other strains based on whole genome sequences (WGSs).
Materials and Methods
Bacterial Isolation
Bacteria were obtained from a collection of microbes originally isolated from a pepper rhizosphere grown in China (31°43′N, 118°46′E). The preliminary screening process is described in Zhang et al. [23]. A potential carbon source utilization of the isolates was assessed by using the BIOLOG(R) GEN III MicroPlate (Biolog Inc., Hayward, CA) according to manufacturer’s instructions. Production of IAA-like auxins, ammonia production, and an in vitro antagonism assay were performed according to references [24]. One bacterial strain NJAU-Z9 was identified based on its morphological, physiological, and biochemical properties according to Bergey’s Manual of Determinative Bacteriology. The 16S rRNA-related taxa of strain NJAU-Z9 and the sequence similarity were analyzed using a global alignment algorithm, implemented at the EzTaxon-e server [25]. A phylogenetic tree of 16S rRNA gene sequences was reconstructed using the maximum-likelihood (ML) [26] algorithms in the MEGA version 7.0 software. Bootstrap values were set as 1000 replications for analysis. Average nucleotide identity (ANI) values of the closest strains were estimated using the algorithm developed by Goris et al. [27], as implemented in the server EzBioCloud available at http://www.ezbiocloud.net/tools/ani [28]. The WGS of closest type strains were downloaded from the GenBank DNA database and have been described in Table S1.
Whole Genome Sequence Analyses
The sequencing of the NJAU-Z9 genome completion map was performed at Novogene Biotechnology Co, Ltd. (Beijing, China) by Single-Molecule, Real-Time (SMRT) technology [29]. The complete genome of B. velezensis NJAU-Z9 is publicly available in the NCBI database under accession number NZ_CP022556.1. It contains a 3,847,297 bp circular chromosome with a GC content of 46.78%, a 17,243 bp circular plasmid-1 with a GC content of 39.52%, and an 8020 bp non-circular plasmid-2 with a GC content of 39.98%. In total, 55,930 reads with 195.78-fold coverage were produced, and there are 3983 predicted genes in the chromosome, including 86 tRNA-encoding genes and 27 rRNA-encoding (5S, 16S, and 23S) genes.
Primer Design, Evaluation, and Target Plasmid Construction
The coding sequence (CDS) from the NJAU-Z9 whole genome was used for primer design. All fragments of the CDS were subjected to a BLASTn search one by one against the NCBI database (GenBank release 214), and 5 fragments without any matching information were obtained. Among them, there are three segments larger than 1000 bp and two segments smaller than 200 bp. Two small fragments were discarded and three large fragments were used to design sets of primer pairs for NJAU-Z9 (positions 3017742–3018779, 3019264–3020556, and 3020642–3022177). The designed primer pairs were analyzed using Oligo 6, synthesized by Nanjing GenScript Biotechnology Co., Ltd. (China) and qualitatively detected by conventional PCR. Eight-pair primers were used as candidates, the optimal amplification conditions for each pair are shown in Table 1 (Fig. S1 describes the design process in detail), and the primer pair Bacillus was used as a positive amplification control [30]. The nine primer pairs were tested for cross amplification against DNA sequences from six other Bacillus species and our target strain as templates (Table S3). Different amplification products were generated by conventional PCR from NJAU-Z9 genomic DNA using the eight primer pairs and subsequently cloned into pMD19-T vectors (TaKaRa). The recombinant plasmids were transformed into E. coli DH5a and extracted and purified with the AxyPrep Plasmid Miniprep Kit (Axygen Inc., USA).
Table 1.
Primer characteristics and parameters evaluated by qPCR
| Genome location or reference | Code | Primer set | Sequence (5′–3′) | Fragment size (bp) | Optimum conditions | R2 | Slope | Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| Chr1:3019264:3020556 | Z9-F | AGACTTATTCGCATTTAGGC | 76 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s and 60 °C for 34 s | 0.992 | − 3.325 | 96.4 | |
| P8 | Z9-R | ATCTTCTGTGCTCTTTCGAC | ||||||
| Z9-1F | ATGCGTCTCATATGGATAGA | 215 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s, and 60 °C for 30 s followed by 72 °C for 30 s | 0.993 | − 3.429 | 95.7 | ||
| P1 | Z9-1R | ATTAGTAAAGTCAGCAACAA | ||||||
| Z9-2F | GACAAGCTAAATAACCACTA | 205 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s and 60 °C for 30 s followed by 72 °C for 30 s | 0.995 | − 3.436 | 102.8 | ||
| P2 | Z9-2R | TAAGATACCTATGATCCAAT | ||||||
| Chr1:3020642:3022177 | Z9-3F | GTGGTAGTAACGCTATTGAA | 143 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s and 60 °C for 30 s followed by 72 °C for 30 s | 0.993 | − 3.409 | 96.5 | |
| P3 | Z9-3R | CCTCTTTACCCACAACTGCA | ||||||
| Z9-4F | GTGGTAGTAACGCTATTGAA | 147 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s and 62 °C for 34 s | 0.997 | − 3.329 | 99.7 | ||
| P4 | Z9-4R | AAGTAACCTCTTTACCCACA | ||||||
| Chr1:3017742:3018779 | Z9-5F | GGCTCGTGGCACAATAGACC | 280 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s and 62 °C for 34 s | 0.996 | − 3.388 | 97.3 | |
| P5 | Z9-5R | AGCGTGTTTAGGTGCTTTGA | ||||||
| Z9-6F | AGGATACAACGATTCTTACA | 123 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s and 62 °C for 34 s | 0.994 | − 3.393 | 97.1 | ||
| P6 | Z9-6R | CACTTTGGAATCTGGTCTAT | ||||||
| Z9-7F | TATCAAAGCACCTAAACACG | 162 | 1 min incubation at 95 °C, 40 cycles consisting of 95 °C for 15 s and 60 °C for 30 s followed by 72 °C for 30 s | 0.998 | − 3.248 | 103.2 | ||
| P7 | Z9-7R | AGATTATCGGCGGTAGCAAA | ||||||
| [30] | Primer Bacillus | |||||||
DNA Extraction and Real-Time Quantitative PCR
Genomic DNA of NJAU-Z9 and soil DNA were extracted using the Bacterial DNA kit (Omega) and PowerSoil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA, USA) following the manufacturer’s instructions. The qPCR amplification and detection were performed in a 20 µl reaction volume. The cycle threshold (CT) value was automatically determined for each sample with the ABI Prism 7500 system. After amplification, the melting curve and amplification efficiency analysis were performed to confirm amplification specificity. The Ct values were determined for all assays, and initial target gene copy numbers in unknown samples were calculated from the standard curves (all gene fragments are single copy). The plate counting was according to Zhang et al. [23]. The natural soil samples’ colony counts were expressed as the number of CFU per gram (dry weight) of soil, and qPCR quantification data were converted to the equivalent number of CFU per gram (dry weight) of soil. In the pot experiment, total numbers of NJAU-Z9 were quantified by qPCR with primers P4, which was selected by the previous work of this experiment. Each sample was performed in 12 replicates, and the results were expressed as log (CFU g−1) dry soil.
Natural Soil Samples, Seeding, and Pot Experimental Layout
Natural soil samples of 10 g were amended in 24 50-ml centrifuge tubes, and half of them were sterilized at 121 °C for 20 min. The NJAU-Z9 cells prepared according to Zhang et al. [31] were inoculated in six sterile and six non-sterile tubes with approximately 107 CFU g−1 soil each and divided into two groups. One of the sterile tubes was amended with cells and detected immediately, another was detected 5 days later, and a control with no inoculation was prepared. The other group contained unsterilized soil amended with cells and detected immediately, unsterilized soil amended with cells detected 5 days later, and a control with no inoculation. All treatments were performed in triplicate (Fig. S2a described the design process in detail). Plant growth-promoting efficiency of strain NJAU-Z9 was evaluated by seedlings nursing and pot experiments in a greenhouse, and the test pepper variety is sweet pepper (Capsicum annuum L.). The seedlings nursing experiment contained one treatment and a control using ordinary nursery substrate amended with or without strain NJAU-Z9 (5% v/w dry weight). Treatment and control were designed with three replicates. Each replicate included eight seedlings. Pepper seedlings were cultivated in the substrates for 40 days, and the agronomic characteristics and biomasses were measured. After that, pot experiments were further performed to validate the plant growth promotion effects of the seedlings cultured from nursery substrate with strain NJAU-Z9. Pepper seedlings were transferred into pots with 5 kg soil and supplemented with 1.5% (w/w dry weight) chicken manure compost. The seedlings of the treatment and control groups with 25 replications were prepared by the nursery substrate produced by strain NJAU-Z9 (OFBS) and ordinary substrates (OF), respectively. All pots were placed within a randomized block design. At the end of the experiment, the agronomic characteristics and biomasses of the plants were measured. Pot experiments was repeated twice. The qPCR assay was performed to detect the number of target strain in pepper. The first pot experiment was conducted to verify the ability of NJAU-Z9 to stabilize in rhizosphere throughout the growth period, and in the second pot experiment, the amount was monitored at the mature period (Fig. S2b described the design process in detail).
Data Analysis
Statistical analysis was performed by using the IBM SPSS 18.0 software program (IBM Corporation, New York, USA). All statistical tests performed in this study were considered significant at P < 0.05. The data were subject to the Student t test (to compare means of two treatments) and to analysis of variance (to compare many treatments) with means compared by the Tukey test. Colony counts were expressed as the number of CFU per gram (dry weight), and qPCR quantification data were converted to the equivalent number of CFU per gram (dry weight). Pearson correlation coefficients between the qPCR quantification data of NJAU-Z9 and plant growth index were calculated in R software (Version 3.3.3).
Results
Isolation and Identification of Strain PGPR NJAU-Z9
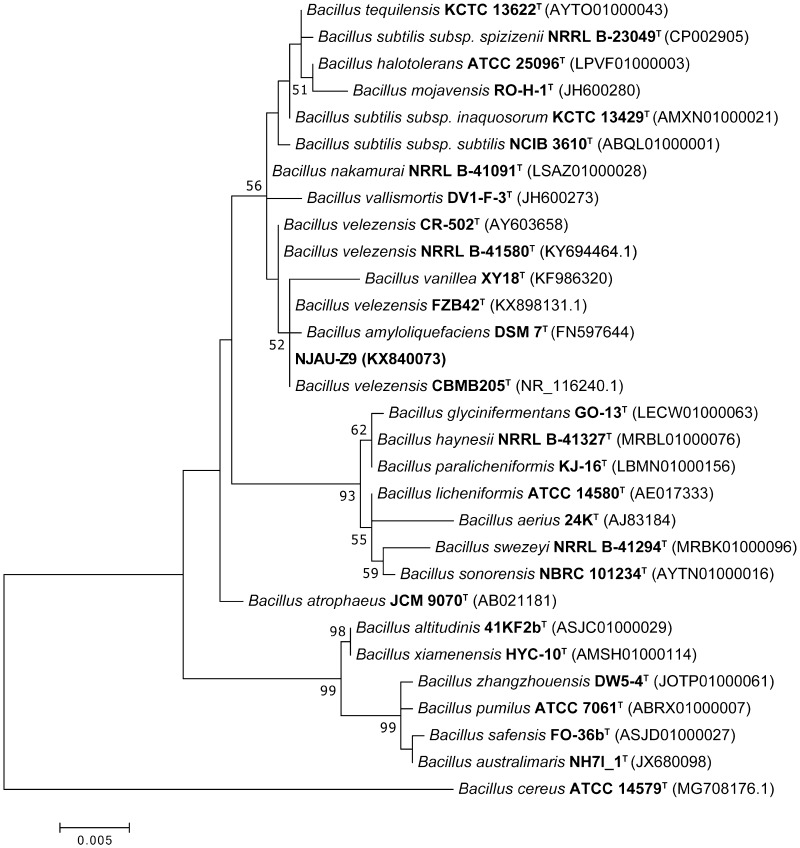
Approximately 150 bacterial strains were isolated from the rhizospheric soil of sweet pepper (Capsicum annuum L.). One strain that was the best at both producing indole acetic acid and NH3 simultaneously and acting in antagonism against Fusarium oxysporum f.sp. niveum and Ralstonia solanacearum (data not shown) was named NJAU-Z9. Strain NJAU-Z9 is a rod-shaped, Gram-positive bacterium with peritrichous flagella and able to form oval endospores. Characteristics detected by BIOLOG GEN III MicroPlate are shown in Table S2. Strain NJAU-Z9 was automatically classified into Bacillus sp. The 16S rRNA gene sequence similarity calculations indicated that strain NJAU-Z9 was closely related to the species of the genus Bacillus and shared the highest sequence similarity with B. velezensis CBMB205T (100%), and B. velezensis FZB42T (100%), followed by B. amyloliquefaciens DSM 7T (99.92%) and B. velezensis CR-502T (99.92%). Phylogenetic analysis based on 16S rRNA gene sequences showed that strain NJAU-Z9 belonged to the genus Bacillus and formed a subclade with B. velezensis CBMB205T and B. velezensis FZB42T (Fig. 1) with a robust bootstrap support (value 100%) in the maximum-likelihood tree. In addition, the ANI values between NJAU-Z9 and the type strains B. velezensis CBMB205T, B. velezensis FZB42T, and B. amyloliquefaciens DSM 7T are all greater than 94%, and B. velezensis CBMB205T is the closest one with ANI value of 97.94%. Moreover, these strains all have highly similar GC contents to NJAU-Z9 (Table S1). Thus, on the basis of morphological, physiological, and biochemical properties combined with 16S rRNA and WGSs analysis, strain NJAU-Z9 was finally identified as B. velezensis.
Fig. 1.
Maximum-likelihood phylogenetic tree based on the 16S rRNA gene sequences of strain NJAU-Z9 and related strains in the genus Bacillus. Bootstrap values are shown as percentages of 1000 replicates; values below 50% are not indicated. Bacillus cereus ATCC 14579T were used as outgroups. Accession numbers are given in parentheses. Bar, 0.005 substitutions per nucleotide position
Primer Design, Evaluation, and Amplification Efficiency
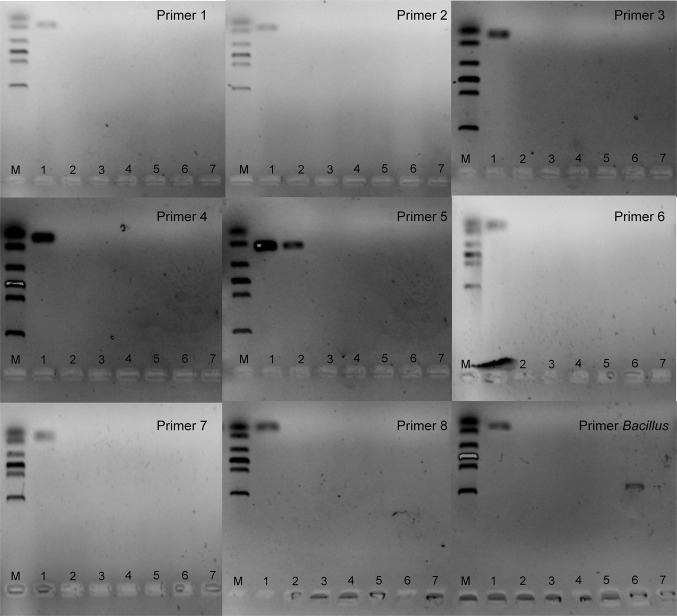
Three coding and intergenic regions from the NJAU-Z9 genome were selected, and a total of 8 primer pairs were designed and tested. Unique amplicons of expected size were observed on agarose gel electrophoresis (Fig. S3b) for each primer with no amplification with any of miscellaneous bands, and the melting point temperatures produced by these primers were approximately 76–83 °C with a single melting peak shape (Fig. S3a). Primer pairs P1, P2, P3, P4, P6, P7, and P8 produced amplicons only when B. velezensis NJAU-Z9 DNA was used as the template and were considered strain primer pairs; primer Bacillus was not able to amplify all Bacillus strains tested and primer pair P5 produced cross-species amplicons (amplification for B. subtilis strain G10 was observed), which was discarded from further analyses (Fig. 2). In addition, amplification of all Bacillus strains using universal primers for the 16S rRNA gene was used as another positive control, however, as expected, 16S rRNA gene universal primers (27F 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R 5′-GGTTACCTTGTTACGACTT-3′) was able to amplify all Bacillus strains tested (Fig. S4).
Fig. 2.
Specificities of the primer pairs designed to amplify different Bacillus species. Lane M, DL2000 DNA Marker; Lane 1, strain NJAU-Z9; 2, B. subtilis NJAU-G10; 3, B. amyloliquefaciens SQR-9; 4, B. amyloliquefaciens T-5; 5, B. pumilus LZ-8; 6, B. amyloliquefaciens NJN-6; and 7, B. vallismortis NJAU-N23. Primers 1 to 8 were designed from the genome of B. velezensis NJAU-Z9; Primer Bacillus was used as a positive amplification control
Plant Growth Promotion Effect
Except for the SPAD and leaf number, the other agronomic traits of the pepper seedlings growing in the nursery substrate amended with strain NJAU-Z9 were significantly higher than those in the control in all three replicates of the seedling cultivation experiments. These results indicate that the functional strain NJAU-Z9 is beneficial for improving pepper seedling growth (Table 2 and Fig. S5). In the second season of the pot experiments, the plant height and stem diameter of the pepper trees were greatly enhanced in the treatment (OFBS) inoculated strain NJAU-Z9, especially for the yields, which were significantly increased (P < 0.05) by 36.9% and 32.8%, respectively, when compared to the OF (Table 3 and Fig. S5).
Table 2.
Effects of the inoculation of nursery substance with strain NJAU-Z9 on pepper seedling growth
| Treatments | Plant height (cm) | Stem diameter (mm) | Leaf size (cm2) | SPAD | Leaf number |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| NJAU-Z9 | 9.17 ± 0.41* | 1.78 ± 0.07* | 5.02 ± 0.12* | 44.4 ± 0.89 | 5 |
| CK | 8.12 ± 0.24 | 1.68 ± 0.02 | 4.34 ± 0.09 | 43.57 ± 0.12 | 4 |
| Experiment 2 | |||||
| NJAU-Z9 | 13.06 ± 0.37* | 2.33 ± 0.01* | 13.19 ± 0.22* | 43.63 ± 0.84 | 5 |
| CK | 12.11 ± 0.2 | 2.19 ± 0.02 | 11.61 ± 0.23 | 42.97 ± 0.34 | 5 |
| Experiment 3 | |||||
| NJAU-Z9 | 10.02 ± 0.22* | 1.76 ± 0.03* | 6.1 ± 0.05* | 41.37 ± 0.52* | 4 |
| CK | 9.15 ± 0.38 | 1.41 ± 0.05 | 5.27 ± 0.14 | 39.13 ± 0.11 | 4 |
Values are means ± standard deviation
*Represent significant difference (t test, P < 0.05)
Table 3.
Agronomic traits of pepper after transplanting for 40 days in the pot experiment
| Treatments | Plant height (cm) | Stem diameter (mm) | Biomass (g) | SPAD |
|---|---|---|---|---|
| Experiment 1 | ||||
| NJAU-Z9 | 51.67 ± 1.76* | 6.35 ± 0.82 | 1282.38 ± 41.89* | 46.4 ± 0.54 |
| CK | 45.33 ± 4.68 | 5.68 ± 0.33 | 936.46 ± 62.41 | 47.57 ± 0.69 |
| Experiment 2 | ||||
| NJAU-Z9 | 45.26 ± 6.89* | 5.33 ± 0.41* | 972.45 ± 43.21* | 43.28 ± 1.24 |
| CK | 38.42 ± 2.66 | 4.19 ± 0.22 | 731.83 ± 27.69 | 44.51 ± 0.33 |
Values are means ± standard deviation
*Represent significant difference (t test, P < 0.05)
Quantification of NJAU-Z9
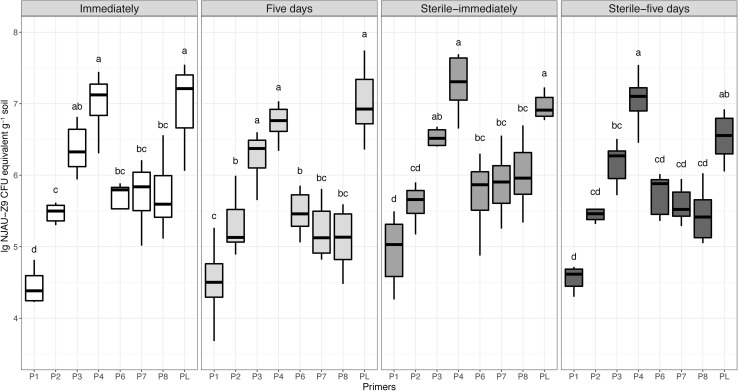
As shown in Fig. 3, the amounts of NJAU-Z9 in all sterile soil samples were slightly higher than that in the non-sterile soils regardless of measured on the first day (immediately) or the fifth day, while in non-sterile soils, the amounts of NJAU-Z9 in the fifth day were slightly lower than that on the first day. Moreover, among all primer pairs, the amounts from P1 were lower than others, the values of P4 were significantly higher than those of P2, P6, P7, and P8, and no significant difference was observed between P4 and P3 except on the fifth day in the sterile soil. In addition, the amounts from P3 and P4 showed no significant difference with plate counts (PL) (Fig. 3). Since the target gene fragments of all primers are single copies, based on the plate counts data as a reference, it can be concluded that the detection values from P3 and P4 were close to the true values (approximately 107 CFU g−1 soil). Moreover, soil samples from the sterile-control and control groups (immediately and five days, respectively) were also analyzed by using qPCR and plate counts method, and the CT values were both above 32, with no strains observed on the plates, indicating that the original soil did not contain the target strain (data not shown).
Fig. 3.
Detection of strain NJAU-Z9 by qPCR and plate counting (PL) methods in sterile and non-sterile soils. The values for qPCR are the means from three experiments using different strain primers. Sterile-immediately: immediately detected under sterile soil; sterile-five days: detection was performed after 5 days under sterile soil; immediately: immediately detected under non-sterile soil; 5 days: detection was performed after 5 days under non-sterile soil. The bars are the respective standard deviations (n = 12). The letters indicate significant differences among primers determined by the Tukey test
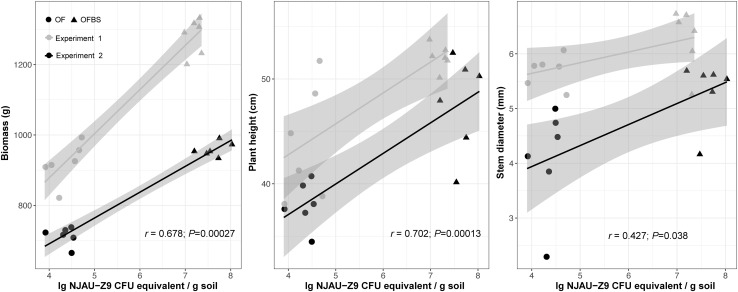
In two seasons of the pot experiments, the number of strain NJAU-Z9 was effectively detected in the OFBS treatment-transplanted seedlings cultivated in the nursing substrates and amended with strain NJAU-Z9. In the first pot experiment, the number of strain NJAU-Z9 exhibited a stable rhizosphere colonization during the entire growing period of pepper (Fig. S6). The number was significantly higher (P < 0.05) than that detected in the OF control regardless of the different stages in the first or second pot experiment. Most importantly, Pearson correlation coefficients were also calculated between the number of strain NJAU-Z9 at the mature stage in two pot experiments and the plant growth index significantly positively correlated with biomass (r = 0.678, P = 0.000027), plant height (r = 0.702, P = 0.00013), and stem diameter (r = 0.427, P = 0.038) (Fig. 4).
Fig. 4.
Pearson correlations (r) between the qPCR quantification data of strain NJAU-Z9 and plant growth index of mature period in two pot experiments
Discussion
In accordance with our results that pepper seeds inoculated with B. velezensis strain NJAU-Z9 obviously grew better than the control, all plant parameters showed enhancement when Cajanus cajan seeds were inoculated with functional microbes [32], and an increase of biomass after inoculation with B. phytofirmans PsJN has been reported in maize, wheat, and Acacia ampliceps [33, 34].
To date, a certain amount of microbial species such as Paecilomyces lilacinus, Lactobacillus, and Enterobacter radicincitans, were successfully monitored in various environments by qPCR using species-specific PCR primers [14, 35–37]. In order to solve the quantitative and dynamic monitoring of inoculated strains, we developed a strain qPCR protocol based on whole genome analysis to quantify B. velezensis strain NJAU-Z9. Eight-pair primers were designed by in silico comparison of the fragments from the WGS of B. velezensis NJAU-Z9, and the optimum amplification conditions were obtained by analyzing their dissolution curves and amplification efficiencies. Similarly, different methods, usually based on different experimental approaches, were used to design taxon-specific primers. Briefly, sequence-characterized amplified region (SCAR) markers obtained from BOX-PCR, enterobacterial repetitive intergenic consensus sequence-PCR, and randomly amplified polymorphic DNA (RAPD)-PCR fragments were recently applied to design primers for the qPCR quantification of Azospirillum brasilense and Azospirillum lipoferum at the strain level [38, 39]. However, these methods are more time consuming and insensitive compared to the method based on the WGS of the target species, which has been proven to be a rapid and reliable approach to provide comprehensive information about an organism and to detect specific DNA regions to be used as strain genetic markers for the quantitative detection of bacterial strains [40]. Primer verification assays have been performed using genomic DNA sequences from six other Bacillus species and our target strain as templates, and except primers P5, the others are strain specific which cannot amply any gene segment. In accordance with our results, four primer pairs were designed from Azospirillum brasilense FP2 whole genome, and after verification assays, three were left as strain primer pairs [41]. Thus, WGS offers genetic information with the highest resolution to explore novel strategies for monitoring the target microbes.
Results from natural soil using qPCR based on the strain primers showed that P3 and P4 primers were identified as NJAU-Z9-specific quantitative PCR primers in combination with the plate counts. Similar to our results, Stets et al. [41] also used the plate count data as a reference for determining primer pairs when monitoring Azospirillum brasilense FP2 on wheat roots in sterile and non-sterile conditions. For bacteria, there is a similar relationship between the number of single-copy genes and the number of colonies (CFU), and in the short-term inoculation experiment, the use of plate count data is a powerful reference for the effectiveness of specific primers [42]. Besides, in ecological research, the soil biodiversity of non-sterile soils is significantly different from that of sterile soils [43], our results also showed that the amount of B. velezensis NJAU-Z9 in sterile soil is slightly higher than that in non-sterile soil.
Pot experiments results showed that the inoculation of B. velezensis NJAU-Z9 significantly promoted plant growth. Similar to our results, strain B. velezensis NKG-1 was isolated and reported to suppress mycelial growth and conidial germination and to promote tomato plant growth [44]. Additionally, strain B. velezensis RC218 was reported to reduce Fusarium head blight and deoxynivalenol accumulation [45]. During the pot experiments, B. velezensis strain NJAU-Z9 stably colonized the rhizosphere, and the number present at the mature stage was positively correlated with plant growth. The results were consistent with common growth-promoting effects among numerous PGPR in that a gradual increase in equivalent cell numbers of PGPR detected by qPCR was observed over time, along with a simultaneous increase in plant growth promotion [46].
In conclusion, inoculation of B. velezensis strain NJAU-Z9 in the ordinary nursery substrate enhanced the plant growth, regardless in the seed nursing and subsequently transplanting stages. Two primer pairs from WGS of B. velezensis strain NJAU-Z9 were successfully designed and tested to monitor the fluctuation in the population of this strain by qPCR after inoculation into soil samples under sterile and non-sterile conditions. The detection efficiency was also successfully verified in two seasonal pot experiments, and the results demonstrated that B. velezensis strain NJAU-Z9 maintained high numbers during different growing stages, which were positively correlated with plant growth. In summary, the strategy for the design of strain primers described here may theoretically be used for different microbes for which the WGS is available in a database, and a qPCR method with strain primer pairs can be effectively applied to quantitatively monitor the population of PGPR in different environments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the National Key Research and Development Program of China (2017YFD0200805, 2016YFD0800605, and 2016YFE0101100), the Scientific and technological projects of Jiangsu, China (BY2016077-05), the Natural Science Foundation of Jiangsu (BK20160710 and BK20150059), the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD), the 111 project (B12009), the China Scholarship Council (award to Rong Li for 1 year’s abroad study), and Top-notch Academic Programs Project of Jiangsu Higher Education Institution (PPZY2015A061).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. In: Proceedings of the 4th international conference on plant pathogenic bacteria. Tours, pp 879–882
- 2.Collins DP, Jacobsen BJ. Optimizing a Bacillus subtilis isolate for biological control of sugar beet cercospora leaf spot. Biol Control. 2003;26(2):153–161. doi: 10.1016/S1049-9644(02)00132-9. [DOI] [Google Scholar]
- 3.Lópezbucio J, Camposcuevas JC, Hernándezcalderón E, Velásquezbecerra C, Faríasrodríguez R, Macíasrodríguez LI, Valenciacantero E. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant Microbe Interact. 2007;20(2):207–217. doi: 10.1094/MPMI-20-2-0207. [DOI] [PubMed] [Google Scholar]
- 4.Ramos B, GarcíA JAL, Probanza AN, Barrientos ML, Mañero FJG. Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus icheniformis. Environ Exp Bot. 2003;49(1):61–68. doi: 10.1016/S0098-8472(02)00059-X. [DOI] [Google Scholar]
- 5.Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001;20(1):1–11. doi: 10.1016/S0261-2194(00)00056-9. [DOI] [Google Scholar]
- 6.Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Okon Y, Vanderleyden J. Effect of inoculation with wild type Azospirillum brasilense and A. irakense strains on development and nitrogen uptake of spring wheat and grain maize. Biol Fertil Soils. 2002;36(4):284–297. doi: 10.1007/s00374-002-0534-9. [DOI] [Google Scholar]
- 7.Bashan Y, Gonzalez LE. Long-term survival of the plant-growth-promoting bacteria Azospirillum brasilense and Pseudomonas fluorescens in dry alginate inoculant. Appl Microbiol Biotechnol. 1999;51(2):262–266. doi: 10.1007/s002530051391. [DOI] [Google Scholar]
- 8.Ritz K. The Plate debate: cultivable communities have no utility in contemporary environmental microbial ecology. FEMS Microbiol Ecol. 2007;60(3):358–362. doi: 10.1111/j.1574-6941.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 9.Streit WR, Schmitz RA. Metagenomics-the key to the uncultured microbes. Curr Opin Microbiol. 2004;7(5):492–498. doi: 10.1016/j.mib.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59(1):143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelles L, Bai Q, Beck T, Beese F. Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol Biochem. 1992;24(4):317–323. doi: 10.1016/0038-0717(92)90191-Y. [DOI] [Google Scholar]
- 12.Gattinger A, Ruser R, Schloter M, Munch JC. Microbial community structure varies in different soil zones of a potato field. J Plant Nutr Soil Sci. 2002;165(4):421–428. doi: 10.1002/1522-2624(200208)165:4<421::AID-JPLN421>3.0.CO;2-N. [DOI] [Google Scholar]
- 13.Rosado A, Seldin L, Wolters A, Van Elsas J. Quantitative 16S rDNA-targeted polymerase chain reaction and oligonucleotide hybridization for the detection of Paenibacillus azotofixans in soil and the wheat rhizosphere. Fems Microbiol Ecol. 1996;19(3):153–164. doi: 10.1111/j.1574-6941.1996.tb00208.x. [DOI] [Google Scholar]
- 14.Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Valori F. Effects of root exudates in microbial diversity and activity in rhizosphere soils. In: Nautiyal CS, Dion P, editors. Molecular mechanisms of plant and microbe coexistence. Berlin: Springer; 2008. pp. 339–365. [Google Scholar]
- 15.Madhaiyan M, Poonguzhali S, Soonwo K, Tongmin S. Bacillus methylotrophicus sp. nov., a methanol-utilizing, plant-growth-promoting bacterium isolated from rice rhizosphere soil. Int J Syst Evol Microbiol. 2010;60:2490–2495. doi: 10.1099/ijs.0.015487-0. [DOI] [PubMed] [Google Scholar]
- 16.Ruizgarcía C, Béjar V, Martínezcheca F, Llamas I, Quesada E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Velez in Malaga, southern Spain. Int J Syst Evol Microbiol. 2005;55(1):191–195. doi: 10.1099/ijs.0.63310-0. [DOI] [PubMed] [Google Scholar]
- 17.Borriss R, Chen XH, Rückert C, Blom J, Becker A, Baumgarth B, et al. Relationship of clades associated with strains DSM 7 and FZB42: a proposal for nov. and nov. based on their discriminating complete genome sequences. Int J Nurs Stud. 2011;17(4):221–234. [Google Scholar]
- 18.Ma L, Cao YH, Cheng MH, Huang Y, Mo MH, Wang Y, Yang JZ, Yang FX. Phylogenetic diversity of bacterial endophytes of Panax notoginseng with antagonistic characteristics towards pathogens of root-rot disease complex. Antonie Van Leeuwenhoek. 2013;103(2):299–312. doi: 10.1007/s10482-012-9810-3. [DOI] [PubMed] [Google Scholar]
- 19.Chung EJ, Hossain MT, Khan A, Kim KH, Che OJ, Chung YR. Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. Plant Pathol J. 2015;31(2):152–164. doi: 10.5423/PPJ.OA.12.2014.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunlap CA, Kim SJ, Kwon SW, Rooney AP. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int J Syst Evol Microbiol. 2016;66(3):1212–1217. doi: 10.1099/ijsem.0.000858. [DOI] [PubMed] [Google Scholar]
- 21.Idris ESE, Iglesias DJ, Talon M, Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact. 2007;20(6):619. doi: 10.1094/MPMI-20-6-0619. [DOI] [PubMed] [Google Scholar]
- 22.Wu B, Wang X, Yang L, Yang H, Zeng H, Qiu Y, Wang C, Yu J, Li J, Xu D. Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. Appl Soil Ecol. 2016;103:1–12. doi: 10.1016/j.apsoil.2016.03.002. [DOI] [Google Scholar]
- 23.Zhang Y, Wen CY, Zhao MQ, Zhang M, Gao Q, Li R, Shen QR. Isolation of plant growth promoting rhizobacteria from pepper and development of bio-nursery substrates. J Nanjing Agric Univ. 2015;38(6):950–957. [Google Scholar]
- 24.Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61(2):793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 26.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 27.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 28.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek. 2017;110(10):1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 29.Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B. Real-Time DNA sequencing from single polymerase molecules. Methods Enzymol. 2009;323(5910):133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 30.Vardhan S, Kaushik R, Saxena AK, Arora DK. Restriction analysis and partial sequencing of the 16S rRNA gene as index for rapid identification of Bacillus species. Antonie Van Leeuwenhoek. 2011;99(2):283–296. doi: 10.1007/s10482-010-9487-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Li R, Cao L, Shi J, Liu H, Huang Y, Shen Q. Algal sludge from Taihu Lake can be utilized to create novel PGPR-containing bio-organic fertilizers. J Environ Manage. 2014;132:230–236. doi: 10.1016/j.jenvman.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R, Bisaria VS, Sharma S. Effect of agricultural amendments on Cajanus cajan (Pigeon Pea) and its rhizospheric microbial communities—a comparison between chemical fertilizers and bioinoculants. PLoS ONE. 2015;10(7):e0132770. doi: 10.1371/journal.pone.0132770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afzal M, Shabir G, Tahseen R, Islam EU, Iqbal S, Khan QM, Khalid ZM. Endophytic Burkholderia sp. strain PsJN improves plant growth and phytoremediation of soil irrigated with textile effluent. CLEAN. 2014;42(9):1304–1310. [Google Scholar]
- 34.Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot. 2014;97:30–39. doi: 10.1016/j.envexpbot.2013.09.014. [DOI] [Google Scholar]
- 35.Atkins SD, Clark IM, Pande S, Hirsch PR, Kerry BR. The use of real-time PCR and species-specific primers for the identification and monitoring of Paecilomyces lilacinus. Fems Microbiol Ecol. 2005;51(2):257–264. doi: 10.1016/j.femsec.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Haarman M, Knol J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2006;72(4):2359–2365. doi: 10.1128/AEM.72.4.2359-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruppel S, Rühlmann J, Merbach W. Quantification and localization of bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil. 2006;286(1):21–35. doi: 10.1007/s11104-006-9023-5. [DOI] [Google Scholar]
- 38.Couillerot O, Bouffaud ML, Baudoin E, Muller D, Caballero-Mellado J, Moënne-Loccoz Y. Development of a real-time PCR method to quantify the PGPR strain Azospirillum lipoferum CRT1 on maize seedlings. Soil Biol Biochem. 2010;42(12):2298–2305. doi: 10.1016/j.soilbio.2010.09.003. [DOI] [Google Scholar]
- 39.Couillerot O, Poirier MA, Prigent-Combaret C, Mavingui P, Caballero-Mellado J, Moënne-Loccoz Y. Assessment of SCAR markers to design real-time PCR primers for rhizosphere quantification of Azospirillum brasilense phytostimulatory inoculants of maize. J Appl Microbiol. 2010;109(2):528–538. doi: 10.1111/j.1365-2672.2010.04673.x. [DOI] [PubMed] [Google Scholar]
- 40.Havlak P, Chen R, Durbin KJ, Egan A, Ren Y, Song XZ, Weinstock GM, Gibbs RA. The Atlas genome assembly system. Genome Res. 2004;14(4):721–732. doi: 10.1101/gr.2264004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stets MI, Alqueres SM, Souza EM, Pedrosa FO, Schmid M, Hartmann A, Cruz LM. Quantification of Azospirillum brasilense FP2 bacteria in wheat roots by strain-specific quantitative PCR. Appl Environ Microbiol. 2015;81(19):6700–6709. doi: 10.1128/AEM.01351-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Qian K, Lu JX, Zou LL, Chen D, Sun BC. Realtime quantitative polymerase chain reaction determining bla CTX-M-14 gene in bacterium. Chin J Antibiot. 2009;34(8):505–508. [Google Scholar]
- 43.Wagg C, Bender SF, Widmer F, Mg VDH. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci USA. 2014;111(14):5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge B, Liu B, Nwet TT, Zhao W, Shi L, Zhang K. Bacillus methylotrophicus strain NKG-1, isolated from Changbai Mountain, China, has potential applications as a biofertilizer or biocontrol agent. PLoS ONE. 2016;11(11):e0166079. doi: 10.1371/journal.pone.0166079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palazzini JM, Dunlap CA, Bowman MJ, Chulze SN. Bacillus velezensis RC 218 as a biocontrol agent to reduce fusarium head blight and deoxynivalenol accumulation: genome sequencing and secondary metabolite cluster profiles. Microbiol Res. 2016;192:30–36. doi: 10.1016/j.micres.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Kalam S, Das SN, Basu A, Podile AR. Population densities of indigenous Acidobacteria change in the presence of plant growth promoting rhizobacteria (PGPR) in rhizosphere. J Basic Microbiol. 2017;57(5):376–385. doi: 10.1002/jobm.201600588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.