Abstract
In patients with a pediatric rheumatic disease (PRD), chronic musculoskeletal pain (CMP) can have a major impact on functioning and social participation. Because CMP is not always alleviated solely by the use of pharmacological approaches, the aim was to systematically review the available evidence regarding non-pharmacological treatment options for reducing CMP in patients with PRD. PubMed, Embase, PsycINFO, and the Cochrane Library were systematically searched for (non-)randomized trials investigating non-pharmacological treatments for CMP in PRD published through October 25, 2017. The GRADE approach was used to assess the quality of evidence. The search yielded 11 studies involving 420 children 5–18 years of age. All studies were relatively small and short-term, and the quality of evidence ranged from very low to moderate. The main modalities within non-pharmacology therapy were psychological interventions and exercise-based interventions. Some studies show modest positive short-term results for psychological and exercise-based interventions. Psychological and exercise-based interventions can have a modest positive result in PRD, with no evidence of side effects. Non-pharmacological therapies are a promising option to alleviate pain in PRD and improve functioning, which can be used as an alternative for or in addition to pharmacological therapies. Because chronic pain can differ etiologically from acute pain in PRD, non-pharmacological therapies might have different effects in patients with or without active inflammation. To best determine the effect of non-pharmacological therapies, future studies should take this difference into account.
Electronic supplementary material
The online version of this article (10.1007/s00296-018-4136-8) contains supplementary material, which is available to authorized users.
Keywords: Rheumatic diseases, Musculoskeletal pain, Chronic pain, Autoimmune diseases, Pediatrics, Juvenile idiopathic arthritis
Introduction
Pediatric rheumatic diseases (PRDs) are a group of chronic inflammatory conditions characterized by periods of disease flare-ups and often accompanied by pain [1]. Pain in PRD is a common problem, with a prevalence up to 86% in children with juvenile idiopathic arthritis (JIA) [2, 3]. Adolescents with pain reported experiencing reduced levels of physical functioning compared to patients with either mild or no pain, and they reported a significantly higher school absenteeism over the previous 6 months [4]. Acute musculoskeletal pain in PRD can often be attributed primarily to local inflammation; therefore, an anti-inflammatory treatment regime is a key therapeutic feature [5]. However, acute pain can progress into chronic musculoskeletal pain (CMP), even if the disease activity score is low [6, 7].
Once musculoskeletal pain becomes chronic, it often persists into adulthood [1, 8–10]. Children with CMP experience high levels of stress and are prone to anxiety and depression, which can in turn lead to increased pain and disability [11–15]. In addition, children with CMP often report sleep difficulties, including a lack of refreshing sleep and increased fatigue, further disrupting their social and academic development [4, 16–20]. Moreover, the impact of CMP is not confined to the individual patient, but can extend to the entire family and can have significant societal costs [21–24].
Pain can negatively influence our behavior, activity, and participation; although this might be initially helpful, it can lose purpose if the pain becomes chronic. Acute pain is induced by local inflammation or injury. After that, peripheral and central sensitization contributes to an amplification of a new pain stimulus [25]. Finally, endogenous pain modulatory pathways determine pain responses by the influence of attention, suggestion, expectation, stress, anxiety, context and past experience [25]. While most pharmacological interventions are targeted on treatment of inflammation, alleviation of chronic pain might need another approach [3, 6, 7, 16]. Several studies found that in addition to biological processes, psychosocial factors such as coping and cognitive health beliefs can determine the experience and impact of chronic pain, giving rise to the so-called biopsychosocial model [15, 21, 26]. Expanding our knowledge beyond pharmacological solutions to include non-pharmacological interventions, such as psychological or exercise-based interventions, may, therefore, provide a promising means to alleviate pain and improve functioning in children with PRD.
Psychological therapies and exercise-based therapies have been shown to be beneficial for children with widespread chronic pain, who did not have PRD [27–30]. These therapies have also been shown to exert modest beneficial effects in adults with rheumatic disease [31]. In their review published in 2013, Cunningham and Kashikar-Zuck proposed that a multidisciplinary approach consisting of carefully selected pharmacological and non-pharmacological interventions based upon a biopsychosocial framework may provide the most effective approach to treating pain [31].
Although non-pharmacological therapies may represent a promising addition and/or alternative to pharmacological therapies for alleviating chronic pain, evidence with respect to using non-pharmacological therapies for CMP in children and adolescents with PRD is extremely limited. In this review, the aim is to provide an overview of published non-pharmacological therapies for CMP in patients with PRD. This overview may serve as a stepping stone for future research and for the implementation of non-pharmacological therapies in clinical practice.
Methods
In our review, both randomized controlled trials (RCTs) and non-randomized controlled trials were eligible. The primary outcome measure was pain intensity, and the secondary outcome measures were functional disability and quality of life. We performed a systematic search of PubMed (both MEDLINE-indexed and PMC-archived items), Embase (Scopus), PsycINFO, and the Cochrane Library, with no date or language restrictions. The search terms included terms related to musculoskeletal pain or dysfunction, non-pharmacological treatment modalities, children, and pediatric rheumatic diseases. In addition, the reference lists of the retrieved papers were manually cross-referenced, and Scopus was used to search for additional relevant studies. The following inclusion criteria were used: (1) children 5–18 years of age with PRD and chronic musculoskeletal pain (defined as ≥ 3 months in duration) not associated with active disease; (2) it concerned primary research and was available in full-text; (3) the study included at least one non-pharmacological intervention arm such as exercise, physiotherapy, cognitive behavioral therapy (CBT), occupational therapy, biofeedback, or complementary and alternative medicine; and (4) the study outcomes included pain intensity. Exclusion criteria were: (1) Treatment arm with < 5 patients at the end of treatment; (2) studies on complex regional pain syndrome (CRPS); and (3) CMP associated with a malignant disease process.
The methodological quality of each included study was independently assessed by two authors (LNN and MMN) based on the Cochrane risk of bias tool, and the quality of evidence was assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach [32]. GRADE was developed to assess pooled data from studies in comparable settings, with comparable outcome parameters. In our review, however, the studies were separately assessed due to the limited numbers of studies available and the heterogeneity among these studies. Quality of evidence was categorized as very low, low, moderate, or high [32].
Results
Search results
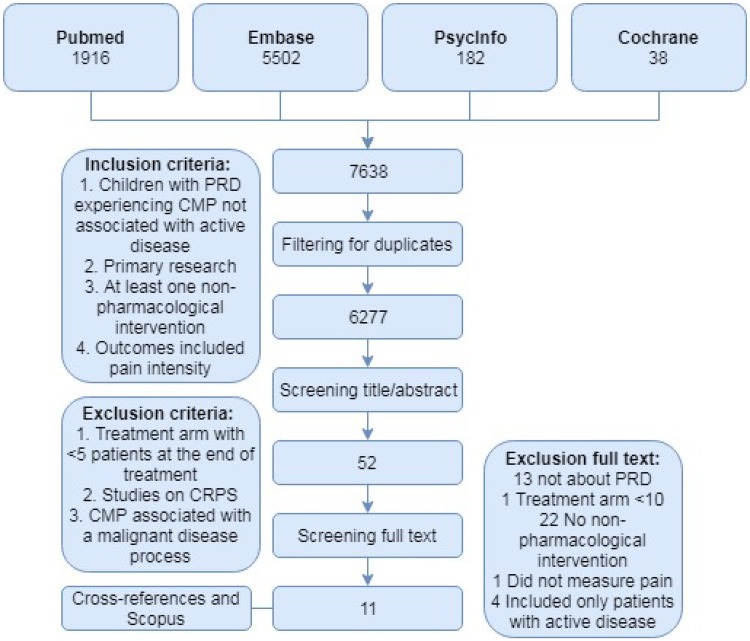
The databases PubMed, Embase, PsycINFO, and the Cochrane Library were systematically searched for articles published through October 25, 2017, yielding a total of 7638 publications. After adjusting for duplicates, 6277 publications remained, of which 6225 were excluded after reviewing the title and/or abstract. After screening the full-text articles for the remaining 52 publications, we identified eleven randomized/non-randomized controlled trials that described non-pharmacological therapies for treating chronic pain in PRD (Fig. 1) [33–43]. Ten of these studies involved children with juvenile idiopathic arthritis (JIA), and one study involved children with systemic lupus erythematosus (SLE). The characteristics of the included studies are summarized in Supplementary Table S1.
Fig. 1.
Flow-chart depicting the search strategy, inclusion and exclusion criteria, and studies included in the final analysis
Next, we attempted to differentiate between patients who had chronic pain with active disease and patients who had chronic pain in the absence of active disease. In our differentiation, we excluded the studies published by Epps et al. (2008), Singh-Grewal et al. (2007), Sandstedt et al. (2013), and Ramelet et al. (2017), as they included patients who were recently diagnosed or had active inflammation at the time of the intervention [44–47]. However, the study by Baydogan et al. included patients with active disease; in contrast, the description of active disease was not always conclusive for the remaining studies [35].
Quality of the evidence
The eleven studies included in our review involved a total of 420 participants. The mean percentage of female patients in the studies was 71% (range 54–100%). All eleven studies had a relatively small cohort and were short-term studies; only two studies included more than 50 participants, and only one study reported follow-up data. Table 1 summarizes the GRADE evidence profiles. Overall, the quality of evidence ranged from very low to moderate.
Table 1.
GRADE evidence profiles per study
| No of participants included in the analysis | Summary of findings for pain | P value (posttreatment between groups) | Quality assessment | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference pre-post treatment (VAS score 0–10) | P value (pre-posttreatment per intervention) | Risk of bias | Indirectness | Imprecision | Overall quality of evidence | ||||||||||||||||||||
| Physical therapy | |||||||||||||||||||||||||
| Field et al. [42] massage therapy vs. relaxation therapy | |||||||||||||||||||||||||
| Massage N = 10 | − 3.0 | < 0.005 | Not given | Very seriousa | Seriousf,g | Seriousj | ⨁◯◯◯ Very low |
||||||||||||||||||
| Relaxation N = 10 | − 0.5 | NS | |||||||||||||||||||||||
| Klepper [38] physical conditioning program vs. waiting list | |||||||||||||||||||||||||
| Physical conditioning N = 25 | − 0.5 | NS | Not applicable (within subjects design) | Very seriousb | Not serious | Seriousk | ⨁◯◯◯ Very low |
||||||||||||||||||
| Waiting list N = 25 | − 0.7 | NS | |||||||||||||||||||||||
| Tarakci et al. [39] land-based home exercise vs. waiting list | |||||||||||||||||||||||||
| Land-based home exercise N = 43 | − 0.9 | < 0.001 | 0.29 | Not serious | Not serious | Seriousk | ⨁⨁⨁◯ Moderate |
||||||||||||||||||
| Waiting list N = 38 | − 0.7 | 0.002 | |||||||||||||||||||||||
| Mendonca et al. [41] pilates exercise vs. conventional exercise program | |||||||||||||||||||||||||
| Pilates exercise N = 25 | − 2.3 | < 0.01 | < 0.0001 | Not serious | Not serious | Seriousk | ⨁⨁⨁◯ Moderate |
||||||||||||||||||
| Conventional exercise program N = 25 | + 0.2 | NS | |||||||||||||||||||||||
| Baydogan et al. [35] strengthening vs. balance-proprioceptive exercise | |||||||||||||||||||||||||
| Strengthening exercise N = 15 | − 1 | < 0.001 | 0.502 | Seriousc | Seriousg | Seriousl | ⨁◯◯◯ Very low |
||||||||||||||||||
| Balance-proprioceptive exercise N = 15 | − 1 | < 0.001 | |||||||||||||||||||||||
| Elnaggar and Elshafey [37] resistive underwater exercise vs. traditional physical therapy | |||||||||||||||||||||||||
| Resistive underwater exercise N = 15 | − 4.4 | 0.001 | 0.001 | Not serious | Serioush | Not serious | ⨁⨁⨁◯ Moderate |
||||||||||||||||||
| Traditional physical therapy N = 15 | − 1.1 | 0.001 | |||||||||||||||||||||||
| Psychological interventions | |||||||||||||||||||||||||
| Stinson et al. [40] managing arthritis online program vs. attention control | |||||||||||||||||||||||||
| Managing arthritis online N = 22 | − 0.6 | Not given | 0.03 | Not serious | Seriousf,g | Not serious | ⨁⨁⨁◯ Moderate |
||||||||||||||||||
| Attention control N = 24 | + 0.5 | Not given | |||||||||||||||||||||||
| Brown et al. [43] cognitive behavioral therapy vs. education only vs. no contact | |||||||||||||||||||||||||
| Cognitive behavioral therapy N = 22 | Not given | Not given | CBT and education only vs. no-contact P = 0.68 | Seriousd | Seriousf,g | Seriousl | ⨁◯◯◯ Very low |
||||||||||||||||||
| Education only N = 10 | Not given | Not given | |||||||||||||||||||||||
| No-contact control N = 16 | Not given | Not given | |||||||||||||||||||||||
| Lomholt et al. [34] cognitive behavioral therapy vs. waiting list | |||||||||||||||||||||||||
| Cognitive behavioral therapy N = 9 | + 0.4 | Not given | 0.81 | Not serious | Seriousi,g | Seriousj | ⨁⨁◯◯ Low |
||||||||||||||||||
| Waiting list N = 10 | + 0.5 | Not given | |||||||||||||||||||||||
| Eid et al. [36] physical therapy with biofeedback vs. physical therapy | |||||||||||||||||||||||||
| Physical therapy with biofeedback N = 18 | − 3.7 | 0.0001 | 0.001 | Not serious | Seriousi,g | Not serious | ⨁⨁⨁◯ Moderate |
||||||||||||||||||
| Conventional physical therapy N = 18 | − 2.2 | 0.0001 | |||||||||||||||||||||||
| Spiegel et al. [33] peer support vs. waiting list | |||||||||||||||||||||||||
| Peer support N = 16 | − 0.3 | Not given | 0.63 | Seriouse | Seriousf,g | Seriousk | ⨁⨁◯◯ Low |
||||||||||||||||||
| Waiting list N = 14 | − 0.2 | Not given | |||||||||||||||||||||||
NS not significant
aRandom sequence generation, allocation concealment, attrition bias and reporting bias not described
bThere was risk of selection, attrition and detection bias
cThere was risk of detection bias, allocation concealment was not described
dThere was risk of attrition bias, allocation concealment was not described
eThere was risk of attrition bias, blinding of outcome assessment was not described
fOnly adolescents
gThe vast majority was female
hNo information was given concerning age range and gender of participants
iLimited age range
jSmall sample size
kVery large standard deviation. l. no information was given regarding standard deviation or confidence interval
Effectiveness of psychological interventions
Several studies reported a moderate reduction in pain with the addition of biofeedback to physical therapy and an online program online for self-management and education; these studies assessed pain using a visual analog scale (VAS) [36, 40]. In contrast, participating in a peer-support program did not result in a significant decrease in pain (measured using the recalled pain inventory) compared to control patients [33]. Two studies measured the effect of cognitive behavioral therapy (CBT), in patients with JIA and patients with SLE and found no difference in either pain or quality of life compared to the respective control groups; both of these studies assessed pain using a VAS, and one additionally used the McGill Pain Questionnaire [34, 43]. With respect to studies that reported functional disability (assessed using Child Health Assessment Questionnaire, the Functional Disability Inventory, or the Juvenile Arthritis Functional Assessment Report), functioning was improved with biofeedback, but not with CBT or telephone consultation with a nurse [36, 40]. Quality of life (measured using the PedsQL or the Juvenile Arthritis Quality of life Questionnaire) did not differ between patients who received peer support, or the online arthritis managing program compared to patients in the respective control groups [33, 40].
Effectiveness of physical therapy
A significant decrease in pain was reported following massage therapy, but not following relaxation therapy [42]. In addition, Tarakci et al. reported that although a 12-week exercise-based intervention significantly reduced pain (the mean change in VAS was − 0.9), the control group had a similar reduction in pain (with a mean change in VAS of − 0.7); however, exercise was more effective at improving both functional capacity and quality of life [39]. They used a combination of strengthening, stretching and postural exercises and functional activities (walking, squat and stair-climbing). In contrast, Klepper reported that pain levels did not change following an exercise intervention, using low-impact aerobic exercise to improve aerobic endurance, muscular strength, and flexibility [38]. Both strength-building exercises (focused on the quadriceps femoris and hamstrings) and balance-proprioceptive exercises were equally effective at reducing pain and functional disability [35]. Another study found that Pilates exercise, but not conventional exercise (described as mainly stretching exercises and improving core stability), significantly reduced pain, improved function, and increased quality of life [41]. Combined resistive underwater exercise and traditional physical therapy were both found to reduce pain, but the underwater exercises were more effective [37]. Traditional physical therapy consisted of hot packs, range-of motion, isometric and stretching exercises, and fitness exercises such as cycling and treadmill walking. All of the above-mentioned studies used a VAS pain scale, the CHAQ, and/or the PedsQL questionnaire to measure pain, functional disability, and quality of life, respectively.
Effects at follow-up
Only one study, which involved adolescent girls with SLE, included follow-up data [43]. In their study, the authors found that patients in the CBT group did not differ significantly from patients in the education and no-contact control groups at either the 3-month or 6-month follow-up time points.
Discussion
Main findings
Chronic musculoskeletal pain is relatively common in patients with pediatric rheumatic disease and can be highly debilitating. Despite the high impact that CMP can have on the patient’s functioning and social participation, therapeutic options are limited. The strikingly few studies involving non-pharmacological therapies reported modest beneficial results in response to psychological and exercise-based interventions. On the other hand, some studies found no clear benefits associated with active non-pharmacological treatments with respect to reducing pain or improving function. This discrepancy may have been due—at least in part—to the difficulty differentiating between acute and chronic pain among the patients in the included studies. Importantly, none of the studies reported side effects associated with the non-pharmacological therapies, making this approach a potentially promising alternative or addition when pharmacological therapies are insufficient for alleviating pain.
Comparison with previous reports
Various aspects of non-pharmacological therapies for CMP in patients with PRD have been discussed previously. For example, Cohen et al. (2017) recently performed a meta-analysis to review the effect of psychosocial therapies on pain in PRD [48]. However, their search was focused on psychosocial interventions and included five articles, two of which were studies involving fibromyalgia. The authors concluded that the results were, therefore, too limited to draw any meaningful conclusions. Similarly, both Takken et al. (2008) and Kuntz et al. (2018) published a review regarding the effect of exercise therapy in JIA [49, 50]. Although both groups reported that exercise therapy appears to be well tolerated and beneficial in terms of reducing pain and improving the function and quality of life among patients with JIA, they noted that specific clinical recommendations may be premature. One possible explanation for their inconclusive results is the heterogeneity among the patients included in the studies.
Strengths and limitations
In all three above-mentioned reviews, no distinction was made between acute and chronic pain or between the presence or absence of active inflammation; importantly, this differentiation might provide insight into which approach might be most effective. In this respect, a strength of our review is our attempt to distinguish among these different patient groups, as these different groups may require different approaches. One limitation is that non-pharmacological therapies include a broader range of interventions than just psychological and exercise-based interventions. Many areas of non-pharmacological treatment modalities such as chiropractic treatment and mindfulness have not been evaluated in a controlled trial or focus on non-specific generalized chronic pain and, therefore, were not included in our systematic review [51, 52]. Second, the treatment effect of some interventions could not be addressed fully, as in some cases they were compared to another intervention (for example, one form of exercise vs. another form of exercise). Third, the currently available evidence was too limited to pool the data or perform a meta-analysis. Finally, we were unable to adjust our result for the sex or age of the patients in the included studies. In general, pain tends to be more prevalent among girls, and this increases with age [53]. It is possible that there are age or gender specific differences in the responses to non-pharmacological therapies that we are not aware of.
Implications for future research
With regard to future research, several aspects are worth mentioning. First, future studies should differentiate between acute and chronic pain, as these two types of pain differ with respect to the underlying pathophysiology. Second, studies that combine a graded exercise therapy with cognitive behavioral interventions to achieve change in the perception of pain might be a feasible approach for restoring functional capacity, increasing social participation, and reducing pain [31]. Third, based on the biopsychosocial model, we hypothesized that CBT may be highly effective; however, we were unable to test this hypothesis due to the limited number of studies available. Thus, gaining further insight into the relationship between an individual child’s thinking, feeling, and behavior might be necessary to tailor the cognitive behavioral intervention to that particular child for the psychological intervention to be effective. Finally, even though follow-up studies are extremely important, only one of the eleven studies in our analysis reported follow-up data (in this case, 6 months of follow-up). An improvement in functioning often precedes a reduction in pain [54]. Therefore, any reduction in pain might be more evident at later time points than immediately following treatment. Given that most of the studies in our analysis were published in 2010 or more recently, follow-up data may still be on the way, and opportunities for future research are numerous in this still-evolving field.
Conclusions
Both psychological and exercise-based interventions have been shown to have modest beneficial effects on CMP in PRD. Moreover, non-pharmacological therapies are not associated with side effects. When pharmacological therapy is insufficient to alleviate pain in PRD, non-pharmacological therapies may serve as a suitable alternative and/or addition for reducing CMP and improving function. Importantly, chronic pain and acute pain may be etiologically different in PRD, and future studies should take this difference into account to identify the optimal therapeutic window for non-pharmacological approaches. Finally, studies are needed that specifically investigate chronic pain in PRD and are designed to improve social participation in children with PRD-related chronic pain, particularly with respect to the long-term effectiveness of these interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The authors acknowledge Dr. C.F. Barrett for his assistance with English language editing.
Abbreviations
- CBT
Cognitive behavioral therapy
- CHAQ
Child Health Assessment Questionnaire
- CI
Confidence interval
- CMP
Chronic musculoskeletal pain
- FDI
Functional disability inventory
- JAFAR-C
Juvenile Arthritis Functional Assessment Report
- JAQQ
Juvenile Arthritis Quality of Life Questionnaire
- JIA
Juvenile idiopathic arthritis
- JRA
Juvenile rheumatoid arthritis
- PedsQL
Pediatric quality of life inventory
- PRD
Pediatric rheumatic disease
- RCT
Randomized controlled trial
- SLE
Systemic lupus erythematous
- VAS
Visual analog scale
Author contributions
LNN and MMN collected and analyzed the data and drafted the paper. SLN and EMvdP designed and supervised the study. AvR provided a critical review of the manuscript. All authors have read and approved the final manuscript and take responsibility for all aspects of the systematic review.
Funding
None.
Conflict of interest
Author Linde N. Nijhof declares that she has no conflict of interest. Author Merel M. Nap-van der Vlist declares that she has no conflict of interest. Author Elise M. van de Putte declares that she has no conflict of interest. Author Annet van Royen-Kerkhof declares that she has no conflict of interest. Author Sanne L. Nijhof declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Contributor Information
Linde N. Nijhof, Phone: +31 88 75 545 55, Email: L.N.Nijhof@umcutrecht.nl
Merel M. Nap-van der Vlist, Email: M.M.vanderVlist-3@umcutrecht.nl
Elise M. van de Putte, Email: E.vandePutte@umcutrecht.nl
Annet van Royen-Kerkhof, Email: A.vanRoyen@umcutrecht.nl.
Sanne L. Nijhof, Email: S.L.Nijhof@umcutrecht.nl
References
- 1.Schanberg LE, Lefebvre JC, Keefe FJ, Kredich DW, Gil KM. Pain coping and the pain experience in children with juvenile chronic arthritis. Pain. 1997;73(2):181–189. doi: 10.1016/S0304-3959(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 2.Bromberg MH, Connelly M, Anthony KK, Gil KM, Schanberg LE. Self-reported pain and disease symptoms persist in juvenile idiopathic arthritis despite treatment advances: an electronic diary study. Arthritis Rheumatol. 2014;66(2):462–469. doi: 10.1002/art.38223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giancane G, Alongi A, Rosina S, Calandra S, Consolaro A, Ravelli A. Open issues in the assessment and management of pain in juvenile idiopathic arthritis. Clin Exp Rheumatol. 2017;35(5 Suppl 1):123–126. [PubMed] [Google Scholar]
- 4.Nijhof LN, Van De Putte EM, Wulffraat NM, Nijhof SL. Prevalence of severe fatigue among adolescents with pediatric rheumatic diseases. Arthritis Care Res. 2016;68(1):108–114. doi: 10.1002/acr.22710. [DOI] [PubMed] [Google Scholar]
- 5.Vanoni F, Minoia F, Malattia C. Biologics in juvenile idiopathic arthritis: a narrative review. Eur J Pediatr. 2017;176(9):1147–1153. doi: 10.1007/s00431-017-2960-6. [DOI] [PubMed] [Google Scholar]
- 6.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum. 2013;65(2):291–302. doi: 10.1002/art.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss JE, Luca NJC, Boneparth A, Stinson J. Assessment and management of pain in juvenile idiopathic arthritis. Paediatr Drugs. 2014;16(6):473–481. doi: 10.1007/s40272-014-0094-0. [DOI] [PubMed] [Google Scholar]
- 8.Varni JW, Wilcox KT, Hanson V, Brik R. Chronic musculoskeletal pain and functional status in juvenile rheumatoid arthritis: an empirical model. Pain. 1988;32(1):1–7. doi: 10.1016/0304-3959(88)90016-4. [DOI] [PubMed] [Google Scholar]
- 9.Oen K, Reed M, Malleson PN, Cabral DA, Petty RE, Rosenberg AM, Cheang M. Radiologic outcome and its relationship to functional disability in juvenile rheumatoid arthritis. J Rheumatol. 2003;30(4):832–840. [PubMed] [Google Scholar]
- 10.Flato B, Lien G, Smerdel A, Vinje O, Dale K, Johnston V, Sorskaar D, Moum T, Ploski R, Forre O. Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol. 2003;30(2):386–393. [PubMed] [Google Scholar]
- 11.Greene JW, Walker LS, Hickson G, Thompson J. Stressful life events and somatic complaints in adolescents. Pediatrics. 1985;75(1):19–22. [PubMed] [Google Scholar]
- 12.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain. 2001;17(4):341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: patterns and predictors across different domains of functioning. Pain. 2007;131(1–2):132–41. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Kashikar-Zuck S, Johnston M, Ting TV, Graham BT, Lynch-Jordan AM, Verkamp E, Passo M, Schikler KN, Hashkes PJ, Spalding S, Banez G, Richards MM, Powers SW, Arnold LM, Lovell D. Relationship between school absenteeism and depressive symptoms among adolescents with juvenile fibromyalgia. J Pediatr Psychol. 2010;35(9):996–1004. doi: 10.1093/jpepsy/jsq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthony KK, Schanberg LE. Assessment and management of pain syndromes and arthritis pain in children and adolescents. Rheum Dis Clin North Am. 2007;33(3):625–660. doi: 10.1016/j.rdc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Sherry DD, Malleson PN. The idiopathic musculoskeletal pain syndromes in childhood. Rheum Dis Clin North Am. 2002;28(3):669–685. doi: 10.1016/S0889-857X(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 17.de Blecourt ACE, Schiphorst Preuper HR, Van Der Schans CP, Groothoff JW, Reneman MF. Preliminary evaluation of a multidisciplinary pain management program for children and adolescents with chronic musculoskeletal pain. Disabil Rehabil. 2008;30(1):13–20. doi: 10.1080/09638280601178816. [DOI] [PubMed] [Google Scholar]
- 18.Valrie CR, Bromberg MH, Palermo T, Schanberg LE. A systematic review of sleep in pediatric pain populations. J Dev Behav Pediatr. 2013;34(2):120–128. doi: 10.1097/DBP.0b013e31827d5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen MN, Sherry DD, Boyne K, McCue R, Gallagher PR, Brooks LJ. Relationship between sleep and pain in adolescents with juvenile primary fibromyalgia syndrome. Sleep. 2013;36(4):509–516. doi: 10.5665/sleep.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews NE, Strong J, Meredith PJ. Activity pacing, avoidance, endurance, and associations with patient functioning in chronic pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2012;93(11):2109–2121.e7. doi: 10.1016/j.apmr.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Palermo TM, Valrie CR, Karlson CW. Family and parent influences on pediatric chronic pain: a developmental perspective. Am Psychol. 2014;69(2):142–152. doi: 10.1037/a0035216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinch J, Eccleston C. Chronic musculoskeletal pain in children: assessment and management. Rheumatology (Oxford) 2009;48(5):466–474. doi: 10.1093/rheumatology/kep001. [DOI] [PubMed] [Google Scholar]
- 23.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J Dev Behav Pediatr. 2000;21(1):58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Sleed M, Eccleston C, Beecham J, Knapp M, Jordan A. The economic impact of chronic pain in adolescence: Methodological considerations and a preliminary costs-of-illness study. Pain. 2005;119(1–3):183–190. doi: 10.1016/j.pain.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Munro J, Singh-Grewal D. Juvenile idiopathic arthritis and pain—more than simple nociception. J Rheumatol. 2013;40(7):1037–1039. doi: 10.3899/jrheum.130557. [DOI] [PubMed] [Google Scholar]
- 26.Stinson JN, Luca NJC, Jibb LA. Assessment and management of pain in juvenile idiopathic arthritis. Pain Res Manag. 2012;17(6):391–396. doi: 10.1155/2012/237258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eccleston C, Palermo TM, Williams ACC, Lewandowski Holley A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher E, Law E, Palermo TM, Eccleston C. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2015;3:CD011118. doi: 10.1002/14651858.CD011118.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S, L. A.S. PTM,EC, Palermo WACdC,MS,TM, Eccleston C, Lewandowski AS, de Williams ACC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review.[Review] [42 refs] Pain. 2010;148(3):387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landry BW, Fischer PR, Driscoll SW, Koch KM, Harbeck-Weber C, Mack KJ, Wilder RT, Bauer BA, Brandenburg JE. Managing chronic pain in children and adolescents: a clinical review. PMR. 2015;7(11):S295–S315. doi: 10.1016/j.pmrj.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham NR, Kashikar-Zuck S. Nonpharmacological treatment of pain in rheumatic diseases and other musculoskeletal pain conditions. Curr Rheumatol Rep. 2013;15(2):306. doi: 10.1007/s11926-012-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel L, Stinson J, Campillo S, Cellucci T, Dancey P, Duffy C, Ellsworth J, Feldman B, Huber A, Johnson N, McGrath P, Rosenberg A, Shiff N, Tse S, Tucker L, Victor C, Luca S. An internet-based self-management program for adolescents with juvenile idiopathic arthritis (JIA): a randomized controlled trial (RCT) Pediatr Rheumatol. 2017;15:133. [Google Scholar]
- 34.Lomholt JJ, Thastum M, Christensen AE, Leegaard A, Herlin T. Cognitive behavioral group intervention for pain and well-being in children with juvenile idiopathic arthritis: a study of feasibility and preliminary efficacy. Pediatr Rheumatol Online J. 2015;13(1):35. doi: 10.1186/s12969-015-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baydogan SN, Tarakci E, Kasapcopur O. Effect of strengthening versus balance-proprioceptive exercises on lower extremity function in patients with juvenile idiopathic arthritis: a randomized, single-blind clinical trial. Am J Phys Med Rehabil. 2015;94(6):417–424. doi: 10.1097/PHM.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 36.Eid MAM, Aly SM, El-Shamy SM. Effect of electromyographic biofeedback training on pain, quadriceps muscle strength, and functional ability in juvenile rheumatoid arthritis. Am J Phys Med Rehabil. 2016;95:921–930. doi: 10.1097/PHM.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 37.Elnaggar RK, Elshafey MA. Effects of combined resistive underwater exercises and interferential current therapy in patients with juvenile idiopathic arthritis: a randomized controlled trial. Am J Phys Med Rehabil. 2016;95(2):96–102. doi: 10.1097/PHM.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 38.Klepper SE. Effects of an eight-week physical conditioning program on disease signs and symptoms in children with chronic arthritis. Arthritis Care Res. 1999;12(1):52–60. doi: 10.1002/1529-0131(199902)12:1<52::AID-ART9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Tarakci E, Yeldan I, Baydogan S, Olgar S, Kasapcopur O. The efficacy of land-based home exercise program in patients with juvenile idiopathic arthritis: a randomized-controlled, single-blind study. Ann Rheum Dis. 2013;71:750. doi: 10.1136/annrheumdis-2012-eular.2933. [DOI] [PubMed] [Google Scholar]
- 40.Stinson JN, McGrath PJ, Hodnett ED, Feldman BM, Duffy CM, Huber AM, Tucker LB, Hetherington CR, Tse SML, Spiegel LR, Campillo S, Gill NK, White ME. An internet-based self-management program with telephone support for adolescents with arthritis: a pilot randomized controlled trial. J Rheumatol. 2010;37(9):1944–1952. doi: 10.3899/jrheum.091327. [DOI] [PubMed] [Google Scholar]
- 41.Mendonça M, Terreri MT, Silva CH, Pinto M, Natour J, Neto MB, Len CA. Effects of pilates exercises on health-related quality of life in individuals with juvenile idiopathic arthritis. Arch Phys Med Rehabil. 2013;94:2093–2102. doi: 10.1016/j.apmr.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Field T, Hernandez-Reif M, Seligman S, Krasnegor J, Sunshine W, Rivas-Chacon R, Schanberg S, Kuhn C. Juvenile rheumatoid arthritis: benefits from massage therapy. J Pediatr Psychol. 1997;22(5):607–617. doi: 10.1093/jpepsy/22.5.607. [DOI] [PubMed] [Google Scholar]
- 43.Brown RT, Shaftman SR, Tilley BC, Anthony KK, Kral MC, Maxson B, Mee L, Bonner MJ, Vogler LB, Schanberg LE, Connelly MA, Wagner JL, Silver RM, Nietert PJ. The health education for lupus study: a randomized controlled cognitive-behavioral intervention targeting psychosocial adjustment and quality of life in adolescent females with systemic lupus erythematosus. Am J Med Sci. 2012;344(4):274–282. doi: 10.1097/MAJ.0b013e3182449be9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, Woo P. Is hydrotherapy cost effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis. Health Technol Assess (Rockv) 2005;9(39):76. doi: 10.3310/hta9390. [DOI] [PubMed] [Google Scholar]
- 45.Singh-Grewal D, Wright V, Bar-Or O, Feldman BM. Pilot study of fitness training and exercise testing in polyarticular childhood arthritis. Arthritis Rheum. 2006;55(3):364–372. doi: 10.1002/art.21996. [DOI] [PubMed] [Google Scholar]
- 46.Sandstedt E, Fasth A, Eek MN, Beckung E. Muscle strength, physical fitness and well-being in children and adolescents with juvenile idiopathic arthritis and the effect of an exercise programme: a randomized controlled trial. Pediatr Rheumatol Online J. 2013;11(1):7. doi: 10.1186/1546-0096-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramelet A-S, Fonjallaz B, Rio L, Zoni S, Ballabeni P, Rapin J, Gueniat C, Hofer M. Impact of a nurse led telephone intervention on satisfaction and health outcomes of children with inflammatory rheumatic diseases and their families: a crossover randomized clinical trial. BMC Pediatr. 2017;17(1):168. doi: 10.1186/s12887-017-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen EM, Morley-Fletcher A, Mehta DH, Lee YC. A systematic review of psychosocial therapies for children with rheumatic diseases. Pediatr Rheumatol Online J. 2017;15(1):6. doi: 10.1186/s12969-016-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takken T, Van Brussel M, Engelbert RHH, Van Der Net J, Kuis W, Helders PJM. Exercise therapy in juvenile idiopathic arthritis: a cochrane review. Eur J Phys Rehabil Med. 2008;44(3):287–297. [PubMed] [Google Scholar]
- 50.Kuntze G, Nesbitt C, Whittaker JL, Nettel-Aguirre A, Toomey C, Esau S, Doyle-Baker PK, Shank J, Brooks J, Benseler S, Emery CA. Exercise therapy in juvenile idiopathic arthritis: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2016;99(1):178–193e1. doi: 10.1016/j.apmr.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Hestbaek L, Stochkendahl MJ. The evidence base for chiropractic treatment of musculoskeletal conditions in children and adolescents: the emperor’s new suit? Chiropr Osteopat. 2010;18:15. doi: 10.1186/1746-1340-18-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali A, Weiss TR, Dutton A, McKee D, Jones KD, Kashikar-Zuck S, Silverman WK, Shapiro ED. Mindfulness-Based Stress reduction for adolescents with functional somatic syndromes: a pilot cohort study. J Pediatr. 2017;183:184–190. doi: 10.1016/j.jpeds.2016.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Lynch-Jordan A, Sil S, Peugh J, Cunningham N, Kashikar-Zuck S, Goldschneider K. Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. PAIN®. 2014;155(10):1955–1961. doi: 10.1016/j.pain.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.