Abstract
Purpose of review
This article provides a brief overview of natural phytoprotective products of allium with a special focus on the therapeutic potential of diallyl polysulfanes from garlic, their molecular targets and their fate in the living organisms. A comprehensive overview of antimicrobial and anticancer properties of published literature is presented for the reader to understand the effective concentrations of polysulfanes and their sensitivity towards different human pathogenic microbes, fungi, and cancer cell lines.
Recent findings
The article finds polysulfanes potentials as new generation novel antibiotics and chemo preventive agent. The effective dose rates of polysulfanes for antimicrobial properties are in the range of 0.5–40 mg/L and for anticancer 20–100 μM. The molecular targets for these redox modulators are mainly cellular thiols as well as inhibition and/or activation of certain cellular proteins in cancer cell lines.
Summary
Antimicrobial and anticancer activities of polysulfanes published in the literature indicate that with further development, they could be promising candidates for cancer prevention due to their selectivity towards abnormal cells.
Keywords: Garlic, Allium sativum, Polysulfanes, Reactive sulfur species, Glutathione, Redox modulators, Chemo-prevention
Introduction
Sulfur plays a major role in biology and is found in numerous peptides, proteins, and low molecular weight metabolites. Among the sulfur compounds found in plants, bacteria, fungi, and animals are many agents with unique chemical and biochemical properties linked to redox processes, metal binding, and catalytic reactions to name a few.
Nature provides a range of sulfur redox modulators from plants, fungi, bacteria, and animals that have been investigated to determine their therapeutic potential. Of which the genus allium presents a range of sulfur-based natural products with many benefits to human health from antimicrobial to anticancer effects. These natural products are particularly prominent in garlic (Allium sativum) and onion (Allium cepa) mainly consisting of thiosulfinates and polysulfanes. The biological activity of such compounds is often associated with a broad spectrum of (bio)chemical properties. Their modes of action are often associated with redox activity, catalysis, metal binding, enzyme inhibition, and/or radical generation allowing these reactive sulfur species (RSS) to interact with oxidative stressors, to affect the function of redox-sensitive cysteine proteins, and to disrupt the integrity of DNA and cellular membranes. This has been discussed in various reviews previously [1, 2]. In some cases, the biological activity of sulfur-containing plant products depends on initial enzymatic activation, which allows thiosulfinates to be generated with high target selectivity. The antibiotic and anticancer activities of RSS make them interesting from a pharmacological perspective. Not surprisingly, research into the biochemical and pharmacological properties of these sulfur chemotypes is advancing rapidly especially as anticancer agents [3].
In order to understand how these sulfur compounds develop their biological activities, we need to consider the rather complicated chemistry of various sulfur chemotypes and their biochemical transformations. Sulfur redox networks provide a glimpse of sulfur-centered formation and transformation pathways in vivo [4]. Although such networks are continuously expanding, they serve as a snapshot of the sulfur redox chemistry known to date and illustrate the complexity of redox-active sulfur species. For full details on sulfur redox mechanisms and pathways, a previous review article by Jacob is worth reading to understand this chemistry [2].
Redox Sulfanes of Allium
Sulfur metabolism in plants provides a treasure of reactive sulfur species (RSS) that includes several chemically unusual substances, such as thiosulfinates and polysulfanes from Alliums.
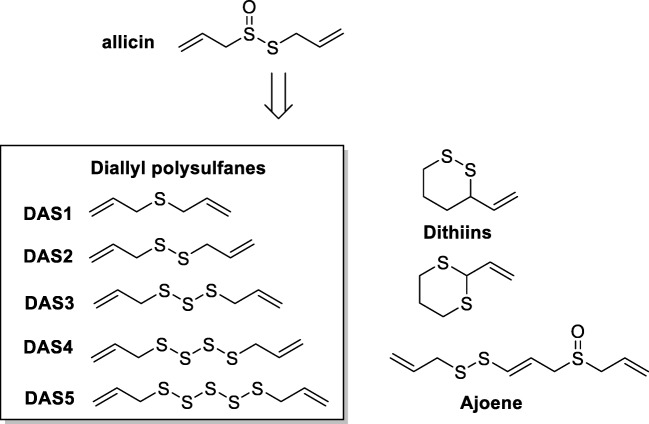
Polysulfanes are the most abundant organosulfur metabolites produced by garlic (Fig. 1) and are the result of an enzymatic reaction involving the non-protein amino acid alliin (S-allyl-l-cysteine sulfoxide), stored in large amounts in the cytosol of the plant cells (5–14 mg/g fresh dry weight, 1.4% of fresh weight) and an enzyme, allinase, present in the vacuole [6]. Upon crushing the enzyme substrate reaction produces and intermediate thiosulfinates, allicin which is not very stable at room temperature (half-life 3.1 h at 20 °C) [7].
Fig. 1.
A selection of the redox-sulfur chemistry found in garlic. Garlic produces various other chemicals which are not part of this review. The polysulfanes produced by garlic on crushing vastly depend on methods of extraction and temperature [5]. In figure DAS1 (diallyl sulfane), DAS2 (diallyl disulfane), DAS3 (diallyl trisulfane), DAS4 (diallyl tetrasulfane), DAS5 (diallyl pentasulfane)
Upon heating, allicin undergoes a cascade of further chemical rearrangements leading to other organosulfur molecules such as ajoene, dithiines, and predominantly diallyl polysulfanes (Fig. 1). The diallyl polysulfanes with DAS1-DAS4 constitute a major part of garlic extract and oils with others like methylated polysulfanes [8]. In this article, we will limit discussion to the diallyl polysulfanes mentioned in Fig. 1.
These diallyl polysulfanes (DAS1-DAS6) depicted in Fig. 1 exhibit distinct redox properties, which provide an interesting spectrum of biological activities in vivo, such as antibiotic, fungicidal, pesticidal, or anticancer activity. The last decade has provided an insight into the molecular basis for such activity and has achieved a better knowledge of the in vitro properties of diallyl polysulfanes. This has led to an improved understanding of their impact on intracellular redox signaling and control pathways in living cells.
The full impact of sulfur in living systems becomes apparent by considering the diversity of sulfur species and their reactions. The biological activity of RSS can be attributed to sulfur and its uniqueness to exist in various oxidation states that exist naturally and able to transform in in vivo environments to plethora of different chemotypes. The oxidation states and various chemotypes have been discussed in a detailed review previously [2]. The redox-active sulfur species are able to oxidize thiols to generate oxidative stressors (e.g., peroxides, hydroxyl radicals, hydropersulfides, persulfides) which in turn results in cocktail of RSS that can adopt various pathways in the cell.
Medicinal/Pharmacological Properties of Sulfanes
Antimicrobial Activity
The key studies accredited allicin as the main contributor for the antimicrobial activity of garlic [9]. Allicin was found to inhibit bacterial growth as a vapor of lung pathogenic bacteria from the genera Pseudomonas, Streptococcus, and Staphylococcus, including multi-drug-resistant (MDR) strains, suggesting that it could be used to combat bacterial lung infections via direct inhalation; currently, there are no volatile antibiotics available to treat pulmonary infections [10]. Growth inhibition of Escherichia coli during allicin exposure coincides with a depletion of the glutathione pool and S-allylmercapto-modification of proteins, resulting in an overall decreased total sulfhydryl levels, which is accompanied by the induction of the oxidative and heat stress response. The mode of action of allicin is a combination of a decrease of glutathione levels, unfolding stress, and inactivation of crucial metabolic enzymes through S-allylmercapto-modification of cysteines [11]. It is suggested that allicin’s ability to permeabilize cell membranes may contribute to its antimicrobial activity independently of its activity as a thiol reagent [12].
Garlic oil and its major diallyl polysulfanes constituents, as well as garlic extract, and allicin possess significant activity against H. pylori [13]. DAS2 and DAS3 were found to be the most abundant components in garlic oil. Interestingly, DAS4 also inhibits H. pylori, an activity which might be relevant in the context of garlic consumption and stomach ulcers. The minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of DAS4 against the NCTC 11637, and 107018 B strains of H. pylori are between 3 and 6 μg/mL, i.e., even lower than the values for allicin (6–12 μg/mL). DAS1, DAS2, and DAS3 were less active, with MIC values of 2074–4148, 100–200, and 13–25 μg/mL, respectively [14].
There have been many studies using garlic to combat clinically important bacteria and fungi. Garlic extract was tested against three major antibiotic resistant pathogens: C. albicans, MRSA, and P. aeruginosa. Interestingly, a synergism effect was reported of garlic extract improving the effectiveness of the antibiotics [15]. A separate study on 30 clinical isolates of MRSA found that allicin, extracted from garlic, caused 88% of the strains tested to have MICs of 16 μg/mL and all strains were inhibited at 32 μg/mL [16]. Garlic extract was used in a study on the drug resistant pathogens E. coli, P. aeruginosa, B. subtilis, S. aureus, K. pneumoniae, S. sonnei, S. epidermidis, and S. typhi. All pathogens showed high susceptibility to garlic extract where the lowest MIC was 0.05 mg/mL [17]. Studies using synthetic allicin, which included multi-drug resistant (MDR) strains, showed that the growth of the majority of Pseudomonas, Streptococcus, and Staphylococcus isolates was completely inhibited by 64 μg/mL allicin. S. pyogenes (SNo 67467), S. pneumoniae (SNo 68668), and S. aureus (ATCC 43300) were completely inhibited by 32 μg/mL allicin and all A. baumannii isolates were completely inhibited by 16 μg/mL. However, K. pneumoniae isolates were slightly more resistant, with a MIC of 128 μg/mL. P. aeruginosa (DSM2659) showed high resistance to allicin (MIC 512 μg/mL) compared to P. aeruginosa (PAO1 SBUG8 and PAO25), MIC 64 μg/mL. MDR and non-MDR S. pneumoniae strains tested were equally susceptible to allicin and showed MICs from 32 to 64 μg/mL allicin and MBCs from 64 to 128 μg/mL allicin, respectively. This study shows that different strains have different susceptibilities to garlic and its constituents [10].
Antifungal studies found that garlic oil can penetrate the cell membrane of C. albicans as well as organelle membranes, such as mitochondria, which would result in destruction of the organelle and ultimately cell death. Due to their lipophilic nature, it is likely that many of the diallylpolysulfanes can pass through membranes of various organisms [18] and they have been shown to interact with membrane lipids to modify membrane fluidity [19].
Garlic oil has been shown to induce differential expression of important genes such as those involved in pathogenesis, oxidation-reduction, and cellular response to drugs and starvation [20]. Allicin and aged garlic extracts exhibit antimicrobial properties against the Burkholderia cepacia complex (Bcc), an intrinsically multi-resistant and life-threatening human pathogen showing the modification of cysteine residue which suggest allicin ability as a general electrophilic reagent targeting protein thiols [21, 22].
An important observation, which is demonstrated in Tables 1 and 2, is the different susceptibilities of pathogens to the different garlic constituents. DAS3 and DAS4 from garlic exhibit a wide spectrum of antimicrobial, antibacterial and antifungal activities For example, DAS3 and DAS4 both inhibit S. aureus (MIC 2.0 and 0.5 μg/mL, respectively), S. aureus (MRSA) (MIC 8.0 and 2.0 μg/mL, respectively), C. albicans (MIC 1.0 and 0.5 μg/mL, respectively), C. krusei (MIC 8.0 and 4.0 μg/mL, respectively), C. glabrate (MIC 4.0 and 2.0 μg/mL, respectively), A. niger (MIC 2.0and 1.0 μg/mL, respectively), A. fumigatus (MIC 8.0 and 4.0 μg/mL, respectively), and A. flavus (MIC 4.0 and 2.0 μg/mL, respectively). While the DAS3 is consistently less active, DAS4 possesses an antibiotic activity comparable to that of allicin. For example, against H. pylori, DAS4 has a MIC of 3–6 mg/L and allicin has a MIC of 6–12 mg/L [23].
Table 1.
MIC values (mg/L) of polysulfanes against different human pathogenic bacteria. MIC values have been converted to mg/L where other concentration units were reported in the literature
| Garlic component/preparation | Organism | MIC (mg/L) | Reference |
|---|---|---|---|
| Allicin | H. pylori | 6–12 | [23] |
| Pseudomonas spp. | 64 | [10] | |
| Streptococcus spp. | 64 | ||
| Staphylococcus spp. | 64 | ||
| P. aeruginosa | 64 | ||
| S. pneumonia | 32 | ||
| S. pyogenes | 32 | ||
| S. aureus | 32 | ||
| A. baumannii | 16 | ||
| E. coli | 23 | [11] | |
| DAS1 | H. pylori | 2074–4148 | [23] |
| B. cereus | 64 | [24] | |
| C. jejuni | 56 | ||
| C. botulinium | 64 | ||
| E. coli | 72 | ||
| L. monocytogenes | 48 | ||
| S. enteric | 54 | ||
| S. aureus | 64 | ||
| V. cholerae | 72 | ||
| DAS2 | S. aureus | 2 | [23] |
| H. pylori | 100 | ||
| B. cereus | 14 | [24] | |
| C. jejuni | 12 | ||
| C. botulinium | 20 | ||
| E. coli | 20 | ||
| L. monocytogenes | 8 | ||
| S. enteric | 12 | ||
| S. aureus | 16 | ||
| V. cholerae | 24 | ||
| DAS3 | S. aureus | 0.5 | [23] |
| H. pylori | 13–25 | ||
| B. cereus | 4 | [24] | |
| C. jejuni | 2 | ||
| C. botulinium | 4 | ||
| E. coli | 12 | ||
| L. monocytogenes | 2 | ||
| S. enteric | 2 | ||
| S. aureus | 8 | ||
| V. cholera | 12 | ||
| M. tuberculosis | 2.5 | [25] | |
| DAS4 | H. pylori | 3–6 | [23] |
| B. cereus | 1 | [24] | |
| C. jejuni | 1 | ||
| C. botulinium | 1 | ||
| E. coli | 4 | ||
| L. monocytogenes | 0.5 | ||
| S. enteric | 0.5 | ||
| S. aureus | 2 | ||
| V. cholerae | 4 | ||
| Garlic oila | H. pylori | 8–32 | [23] |
| B. cereus | 40 | [24] | |
| C. jejuni | 36 | ||
| C. botulinium | 32 | ||
| E. coli | 48 | ||
| L. monocytogenes | 20 | ||
| S. enteric | 32 | ||
| S. aureus | 36 | ||
| V. cholera | 40 | ||
| S. boydii | 2.75 | [26] | |
| S. flexnar | 2.75 | ||
| S. fluvialis | 2.75–5.5 | ||
| V. metschnikovii | 0.34 | ||
| V. parahaemoyticus | 0.08 | ||
| V. enterocolittica | 0.68 | ||
| C. coli | 0.49 | ||
| C. lari | 0.49 | ||
| B. fragilis | 0.04 | ||
| B. subtilis | 0.17–0.68 | ||
| E. aerogenes | 0.68 | ||
| E. faecalis | 0.34 | ||
| K. aerogenes | 0.17 | ||
| P. vulgaris | 2.74 | ||
| L. acidophilus | 0.34–2.75 | ||
| S. faecalis | 0.34 | ||
| S. mutans | 0.08 | ||
| S. pyogenes | 0.04 | ||
| Garlic extracta | P. gingivalis | 16.6 | [27] |
| P. aeruginosa | 6 | ||
| A. actinomycetemcomitans | 62.5 | ||
| S. aureus | 4 | [28] | |
| E.coli | 7 | ||
| B. subtilis | 0.1 | [29] | |
| K. pneumonia | 0.2 | ||
| S. epidermidis | 0.9 | ||
| S. typhi | 0.02 | ||
| Proteus spp. | 7–21 | [30] | |
| H. pylori | 2–5 | [31] | |
| S. epidermidis | 22.9 | [32] | |
| S. pneumoniae | 30.3 | ||
| S. pyogenes | 33 | ||
| H. influenzae | 30.5 | ||
| Shigella spp. | 15.6 | ||
| P. aeruginosa | 3.5 | ||
| S. mutans | 4–32 | [33] |
aActivity depends on how garlic oil and garlic extract are manufactured. Various papers depicting the biological activity of polysulfanes did not mention the characterization of such preparations. The concentrations of individual polysulfanes in an extract or oil widely depend on method of extraction or distillation
Table 2.
MIC (mg/L) of polysulfanes against different pathogenic fungal species. MIC values have been converted to mg/L where other concentration units were reported in the literature
| Garlic component/preparation | Organism | MIC (mg/L) | Percentage inhibition at MIC (%) | Reference |
|---|---|---|---|---|
| Allicin | C. albicans | 0.8 | 100 | [34] |
| C. neoformans | 0.3 | |||
| C. parapsilosis | 0.15 | |||
| C. tropicalis | 0.3 | |||
| C. krusei | 0.3 | |||
| T. glabrata | 0.3 | |||
| Aspergillus spp. | 8–32 | 100 | [35] | |
| T. rubrum | 12.5 | 90 | [36] | |
| DAS2 | C. krusei | 8 | 100 | [14] |
| C. albicans | 1 | |||
| C. krusei | 8 | |||
| C. glabrate | 4 | |||
| A. niger | 2 | |||
| A. fumigates | 8 | |||
| A. flavus | 4 | |||
| DAS3 | C. krusei | 2 | 100 | [14] |
| C. albicans | 0.5 | |||
| C. krusei | 4 | |||
| C. glabrate | 2 | |||
| A. niger | 1 | |||
| A. fumigates | 4 | |||
| A. flavus | 2 | |||
| Garlic oila | C. albicans | 0.35 | 100 | [20] |
| P. funiculosum | 0.69 | |||
| Garlic extracta | Candida sp. | 14.9 | 100 | [32] |
aActivity depends on how garlic oil and garlic extract are manufactured. Various papers depicting the biological activity of polysulfanes did not mention the characterization of such preparations. The concentrations of polysulfanes in an extract or oil widely depend on method of extraction or distillation
Garlic was tested for synergistic effects with antibiotics (levofloxacin, gentamicin, azithromycin, and doxycycline) against Pseudomonas and Acinetobacter genera. This results in a decrease in the antibiotic MIC of 4-≥32, 4-≥2048, 2-≥2048 and 2-≥128 fold, respectively. The garlic increased the rate of lethality of the antibiotics against the bacteria. While these results show a potential for the synergistic use of garlic with antibiotics, a notable weakness of this study is it does not provide any details of the garlic preparation that is used [37, 38]. This unfortunately limits the meaningfulness of this piece of work.
Anticancer Activities
Polysulfanes have also been studied as a potential anticancer agent. Most studies have been conducted on DAS3 which shows this molecule as promising chemopreventive therapy for cancer. Studies with polysulfanes on different cancer lines and effective dose rates are summarized in Table 3. This research area has developed in the last decade enormously and various research groups have identified different targets in different types of cancer cell lines. The details of molecular targets identified by polysulfanes are highlighted in next section. This was noticed that some cancer cell lines are more sensitive to polysulfanes than others (Table 3). For example, DAS3 was effective at very low concentration (~ 2–9 mg/mL) in colon and breast cancer compared to gastric and skin (~ 29 mg/mL).
Table 3.
Effect of polysulfanes on different human cancer cell lines and their molecular targets. The concentration units reported in the literature have all been converted to mg/mL so they can be compared with the antimicrobial activities in Tables 1 and 2
| Redox modulator | Cancer type/cell line | Dose (mg/mL) | Target | Effect | Reference |
|---|---|---|---|---|---|
| Ajoene | Leukemia | 9.36 | Bcl-2 | Inhibition of proliferation and induction of apoptosis | [39] |
| HL-60, U937, HEL and OCIM-1 | Caspase-3 | ||||
| DAS2 | Breast cancer | 2.6 | Estrogen receptor (ER)-positive (KPL-1 and MCF-7) and -negative (MDA-MB-231 and MKL-F) | Growth inhibition of cancer cells by inducing apoptosis | [40] |
| MDA-MB-231 | |||||
| DAS2 | Breast | 29.2 | Kinase protein | Inhibited proliferation of MCF-7 cells and increased apoptotic ratio | [41] |
| MCF-7 | |||||
| Caspase-3 | |||||
| DAS2 | Colon | 29.2 | Histone H3 and H4 | Inhibition of caner proliferation by interaction with HDAC pathway | [42] |
| Caco-2, HT-29 | |||||
| DAS3 | Liver | 35 | Caspase-3 | Increased H2O2 levels, lowered thiol levels and inhibited cell proliferation | [3] |
| HepG2 | |||||
| DAS3 | Colon | 2.0 | Tubulin | Suppression of cell growth | [43] |
| HCT-15 | 2.3 | ||||
| DLD-1 | |||||
| DAS3 | Prostate | 7.1 | CDK1 | Inhibition of cells by dose dependent manner. | [44] |
| PC-3 | |||||
| DAS3 | Gastric | 20.5 | Bcl-2 | Inhibited viability of BCG-823 in vitro and modulated Bcl-2. | [45] |
| BGC-823 | |||||
| DAS3 | Breast | 1.78 | MMP2/9 | Suppressed metastasis | [46] |
| MDA-MB-231 | |||||
| HS 578t | |||||
| DAS3 | Colon | 8.91 | Focal adhesion kinase (FAK | Inhibition of angiogenesis | [47] |
| HT29 | |||||
| DAS3 | A375 | 17.8 | Mitochondrial caspase pathway | Increase in ROS | [48] |
| Skin | |||||
| DAS3 | Lymphoma | 3.56 | NF-κB | Apoptosis in primary effusion lymphoma [PEL] | [49] |
| BC2, BC3, | |||||
| BCBL1, HBL6 | |||||
| DAS3 | Prostate | 3.56 | Androgen receptor (AR) | Decrease in AR levels | [50] |
| LNCaP, C4-2, TRAMP-C1 | |||||
| DAS3 | Glioblastoma | 17.8 | Bcl-2 | Inhibition and proliferation | [51] |
| U87MG | |||||
| Neuroblastoma | |||||
| SH-SY5Y |
General reviews have been published on polysulfanes as chemopreventives with a special focus on DAS3 for pediatric cancer treatment. A recent review indicated that DAS3 is of importance not only for its remarkable antitumor and cancer preventive effects, as suggested by many in vitro and in vivo studies, but also because of its many health benefits, like improvements to immune-system function, radioprotection, and protection against microbial infections. These features make it a potential candidate for the treatment of pediatric cancers. Herman-Antosiewicz and Yi concluded the molecular targets of polysulfanes in cellular environment showing that it selectively targets the cancer cells [52, 53].
DAS3 is more potent than mono- and disulfides against skin cancer. DAS3 inhibits cell growth of human melanoma A375 cells and basal cell carcinoma (BCC) cells by increasing the levels of intracellular reactive oxygen species (ROS) and DNA damage and by inducing G2/M arrest, endoplasmic reticulum (ER) stress. A recent review focuses on the molecular mechanisms of garlic-derived allyl sulfides on skin cancer prevention [54]. Similar findings were observed in human colon cancer cells HCT-15 and DLD-1. The growth of the cells was significantly suppressed by DAS3, but neither DAS nor DAS2 showed such an effect [55]. An interesting paper depicts that allyl group in polysulfanes are responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells [43]. The effective dose rate in different cancer cell lines and their molecular targets are summarized in Table 3 below.
Molecular Targets and Metabolism of Sulfanes
Low molecular weight (LMW) thiols such as glutathione (GSH) serve as intracellular redox buffers in most aerobic organisms. They play a central role in the essential maintenance of an intracellular reducing environment and neutralize the damaging effects of toxic oxidants. When the cellular concentrations of these LMW thiols are dramatically reduced, the ability to defend against, and survive, oxidative stress is severely impaired [56].
Upon entering the target (i.e., microbes and cancer cells) polysulfanes undergo rapid thiol-polysulfide exchange reactions with these LMW thiols. The implications of this process are twofold: (a) cellular LMW thiol concentrations decrease making them more susceptible to oxidative stresses and (b) in parallel, RSS such as allyl persulfide, hydropolysulfide species are formed, which can enhance the production of toxic oxidants (e.g., hydrogen peroxide, superoxide) thereby increasing oxidative stress [2].
In addition, polysulfanes can also react with exposed cysteine thiols on proteins to form S-allyl modified proteins. Such cysteinyl-S-allylations processes can result in altered/impaired protein function [57]. In addition to the redox activity of these molecules, their lipophilicity may contribute to their biological activity (e.g., by interrupting membrane integrity, binding to hydrophobic sites on proteins) [18, 58]. These different modes of action are summarized in Fig. 2.
Fig. 2.
A summary of diallyl polysulfane reactions/interactions in vivo and their physiological consequences [1]. Once polysulfanes enter the target cell, they can react with thiols (GSH) to produce hydropolysulfane (RSxH), which upon oxidation (ROS) can produce perthiyl radicals (RSS•). Perthiyl radicals can decompose into thiyl radicals (RS•), after accepting an electron they can result in formation of thiols. The pathways of such species have been discussed previously, and hence, will not be presented here [2, 4]. Diallyl polysulfanes can either enhance or suppress cytochrome P450s, which are involved in the detoxification process [59]. It is hypothesized that reduction of polysulfanes leads to the production of allyl mercaptan (AM) which can be further methylated by S-adenosyl methionine synthetase into allyl methyl sulfide (AMS) [60, 61]. AM and AMS have been determined as DAS1 and DAS2 metabolites [62]. SAM S-adenosyl methionine, SAH S-adenosyl homocysteine
In pathological conditions, it has been shown that organosulfur compounds from garlic have protective effects, which are mostly associated with antioxidant properties. Because of the ability of polysulfanes to target multiple biochemical pathways, many researchers have studied their roles. The major metabolic pathways of sulfanes in mammals include methylation, oxidation, glutathione, and N-acetyl conjugations, and in certain instances, after ingestion of raw garlic by a human, allyl methyl sulfide, allyl methyl disulfide, DAS2, and DAS3 were discovered in the breath of tested volunteers [63], therefore providing an early example of their biochemical transformations within the body.
Recent studies in cancer cell lines investigated various pathways of polysulfanes within cell that includes proliferation, G2/M phase arrest, and radical and triggering antioxidant response [41, 52, 64–66]. Polysulfanes can also activate other biochemical pathways, i.e., inducing caspase-3 activity, enhancing H2O2 levels, and strongly decreasing glutathione levels, inhibiting the expression of estrogen proteins [67–71], modulation of Bcl-2 family proteins [48], inhibition of HDAC pathways [72], and angiogenesis [47].
The metabolic and fate of polysulfanes have not been widely studied, but this subject has gathered attention in the last few years. In relation to DAS1, it can form conjugates with GSH and modified glutathione S-transferase, glutathione peroxidase, and glutathione reductase activities [73]. Some studies in rats conclude that DAS2 quickly produces allyl mercaptan, allyl methyl sulfide, allyl methyl sulfoxide, and allyl methyl sulfone as major metabolites in rat liver. Similarly, allyl mercaptan was isolated from rat liver and extracellular fluid of primary rat hepatocytes when perfused with DAS2 (1 mM) after 90 min [74].
DAS2 and DAS3 can induce NAD(P)H:quinone oxidoreductases 1 (NQO1) via nuclear factor erythroid 2 (Nrf2) activation [75–78]. It has also been shown that DAS3 can induce intracellular ROS accumulation, which would activate Nrf2 by oxidation of cysteine residues [77]. DAS3 can also elevate intracellular ROS levels and the redox-regulatory proteins, such as glutaredoxin (GRX) [79]. In human cancer cells, this activates the ASK1-MEK-JNK-Bim transduction-signaling pathway, which subsequently triggers the Bax-dependent mitochondrial apoptotic pathway resulting in apoptosis [80, 81].
As with all polysulfanes, increasing the H2O2 concentration can result in a cascade of molecular events due to specific oxidation of signaling proteins including kinases, transcription factors, and phosphatases. H2O2 can also react with low molecular weight thiols, such as GSH and cysteine, by a nucleophilic attack from the thiolate onto the reactive H2O2. The acidity of the electrostatic environment around the –SH group may increase which increases the reactivity towards H2O2. This would result in a higher fraction of the thiolate form. In cysteine residues, lower stability of the thiolate increases nucleophilicity towards H2O2 of the thiolate [82]. Some studies using a sulfane model suggest an important role for O2•- radicals in inducing cell death (apoptosis) [83].
Polysulfanes have also been investigated as potent H2S donors in the presence of thiols [84, 85]. Preclinical studies have shown that enhancement of endogenous H2S has an impact on vascular reactivity. In CVD models, the administration of H2S prevents myocardial injury and dysfunction. It is hypothesized that these beneficial effects of garlic may be mediated by H2S-dependent mechanisms [86]. Computational and experimental studies have revealed that glutathione and cysteine are capable of releasing H2S from diallyl trisulfide (DAS3), via thiol-polysulfide exchange pathways, but diallyl disulfide (DAS2) is a much poorer H2S donor via an α-carbon nucleophilic substitution pathway [87, 88].
Intraperitoneal (ip) administration of DAS1, DAS2, and DAS3 in mice increased the activity of rhodanese. Moreover, DAS2 and DAS3 increased the total sulfane sulfur level and γ-cystathionase activity in the normal mouse kidney. Aldehyde dehydrogenase activity was inhibited in the kidney after DAS3 administration. The results indicated that none of the studied polysulfanes affected the level of bound sulfur or H2S. Thus, it can be concluded that garlic-derived DAS2 and DAS3 can be a source of sulfane sulfur for renal cells but they are not connected with persulfide formation [89]. DAS1, DAS2, and DAS3 dissolved in corn oil were given intraperitoneally to mice for 10 days. It showed that polysulfanes had a beneficial effect in the mouse liver, decreasing reactive oxygen species and malondialdehyde levels, and increasing glutathione S-transferase activity and non-protein sulfhydryl group level. Moreover, DAS2 and DAS3 elevated the total sulfane sulfur pool and activity suggesting its antioxidant and regulatory capacities [90].
Some studies of DAS4 have shown that it induces reactive oxygen species (ROS) in normal cells similar to cancer cells in a time (0 to 60 min) and dose-dependent manner (0 to 50 μM) [91]; it also activates both the eIF2α and Nrf2/HO-1 pathways [92].
Conclusion
This review summarizes the numerous biological activities garlic diallyl polysulfanes are involved in. While there is extensive evidence that show the various medicinal benefits of garlic polysulfanes, there is a critical need for controlled clinical studies to strictly evaluate the safety and efficacy of these compounds for establishing sufficient application methods before medical use. Furthermore, polysulfanes are emerging as promising, environmentally benign pesticides which are not only safe for humans, but also various non-target species endangered by synthetic chemical pesticides [8, 93]. With further work, diallyl polysulfanes could provide the basis for the innovative development of novel antibiotics, fungicides, pesticides, and anticancer agents.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of theauthors.
Footnotes
This article is part of the Topical Collection on Redox Modulators
References
- 1.Münchberg U, Anwar A, Mecklenburg S, Jacob C. Polysulfides as biologically active ingredients of garlic. Org Biomol Chem. 2007;5:1505–1518. doi: 10.1039/b703832a. [DOI] [PubMed] [Google Scholar]
- 2.Jacob C. A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat Prod Rep. 2006;23:851–863. doi: 10.1039/b609523m. [DOI] [PubMed] [Google Scholar]
- 3.Iciek M, Kwiecien I, Wlodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50(3):247–265. doi: 10.1002/em.20474. [DOI] [PubMed] [Google Scholar]
- 4.Filomeni G, Rotilio G, Ciriolo MR. Molecular transduction mechanisms of the redox network underlying the antiproliferative effects of allyl compounds from garlic. J Nutr. 2008;138(11):2053–2057. doi: 10.1093/jn/138.11.2053. [DOI] [PubMed] [Google Scholar]
- 5.Block E, editor. Garlic and other Alliums, the Lore and the Science. Cambridge: RSC Publishing; 2010. [Google Scholar]
- 6.Lawson LD. Garlic: a review of its medicinal effects and indicated active compounds. Phytomedicines of Europe. ACS Symposium Series, vol 691: American Chemical Society; 1998. p. 176–209.
- 7.Fujisawa H, Suma K, Origuchi K, Seki T, Ariga T. Thermostability of allicin determined by chemical and biological assays. Biosci Biotechnol Biochem. 2008;72(11):2877–2883. doi: 10.1271/bbb.80381. [DOI] [PubMed] [Google Scholar]
- 8.Anwar A, Gould E, Tinson R, Groom M, Hamilton C. Think yellow and keep green—role of sulfanes from garlic in agriculture. Antioxidants. 2017;6(1):3. doi: 10.3390/antiox6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavallito CJ, Bailey JH. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J Am Chem Soc. 1944;66(11):1950–1951. doi: 10.1021/ja01239a048. [DOI] [Google Scholar]
- 10.Reiter Jana, Levina Natalja, van der Linden Mark, Gruhlke Martin, Martin Christian, Slusarenko Alan. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules. 2017;22(10):1711. doi: 10.3390/molecules22101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller A, Eller J, Albrecht F, Prochnow P, Kuhlmann K, Bandow JE, et al. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J Biol Chem. 2016;291(22):11477–11490. doi: 10.1074/jbc.M115.702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruhlke MC, Hemmis B, Noll U, Wagner R, Luhring H, Slusarenko AJ. The defense substance allicin from garlic permeabilizes membranes of Beta vulgaris, Rhoeo discolor, Chara corallina and artificial lipid bilayers. Biochim Biophys Acta. 2015;1850(4):602–611. doi: 10.1016/j.bbagen.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Zardast M, Namakin K, Esmaelian Kaho J, Hashemi SS. Assessment of antibacterial effect of garlic in patients infected with Helicobacter pylori using urease breath test. Avicenna J Phytomed. 2016;6(5):495–501. [PMC free article] [PubMed] [Google Scholar]
- 14.O'Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 2000;66(5):2269–2273. doi: 10.1128/AEM.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Ma X, Deng L, Zhao X, Wei Y, Gao Z, Jia J, Xu J, Sun C. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J Microbiol. 2015;8(5):e14814. doi: 10.5812/jjm.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutler RR, Wilson P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br J Biomed Sci. 2004;61(2):71–74. doi: 10.1080/09674845.2004.11732646. [DOI] [PubMed] [Google Scholar]
- 17.Gull I, Saeed M, Shaukat H, Aslam SM, Samra ZQ, Athar AM. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann Clin Microbiol Antimicrob. 2012;11:8. doi: 10.1186/1476-0711-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anwar A, Burkholz T, Scherer C, Abbas M, Lehr C-M, Diederich M, et al. Naturally occurring reactive sulfur species, their activity against Caco-2 cells, and possible modes of biochemical action (vol 28, pg 251, 2008) J Sulfur Chem. 2008;29(5):573. doi: 10.1080/17415990802519103. [DOI] [Google Scholar]
- 19.Tsuchiya H, Nagayama M. Garlic allyl derivatives interact with membrane lipids to modify the membrane fluidity. J Biomed Sci. 2008;15(5):653–660. doi: 10.1007/s11373-008-9257-8. [DOI] [PubMed] [Google Scholar]
- 20.Li WR, Shi QS, Dai HQ, Liang Q, Xie XB, Huang XM, Zhao GZ, Zhang LX. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci Rep. 2016;6:22805. doi: 10.1038/srep22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallock-Richards D, Doherty CJ, Doherty L, Clarke DJ, Place M, Govan JRW, Campopiano DJ. Garlic revisited: antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS One. 2014;9(12):e112726. doi: 10.1371/journal.pone.0112726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Qing, Meng Xiao, Li Ya, Zhao Cai-Ning, Tang Guo-Yi, Li Hua-Bin. Antibacterial and Antifungal Activities of Spices. International Journal of Molecular Sciences. 2017;18(6):1283. doi: 10.3390/ijms18061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 2000;66(5):2269–2273. doi: 10.1128/AEM.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rattanachaikunsopon P, Phumkhachorn P. Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria of chives (Allium schoenoprasum) Biosci Biotechnol Biochem. 2008;72(11):2987–2991. doi: 10.1271/bbb.80482. [DOI] [PubMed] [Google Scholar]
- 25.Oosthuizen C, Arbach M, Meyer D, Hamilton C, Lall N. Diallyl polysulfides from Allium sativum as immunomodulators, hepatoprotectors, and antimycobacterial agents. J Med Food. 2017;20(7):685–690. doi: 10.1089/jmf.2016.0137. [DOI] [PubMed] [Google Scholar]
- 26.Ross ZM, O'Gara EA, Hill DJ, Sleightholme HV, Maslin DJ. Antimicrobial properties of garlic oil against human enteric bacteria: evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl Environ Microbiol. 2001;67(1):475–480. doi: 10.1128/AEM.67.1.475-480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shetty S, Thomas B, Shetty V, Bhandary R, Shetty RM. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: a microbiological study. Ayu. 2013;34(4):445–451. doi: 10.4103/0974-8520.127732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha SK, Saha S, Akhter SM, Khatun S, Islam MM, Roy P. In vitro determination of minimum inhibitory concentration of aqueous garlic extract and imipenem against Staphylococcus aureus and Escherichia coli. Mymensingh Med J. 2016;25(3):477–484. [PubMed] [Google Scholar]
- 29.Gull I, Saeed M, Shaukat H, Aslam SM, Samra ZQ, Athar AM. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann Clin Microbiol Antimicrob. 2012;11:8. doi: 10.1186/1476-0711-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durairaj, Srinivasan, Lakshmanaperumalsamy. In vitro antibacterial activity and stability of garlic extract at different pH and temperature. Electron J Biol. 2009;5(1).
- 31.Cellini L, Di Campli E, Masulli M, Di Bartolomeo S, Allocati N. Inhibition of helicobacter pylori by garlic extract (Allium sativum) FEMS Immunol Med Microbiol. 1996;13(4):273–277. doi: 10.1111/j.1574-695X.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 32.Iwalokun BA, Ogunledun A, Ogbolu DO, Bamiro SB, Jimi-Omojola J. In vitro antimicrobial properties of aqueous garlic extract against multidrug-resistant bacteria and Candida species from Nigeria. J Med Food. 2004;7(3):327–333. doi: 10.1089/jmf.2004.7.327. [DOI] [PubMed] [Google Scholar]
- 33.Fani MM, Kohanteb J, Dayaghi M. Inhibitory activity of garlic (Allium sativum) extract on multidrug-resistant Streptococcus mutans. J Indian Soc Pedod Prev Dent. 2007;25(4):164–168. doi: 10.4103/0970-4388.37011. [DOI] [PubMed] [Google Scholar]
- 34.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1(2):125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 35.Shadkchan Y, Shemesh E, Mirelman D, Miron T, Rabinkov A, Wilchek M, Osherov N. Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro, and in a murine model of disseminated aspergillosis. J Antimicrob Chemother. 2004;53(5):832–836. doi: 10.1093/jac/dkh174. [DOI] [PubMed] [Google Scholar]
- 36.Aala F, Yusuf UK, Jamal F, Rezaie S. Antimicrobial effects of allicin and ketoconazole on trichophyton rubrum under in vitro condition. Braz J Microbiol. 2012;43(2):786–792. doi: 10.1590/s1517-83822012000200044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abouelfetouh M. Enhancement of antimicrobial activity of four classes of antibiotics combined with garlic. Asian J Plant Sci. 2012;11:148–152. doi: 10.3923/ajps.2012.148.152. [DOI] [Google Scholar]
- 38.Li G, Ma X, Deng L, Zhao X, Wei Y, Gao Z, Jia J, Xu J, Sun C. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J Microbiol. 2015;8(5):e14814. doi: 10.5812/jjm.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassan HT. Ajoene (natural garlic compound): a new anti-leukaemia agent for AML therapy. Leuk Res. 2004;28(7):667–671. doi: 10.1016/j.leukres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa H, Kiyozuka Y, Uemura Y, Senzaki H, Shikata N, Hioki K, Tsubura A. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol. 2001;127(4):258–264. doi: 10.1007/s004320000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei X-y, Yao S-q, Zu X-y, Huang Z-x, Liu L-j, Zhong M, et al. Apoptosis induced by diallyl disulfide in human breast cancer cell line MCF-7. Acta Pharmacol Sin. 2008;29:1233. doi: 10.1111/j.1745-7254.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- 42.Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duée P-H, Martel P, Chaumontet C. Diallyl disulfide (DADS) increases histone acetylation and p21 waf1/cip1 expression in human colon tumor cell lines. Carcinogenesis. 2004;25(7):1227–1236. doi: 10.1093/carcin/bgh123. [DOI] [PubMed] [Google Scholar]
- 43.Hosono T, Hosono-Fukao T, Inada K, Tanaka R, Yamada H, Iitsuka Y, Seki T, Hasegawa I, Ariga T. Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells. Carcinogenesis. 2008;29(7):1400–1406. doi: 10.1093/carcin/bgn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arunkumar A, Vijayababu MR, Kanagaraj P, Balasubramanian K, Aruldhas MM, Arunakaran J. Growth suppressing effect of garlic compound diallyl disulfide on prostate cancer cell line (PC-3) <i>in vitro</i>. Biol Pharm Bull. 2005;28(4):740–743. doi: 10.1248/bpb.28.740. [DOI] [PubMed] [Google Scholar]
- 45.Jiang XY, Zhu XS, Xu HY, Zhao ZX, Li SY, Li SZ, Cai JH, Cao JM. Diallyl trisulfide suppresses tumor growth through the attenuation of Nrf2/Akt and activation of p38/JNK and potentiates cisplatin efficacy in gastric cancer treatment. Acta Pharmacol Sin. 2017;38(7):1048–1058. doi: 10.1038/aps.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Zhu P, Wang Y, Wei Z, Tao L, Zhu Z, Sheng X, Wang S, Ruan J, Liu Z, Cao Y, Shan Y, Sun L, Wang A, Chen W, Lu Y. Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NF-kappaB and ERK/MAPK signaling pathways. PLoS One. 2015;10(4):e0123781. doi: 10.1371/journal.pone.0123781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai KC, Hsu SC, Yang JS, Yu CC, Lein JC, Chung JG. Diallyl trisulfide inhibits migration, invasion and angiogenesis of human colon cancer HT-29 cells and umbilical vein endothelial cells, and suppresses murine xenograft tumour growth. J Cell Mol Med. 2015;19(2):474–484. doi: 10.1111/jcmm.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang HC, Yang JH, Hsieh SC, Sheen LY. Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/M arrest and apoptosis. J Agric Food Chem. 2010;58(11):7096–7103. doi: 10.1021/jf100613x. [DOI] [PubMed] [Google Scholar]
- 49.Shigemi Z, Furukawa Y, Hosokawa K, Minami S, Matsuhiro J, Nakata S, et al. Diallyl trisulfide induces apoptosis by suppressing NF-kappaB signaling through destabilization of TRAF6 in primary effusion lymphoma. Int J Oncol. 2016;48(1):293–304. doi: 10.3892/ijo.2015.3247. [DOI] [PubMed] [Google Scholar]
- 50.Stan SD, Singh SV. Transcriptional repression and inhibition of nuclear translocation of androgen receptor by diallyl trisulfide in human prostate cancer cells. Clin Cancer Res. 2009;15(15):4895–4903. doi: 10.1158/1078-0432.Ccr-09-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jurkowska H, Wróbel M, Kaczor-Kamińska M, Jasek-Gajda E. A possible mechanism of inhibition of U87MG and SH-SY5Y cancer cell proliferation by diallyl trisulfide and other aspects of its activity. Amino Acids. 2017;49(11):1855–1866. doi: 10.1007/s00726-017-2484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antosiewicz J, Ziolkowski W, Kar S, Powolny AA, Singh SV. Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med. 2008;74(13):1570–1579. doi: 10.1055/s-2008-1081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi L, Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem Toxicol. 2013;57:362–370. doi: 10.1016/j.fct.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Xia Q, Cui J, Diao Y, Li J. Reversion of P-glycoprotein-mediated multidrug resistance by diallyl trisulfide in a human osteosarcoma cell line. Oncol Rep. 2014;31(6):2720–2726. doi: 10.3892/or.2014.3154. [DOI] [PubMed] [Google Scholar]
- 55.Seki T, Hosono T, Hosono-Fukao T, Inada K, Tanaka R, Ogihara J, et al. Anticancer effects of diallyl trisulfide derived from garlic. Asia Pac J Clin Nutr. 2008;17(Suppl 1):249–252. [PubMed] [Google Scholar]
- 56.Masip L, Veeravalli K, Georgiou G. The many faces of glutathione in bacteria. Antioxid Redox Signal. 2006;8(5–6):753–762. doi: 10.1089/ars.2006.8.753. [DOI] [PubMed] [Google Scholar]
- 57.Ariga Toyohiko, Seki Taiichiro. Antithrombotic and anticancer effects of garlic-derived sulfur compounds: A review. BioFactors. 2006;26(2):93–103. doi: 10.1002/biof.5520260201. [DOI] [PubMed] [Google Scholar]
- 58.Schneider T, Ba LA, Khairan K, Zwergel C, Bach ND, Bernhardt I, Brandt W, Wessjohann L, Diederich M, Jacob C. Interactions of polysulfanes with components of red blood cells. Med Chem Commun. 2011;2(3):196–200. doi: 10.1039/c0md00203h. [DOI] [Google Scholar]
- 59.Davenport DM, Wargovich MJ. Modulation of cytochrome P450 enzymes by organosulfur compounds from garlic. Food Chem Toxicol. 2005;43(12):1753–1762. doi: 10.1016/j.fct.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Nian H, Delage B, Pinto JT, Dashwood RH. Allyl mercaptan, a garlic-derived organosulfur compound, inhibits histone deacetylase and enhances Sp3 binding on the P21WAF1 promoter. Carcinogenesis. 2008;29(9):1816–1824. doi: 10.1093/carcin/bgn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawson LD, Wang ZJ. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: use in measuring allicin bioavailability. J Agric Food Chem. 2005;53(6):1974–1983. doi: 10.1021/jf048323s. [DOI] [PubMed] [Google Scholar]
- 62.Germain E, Auger J, Ginies C, Siess MH, Teyssier C. In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotica. 2002;32(12):1127–1138. doi: 10.1080/0049825021000017902. [DOI] [PubMed] [Google Scholar]
- 63.Taucher J, Hansel A, Jordan A, Lindinger W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J Agric Food Chem. 1996;44(12):3778–3782. doi: 10.1021/jf960640e. [DOI] [Google Scholar]
- 64.Xiao D, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Brisson M, Lazo JS, Singh SV. Diallyl trisulfide-induced G(2)-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc25C. Oncogene. 2005;24(41):6256–6268. doi: 10.1038/sj.onc.1208759. [DOI] [PubMed] [Google Scholar]
- 65.Ha MW, Ma R, Shun LP, Gong YH, Yuan Y. Effects of allitridi on cell cycle arrest of human gastric cancer cells. World J Gastroenterol. 2005;11(35):5433–5437. doi: 10.3748/wjg.v11.i35.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu KL, Chen HW, Wang RY, Lei YP, Sheen LY, Lii CK. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW 264.7 macrophages. J Agric Food Chem. 2006;54(9):3472–3478. doi: 10.1021/jf060043k. [DOI] [PubMed] [Google Scholar]
- 67.Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269(2):305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puccinelli Michael, Stan Silvia. Dietary Bioactive Diallyl Trisulfide in Cancer Prevention and Treatment. International Journal of Molecular Sciences. 2017;18(8):1645. doi: 10.3390/ijms18081645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiesel VA, Stan SD. Diallyl trisulfide, a chemopreventive agent from Allium vegetables, inhibits alpha-secretases in breast cancer cells. Biochem Biophys Res Commun. 2017;484(4):833–838. doi: 10.1016/j.bbrc.2017.01.184. [DOI] [PubMed] [Google Scholar]
- 70.Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iranian J Basic Med Sci. 2013;16(10):1031–1048. [PMC free article] [PubMed] [Google Scholar]
- 71.Chandra-Kuntal K, Lee J, Singh SV. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res Treat. 2013;138(1):69–79. doi: 10.1007/s10549-013-2440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallace GC, Haar CP, Vandergrift WA, 3rd, Giglio P, Dixon-Mah YN, Varma AK, et al. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J Neuro-Oncol. 2013;114(1):43–50. doi: 10.1007/s11060-013-1165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang CS, Chhabra SK, Hong JY, Smith TJ. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr. 2001;131(3s):1041S–1045S. doi: 10.1093/jn/131.3.1041S. [DOI] [PubMed] [Google Scholar]
- 74.Gao, Jiang, Wang, Zhao, Wang. Drug metabolism and pharmacokinetics of organosulfur compounds from garlic. J Drug Metab Toxicol 2013;4(159).
- 75.Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Tony Kong AN. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37(10):1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 76.Gong P, Hu B, Cederbaum AI. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys. 2004;432(2):252–260. doi: 10.1016/j.abb.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 77.Kim S, Lee HG, Park SA, Kundu JK, Keum YS, Cha YN, Na HK, Surh YJ. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One. 2014;9(1):e85984. doi: 10.1371/journal.pone.0085984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35(6):995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 79.Lee BC, Park BH, Kim SY, Lee YJ. Role of Bim in diallyl trisulfide-induced cytotoxicity in human cancer cells. J Cell Biochem. 2011;112(1):118–127. doi: 10.1002/jcb.22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das A, Banik NL, Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007;110(5):1083–1095. doi: 10.1002/cncr.22888. [DOI] [PubMed] [Google Scholar]
- 81.Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6(5):1599–1609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrer-Sueta G, Manta B, Botti H, Radi R, Trujillo M, Denicola A. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem Res Toxicol. 2011;24(4):434–450. doi: 10.1021/tx100413v. [DOI] [PubMed] [Google Scholar]
- 83.Allah DR, Schwind L, Asali IA, Nasim J, Jacob C, Gotz C, et al. A scent of therapy: synthetic polysulfanes with improved physico-chemical properties induce apoptosis in human cancer cells. Int J Oncol. 2015;47(3):991–1000. doi: 10.3892/ijo.2015.3093. [DOI] [PubMed] [Google Scholar]
- 84.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A. 2007;104(46):17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang F, Jin H, Wu L, Shao J, Zhu X, Chen A, Zheng S. Diallyl trisulfide suppresses oxidative stress-induced activation of hepatic stellate cells through production of hydrogen sulfide. Oxidative Med Cell Longev. 2017;2017:1406726–1406713. doi: 10.1155/2017/1406726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bradley JM, Organ CL, Lefer DJ. Garlic-derived organic polysulfides and myocardial protection. J Nutr. 2016;146(2):403s–409s. doi: 10.3945/jn.114.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai YR, Hu CH. Computational study of H2S release in reactions of diallyl polysulfides with thiols. J Phys Chem B. 2017;121(26):6359–6366. doi: 10.1021/acs.jpcb.7b03683. [DOI] [PubMed] [Google Scholar]
- 88.Liang D, Wu H, Wong MW, Huang D. Diallyl trisulfide is a fast H2S donor, but diallyl disulfide is a slow one: the reaction pathways and intermediates of glutathione with polysulfides. Org Lett. 2015;17(17):4196–4199. doi: 10.1021/acs.orglett.5b01962. [DOI] [PubMed] [Google Scholar]
- 89.Iciek Małgorzata, Bilska-Wilkosz Anna, Górny Magdalena, Sokołowska-Jeżewicz Maria, Kowalczyk-Pachel Danuta. The Effects of Different Garlic-Derived Allyl Sulfides on Anaerobic Sulfur Metabolism in the Mouse Kidney. Antioxidants. 2016;5(4):46. doi: 10.3390/antiox5040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iciek MB, Kowalczyk-Pachel D, Kwiecien I, Dudek MB. Effects of different garlic-derived allyl sulfides on peroxidative processes and anaerobic sulfur metabolism in mouse liver. Phytother Res. 2012;26(3):425–431. doi: 10.1002/ptr.3572. [DOI] [PubMed] [Google Scholar]
- 91.Saidu NEB, Abu Asali I, Czepukojc B, Seitz B, Jacob C, Montenarh M. Comparison between the effects of diallyl tetrasulfide on human retina pigment epithelial cells (ARPE-19) and HCT116 cells. Biochim Biophys Acta Gen Subj. 2013;1830(11):5267–5276. doi: 10.1016/j.bbagen.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Saidu NEB, Touma R, Asali IA, Jacob C, Montenarh M. Diallyl tetrasulfane activates both the eIF2α and Nrf2/HO-1 pathways. Biochim Biophys Acta Gen Subj. 2013;1830(1):2214–2225. doi: 10.1016/j.bbagen.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Anwar A, Groom M, Arbach M, Hamilton CJ. How to turn the chemistry of garlic into a ‘botanical’ pesticide. In: Jacob C, Kirsch G, Slusarenko A, Winyard PG, Burkholz T, editors. Recent advances in redox active plant and microbial products: from basic chemistry to widespread applications in medicine and agriculture. Dordrecht: Springer Netherlands; 2014. pp. 323–341. [Google Scholar]