Abstract
Purpose
We first assessed whether the pattern of referrals to a nuclear medicine clinic improved as experience with 223Ra-dichloride increased, and whether referral patterns affected patient outcomes, and second assessed the value of bone scintigraphy, total alkaline phosphatase (tALP) and lymphadenopathy as prognostic factors in patients receiving 223Ra-dichloride.
Methods
A total of 57 patients eligible to receive 223Ra-dichloride over a 2-year period (March 2014 to March 2016) were retrospectively assessed and prospectively followed (median follow up 298 days). 223Ra-Dichloride was administered at 4-week intervals for a maximum of six injections. The numbers of patients in years 1 and 2 referred in relation to extent of bone disease (EOBD) category and overall survival (OS) were determined. The prognostic factors EOBD category, baseline tALP (tALPBL), tALP response, greatest percentage reduction in tALP from baseline in any treatment cycle (ALPmax; among patients with elevated ALPBL), and the presence of lymphadenopathy were assessed as predictors of OS.
Results
The proportion of patients with EOBD1 was higher in year 2 than in year 1 (29% and 4%, respectively), and in year 2 there was a lower rate of symptomatic skeleton-related events, a higher proportion of patients completing six cycles, and longer (albeit nonsignificant) OS (p = 0.55). There were significant differences in OS between EOBD4 patients and those in all other groups and between EOBD1 and EOBD3 patients (p < 0.05). OS was longer in patients with normal tALPBL than in those with elevated tALPBL (p = 0.01), in ALP responders than in nonresponders (p < 0.05), and in patients without lymphadenopathy than in those with lymphadenopathy (p = 0.29). OS was correlated with ALPmax (r2 = 0.24).
Conclusion
A collaborative multidisciplinary referrals pathway, together with increased experience with 223Ra-dichloride, led to improved outcomes. In patients with elevated tALPBL, tALP dynamics may be useful for monitoring response and predicting OS. Imaging and prognostic markers may therefore be of value for individualizing 223Ra-dichloride treatment and planning retreatment; however, further studies are required.
Keywords: Alkaline phosphatase, Bone metastases, Metastatic castration-resistant prostate cancer, 223Ra-Dichloride, Referral patterns, Radium
Introduction
Castration-resistant prostate cancer (CRPC) is defined as disease progression with rising serum prostate-specific antigen (PSA) levels or radiological progression despite documented castrate levels of testosterone [1]. While 4% of newly diagnosed prostate cancer patients under the age of 75 years present with metastases [2], 85% of patients have proven evidence of metastases on diagnosis of castration resistance [3]. Following the development of CRPC, the incidence of metastatic disease and/or radiotherapy (a surrogate for disease progression) has been reported to be 6.4 per 100 person-years [4].
As there is no cure for metastatic CRPC (mCRPC), the goal of treatment is to prolong life and improve quality of life (QoL) [5]. According to the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) guidelines, treatment options for mCRPC include: sipuleucel-T (for asymptomatic or minimally symptomatic mCRPC; available in the USA only), androgen-targeted therapies including abiraterone with prednisone or enzalutamide (for asymptomatic or minimally symptomatic mCRPC), cytotoxic chemotherapies including docetaxel or cabazitaxel (for symptomatic mCRPC, often but not exclusively with visceral metastases), 223Ra-dichloride (for symptomatic mCRPC with bone metastases and without visceral metastases), and zoledronic acid or denosumab (for patients with bone metastases to prevent skeleton-related events). Continued concurrent androgen-deprivation therapy (ADT) is also recommended [5, 6]. Treatment recommendations are dependent on tumour burden, prior therapy, whether the patient is asymptomatic, minimally symptomatic or symptomatic, the presence of visceral or bone metastases, and Eastern Cooperative Oncology Group (ECOG) performance status [5, 6]. In the UK, the 2014 National Institute for Health and Care Excellence (NICE) guidelines recommend docetaxel (for patients with a Karnofsky performance status of ≥60%), corticosteroids such as dexamethasone (if ADT and antiandrogen therapy have failed), and bone-targeted therapies including bisphosphonates (for pain relief when other treatments have failed), strontium-89 (in patients with painful bone metastases) [7] and, more recently (2016), 223Ra-dichloride (in patients with mCRPC, symptomatic bone metastasis and no evidence of visceral metastasis [8]).
223Ra-Dichloride is a targeted alpha-emitter therapy that mimics calcium and targets areas of increased bone turnover. Alpha particles have a very short range (<100 μm), so damage to surrounding tissues is minimal [9]. Prior to the introduction of 223Ra-dichloride, bone-targeting therapies did not lead to longer overall survival (OS); the benefits were primarily limited to pain relief and delay of skeletal events [10]. 223Ra-Dichloride is approved in the UK for the ‘treatment of adults with CRPC, symptomatic bone metastases and no known visceral metastases’ [8, 11]. The ALSYMPCA study investigated the efficacy of 223Ra-dichloride in 921 patients with mCRPC and symptomatic bony metastases [12]. The primary endpoint was OS; secondary efficacy endpoints included time to the first symptomatic skeletal event and various safety and QoL endpoints. Overall, 223Ra-dichloride showed benefits in terms of OS and QoL compared with placebo. Despite these very positive results, to date, few reliable prognostic factors have been identified in mCRPC patients undergoing treatment with 223Ra-dichloride [13].
The purpose of the current study was to describe real-world experience with 223Ra-dichloride at a single centre in the UK (Brighton and Sussex University Hospital), and to assess bone scintigraphy, a biochemical biomarker (total alkaline phosphatase, tALP) and coexisting lymph node disease as prognostic factors. The study also aimed to assess whether the pattern of referrals to the nuclear medicine clinic improved as experience with 223Ra-dichloride increased, and to evaluate whether referral patterns influenced patient outcomes.
Materials and methods
Study design
This study was a retrospective and prospective review of all patients who were referred to the Nuclear Medicine Department of Brighton and Sussex University Hospital over a 2-year period (March 2014 to March 2016) who were eligible to receive treatment with 223Ra-dichloride. These patients were defined as those with mCRPC, symptomatic bone metastases and no known visceral metastatic disease. Patient records were reviewed using patient notes, ChemoCare electronic records (e.g. treatment schedule), the picture and archiving system (imaging), and laboratory blood test data. All patients gave written informed consent for their diagnostic and therapeutic management and follow up.
Treatment schedule
223Ra-Dichloride was administered via intravenous injection at 4-week intervals for a maximum of six injections (in accordance with prescribing information). The administered volume was calculated as described in the prescribing information [11]:
Patients who developed visceral metastases, as identified by computed tomography (CT) prompted by a sudden change in clinical presentation and a marked increase in biochemical markers (tALP and/or PSA), were required to discontinue treatment.
Referral patterns
Various practical strategies were put in place at the institution to improve clinician-to-patient communication and establish a more coherent interface between uro-oncology and nuclear medicine, with a view to optimizing the patient pathway and improving patient outcomes. These strategies have been discussed in more detail elsewhere [14]. Improvements in referral patterns were assessed by comparing the proportions of patients referred to the Nuclear Medicine Department in year 1 and year 2 for each extent of bone disease (EOBD) category. EOBD was assessed using bone scintigraphy, and the categories were as follows: 1 ≥2 but <6 metastases, 2 6–20 metastases, 3 >20 metastases but less than a superscan, 4 superscan (diffuse involvement i.e. more than 75% of the ribs, vertebrae and pelvic bones) [12, 15].
Effect of referral patterns on outcomes
OS, defined as the time from first 223Ra-dichloride treatment in the nuclear medicine clinic to the date of death from any cause, was assessed in the year-1 and year-2 patients. The incidence of symptomatic skeleton-related events (SSREs) was also determined. An SSRE was defined as any skeleton-related adverse event or any radiotherapy to bone.
Prognostic factors
EOBD category
OS and number of completed treatment cycles were recorded according to EOBD status.
Alkaline phosphatase
Total ALP (tALP) was assessed at baseline (i.e. before treatment; ALPBL) and before every subsequent cycle. Normal ALPBL was defined as ≤130 U/L, and elevated ALPBL was defined as >130 U/L. ALP response was defined as a reduction from baseline of ≥30%, confirmed ≥4 weeks later [12]; ALP nonresponse was defined as a change of <30% or an increase of ≥25% in ALP from baseline. In an additional exploratory analysis among patients with elevated tALPBL, ALP response was defined as a reduction of ≥10% from baseline and ALP nonresponse was defined as a change of <10% or an increase from baseline.
OS was assessed in relation to ALPBL comparing those with normal ALPBL and those with elevated ALPBL, and comparing ALP responders (≥30% reduction from baseline or ≥10% reduction from baseline among patients with elevated tALPBL) and ALP nonresponders. ALPmax was the greatest percentage reduction in ALP from baseline in any treatment cycle, and was assessed in patients with elevated tALPBL only (patients with normal ALPBL were excluded because they would not be expected to show a further significant reduction). The correlation between ALPmax and OS (r2) was also determined.
Lymph node disease
The presence of coexisting bone and lymph node metastases was determined using cross-sectional imaging. Lymph node disease categories were as follows: group 1 baseline lymph node disease, group 1a lymph node disease progression during treatment, group 2 no evidence of lymph node disease at any stage, and group 3 development of new lymph node disease during treatment. For each lymph node disease category, OS and EOBD category were determined.
Statistical methods
Analyses were performed using Excel and Statsdirect. OS was analysed using Kaplan-Meier survival curves. Peto’s log-rank test was used to determine whether the survival curves were significantly different between groups.
Results
Patient population
In year 1 (March 2014–2015), 26 patients were included, and in year 2 (March 2015–2016), 31 patients were included. Baseline characteristics of the patients are shown in Table 1. Overall, 14 of the 26 patients (54%) completed five or six cycles in year 1, and 17 of the 31 patients (55%) completed five or six cycles in year 2. Median follow-up was 298 days.
Table 1.
Baseline characteristics of the 57 patients included in this study and the 614 patients included in the 223Ra-dichloride group of the ALSYMPCA trial [12]
| Characteristic | This study | ALSYMPCA trial |
|---|---|---|
| Age (years), median (range) | 74 (48–90) | 71 (49–90) |
| Age >75 years, n (%) | 23 (40) | 171 (28) |
| Any prior use of docetaxel, n (%) | ||
| Yes | 32 (56) | 352 (57) |
| No | 25 (44) | 262 (43) |
| Total alkaline phosphatase level (U/L), n (%) | ||
| <220 | 28 (54) | 348 (57) |
| ≥220 | 24 (46) | 266 (43) |
| 0–130 | 16 (28) | NR |
| 130–260 | 15 (26) | NR |
| 260–1,000 | 21 (3) | NR |
| >1,000 | 4 (7) | NR |
| Eastern Cooperative Oncology Group performance status, n (%) | ||
| 0 | 1 (2) | 165 (27) |
| 1 | 34 (60) | 371 (60) |
| ≥2 | 22 (38) | 77 (13) |
| Extent of bone disease (EOBD category), n (%) | ||
| <6 metastases (EOBD1) | 10 (18) | 100 (16) |
| 6–20 metastases (EOBD2) | 16 (28) | 262 (43) |
| >20 metastases (EOBD3) | 17 (30) | 195 (32) |
| Superscan (EOBD4) | 14 (25) | 54 (9) |
| Baseline blood investigations, median (range) | ||
| Haemoglobin (g/dL) | 12.1 (7.6–14.6) | 12.2 (8.5–15.7) |
| Total alkaline phosphatase (U/L) | 240 (58–2,805) | 211 (32–6,431) |
| Prostate-specific antigen (μg/L) | 223.9 (0.61–1,747) | 146 (4–6,026) |
| Other treatments, n (%) | ||
| Adjuvant therapy during 223Ra-dichloride | 50 (88) | NR |
| Antiandrogen | 40 (70) | NR |
| Steroids | 34 (60) | NR |
| External beam radiation therapy | 12 (21) | 99 (16)a |
| Abiraterone | 7 (12) | NR |
| Enzalutamide | 7 (12) | NR |
| Bisphosphonates | NR | 250 (41)b |
NR not reported
aWithin 12 weeks of therapy
b''Current use'
Referral patterns
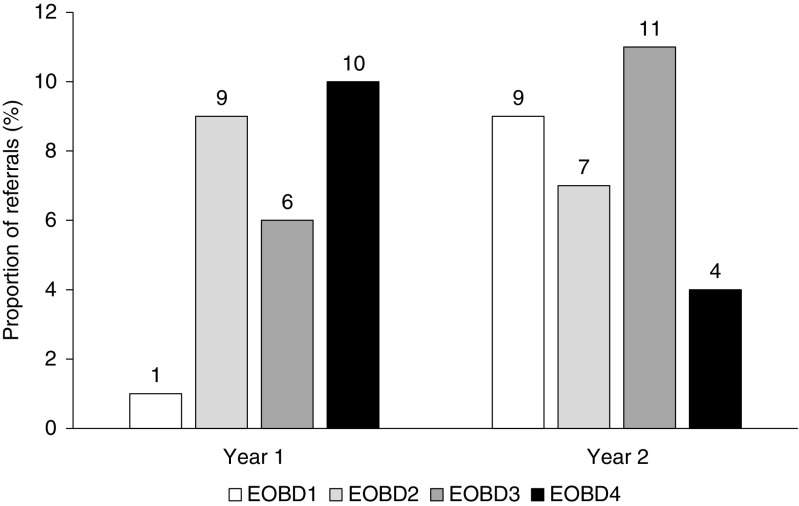
The proportions of patients with EOBD1 referred to the Nuclear Medicine Department were 4% ( 1/26 patients) in year 1 and 29% (9/31 patients) in year 2, an increase of 25%. The proportions of patients with EOBD4 referred were 38% (10/26 patients) in year 1 and 13% (4/31 patients) in year 2, a decrease of 25%. Overall, referral rates of patients with EOBD2 and EOBD3 remained largely similar (Fig. 1).
Fig. 1.
Pattern of referrals from uro-oncology to nuclear medicine in year 1 and year 2, in relation to the extent of bone disease (EOBD) category among the 57 included patients
Effect of referral patterns on outcomes
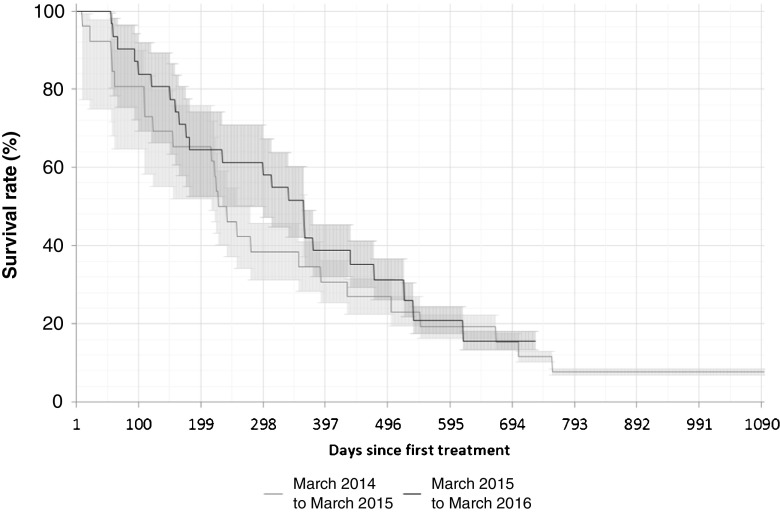
In the total population, OS in year 2 was longer than in year 1, but the difference was not statistically significant (227 vs. 266 days, p = 0.55; Fig. 2). In parallel with a lower bone disease burden, the incidence of SSREs was lower in year 2 than in year 1 (9.6% and 19.2%, respectively; Table 2).
Fig. 2.
Overall survival in 26 patients in year 1 and 31 patients in year 2. Median survival times: 233 days in the total population, 227 days in year-1 patients, and 266 days in year-2 patients. Median follow-up times: 298 days in the total population, 234 days in year-1 patients, and 363 days in year-2 patients (p = 0.55, year 1 vs. year 2)
Table 2.
Incidence of symptomatic skeleton-related events in year 1 and year 2 in relation to extent of bone disease (EOBD) category
| Event | EOBD category |
|---|---|
| Year 1 (5/26 [19.2%]) | |
| External radiation therapy for pain (spine) | 2 |
| Hip fracture | 3 |
| MSCC and skull metastases with symptoms | 3 |
| MSCC | 4 |
| MSCC and femoral fracture | 4 |
| Year 2 (3/31 [9.6%]) | |
| Lumbar spine pain causing admission | 2 |
| MSCC | 3 |
| External radiation therapy for pain (spine and pelvis) | 3 |
MSCC metastatic spinal cord compression
Prognostic factors
EOBD category
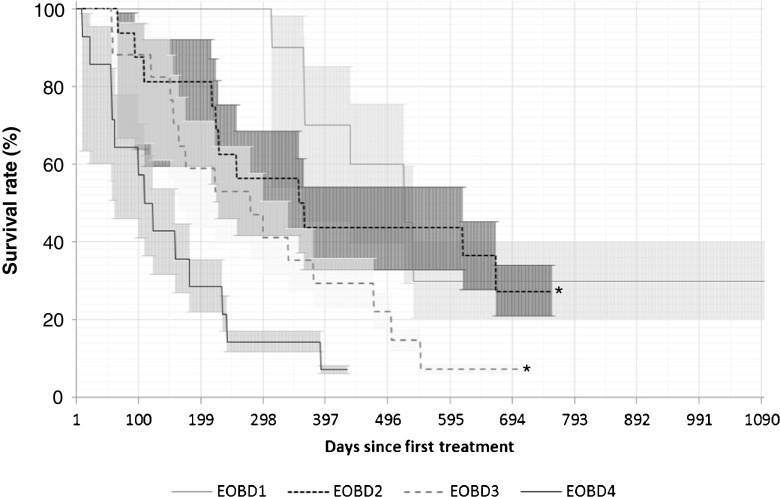
OS curves for patients with each EOBD category are shown in Fig. 3. The numbers of patients in each group who died were as follows: 7 (70%) with EOBD1, 12 (75%) with EOBD2, 16 (94%) with EOBD3 and 14 (100%) with EOBD4. There were significant differences in OS between patients with EOBD4 and all other groups (EOBD1 vs. EOBD4, p = 0.001; EOBD2 vs. EOBD4, p = 0.002; EOBD3 vs. EOBD4, p = 0.02), and between patients with EOBD1 and those with EOBD3 (p = 0.04). During both year 1 and year 2, the proportions of patients completing six cycles of treatment were far higher in patients with EOBD1 (100% and 89%, respectively) than in those with EOBD4 (20% and 25%, respectively; Table 3).
Fig. 3.
Overall survival in relation to extent of bone disease (EOBD) category: 10 patients with EOBD1, 16 with EOBD2, 17 with EOBD3 and 14 with EOBD4. Median survival times: 437 days, 242 days, 250 days, and 116 days in patients with EOBD1, EOBD2, EOBD3 and EOBD4, respectively. Median follow-up times: 485 days, 359 days, 278 days, and 116 days, respectively. *p < 0.05 vs. EOBD4
Table 3.
Number (%) of patients completing 223Ra-dichloride treatment cycles in year 1 and year 2, in relation to extent of bone disease (EOBD) category
| Number of treatment cycles | Total | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Year 1 | |||||||
| EOBD1 | 1 (100) | 1 | |||||
| EOBD2 | 3 (33) | 1 (11) | 1 (11) | 4 (44) | 9 | ||
| EOBD3 | 1 (17) | 1 (17) | 4 (67) | 6 | |||
| EOBD4 | 4 (40) | 2 (20) | 1 (10) | 1 (10) | 2 (20) | 10 | |
| Total | 4 (15) | 2 (8) | 5 (19) | 1 (4) | 3 (12) | 11 (42) | 26 |
| Year 2 | |||||||
| EOBD1 | 1 (11) | 8 (89) | 9 | ||||
| EOBD2 | 1 (14) | 1 (14) | 2 (29) | 3 (43) | 7 | ||
| EOBD3 | 2 (18) | 2 (18) | 2 (18) | 2 (18) | 3 (27) | 11 | |
| EOBD4 | 1 (25) | 2 (50) | 1 (25) | 4 | |||
| Total | 3 (10) | 3 (10) | 3 (10) | 5 (16) | 2 (6) | 15 (48) | 31 |
Alkaline phosphatase
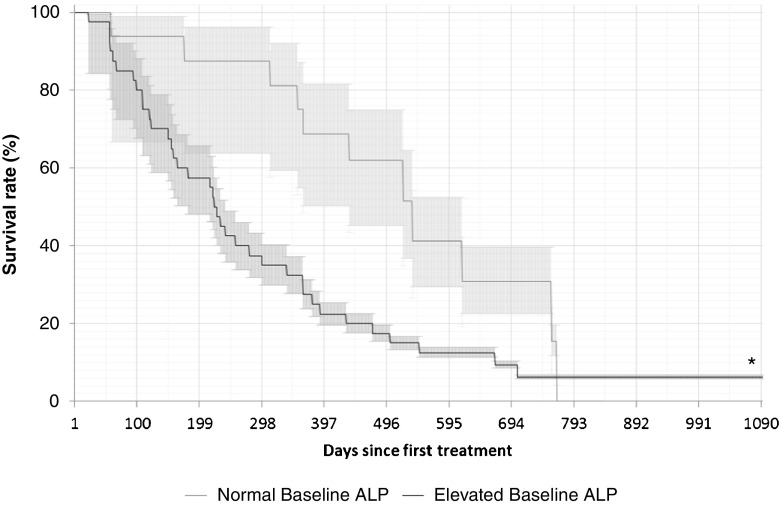
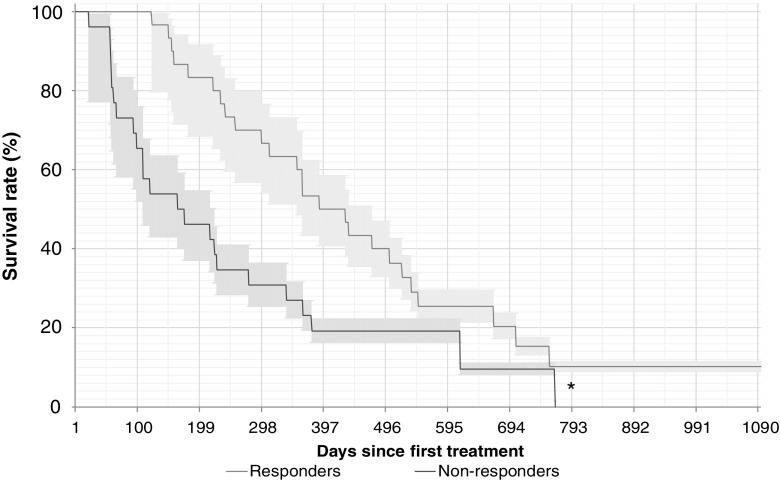
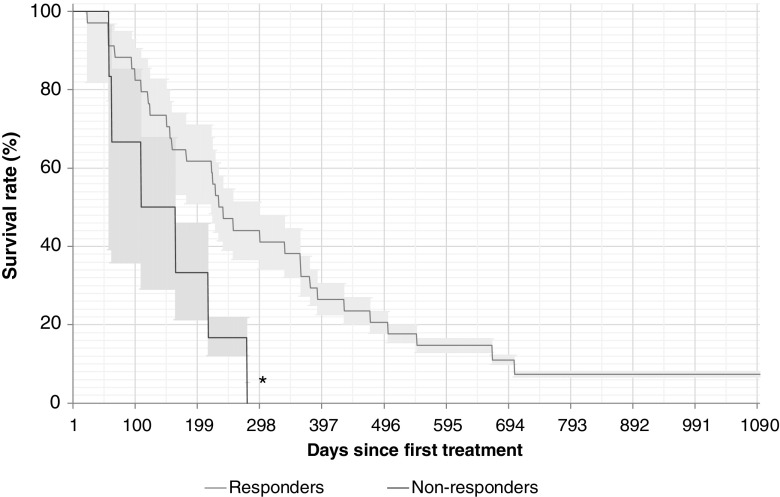
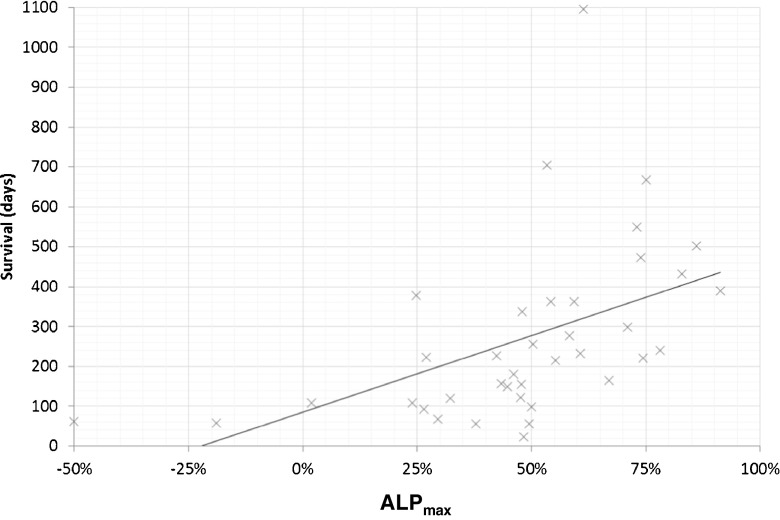
OS was significantly longer among patients with normal ALPBL than among those with elevated ALPBL (401 vs. 222 days, p = 0.01; Fig. 4), and among ALP responders (≥30% reduction) than among ALP nonresponders (363 vs. 115 days, p = 0.01; Fig. 5). When ALP response was defined as a reduction of ≥10% from baseline (exploratory analysis among patients with elevated ALPBL only), OS was significantly longer among ALP responders than among ALP nonresponders (256 vs. 137 days, p = 0.03; Fig. 6). Among patients with elevated ALPBL, a correlation was also observed between OS (233 days) and ALPmax (r2 = 0.24; Fig. 7). The correlation between OS and ALPmax was weaker among patients with normal ALPBL (r2 = 0.17).
Fig. 4.
Overall survival in relation baseline alkaline phosphatase (ALPBL) status (normal in 16 patients, elevated in 40; one patient did not have any baseline data). Median survival times: 401 days in patients with normal ALPBL, and 222 days in those with elevated ALPBL. Median follow-up times: 468 days in patients with normal ALPBL, and 234 days in those with elevated ALPBL. ,. *p = 0.01 vs. normal ALPBL
Fig. 5.
Overall survival in relation to alkaline phosphatase (ALP) response (≥30% vs. <30% reduction from baseline; 30 responders, 26 nonresponders). Median survival times: 363 days in ALP responders, and 115 days in ALP nonresponders. Median follow-up times: 411 days in ALP responders, and 170 days in ALP nonresponders. *p = 0.01 vs. ALP responders
Fig. 6.
Overall survival in relation to alkaline phosphatase (ALP) response among patients with elevated total ALP at baseline (≥10% vs. <10% reduction from baseline; 34 responders, 6 nonresponders). Median survival times: 256 days in ALP responders, and 137 days in ALP nonresponders. Median follow-up times: 325 days in ALP responders, and 216 days in ALP nonresponders. *p = 0.03 vs. ALP responders
Fig. 7.
Correlation between overall survival and greatest percentage reduction in total alkaline phosphatase (tALP) from baseline in any treatment cycle (ALPmax) among 40 patients with elevated tALP at baseline (r2 = 0.24)
Lymph node disease
Recent cross-sectional imaging data (CT and/or magnetic resonance imaging, MRI) were available in 46 patients who were included in the analysis of lymph node disease. Over a quarter of the patients had baseline lymph node disease (group 1; 13/46 patients, 28%). Of these, six patients progressed during treatment (group 1a; 6/13, 46%). There was no evidence of lymph node disease at baseline or during treatment in 30 patients (group 2; 30/46, 65%). New lymph node disease developed in three patients (group 3; 3/46, 7%).
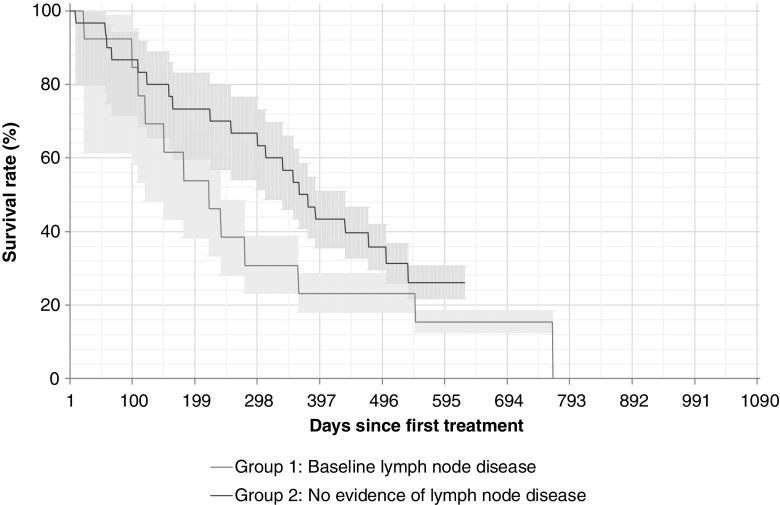
The presence of coexisting lymph node disease appeared to correlate with bone disease burden: in lymph node disease group 1, more patients had EOBD3 (5/13 patients, 38%) or EOBD4 (4/13, 31%) than EOBD1 (2/13, 15%) or EOBD2 (2/13, 15%). The same was true of lymph node disease group 1a. OS was shorter among patients with coexisting lymph node metastasis at baseline or with lymph node progression than in those without lymphadenopathy at any stage (181 days vs. 325 days, p = 0.29; Fig. 8).
Fig. 8.
Overall survival in relation to the presence lymph node disease (13 patients with lymph node disease, 30 patients without). Median survival times: 181 days in those with lymph node disease, and 325 days in those without lymphadenopathy at any stage. Median follow-up times: 221 days in those with lymph node disease, and 371 days in those without lymphadenopathy at any stage. p = 0.29 for presence vs. absence of lymph node disease
Discussion
Prior to this study, patients were often referred to the Nuclear Medicine Department at this centre with very late-stage disease and severe metastatic bone burden; indeed, many clinicians were waiting for 223Ra-dichloride clinical approval (which was granted on the basis of the ALSYMPCA trial findings) before referring patients. At baseline, the prevalence of diffuse bone metastases (so-called ‘superscan’) at referral in the overall population during both year 1 and year 2 was 25% (10 of 26 patients, 38%, in year 1; 4 of 31 patients, 13%, in year 2; i.e. 14 of 57 patients, 25%, in total), compared with 9% in the ALSYMPCA trial [12]. These patients with a superscan would often not complete the treatment course. Our study showed that the pattern of referrals reversed from year 1 to year 2 of 223Ra-dichloride service, with a marked increase in referrals of patients with EOBD1 and a decrease in referrals of those with EOBD4. The pattern of referrals in patients with EOBD2/3 remained similar between year 1 and year 2. The implementation of a strong collaborative uro-oncology/nuclear medicine multidisciplinary referral pathway led to referring clinicians having increased confidence in earlier intervention and greater success of treatment with 223Ra-dichloride.
In this analysis, EOBD (as determined by bone scintigraphy) was shown to be a predictor of OS in patients treated with 223Ra-dichloride. Treating more patients with a lower bone burden resulted in improved overall outcomes, including a reduction in the incidence of SSREs and an increase in OS. The rates of SSREs observed in this study (19.2% in year 1, 9.6% in year 2) were much lower than that found in the ALSYMPCA trial (35%), in spite of the fact that the studies used the same definition of SSRE. This may be due to a longer follow-up in the ALSYMPCA trial and/or further advances in CRPC treatment since the ALSYMPCA trial and overall better bone health in the more recently recruited patients, but this interesting aspect requires further investigation in prospective studies. We also expect that SSREs may increase over time due to the natural course of the disease. In our sample, SSREs were observed in patients with EOBD2, 3 and 4 in year 1, and in patients with EOBD2 and 3 in year 2.
EOBD was also shown to be predictive of survival in patients receiving 223Ra-dichloride treatment in the ALSYMPCA trial. The median OS, however, was shorter in our study (233 days) than in the ALSYMPCA trial (14.9 months). This may have been due to the higher burden of disease in our patients than in the ALSYMPCA trial patients: our patients included a higher proportion of with a superscan (overall 25% for both years vs. 9%), a much lower proportion with EOBD2 (28% vs. 43%), a higher proportion with ECOG performance status ≥2 (38% vs.13%) and a lower proportion with ECOG performance status 0 (2% vs. 27%), and the median baseline PSA level was higher in our patients (223.9 vs. 146 μg/L; Table 1). It may also have been a result of differences in the definition of OS: in our study, OS was defined from the first dose (i.e. first visit to the nuclear medicine clinic), whereas in the ALSYMPCA trial OS was defined from randomization, which may or may not have been when the first 223Ra-dichloride dose was administered [12]. A small case series (15 patients) showed differing results, indicating that baseline EOBD is not indicative of response to 223Ra-dichloride therapy. However, the authors did note a trend towards an unfavourable response to treatment among those presenting with EOBD4 [16].
In our study, tALP was useful as a predictor of OS in patients receiving 223Ra-dichloride. ALP responders had significantly longer OS than ALP nonresponders (363 vs. 115 days for a reduction of ≥30% from baseline and 256 vs. 137 days for a reduction of ≥10% from baseline among patients with elevated ALPBL; p < 0.05 for both). In addition, among patients with elevated ALPBL, those with the greatest reductions in ALP in any treatment cycle (ALPmax) generally had a longer OS. We have previously hypothesized that tALP dynamics and ALPmax are more relevant in patients with elevated tALPBL than in those with normal ALPBL [17]. In the present study, patients with normal tALPBL had a longer OS than those with elevated tALPBL (401 vs. 222 days; p = 0.01). Changes in tALP were less clear in those with normal tALPBL, as demonstrated by the fact that the correlation between OS and ALPmax was stronger among patients with elevated ALPBL than normal ALPBL (r2 = 0.24 and r2 = 0.17, respectively). Similar trends were observed in the ALSYMPCA trial: in patients with elevated tALPBL (defined as ≥220 U/L), 223Ra-dichloride treatment was associated with longer OS than placebo treatment. In patients with tALPBL <220 U/L, there was a trend towards a longer OS with 223Ra-dichloride than with placebo, but the confidence intervals crossed the line of unity [12].
In a recent study, a significant reduction in tALP occurred as early as 4 weeks after initiating 223Ra-dichloride therapy [18]. Reductions in tALP in week 12 correlated with longer OS, but did not meet statistical requirements for use as a surrogate marker of survival. The authors suggested, therefore, that dynamic changes in tALP may be useful for monitoring but not as a surrogate marker. It is of note, however, that dynamic changes were analysed in all patients, including those with normal baseline tALP – in contrast to our study in which changes were analysed only in patients with elevated tALPBL. The utility of tALP alone is somewhat limited by the fact that it may increase for reasons other than treatment failure, for example the development of liver metastases [19]. However, ALP response has also been shown to be associated with survival in patients receiving chemotherapy [18, 20]. In the TAX327 trial among men with high baseline ALP receiving docetaxel or mitoxantrone, normalization of ALP was associated with longer OS, while an increase in ALP was associated with poor OS. This was independent of changes in PSA levels [21].
Regarding co-existent lymph node and bone metastases and survival, the site of metastases may have prognostic implications. Patients with bone metastases have shorter survival than patients with lymph node metastases only, and mortality has been shown to increase as the disease progresses from lymph nodes to bone to visceral tissue [22]. However, prostate cancer often metastasizes to bone first, with lymph node disease developing later. Our data suggest that coexistent lymph node disease or lymph node disease progression during 223Ra-dichloride treatment may be predictive of a shorter OS. Lymph node disease appears to be associated with higher bone disease burden, but may be present even in patients with EOBD1 or EOBD2. Patients with coexisting bone and lymph node metastases should therefore be considered for combined 223Ra-dichloride treatment with, for example, chemotherapy or beta-emitting 177Lu-PSMA-617 at an earlier stage. Large prospective studies are required to further assess the effect of 223Ra-dichloride on outcomes in this patient group, and to determine whether coexisting lymph node disease may be an indicator of survival [23].
In the ENTHUSE M0 trial, more than 30% of patients whose CRPC was thought to be nonmetastatic (M0) in fact had metastatic disease when assessed by MRI, CT or bone scan. This unexpectedly high rate of metastatic disease suggests that regular imaging should be considered in CRPC, even in the absence of symptoms [24]. In one of our previous studies, cross-sectional imaging within 3 months of initiating 223Ra-dichloride therapy improved completion rates. This is an issue that needs to be addressed in treatment guidelines [25].
There were several limitations in this analysis. While OS was, to a certain degree, longer in year 2 than in year 1, the difference was not statistically significant, and this may have been due to the relatively small sample size. The proportion of patients with EOBD2 was far lower than that in the ALSYMPCA trial (28% vs. 43% [12]) and was slightly lower in year 2 than in year 1; this too may have had an inverse impact on the OS. Furthermore, to receive 223Ra-dichloride, patients were required to be castration-resistant with symptomatic bone metastases, irrespective of when they had been labelled as castration-resistant. As a result, in some patients the time since becoming castration-resistant was longer, and hence these patients were likely to have had a higher metastatic burden and poorer outcomes. It is also not clear whether patients were truly being referred earlier (i.e. whether patients with earlier stage disease, who may not previously have been considered eligible for referral, were being referred in year 2) or whether patients with later stage disease (i.e. superscan patients) were no longer being referred.
This study has helped identify a number of opportunities for further research. These include the following:
Selection of patients for retreatment with 223Ra-dichloride.
The impact of intervention at earlier stages of bone disease.
The use of 223Ra-dichloride in asymptomatic patients (not currently on-label).
The efficacy of 223Ra-dichloride and abiraterone and/or enzalutamide. Note that the combination of 223Ra-dichloride, abiraterone and prednisone/prednisolone is currently contraindicated based on evidence suggesting increased mortality and fracture risk with this combination in the ERA 223 trial in patients with mCRPC, bone metastases and mild or no symptoms. It is important to note that the safety and efficacy of 223Ra-dichloride in combination with second-generation androgen receptor antagonists, such as enzalutamide, have not yet been established; however, to date, no safety issues have been reported [26].
The efficacy of an as-yet-unexplored combination or ‘cocktail’ of alpha-emitting radium and beta-emitting 177Lu-PSMA radioligand therapy (due to a better response of lymph node metastases than bone metastases [27], but transient side effects on the salivary and lacrimal glands in contrast to alpha-emitting PSMA treatment, but also due to a possible synergistic effect of the two different types of emission).
The identification of the best imaging and biochemical markers for assessing response to 223Ra-dichloride.
Conclusion
In this study, the increased confidence of referring clinicians following the introduction of a strong collaborative multidisciplinary referral pathway led to earlier intervention and improved outcomes in patients treated with 223Ra-dichloride. Monitoring dynamic changes in tALP is more relevant in patients with elevated tALPBL than in those with normal tALPBL; patients with normal tALPBL had a longer OS than those with elevated tALPBL, but changes in tALP were less clear in those with normal tALPBL. Imaging and prognostic markers may be of value for individualizing 223Ra-dichloride treatment and planning retreatment, and their integration into treatment guidelines should be considered.
Acknowledgments
Medical writing support was provided by AS&K Communications and funded by Bayer.
Funding
The publication of this article was supported by funds of the European Association of Nuclear Medicine (EANM).
Conflicts of interest
Dr. Dizdarevic provides occasional consultancy to Bayer and has received occasional conference/travel sponsorship.
Dr. Robinson provides occasional consultancy to Bayer and has received occasional conference/travel sponsorship.
Ms. Jessop provides occasional consultancy to Bayer and has received occasional conference/travel sponsorship.
Mr. Begley has, on one occasion, received conference/travel sponsorship from Bayer.
Dr. Main has no conflicts of interest to declare.
Ethical approval
Not applicable – the study falls under clinical audit/quality improvement project or service evaluation and is therefore not considered research requiring NHS ethical approval, as determined by the Medical Research Council Health Authority tool (http://www.hra-decisiontools.org.uk/research/). The study has been registered on the Imaging and Nuclear Medicine Department’s Governance Audit Data Base.
Informed consent
All patients gave written informed consent for their diagnostic and therapeutic management and follow up.
References
- 1.Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17:72–79. doi: 10.3747/co.v17i0.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu J, Nguyen P, Mao J, Halpern J, Shoag J, Wright J, et al. Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol. 2017;3:705–707. doi: 10.1001/jamaoncol.2016.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 4.Hirst CJ, Cabrera C, Kirby M. Epidemiology of castration resistant prostate cancer: a longitudinal analysis using a UK primary care database. Cancer Epidemiol. 2012;36:e349–e353. doi: 10.1016/j.canep.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32:3436–3448. doi: 10.1200/JCO.2013.54.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: prostate cancer. 2016. https://www.nccn.org/professionals/physician_gls/default.aspx#site.
- 7.National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management. https://www.nice.org.uk/guidance/cg175/chapter/1-Recommendations. [PubMed]
- 8.National Institute for Health and Care Excellence. Radium-223 dichloride for treating hormone-relapsed prostate cancer with bone metastases (TA412). p. 1–41. https://www.nice.org.uk/guidance/ta412.
- 9.Hague C, Logue JP. Clinical experience with radium-223 in the treatment of patients with advanced castrate-resistant prostate cancer and symptomatic bone metastases. Ther Adv Urol. 2016;8:175–180. doi: 10.1177/1756287216629870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan CJ, Saylor PJ, Everly JJ, Sartor O. Bone-targeting radiopharmaceuticals for the treatment of bone-metastatic castration-resistant prostate cancer: exploring the implications of new data. Oncologist. 2014;19:1012–1018. doi: 10.1634/theoncologist.2013-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer. Xofigo. Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002653/WC500156172.pdf.
- 12.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 13.Wong WW, Anderson EM, Mohammadi H, Daniels TB, Schild SE, Keole SR, et al. Factors associated with survival following radium-223 treatment for metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2017;15:e969–e975. doi: 10.1016/j.clgc.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Jessop M, Staker A, Day K. Role of advanced practitioner in the clinical setting of a multi-disciplinary clinic for alpha emitting 223Radium-dichloride therapy for bone metastases in castration-resistant prostate cancer. Presented at the Annual Congress of the European Association of Nuclear Medicine, 18–22 Oct. 2014, Gothenburg, Sweden.
- 15.Soloway M, Hardeman S, Hickey D. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::AID-CNCR2820610133>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Gayed I, Canfield S, Fanous M, Wan D, Joseph U, Amato R. Optimizing patient selection for therapy with radium 223 dichloride. J Nucl Med. 2017;57:2016–7.
- 17.Dizdarevic S, Begley P, Jessop M, et al. Imaging and biochemical predictive biomarkers of overall survival and treatment response in patients receiving 223Ra-dichloride treatment in clinical practice. J Nucl Med. 2017;5:1457. [Google Scholar]
- 18.Sartor O, Coleman RE, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–1097. doi: 10.1093/annonc/mdx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontana A, Delmas P. Markers of bone turnover in bone metastases. Cancer. 2000;88:2952–2960. doi: 10.1002/1097-0142(20000615)88:12+<2952::AID-CNCR11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Etchebehere EC, Milton DR, Araujo JC, Swanston NM, Macapinlac HA, Rohren EM. Factors affecting 223Ra therapy: clinical experience after 532 cycles from a single institution. Eur J Nucl Med Mol Imaging. 2016;43:8–20. doi: 10.1007/s00259-015-3185-4. [DOI] [PubMed] [Google Scholar]
- 21.Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ, Eisenberger MA, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol. 2012;30:607–613. doi: 10.1016/j.urolonc.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dizdarevic S, Jessop M, Begley P, Robinson A. Co-existing lymph node and bone metastases may be negative predictive marker of survival in patients with CRPC treated with 223Ra-dichloride. Presented at the Annual Congress of the European Association of Nuclear Medicine, 21–25 Oct. 2017, Vienna, Austria.
- 24.Yu Evan Y., Miller Kurt, Nelson Joel, Gleave Martin, Fizazi Karim, Moul Judd W., Nathan Faith E., Higano Celestia S. Detection of Previously Unidentified Metastatic Disease as a Leading Cause of Screening Failure in a Phase III Trial of Zibotentan Versus Placebo in Patients with Nonmetastatic, Castration Resistant Prostate Cancer. The Journal of Urology. 2012;188(1):103–109. doi: 10.1016/j.juro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Main S, Jessop M, Robinson A, et al. Predictors of 223Ra-dichloride treatment outcome in clinical practice in relation to the ALSYMPCA trial. Nucl Med Commun. 2017;38:abstract 62.
- 26.European Medicines Agency. Prostate cancer medicine Xofigo must not be used with Zytiga and prednisone/prednisolone. London: European Medicines Agency; 2018.
- 27.Kulkarni H, Singh A, Baum R. Differential response of bone versus lymph node metastases after Lu-177 labelled PSMA radioligand therapy in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:S37. [Google Scholar]