Abstract
The endoplasmic reticulum (ER) is the main cellular organelle involved in protein synthesis, assembly and secretion. Accumulating evidence shows that across several neurodegenerative and neuroprogressive diseases, ER stress ensues, which is accompanied by over-activation of the unfolded protein response (UPR). Although the UPR could initially serve adaptive purposes in conditions associated with higher cellular demands and after exposure to a range of pathophysiological insults, over time the UPR may become detrimental, thus contributing to neuroprogression. Herein, we propose that immune-inflammatory, neuro-oxidative, neuro-nitrosative, as well as mitochondrial pathways may reciprocally interact with aberrations in UPR pathways. Furthermore, ER stress may contribute to a deregulation in calcium homoeostasis. The common denominator of these pathways is a decrease in neuronal resilience, synaptic dysfunction and even cell death. This review also discusses how mechanisms related to ER stress could be explored as a source for novel therapeutic targets for neurodegenerative and neuroprogressive diseases. The design of randomised controlled trials testing compounds that target aberrant UPR-related pathways within the emerging framework of precision psychiatry is warranted.
Keywords: Neurodegeneration, Neuroprogression, Unfolded protein response, Mood disorders, Endoplasmic reticulum stress, Molecular neurobiology
Introduction
The endoplasmic reticulum (ER) is a cell organelle that plays an indispensable role in protein synthesis, folding and sorting, as well as the delivery of proteins to their ultimate cellular destination. This role is facilitated by the presence of a multitude of chaperone proteins capable of binding to hydrophobic areas of newly synthesised, but as yet unfolded, proteins to facilitate optimal protein folding and prevent protein–protein aggregation. Under physiological conditions, protein folding and function are also facilitated by N-linked glycosylation and the formation of disulphide bonds by reaction mechanisms favoured by the highly oxidative environment of the ER [1, 2].
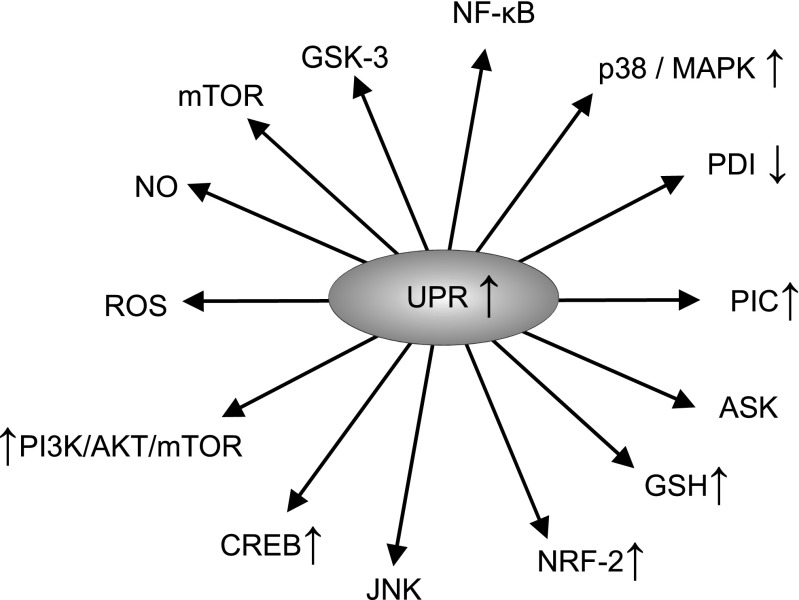
However, in pathophysiological circumstances, the accumulation of misfolded or unfolded proteins may ensue [2, 3]. Several mechanisms may contribute to the accumulation of unfolded proteins, including an excessive biosynthesis of reactive oxygen species (ROS), a lowered efficiency of cellular anti-oxidant defences [2, 4], as well as disturbances in calcium homoeostasis [2, 3]. In addition, in diseases like amyloidosis and Huntington’s disease the accumulation of misfolded proteins appears to be a pivotal pathophysiological event. In such circumstances, the ER initially elicits an adaptive or protective response described as the unfolded protein response (UPR) aimed at restoring homoeostasis within the organelle and the cell through the re-establishment of protein homeostasis [5–7]. Nevertheless, in some pathophysiological situations, the homeostatic capacity of the ER and the UPR may not meet cellular demands and may even become detrimental (vide infra), a condition referred to as ER stress. While severe and prolonged ER stress may trigger apoptotic cell death [8, 9], there is an accumulating body of evidence supporting the proposition that sub-lethal ER stress and the consequent chronic upregulation of the UPR are involved in the pathogenesis and pathophysiology of several diseases [10–12]. Figure 1 summarises the effects of upregulation of the UPR.
Fig. 1.
Effects of the upregulation of the UPR
Exemplars of such illnesses include Alzheimer’s disease [13, 14], Parkinson’s disease [15, 16], multiple sclerosis [17, 18] and amyotrophic lateral sclerosis [19, 20]. More recently, a putative role of ER stress for psychiatric disorders in which neuroprogression may occur, including bipolar disorder [12, 21, 22], major depressive disorder [23, 24] and schizophrenia, [25] has been disputed. It is noteworthy that the chronic upregulation of the UPR may lead to the development of chronic inflammation [26, 27], oxidative stress [11, 28, 29] and multiple dimensions of mitochondrial dysfunction [30–33] and that these elements appear to be shared factors involved in the pathogenesis and pathophysiology of neurodegenerative and neuroprogressive disease, although disease-specific elements also seem to be involved [34–39]. There is also some evidence to suggest that the detrimental effects of ER stress and chronic UPR upregulation could be “druggable” and hence inhibition of pathways involved in the UPR may confer neuroprotection. For example, there are reports demonstrating that inhibition of ER stress pathways could protect against neuronal injury [40–42].
Thus, this review has two overarching aims: first, to detail putative pathways whereby activation of the UPR may instigate or exacerbate chronic inflammation, oxidative/nitrosative stress and multiple dimensions of mitochondrial dysfunction that are observed across neuroprogressive illnesses and, second, to examine therapeutic options targeting ER stress and the UPR as novel neurotherapeutic targets for neuroprogressive diseases. Initially, processes stemming from ER stress and UPR activation which may lead to the initiation or exacerbation of chronic neuroinflammation will be critically examined before moving on to a consideration of putative pathways leading to the initiation or exacerbation of oxidative and nitrosative stress, and multiple dimensions of mitochondrial dysfunction.
ER Stress, Activation of the UPR and the Development of Chronic Inflammation
Processes Involved in the Activation of the UPR
During the UPR, a triad of ER transmembrane protein receptors referred to as protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1α (IRE1α) and activating transcription factor 6 (ATF6), whose activity is negatively regulated by the master ER chaperone GRP78, act as sensors to detect misfolded/mutant proteins [43, 44]. However, in an environment of ER stress, GRP78 binds to the exposed hydrophobic domains of unfolded or misfolded proteins leading to their dissociation from PERK, ATF6 and IRE1α, thus activating these ER signalling pathways [43]. Once activated, each of these receptors may undergo oligomerisation and other conformational changes, thus inducing highly specific downstream signalling cascades [44, 45].
Activation of PERK and the Development of Chronic Inflammation
PERK phosphorylates eukaryotic translation initiation factor-2α (eIF2α) leading to an inhibition of general protein translation and promotion of the preferential translation of transcription factor ATF4 [7, 46]. ATF4 in turn translocates to the nucleus whereupon it induces the transcription of additional UPR target genes and, in an environment of extreme ER stress, ATF4 targets the promoter of the gene that encodes the transcription factor CHOP, which plays a major role in the instigation of apoptotic cell death [47] (see [48] for a review).
Activation of PERK leads to upregulation of the JAK1/STAT3 signalling axis and subsequent increments in the transcription and translation of IL-6 and oncostatin, thus forming a feed-forward loop driving escalating levels of inflammation [49]. It is noteworthy that activation of PERK in astrocytes, and subsequent paracrine activation of microglia, is now recognised as a relevant mechanism in the initiation and perpetuation of neuroinflammation [49]. PERK activation leads to phosphorylation of eIF2α, which also suppresses the translation of IκB, resulting in translocation of the cytosolic transcription factor NF-κB to the nucleus, whereupon it may induce the expression of genes involved in instigating and regulating inflammatory pathways [50]. Furthermore, PERK may also regulate cellular redox homoeostasis via the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and the subsequent upregulation of reduced glutathione [51–53]. It is also noteworthy that PERK-activated ATF4 also regulates the cellular redox state and may also act independently of PERK to induce the production of pro-inflammatory cytokines [50].
Activation of ATF6 and the Development of Chronic Inflammation
Upregulation of monomeric ATF6 also exerts a range of complex, broadly pro-inflammatory effects via the upregulation of NF-κB via mechanisms involving activation of the CREB and PI3K/Akt/mTOR signalling pathways [50, 54]. The upregulation of this UPR pathway also exerts direct effects on inflammation via the upregulation of toll-like receptor activity on macrophages [55].
Activation of IRE1α and the Development of Chronic Inflammation
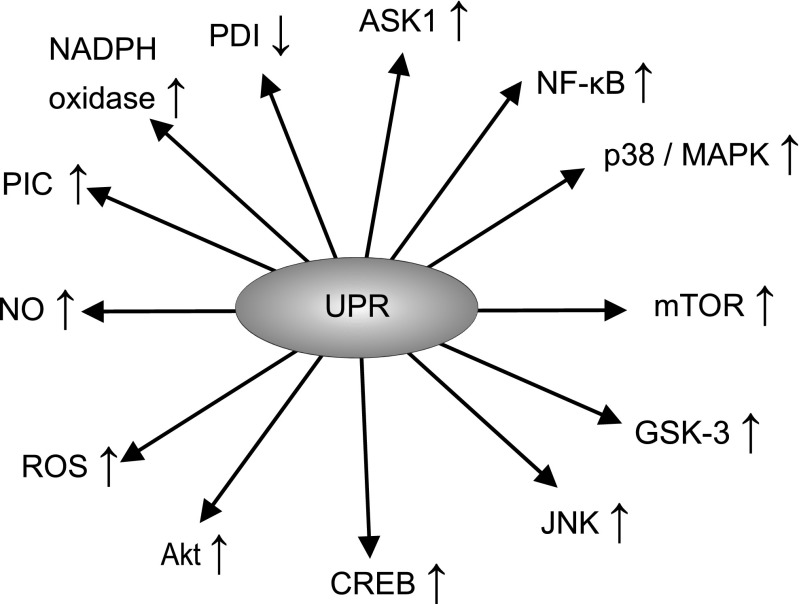
IRE1α functions both as a kinase and as an endonuclease, which is activated via a process of oligomerisation in the absence of GRP78 inhibition. Evidence suggests that this enzyme could play a major role in regulating the splicing of several mRNAs and its activity is an indispensable player in the translation and activation of transcription factor X-box binding protein-1 (XBP-1) [56, 57]. XBP-1, in turn, increases the transcription of several UPR target genes including the one encoding GRP78 [58, 59]. The activated IRE1α can also form a multiprotein complex with apoptosis signal-regulating kinase 1 (ASK1), resulting in the upregulation of various intracellular signalling systems such as c-Jun N-terminal kinase (JNK) [60], p38/MAPK [61, 62], NF-κB [63, 64], glycogen synthase kinase 3 (GSK-3) [65, 66], mammalian target of rapamycin (mTOR) [67, 68] and the phosphatidylinositol 3-kinase/protein kinase B/mTOR (PI3K/AKT/mTOR) pathway [69–71]. These pathways also play a major role in determining the balance between cell survival and cell death, generally promoting cell survival in an environment of chronic oxidative stress. Yet it is important to note that their effects on cell survival are pleiotropic, and activation of these pathways may also drive cellular death in other circumstances, particularly when ER stress is severe [72, 73]. The net effect of these signalling systems is somewhat unpredictable as they engage in a complex pattern of mutual cross-talk with the UPR and each other, and their relative activities appear to influence the balance between cell proliferation and cell death [74–76]. Figure 2 illustrates the actions of the UPR.
Fig. 2.
Actions of the UPR
For example, the UPR activates GSK-3, possibly via a route involving increased autophagy of the inactive kinase phosphorylated at serine 219 [65]. This kinase in turn also appears to play a role in the regulation of the UPR by influencing the phosphorylation status of CHOP and caspase-3 [66, 77]. There is also evidence of a bidirectional feedback between UPR activity and levels of mTOR signalling [78–80]. Similarly, the activation of NF-κB by the UPR also acts to reduce ER stress by accelerating the clearance of misfolded proteins via the modulation of autophagic activity [72]. Readers interested in a detailed consideration of the mechanisms enabling and regulating such “cross-talk”, and how such communication leads to variations in biochemical and immunological profiles over time, are referred to previous scholarly reviews [81, 82]. Importantly, from the perspective of the aims of this paper, changes in the activity of p38/MAPK, JNK, NF-κB, mTOR, GSK-3 and PI3K/AKT have pivotal roles in instigating and/or modulating inflammatory and immune pathways and the activity of peripheral mononuclear blood cells such as macrophages [83–87].
Several research teams have adduced data demonstrating that p38/MAPK is a major player in the promotion and regulation of inflammatory and immune responses in general and that the upregulation of p38/MAPK is a pivotal driver of pro-inflammatory cytokine transcription and translation [88]. Phosphorylation of NF-κB and a range of other transcription factors, such as myocyte enhancer factor-2 (MEF-2), as a result of upregulated p38/MAPK activity induces transcriptional activation of tumour necrosis factor-α (TNF-α), IL-6 and other pro-inflammatory cytokines [83, 89]. Similarly, there is copious evidence that activation of JNK signalling plays a major role in cytokine production and the subsequent development of inflammation [84, 90].
The NF-κB pathway also regulates the production of pro-inflammatory cytokines and several other processes driving the inflammatory response, such as leukocyte recruitment and the survival of peripheral mononuclear blood cells, which are important contributors to the inflammatory response [91, 92]. Furthermore, the complex bidirectional signalling between pro-inflammatory cytokines (notably TNF-α) allows for the development of a self-amplifying inflammatory response [93, 94]. However, it should be noted that the anti-apoptotic activity of NF-κB may protect cells against the ravages of inflammation and in certain circumstances the pro-apoptotic properties of this signalling system can also contribute to the resolution of inflammation by contributing to the immunologically silent destruction of infiltrating leucocytes and macrophages [91].
The activity of GSK-3 influences the balance between the production of pro- and anti-inflammatory cytokines, T cell differentiation, toll-like receptor responses and the proliferation and activity of transcription factors which are known to play a regulatory role in the duration and magnitude of the immune response, such as signal transducer and activator of transcription (STAT), nuclear factor of activated T cells (NFAT), T-box transcription factor (Tbet) and NF-κB [95–97]. A recent review further illustrates the immunoregulatory role of GSK [98]. Much of this regulatory activity occurs in concert with mTOR and PI3K/AKT pathways [99]. These interactions are complex but are essentially connected with the role of mTOR as a metabolic sensor and its capacity to integrate metabolic and immune processes, thereby regulating the activation and proliferation of T cells, B cells and antigen presenting cells. Readers interested in a detailed consideration of the processes involved are invited to consult the work of Powell et al. [100] and Weichhart et al. [85]. It should also be noted that the PI3K/AKT/mTOR signalling axis has a broadly restraining effect on the development of chronic inflammation by limiting the production of type 1 interferons, while increasing the production of IL-10, and hence, its downregulation during chronic UPR activation may contribute to the development and perpetuation of an inflammatory state [86].
Given the above data, accumulating evidence supporting an association between the chronic upregulation of the UPR and the development of chronic inflammation is perhaps unsurprising [26, 65, 70, 101]. It is also noteworthy that the chronic upregulation of pro-inflammatory cytokines in tandem with upregulation of NF-κB and p38/MAPK may enhance the biosynthesis of ROS and nitric oxide (NO), and thus may promote or otherwise aggravate oxidative and nitrosative stress [87, 102–104], and hence provides a mechanism for the development of chronic oxidative stress accompanying acute or chronic upregulation of the UPR [11, 28, 105]. Moreover, the complex interplay of NF-κB, p38/MAPK and ROS may lead to a self-amplifying pattern of redox dyshomoeostasis [106–108]. However, there are a number of other mechanisms which may also contribute to the development of oxidative and nitrosative stress following ER stress and over-activation of the UPR which seem underdiscussed, and we will now turn to a consideration of these factors.
UPR Activation and the Development of Oxidative and Nitrosative Stress
Mechanisms Involved in the Development of Chronic Oxidative and Nitrosative Stress
ER stress and the subsequent activation of the UPR may lead to an increased production of ROS and subsequently to oxidative stress via a number of mechanisms other than the upregulation of MAPK and NF-κB [105]. Such mechanisms involve an upregulation of protein disulphide isomerase (PDI) resulting in the activation of NADPH oxidase isomers, notably NOX-2 and NOX-4 [109], and the upregulation of oxidative protein folding in the ER, which rivals mitochondrial respiration as a source of cellular ROS [110, 111]. Other factors involved in the development of oxidative stress in such circumstances include the oxidation of reduced glutathione (GSH), an increased S-nitrosylation of proteins and an increase in Ca2+ efflux from the ER into the mitochondria [28, 112].
Upregulation of PDI and Activation of NADPH Oxidase Isoforms
The development of ER stress and activation of the UPR may lead to upregulation of PDI [113–116]. This is an important event in the context of the development of oxidative stress, as PDI is associated with NOX isoforms and acts as a redox-sensitive protein which regulates their activation [10, 109, 117]. The change in cellular redox status “sensed” by PDI thus may activate NOX-2 and NOX-4 [10, 118, 119], leading to the production of superoxide ions [120, 121].
The effects of PDI in activating NOX enzymes appear to be of pathophysiological relevance since these enzymes are a major source of ROS in several cell types [122, 123], and ROS production by NOX isoforms may even exceed mitochondria as the prime source of ROS in some cell types [124]. However, while ROS production within mitochondria stems from the integral architecture and membrane organisation, NOX signalling is dependent on multiple protein interactions and post-translational modifications leading to the assembly of a functional NOX complex and the subsequent trafficking to specific subcellular locations [122, 123]. The assembly of subunits and the translocation NOX enzymes to sites of activity appears to be met by the chaperone rather than the isomerase activity of PDI via hydrophobic rather than electrostatic or covalent associations [109, 125]. Nevertheless, both the chaperone and isomerase activities of PDI are required to fulfil its role in the oxidative folding of proteins within the ER [126].
Upregulation of PDI and Increased Rate of ROS and RNS Production from Oxidative Protein Folding
The ER contains numerous molecules whose task is to ensure that proteins secreted from the organelle have acquired the prerequisite post-translational modifications and the correct conformation [127]. One important process involved in ensuring optimal protein folding is the acquisition of disulphide bonds. The interaction between PDI and oxidoreductin-1α (Ero1α) is probably the most important vehicle for oxidative protein folding in the ER [128, 129]. Hence PDI has the capacity to supply, isomerise or, in some circumstances, reduce disulphide bonds in target proteins [130], while its activity is dependent upon the existence of two distinct remote active sites which are directly or indirectly oxidised by Ero1α to form disulphide bridges [130–132]. Such oxidation provokes a conformational change allowing for the entry of unfolded protein substrates in the reduced state [133, 134]. Once in situ, key thiol groups on these proteins are oxidised to form disulphide bridges, resulting in the reduction of PDI; these target proteins then “receive” disulphide bonds from PDI; Ero1α then re-oxidises the reduced PDI and transfers electrons from the reduced PDI to molecular oxygen, which is subsequently reduced to hydrogen peroxide (H2O2) [135, 136], thus resulting in the re-oxidation of the oxidoreductase [128, 137]. The capacity of Ero1α to reduce molecular oxygen is dependent on the existence of a helical structure containing flavin adenine dinucleotide (FAD) sealed by a disulphide bridge between Cys(208)-Cys(241). This “seal” may be disrupted via the formation of a mixed disulphide bridge between PDI and one of these cysteines, which underpins the capacity of this chaperone to regulate the activity of its co-oxidoreductase [135, 136]. In addition, Ero1α activity in the ER is upregulated by the UPR and hence H2O2 levels may increase as a result of ER stress [135, 136]. Initially, such upregulation may have an adaptive purpose as the glutathione peroxidase isoform GPx7 may utilise H2O2 to accelerate the oxidative folding of substrates in vivo [65]. Briefly, evidence suggests that H2O2 oxidises the Cys57 residue of GPx7 to produce sulfonic acid, which in turn may react with its Cys86 to form a disulphide bond. Both the disulphide and the sulfonic acid forms of GPx7 may oxidise PDI to catalyse oxidative folding [138]. However, the accumulation of ROS and reactive nitrogen species (RNS) following activation of the ER [139, 140] leads to S-nitrosylation and the subsequent inactivation of PDI, thus leading to a loss of its chaperone and isomerase activities [140–142]. This loss of activity may have meaningful pathophysiological consequences; the accumulation of misfolded proteins within the ER may further enhance the UPR, leading to self-amplifying increases in inflammation as well as oxidative and nitrosative stress [143, 144]. Importantly, such increases in ROS and RNS may also promote ER stress, which leads to an increase in Ca2+ efflux from the ER into mitochondria [28, 112] which is enabled by tubular channels tethering the organelles described as mitochondrial associated molecular membranes (MAMs) [5, 145]; an increase in Ca2+ within the mitochondria may ultimately lead to the development of multiple dimensions of mitochondrial dysfunction as discussed below.
ER Stress, UPR Activation and Mitochondrial Dysfunction
Initial Increase in Mitochondrial Respiration
The ER and mitochondria are physically connected by highly specialised structures referred to as MAMs. These molecules act as a conduit for the exchange of proteins, lipids, a range of metabolites, various signalling molecules and most importantly Ca2+, and this complex cascade of events appears to influence the balance between cell death and survival [5, 146]. The architecture of a MAM is highly complex and contains a wide array of structural, functional and regulatory proteins, such as the GTPase activating protein for Rab32 [147, 148]. The inositol trisphosphate receptor (IP3R) and the voltage-dependent anion channels (VDACs) are among the most important molecules for enabling and regulating ER–mitochondria Ca2+ transfer, and are located in the ER and mitochondrial sides of MAMs, respectively, and may complex with the chaperone GRP75, thus forming a channel connecting the two organelles and enabling mutual exchange between membrane and luminal components [149, 150]. Mitofusin 2 (Mfn2) is another important protein present on the ER and mitochondrial surfaces, which plays an indispensable role in ER–mitochondria tethering as well as in the modulation of inter-mitochondrial contacts [5, 151, 152]. The composition of MAMs adapts in response to multiple internal and external stimuli [153, 154], while the formation or dissolution of contact areas between mitochondria and the ER is further regulated by other aspects of organelle dynamics [5, 148, 154]. Importantly, in the adaptive phase of ER stress, there is an increased number of physical contacts between the ER and mitochondrial networks at the perinuclear regions enabling increased transfer of Ca2+ from the ER into the mitochondria [5, 43, 146, 155].
An increase in Ca2+ uptake by the mitochondria may increase transmembrane potential and ATP production aimed at promoting cellular survival as part of an adaptive response to ER stress [156]. Such an increase in energy production is accompanied by increases in the production of mitochondrial proteases such as LON, which are induced by the activation of the PERK pathway, which in turn regulates the structural integrity and assembly of cytochrome c oxidase (COX) [157, 158]. In this scenario, elevated expression of LON protease may increase mitochondrial performance by stimulating the assembly and increasing stabilisation of COX II [157, 158]. However, elevated calcium levels may also increase the production of ATP and ROS [159–161], leading to the activation of mitochondrial nitric oxide synthase (mtNOS) [162, 163], and the production of NO, leading to the inhibition of mitochondrial function via a number of direct and indirect mechanisms including the reversible S-nitrosylation of key structural and functional mitochondrial proteins and enzymes [163–165].
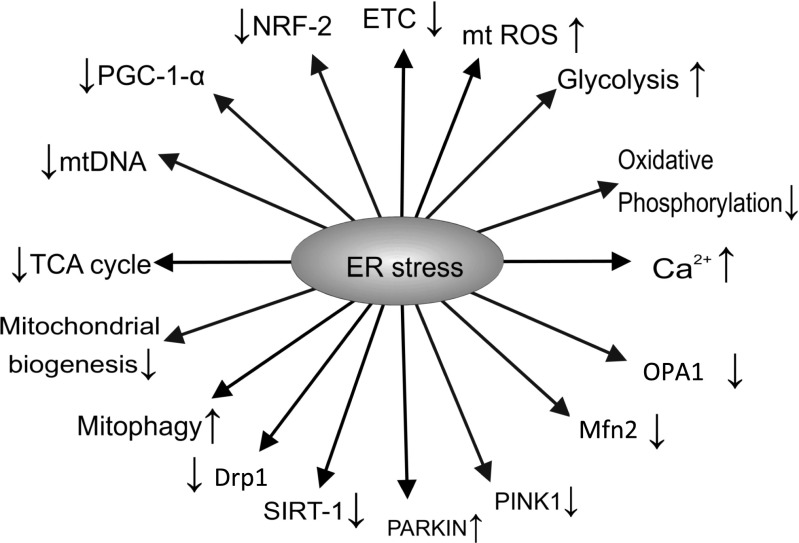
Figure 3 summarises the effects of ER stress.
Fig. 3.
Effects of ER stress
Elevated Levels of NO and Mitochondrial Function
The nitrosylation of mitochondrial structural proteins and enzymes may play a major role in the redox-based regulation of mitochondrial respiration [166, 167]. While nitrosylation in response to modest increases in NO levels may initially act as a defence mechanism aimed at maintaining protein structure and function [168–170], further increases in this RNS may lead to the inhibitory nitrosylation of crucial functional enzymes such as complex I of the electron transport chain [165, 171]. Furthermore, the inhibition of complex I by S-nitrosylation is another initially cytoprotective response, which also leads to decreased ATP production and defects in energy homoeostasis over time [169, 170]. Persistently elevated cellular concentrations of NO may also lead to the inhibitory nitrosylation of crucial functional cysteine thiols of COX and complex II of the electron transport chain, thus leading to chronically suppressed activity of the former and transiently reduced activity of the latter [172, 173]. Such inhibition may ultimately impair oxidative phosphorylation and hence decrease ATP production and GSH levels within the organelle [173–175]. Furthermore, the prolonged inhibition of COX activity also provokes an increase in ATP production via glycolysis in a wide range of cell types as a defensive response aimed at preventing apoptosis or necrosis [176–178]. Importantly, the inhibition of complex III and complex IV by S-nitrosylation may further increase the production of ROS [179, 180], which combined with reduced ATP generation may contribute to the release Ca2+ from the ER [181–183], which may further decrease the biosynthesis of ATP and also increase the generation of ROS in a positive feedback loop [110, 184]. This process is of relevance, as an increased production of ROS may increase the misfolding of mitochondrial proteins, which coupled with impaired oxidative phosphorylation and ATP production may trigger another response aimed at restoring mitochondrial homoeostasis, namely the mitochondrial unfolded protein response (mtUPR) [185–188]. Thus, in the section below, we also discuss the putative pathophysiological relevance of the mtUPR.
Impaired Mitochondrial Performance Following Activation of the mtUPR
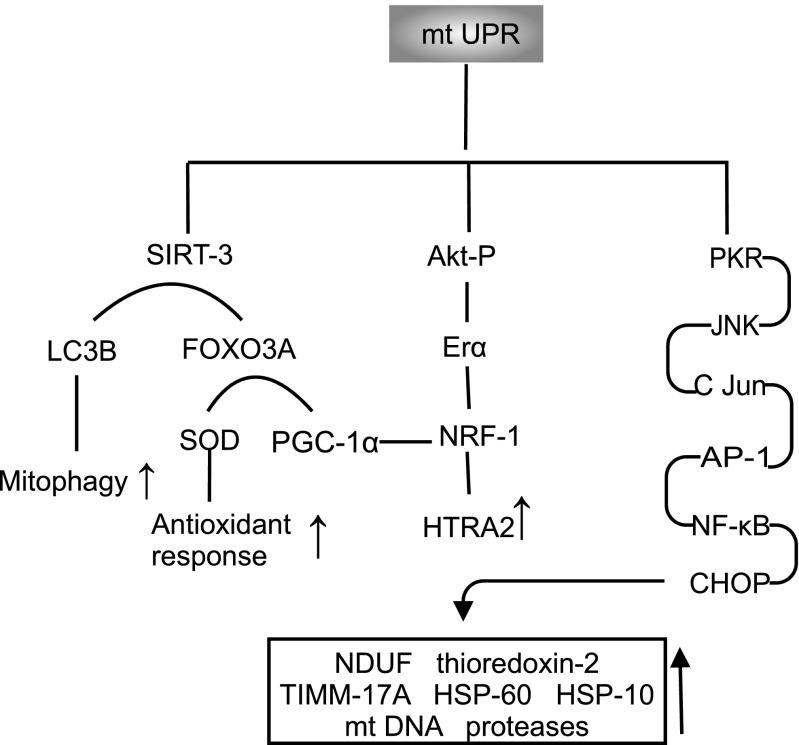
The mtUPR is a multidimensional transcriptional response initiated and maintained by retrograde mitochondrial-to-nuclear signalling following increases in protein misfolding in the mitochondrial matrix and inner membrane space and/or decreased efficiency of protein importation into mitochondria aimed at restoring mitochondrial function and preventing organellar death [189–192].
The initiation of the mtUPR is mediated by sensory quality control proteases with LON or ClpCP being the prime activators in the matrix [191] and the mitochondrial serine protease HTRA2 playing the same role at the inner membrane space [193–195]. Interestingly, the initial upregulation of HTRA2 is provoked by an overproduction of ROS and the subsequent phosphorylation of Akt, which in turn activates the oestrogen receptor in the outer mitochondrial membrane leading to upregulation of the transcription factor nuclear respiratory factor 1 (NRF-1) and ultimately leading to increased mitochondrial production of HTRA-2 [193]. This is an illustrative example of retrograde mitochondrion to nucleus signalling and is similar in principle to the retrograde ER to nucleus signalling that facilitates the UPR response (reviewed in [196]). Another example involves the upregulation of CHOP, which is an indispensable player in the regulation of mitochondrial proteases and chaperones, in an attempt to restore intra-mitochondrial protein folding homoeostasis [197, 198]. However, despite recent evidence suggesting that signals of protein unfolding within mitochondria are transduced to the nucleus via activation of c-Jun, JNK and the activator protein 1 (AP-1) [64, 191], thus sharing some characteristics with the ER UPR, the precise details underpinning this mechanism remain to be elucidated [190]. It should also be noted that while there is some evidence that the factors involved in initiating and regulating the mtUPR are similar in principle to those that regulate the ER UPR, there is another regulatory mechanism governing the mtUPR, namely decreased mitochondrial import efficiency, which is unique to the mtUPR, and some background information is required to understand its genesis and implications.
The vast majority of mitochondrial proteins originate from nuclear DNA and hence must be recruited to the mitochondria and thereafter imported. In most circumstances, this recruitment is initially achieved via the mitochondrial targeting sequence (MTS) [199]. Once in situ at the outer mitochondrial membrane (OMM), the protein is directed via a myriad of regulatory processes to either the OMM, the intermembrane space, the inner mitochondrial membrane (IMM) or the matrix. Importantly, in order to enter the matrix, the protein must cross the IMM via the translocase of inner membrane complex (TIM), which requires the optimal activity of chaperones located at the matrix as well as physiological tricarboxylic acid (TCA) cycle and oxidative phosphorylation activities [199, 200]. Hence, mitochondrial protein import efficiency may provide a proxy measure of diverse aspects of mitochondrial performance [191, 201, 202]. Importantly, a lowered import of proteins into the mitochondria leads to the accumulation in the cytoplasm of proteins normally destined for the organelle [64, 203]. Most such proteins are detected and targeted for proteasomal degradation [204, 205]. However, in lower animals, at least one mitochondrial protein, the transcription factor ATFS-1, which regulates the mtUPR in the worm Caenorhabditis elegans, has both a MTS, which enables its mitochondrial import in normal physiological conditions, and a nuclear localisation sequence (NLS), which enables its translocation to the nucleus in conditions of mitochondrial stress whereupon it activates the mtUPR [187]. There are excellent reviews detailing this process [190, 203]. Notwithstanding that evidence of such a transcription factor in mammals is lacking, Fiorese et al. [206] have recently reported the existence of ATF5 in mammalian cells which is regulated similarly to ATFS-1 and may induce a similar transcriptional response.
The mtUPR is activated by a range of stressors other than the presence of unfolded proteins, which may lead to a decrease in mitochondrial protein import efficiency. In addition to the presence of heavy metals or other substances acting as DNA adducts, contaminants in sulphide bonds, or otherwise, such stressors include a depletion of mtDNA [186, 207], high levels of ROS [185, 186], mitochondrial ribosome impairment [208, 209], inhibition of mitochondrial proteases and chaperones [186], impaired oxidative phosphorylation and ATP production [187, 188] and abnormally high glucose consumption indicating a switch to the glycolytic pathway as a source of energy generation [210].
It should be stressed that while one facet of the mtUPR involves the upregulation of genes aimed at increasing mitochondrial proteases as well as chaperones, thereby promoting protein homoeostasis within the mitochondrial protein folding environment [186, 211], another facet includes changes in the transcription patterns of genes governing cellular metabolism [190]. In particular, the mtUPR may increase the expression of genes governing the rate of glycolysis and the catabolism of amino acids with a concomitant suppression of genes enabling the optimal performance of the TCA cycle and oxidative phosphorylation [212, 213].
Therefore, while aimed at relieving mitochondrial stress and ensuring cellular survival, the over- or chronic activation of the mtUPR may also compromise mitochondrial function and oxidative phosphorylation, thus favouring a switch to aerobic glycolysis as the predominant source of ATP [212, 213]. These changes in cellular metabolism provoked by the activation of the mtUPR could be of interest given data demonstrating that such a response may be regulated by NAD+ and sirtuin (SIRT) deacetylases [193, 209, 213], which are capable of sensing and stimulating metabolic activity by increasing mitochondrial performance via a number of different routes (reviewed by Morris et al. [214]). Importantly, SIRT-1 is inactivated in an environment of nitro-oxidative stress [215] and such inactivation may up-regulate NF-κB [215]. Hence, in a cellular environment of chronic oxidative stress the normal compensatory response to impaired mitochondrial function is negated and the switch to aerobic glycolysis via NF-κB upregulation is preferentially operating [216].
Furthermore, the progressive decline in mitochondrial ATP production and mitochondrial membrane potential in such circumstances coupled with an increase in aerobic glycolysis can activate another very specific mitochondrial quality control mechanism involving retrograde mitochondrion to nucleus signalling referred to as mitophagy [217, 218]. Such a physiologically abnormal elevation in the rate of mitophagy has adverse bioenergetic consequences as this process appears to play a relevant role in the regulation of energy homoeostasis and mitochondrial dynamics [219, 220]. It is also noteworthy that ER stress and the UPR accompanied by increased levels of Ca2+ and ROS can also exert detrimental effects on multiple regulatory processes governing mitochondrial dynamics directly [221–223].
Key reactions and pathways associated with the mtUPR are summarised in Fig. 4.
Fig. 4.
Key reactions and pathways associated with the mtUPR
UPR Activation and Impaired Mitochondrial Dynamics
Background
Accumulating evidence indicates that the balance of activity between pathways regulating mitophagy and those regulating mitochondrial dynamics (mitochondrial biogenesis, fusion, fission and motility) may influence mitochondrial mass, morphology and function and thus the cellular capacity to generate energy and to adjust energy production in the face of changing metabolic demands [219, 224, 225]. In particular, changes in mitochondrial dynamics enable these organelles to maintain a balance between energy production and changes in energy demand by generating highly fused networks of mitochondria or otherwise favouring the formation of more discrete and isolated organelles [226–228]. In addition, pathways and proteins governing mitochondrial dynamics may regulate energy supply and distribution at both the whole organism and cellular levels [229]. Therefore, the targeted manipulation of these processes may open a relevant therapeutic perspective for neuroprogressive disorders. Therefore, facets of mitochondrial dynamics as well as the pathophysiological influence of the chronic upregulation of the UPR on these processes will now be discussed as the final mechanistic section of this paper.
ER Stress and UPR Activation as a Source of Impaired Mitochondrial Mitophagy
Mitophagy is mediated by the cooperative action of the two proteins parkin and PINK. There are excellent reviews detailing the processes involved in the delivery and regulation of mitophagy [203, 219, 225]. Therefore, we provide a brief description of this process in order to explain the putative adverse effects of the UPR upon mitophagy.
PINK1 is a serine/threonine kinase that possesses an N-terminal mitochondria-targeting signal [230] enabling anchorage at the IMM. Under physiological conditions, PINK1 is imported into the mitochondria via TIM and translocase of outer membrane (TOM) protein complexes and is continuously degraded by PARL and matrix processing peptidase [230, 231]. However, following the accumulation of unfolded proteins and/or membrane depolarisation, mitochondrial import efficiency falls and therefore the import of PINK1 to the IMM is compromised [191, 201, 202]. Following such inhibition, the enzyme accumulates at the OMM and forms a large 700-kDa complex with TOM before undergoing activation via autophosphorylation at two serine residues [232, 233]. This activation results in the recruitment of inactive cytosolic parkin onto damaged mitochondria, whereupon the molecule is activated via PINK1-mediated phosphorylation [234]. Following activation, parkin ubiquinates a myriad of mitochondrial substrates as well as itself [235]. These ubiquitinated residues in turn undergo phosphorylation, which is affected by PINK1, thereby triggering further cycles of parkin recruitment in a feed-forward amplification loop [236, 237]. It should be noted that while this process is a prerequisite for the development of mitophagy, it is not sufficient in itself to precipitate the phenomenon, and other mechanisms also appear to play a role. Readers interested in a detailed account of such mechanisms are invited to consult previous work [238–240]. Moreover, mitophagy is also regulated by other processes governing mitochondrial dynamics [241–243], and an imbalance between mitophagy and mitochondrial biogenesis stemming from the activation of the UPR is now thought to play a relevant role in the pathophysiology of several neurodegenerative and neuroprogressive illnesses [225]. This is unsurprising given that the complex cross-talk between these processes is an essential element in regulating cellular energy homoeostasis [244, 245]. Importantly, changes in the rate of mitophagy may deregulate mitochondrial biogenesis [219, 246], thus compromising cellular energy homoeostasis.
UPR Activation and Impaired Mitochondrial Biogenesis
Under physiological conditions, mitochondrial biogenesis is regulated by a sophisticated interplay between the coactivator peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) and the transcription factors NRF-2 and SIRT-1, which enable coupling between changes in cellular metabolism to changes in mitochondrial mass and number [34, 214, 247]. However, in an environment of ER stress and UPR activation, elevated levels of NF-κB, MAPK and PKB/Akt as well as higher NO signalling provoke an increase in PGC-1α, NRF-1, NRF-2 and SIRT-1, which in turn induce an increase in mitochondrial biogenesis as a putative adaptive (i.e. pro-survival) response [248, 249]. However, with increasing levels of inflammation, and increased levels of oxidative and nitrosative stress, the upregulation of PGC-1α is inhibited by TNF-α [104] and the activity of NRF-2, SIRT-1 and NF-κB may be inhibited by S-nitrosylation or over-oxidation of cysteine residues which normally enable their function [171, 214], ultimately leading to a chronic state of decreased mitochondrial biogenesis. The processes governing mitochondrial biogenesis and those governing mitochondrial fusion and fission also engage in a complex bidirectional cross-talk which also plays a role in regulating cellular energy homoeostasis [250], and hence, impaired mitochondrial biogenesis can provoke adverse changes in processes governing mitochondrial fusion and fission which also have the effect of dysregulating cellular energy generation. In addition, the molecular players generating inflammation and oxidative stress also lead to compromised activity of proteins and pathways regulating fusion and fission, which may lead to decreased energy production at cellular and whole organism levels.
UPR Activation and Impaired Activity of Proteins and Processes Governing Fusion and Fission
Background
Mitochondrial fusion and fission processes are regulated and enabled by dynamin family GTPases [251]. Readers interested in a detailed explanation of the mechanisms underpinning the actions of these molecular motors are referred to the work of Ferguson and De Camilli [252]. In mammals, the fusion of OMMs is mediated by Mfn1 and Mfn2, whereas the fusion of inner membranes is mediated via the protein optic atrophy 1 (OPA1) [151, 253–256]. We will focus on their role in mitochondrial respiration and how their activities may be compromised in an environment of ER stress and chronic activation of the UPR.
Role of Mitofusins in Energy Production and Consequences of UPR Upregulation
Mfn2, and to a lesser extent Mfn1, plays pivotal roles in the regulation of mitochondrial respiration and energy homoeostasis [228, 257, 258]. This role is perhaps unsurprising given that Mfn2 is an indispensable player in tethering mitochondria and ER stress and enabling high fidelity and rapid calcium signalling cross-talk between the two organelles in environments of stress and changing metabolic demands for energy [259, 260]. While modulation of calcium signalling appears to be one element underpinning the capacity of Mfn2 to regulate mitochondrial respiration energy and adaptation to increased cellular demands for energy, other mechanisms are clearly involved. Such mechanisms involve the inhibition of ROS production and the regulation of glucose homoeostasis via mechanisms which are not related to effects on calcium signalling, although the precise details of such mechanisms remain to be fully delineated [257, 258]. Crucially, the capacity of this enzyme to adapt the production of ATP by hypothalamic neurones is a major factor in regulating whole body metabolism and whole body energy homoeostasis [257, 258]. In this context, it is of paramount importance that the activity of this enzyme may be inhibited in an environment of chronic inflammation and nitrosative stress. For example, MAPK upregulation suppresses Mfn2 activity [261] and there is some evidence that this enzyme is inhibited in an environment in which the production of pro-inflammatory mediators is elevated [257]. It is also of interest that the capacity of Mfn2 to stimulate mitochondrial function is dependent on the activation of the PI3K/Akt pathway.
Role of OPA1 in Energy Generation and Consequences of UPR Upregulation
There is some evidence to suggest that processing of the mitochondrial dynamin-like GTPase OPA1 is the main regulatory element governing mitochondrial function by modulating IMM potential [262]. Several research teams investigating the effects of OPA1 mutants have adduced evidence indicating that OPA1 activity is an important factor determining the existence of cellular mitochondria as highly fused networks or a myriad of fragmented organelles, which affect the supply of ATP produced by oxidative phosphorylation and influence the capacity to increase cellular energy output in the face of increased metabolic demands for energy as discussed above [263, 264]. These observations have been supported by recent in vitro data supplied by Kao and fellow workers who reported that inactivation of OPA1 results in the fragmentation of established mitochondrial networks as well as a reduction in oxygen consumption, uncoupling of oxidative phosphorylation to ATP production and a shift to aerobic glycolysis as the main mode of energy generation [265].
OPA1 has several other regulatory roles in mitochondrial function such as maintaining the integrity of the quaternary structures of electron transport chain enzymes and preventing depolarisation of the IMM. In addition, OPA1-dependent stabilisation and remodelling of mitochondrial cristae increases the efficiency of energy production by the electron transport chain, while also reducing the production of ROS [266]. OPA1-driven cristae remodelling is another essential factor enabling cells to meet energy production in the context of enhanced energy demands [267]. The importance of OPA1 in this domain is further emphasised by the existence of data demonstrating that its targeted inactivation leads to detrimental changes in crista morphology and reduces the stability and performance of the electron transport chain, thereby compromising oxidative phosphorylation and ATP production [268, 269].
Other roles include stabilising the association between cardiolipin and COX, thereby acting as an anti-apoptotic protein [266, 268–270]. Perhaps predictably, there is experimental evidence that OPA1 transcription and translation are upregulated in an environment of chronic nitro-oxidative stress [271], which is also supported by data demonstrating that mitochondrial dynamics in general, and OPA1 levels in particular, appear to be under the control of the non-canonical NF-κB pathway [272]. In addition, evidence indicates that levels of this enzyme are elevated following activation of the Akt/mTOR pathway [273] and more indirectly by ROS and Ca2+ and by upregulation of PGC-1α [274]. The involvement of AKT and NF-κB in the regulation of OPA1 activity is particularly germane as both molecules are inactivated by S-nitrosylation in an environment of nitro-oxidative stress [275–278], and such inactivation may compromise the ability of mitochondria to cope with elevated cellular requirements for energy.
The Role of Drp1 in Energy Generation and the Consequences of UPR Upregulation
The activity of the mitochondrial fission protein Drp1 is regulated by a plethora of factors such as Ca2+ concentrations, ROS levels and a range of post-translational modifications [279–281] (see [282] for a review). Chronically elevated levels of ROS and RNS lead to changes in Drp1 activity and/or rates of mitochondrial fission via a number of routes. One such route involves the inactivation of Drp1 by AMPK, whose activity is upregulated in an environment of chronically elevated ROS generation [214, 283, 284]. This is of importance as there is evidence that inhibition of this GTPase may disrupt mitochondrial networks, thus leading to adverse changes in organelle morphology accompanied by a reduction in Mfn1 and Mfn2 as well as a compromised proteolytic processing of OPA1 isoforms [285] and hence presents yet another route by which the chronic upregulation of the UPR could compromise energy generation. It is also noteworthy that S-nitrosylation of Drp1 in an environment of chronically upregulated nitro-oxidative stress could increase the rate of mitochondrial fission [286], thereby creating an imbalance between fusion and fission which may lead to detrimental net alterations in mitochondrial morphology and energy production [287–289]. The precise mechanisms underpinning such an increase in fission rates is still a matter of ongoing debate. However, it may not be a direct consequence of nitrosylation-induced increases in the enzymatic activity of Drp1 [290]. Lastly, mitophagy relies on a synergistic interplay between parkin and the dynamin family kinase Drp1, with the fission activity of the latter required to generate small mitochondria, thereby enabling efficient engulfment by autophagosomes [239, 291]. Hence, inhibition of this enzyme may also lead to disrupted mitophagy, which in turn has the capacity to dysregulate mitochondrial dynamics, and ultimately ATP production, further compromising energy generation.
Having reviewed the multiple mechanisms involved in driving the advent or exacerbation of chronic inflammation, oxidative stress and mitochondrial dysfunction, we will now consider possible therapeutic targets for the management of neuroprogressive and neurodegenerative diseases. Based on the mechanisms highlighted, it seems reasonable to suggest that molecules with the capacity to target the mechanisms driving ER stress and the UPR and to ameliorate the adverse downstream events following the activation of these pathways, would be desirable, and this consideration forms the basis of the approaches suggested below.
Possible Neurotherapeutic Targets
Melatonin
Recent evidence indicates that melatonin exerts a regulatory role in the process of mitophagy by stimulating the autophagic clearance of irreparably damaged mitochondria and increasing mitochondrial biogenesis, probably by a route involving the activation of AMPK and SIRT-1 [292–294]. Melatonin administration may also restore calcium homoeostasis, mitochondrial dynamics and mitochondrial permeability transition [295–298]. At least partly, those beneficial effects appear to be related to entry into the organelle, which may be facilitated by Glut-1 or peptide 1 and 2 transporter proteins located in the OMM [299, 300].
Moreover, melatonin may shift the pattern of mitochondrial dynamics towards a decrease in fission and an increase in fusion [296, 298, 301]. This activity has been demonstrated in a wide range of cell types [297, 301]. From a mechanistic perspective, the weight of evidence suggests that melatonin may attenuate the translocation of Fis1, Drp1 and Bax from the cytosol to mitochondria and may also upregulate mitochondrial fusion proteins Mfn1, Mfn2 and OPA1 [295, 300, 302, 303].
Melatonin supplementation also exerts multiple protective effects on mitochondria via a number of different mechanisms which may mitigate against the development of maladaptive processes within these organelles which initially stem from the upregulation of the UPR (i.e. ER stress). Such mechanisms include a reduction of mitochondrial oxidative stress [304, 305]; an increased efficiency of ATP production [306, 307]; a reduction in mitochondrial NOS expression [308, 309]; an amelioration of calcium dyshomoeostasis [310, 311]; the preservation of mitochondrial membrane potential [307, 312]; and a reduced release of cytochrome c into the cytosol accompanied by the inhibition of caspase-3 activity [313]. Several authors have demonstrated protective effects of melatonin supplementation against damage to mitochondria caused by a myriad of different insults including, but not limited to, sepsis [314, 315], ischaemia/reperfusion [316, 317] and challenge with neurotoxic compounds such as 1-methyl-4-phenylpyridinium ion (MPP+) [302], β-amyloid peptide (Aβ 25–35) [318, 319], 4-hydroxynonenal [320] and lipopolysaccharide [309] .
Melatonin therapy also inhibits the DNA binding activity and activation of NF-κB, with concomitant reductions in NLRP3 activity and the synthesis of pro-inflammatory cytokines [321–323]. These anti-inflammatory effects are considered to underpin the promising results obtained from studies investigating the use of melatonin in animal models of neurodegenerative diseases and are the motivation for an increased focus on the use of the molecule as a therapeutic agent targeting the pathogenesis and pathophysiology of diseases such as Alzheimer’s disease and Parkinson’s disease at doses ranging from 50 to 100 mg daily [305, 324]. In addition, it has been proposed that melatonin treatment could be useful for cognitive dysfunction associated with mood disorders [325]. However, evidence remains inconclusive [326].
CoQ10
Converging preclinical and clinical evidence suggest that coenzyme Q10 (CoQ10) supplementation may offer therapeutic benefits in a range of neurodegenerative and neuroprogressive disorders, at least partly owing to its effects on ER stress and adverse downstream effects. For example, Yubero-Serrano et al. [327] reported that supplementation of CoQ10 in tandem with a Mediterranean diet effectively suppressed the expression of genes encoding proteins involved in the UPR. Furthermore, CoQ10 also appeared to exert a positive effect on mitochondrial dynamics by exerting a direct effect on ATP production [328], while this therapeutic target may also preserve the structure of mitochondrial cristae, with an accompanying increase in mitochondrial biogenesis [329]. In addition, CoQ10 may also restore endogenous anti-oxidants, such as vitamin E [330], and is also an essential player in enabling the optimal performance of the electron transport chain and stabilisation of the mitochondrial permeability transition pore [331] (reviewed by [332]).
There is also a considerable and increasing body of evidence demonstrating beneficial effects of CoQ10 on levels of pro-inflammatory cytokines and ROS, which may both act as a trigger of the UPR and be effectors of pathology following activation. For example, the effectiveness of CoQ10 supplementation at a daily dose of 500 mg for 12 weeks reduced inflammation and oxidative stress in a randomised, double-blind, placebo-controlled trial involving participants with relapsing-remitting multiple sclerosis [333, 334]. Controlled data demonstrating the ameliorative effects on inflammation and oxidative stress of CoQ10 supplementation also extend into other disease areas such as coronary artery disease, and there is some evidence that such benefits could be dose related [335]. In addition, evidence suggests that CoQ10 supplementation at doses up to 300 mg/day is safe and well tolerated [336, 337]. The former research team reported a significant reduction in cardiovascular mortality, over and above that seen in patients receiving standard treatment, in an elderly population of 445 patients supplemented with 200 mg of CoQ10 for 4 years [336], while the latter group of researchers found a significant reduction in cardiovascular morbidity and mortality in a population of 420 patients with chronic heart failure supplemented with 300 mg of CoQ10 for 2 years [337]. Human and animal studies have also demonstrated the potential for increased clinical benefit from the use of mitochondrial-targeted CoQ10 (MitoQ) where the active molecule is covalently attached to the lipophilic triphenylphosphonium cation [300, 338]. This mode of delivery allows levels of CoQ10 to accumulate within the mitochondrial matrix, reaching levels several hundred-fold higher than can be achieved via supplementation with the naked coenzyme [338, 339]. Human trials of the use of MitoQ in the treatment of neurodegenerative diseases have produced initial evidence of benefit with particularly encouraging results seen in Parkinson’s disease [340, 341]. In addition, CoQ10 could represent a novel therapeutic target for cognitive dysfunction associated with mood disorders [342], and a recent uncontrolled study found potential benefits for CoQ10 as a treatment for bipolar depression in late-life [343]. Clearly, the field awaits the design of large-scale and well-designed controlled trials testing CoQ10 as a therapeutic target for mood disorders.
NAC
Animal and clinical studies have reported beneficial effects of N-acetylcysteine (NAC) supplementation on levels of ER stress [344–347]. For example, rats supplemented for 2 months with drinking water containing 600 mg NAC per litre displayed reduced levels of PDI and GRP78 compared with rats which did not receive NAC [345]. Similar findings indicate that NAC supplementation at 100 or 300 mg/kg for 20 weeks promote significant reductions in ROS levels [346, 347]. Moreover, NAC-related benefits upon ER stress appear to be dose-dependent, with 20 mmol/L of NAC being more effective than 10 mmol/L in reducing levels of GRP78 and ROS [348]. It should be noted, however, that NAC is a pleiotropic agent and several mechanisms other than direct effects on the UPR may also contribute to its therapeutic effects across neurodegenerative and neuroprogressive diseases [349, 350], while evidence suggests that adjunctive NAC treatment may mitigate cognitive dysfunction in a range of such disorders [351].
Conclusions and Future Directions
This review indicates that pathways related to the UPR may reciprocally interact with immune-inflammatory, neuro-oxidative, neuro-nitrosative, as well as mitochondrial mechanisms, which are thought to play a major shared pathophysiological role across several neuroprogressive and neurodegenerative diseases. Therefore, the chronic upregulation of the UPR may interact with a range of cell death mechanisms underpinning neurodegeneration and neuroprogression [352] and hence represents a novel neurotherapeutic target.
Moreover, this review also opens relevant directions for further research. First, the involvement of mechanistic pathways related to the UPR in separate disorders deserves further investigation. Second, the extent to which effects upon the UPR could contribute to therapeutic benefits of novel therapeutic targets (for example, melatonin, CoQ10 and NAC) is a matter of ongoing research efforts. Lastly, the identification of patients who could benefit from therapies targeting ER stress pathways, taking in account the emerging framework of precision psychiatry [353], could represent a relevant road of research.
Acknowledgements
We should like to thank Myrela O. Machado, MD, PhD, for her kind assistance with the figures.
Authorships
All authors contributed to the writing up of the paper.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65(6):862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 3.Görlach A, Klappa P, Kietzmann DT. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8(9–10):1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 4.Fedoroff N. Redox regulatory mechanisms in cellular stress responses. Ann Bot. 2006;98(2):289–300. doi: 10.1093/aob/mcl128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum–mitochondria connection: one touch, multiple functions. Biochim Biophys Acta. 2014;1837(4):461–469. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Peraza JF, Engel T, Martin-Ibanez R, Sanz-Rodriguez A, Fernandez-Fernandez MR, Esgleas M, Canals JM, Henshall DC, Lucas JJ. Protective neuronal induction of ATF5 in endoplasmic reticulum stress induced by status epilepticus. Brain. 2013;136(Pt 4):1161–1176. doi: 10.1093/brain/awt044. [DOI] [PubMed] [Google Scholar]
- 7.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y, Yang JM. Survival and death of endoplasmic-reticulum-stressed cells: role of autophagy. World J Biol Chem. 2011;2(10):226–231. doi: 10.4331/wjbc.v2.i10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34(3):286–297. [PubMed] [Google Scholar]
- 10.Laurindo FR, Fernandes DC, Amanso AM, Lopes LR, Santos CX. Novel role of protein disulfide isomerase in the regulation of NADPH oxidase activity: pathophysiological implications in vascular diseases. Antioxid Redox Signal. 2008;10(6):1101–1113. doi: 10.1089/ars.2007.2011. [DOI] [PubMed] [Google Scholar]
- 11.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11(10):2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 12.Pfaffenseller B, Wollenhaupt-Aguiar B, Fries GR, Colpo GD, Burque RK, Bristot G, Ferrari P, Ceresér KMM, Rosa AR, Klamt F, Kapczinski F. Impaired endoplasmic reticulum stress response in bipolar disorder: cellular evidence of illness progression. Int J Neuropsychopharmacol. 2014;17(09):1453–1463. doi: 10.1017/s1461145714000443. [DOI] [PubMed] [Google Scholar]
- 13.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viana RJ, Nunes AF, Rodrigues CM. Endoplasmic reticulum enrollment in Alzheimer’s disease. Mol Neurobiol. 2012;46(2):522–534. doi: 10.1007/s12035-012-8301-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxid Redox Signal. 2007;9(5):553–561. doi: 10.1089/ars.2006.1524. [DOI] [PubMed] [Google Scholar]
- 16.Cali T, Ottolini D, Brini M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors. 2011;37(3):228–240. doi: 10.1002/biof.159. [DOI] [PubMed] [Google Scholar]
- 17.Stone S, Lin W. The unfolded protein response in multiple sclerosis. Front Neurosci. 2015;9:264. doi: 10.3389/fnins.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Getts MT, Getts DR, Kohm AP, Miller SD. Endoplasmic reticulum stress response as a potential therapeutic target in multiple sclerosis. Therapy. 2008;5(5):631–640. doi: 10.2217/14750708.5.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lautenschlaeger J, Prell T, Grosskreutz J. Endoplasmic reticulum stress and the ER mitochondrial calcium cycle in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(2):166–177. doi: 10.3109/17482968.2011.641569. [DOI] [PubMed] [Google Scholar]
- 20.Tadic V, Prell T, Lautenschlaeger J, Grosskreutz J. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front Cell Neurosci. 2014;8:147. doi: 10.3389/fncel.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi A, Kasahara T, Kametani M, Toyota T, Yoshikawa T, Kato T. Aberrant endoplasmic reticulum stress response in lymphoblastoid cells from patients with bipolar disorder. Int J Neuropsychopharmacol. 2008;12(01):33. doi: 10.1017/s1461145708009358. [DOI] [PubMed] [Google Scholar]
- 22.Bengesser SA, Fuchs R, Lackner N, Birner A, Reininghaus B, Meier-Allard N, Stracke A, Kapfhammer HP, Reininghaus EZ, Wallner-Liebmann S. Endoplasmic reticulum stress and bipolar disorder—almost forgotten therapeutic drug targets in the unfolded protein response pathway revisited. CNS Neurol Disord Drug Targets. 2016;15(4):403–413. doi: 10.2174/1871527315666160321104613. [DOI] [PubMed] [Google Scholar]
- 23.Gold PW, Licinio J, Pavlatou MG. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-[gamma] systems. Mol Psychiatry. 2013;18(2):154–165. doi: 10.1038/mp.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timberlake MA, Dwivedi Y. Altered expression of endoplasmic reticulum stress associated genes in hippocampus of learned helpless rats: relevance to depression pathophysiology. Front Pharmacol. 2015;6:319. doi: 10.3389/fphar.2015.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38(10):1910–1920. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16(8):469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med. 2012;18(10):589–598. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Eletto D, Chevet E, Argon Y, Appenzeller-Herzog C. Redox controls UPR to control redox. J Cell Sci. 2014;127(Pt 17):3649–3658. doi: 10.1242/jcs.153643. [DOI] [PubMed] [Google Scholar]
- 29.Cao SS, Kaufman RJ. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin Ther Targets. 2013;17(4):437–448. doi: 10.1517/14728222.2013.756471. [DOI] [PubMed] [Google Scholar]
- 30.Rocha M, Diaz-Morales N, Rovira-Llopis S, Escribano-Lopez I, Banuls C, Hernandez-Mijares A, Diamanti-Kandarakis E, Victor VM. Mitochondrial dysfunction and endoplasmic reticulum stress in diabetes. Curr Pharm Des. 2016;22(18):2640–2649. doi: 10.2174/1381612822666160209152033. [DOI] [PubMed] [Google Scholar]
- 31.Morais KL, Pacheco MT, Berra CM, Bosch RV, Sciani JM, Chammas R, de Freitas SR, Iqbal A, Chudzinski-Tavassi AM. Amblyomin-X induces ER stress, mitochondrial dysfunction, and caspase activation in human melanoma and pancreatic tumor cell. Mol Cell Biochem. 2016;415(1–2):119–131. doi: 10.1007/s11010-016-2683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimm S. The ER–mitochondria interface: the social network of cell death. Biochim Biophys Acta. 2012;1823(2):327–334. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Scaini G, Rezin GT, Carvalho AF, Streck EL, Berk M, Quevedo J. Mitochondrial dysfunction in bipolar disorder: evidence, pathophysiology and translational implications. Neurosci Biobehav Rev. 2016;68:694–713. doi: 10.1016/j.neubiorev.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 34.Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13(1):68. doi: 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595–606. doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- 36.Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135(5):373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 38.Lai KSP, Liu CS, Rau A, Lanctot KL, Kohler CA, Pakosh M, Carvalho AF, Herrmann N. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017;88(10):876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- 39.Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, de Andrade NQ, Morris G et al (2017) Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 10.1007/s12035-017-0632-1 [DOI] [PubMed]
- 40.Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66(4):899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- 41.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27(4):901–908. doi: 10.1523/jneurosci.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maly DJ, Papa FR. Druggable sensors of the unfolded protein response. Nat Chem Biol. 2014;10(11):892–901. doi: 10.1038/nchembio.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AFG, Rothermel BA, Lavandero S. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol. 2012;44(1):16–20. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11(9):2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2(6):326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 46.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 47.Lenna S, Trojanowska M (2012) The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr Opin Rheumatol 24 (6). doi:10.1097/BOR.0b013e3283588dbb [DOI] [PMC free article] [PubMed]
- 48.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345(6192):98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meares GP, Liu Y, Rajbhandari R, Qin H, Nozell SE, Mobley JA, Corbett JA, Benveniste EN. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol. 2014;34(20):3911–3925. doi: 10.1128/mcb.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circul Res. 2012;110(1):174–189. doi: 10.1161/circresaha.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, Cavener D, Diehl JA. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29(27):3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279(19):20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 53.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamasaki-Mann M, Demuro A, Parker I. Modulation of endoplasmic reticulum Ca2+ store filling by cyclic ADP-ribose promotes inositol trisphosphate (IP3)-evoked Ca2+ signals. J Biol Chem. 2010;285(32):25053–25061. doi: 10.1074/jbc.m109.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao J, Yue S, Fu Y, Zhu J, Wang X, Busuttil RW, Kupiec-Weglinski JW, Lu L, Zhai Y. ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia reperfusion injury. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2014;14(7):1552–1561. doi: 10.1111/ajt.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 57.Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem. 2014;289(3):1203–1211. doi: 10.1074/jbc.R113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiang C, Wang Y, Zhang H, Han F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis. 2017;22(1):1–26. doi: 10.1007/s10495-016-1296-4. [DOI] [PubMed] [Google Scholar]
- 60.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta. 2014;1843(10):2150–2163. doi: 10.1016/j.bbamcr.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Clarke R, Cook K (2015) Unfolding the role of stress response signaling in endocrine resistant breast cancers. Front Oncol 5(140). 10.3389/fonc.2015.00140 [DOI] [PMC free article] [PubMed]
- 63.Janssens S, Pulendran B, Lambrecht BN. Emerging functions of the unfolded protein response in immunity. Nat Immunol. 2014;15(10):910–919. doi: 10.1038/ni.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jovaisaite V, Auwerx J. The mitochondrial unfolded protein response-synchronizing genomes. Curr Opin Cell Biol. 2015;33:74–81. doi: 10.1016/j.ceb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14(9):581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 66.Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3β in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem. 2002;277(47):44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- 67.Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6(2):239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 68.Kapuy O, Vinod PK, Bánhegyi G. mTOR inhibition increases cell viability via autophagy induction during endoplasmic reticulum stress—an experimental and modeling study. FEBS Open Bio. 2014;4:704–713. doi: 10.1016/j.fob.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qu M, Shen W. Role of PI3K/Akt pathway in endoplasmic reticulum stress and apoptosis induced by saturated fatty acid in human steatotic hepatocytes. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese Journal of Hepatology. 2015;23(3):194–199. doi: 10.3760/cma.j.issn.1007-3418.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Mantuano E, Henry K, Yamauchi T, Hiramatsu N, Yamauchi K, Orita S, Takahashi K, Lin JH, Gonias SL, Campana WM. The unfolded protein response is a major mechanism by which LRP1 regulates Schwann cell survival after injury. J Neurosci. 2011;31(38):13376–13385. doi: 10.1523/JNEUROSCI.2850-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao F, Yang C, Chen SS, Wang YY, Zhou W, Hao Q, Lu T, Hoffer B, Zhao LR, Duan WM, Xu QY. Long-term protective effects of AAV9-mesencephalic astrocyte-derived neurotrophic factor gene transfer in parkinsonian rats. Exp Neurol. 2017;291:120–133. doi: 10.1016/j.expneurol.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Nivon M, Fort L, Muller P, Richet E, Simon S, Guey B, Fournier M, Arrigo AP, Hetz C, Atkin JD, Kretz-Remy C. NFkappaB is a central regulator of protein quality control in response to protein aggregation stresses via autophagy modulation. Mol Biol Cell. 2016;27(11):1712–1727. doi: 10.1091/mbc.E15-12-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Shao Z, Zetoune FS, Zeidler MG, Gowrishankar K, Vincenz C. NRADD, a novel membrane protein with a death domain involved in mediating apoptosis in response to ER stress. Cell Death Differ. 2003;10(5):580–591. doi: 10.1038/sj.cdd.4401208. [DOI] [PubMed] [Google Scholar]
- 74.Homma K, Katagiri K, Nishitoh H, Ichijo H. Targeting ASK1 in ER stress-related neurodegenerative diseases. Expert Opin Ther Targets. 2009;13(6):653–664. doi: 10.1517/14728220902980249. [DOI] [PubMed] [Google Scholar]
- 75.Walter F, Schmid J, Dussmann H, Concannon CG, Prehn JH. Imaging of single cell responses to ER stress indicates that the relative dynamics of IRE1/XBP1 and PERK/ATF4 signalling rather than a switch between signalling branches determine cell survival. Cell Death Differ. 2015;22(9):1502–1516. doi: 10.1038/cdd.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekine Y, Takeda K, Ichijo H. The ASK1-MAP kinase signaling in ER stress and neurodegenerative diseases. Curr Mol Med. 2006;6(1):87–97. doi: 10.2174/156652406775574541. [DOI] [PubMed] [Google Scholar]
- 77.Meares GP, Mines MA, Beurel E, Eom TY, Song L, Zmijewska AA, Jope RS. Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells. Exp Cell Res. 2011;317(11):1621–1628. doi: 10.1016/j.yexcr.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camacho A, Rodriguez-Cuenca S, Blount M, Prieur X, Barbarroja N, Fuller M, Hardingham GE, Vidal-Puig A. Ablation of PGC1 beta prevents mTOR dependent endoplasmic reticulum stress response. Exp Neurol. 2012;237(2):396–406. doi: 10.1016/j.expneurol.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abelaira HM, Reus GZ, Ignacio ZM, Dos Santos MA, de Moura AB, Matos D, Demo JP, da Silva JB, Michels M, Abatti M, Sonai B, Dal Pizzol F, Carvalho AF, Quevedo J. Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. J Psychiatr Res. 2017;87:81–87. doi: 10.1016/j.jpsychires.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Abelaira HM, Reus GZ, Ignacio ZM, Dos Santos MAB, de Moura AB, Matos D, Demo JP, da Silva JBI, Danielski LG, Petronilho F, Carvalho AF, Quevedo J. Ketamine exhibits different neuroanatomical profile after mammalian target of rapamycin inhibition in the prefrontal cortex: the role of inflammation and oxidative stress. Mol Neurobiol. 2017;54(7):5335–5346. doi: 10.1007/s12035-016-0071-4. [DOI] [PubMed] [Google Scholar]
- 81.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5(3):a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pereira CFM. Crosstalk between endoplasmic reticulum stress and protein misfolding in neurodegenerative diseases. ISRN Cell Biol. 2013;2013:22. doi: 10.1155/2013/256404. [DOI] [Google Scholar]
- 83.Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, Wang Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111(19):2494–2502. doi: 10.1161/01.cir.0000165117.71483.0c. [DOI] [PubMed] [Google Scholar]
- 84.Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 2009;48(3):161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15(10):599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weichhart T, Saemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann Rheum Dis. 2008;67(Suppl 3):iii70–iii74. doi: 10.1136/ard.2008.098459. [DOI] [PubMed] [Google Scholar]
- 87.Lucas K, Morris G, Anderson G, Maes M. The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets. 2015;14(7):838–854. doi: 10.2174/1871527314666150317224645. [DOI] [PubMed] [Google Scholar]
- 88.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773(8):1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 89.Bachstetter AD, Van Eldik LJ. The p38 MAP kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1(3):199–211. [PMC free article] [PubMed] [Google Scholar]
- 90.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morris G, Maes M. Increased nuclear factor-kappaB and loss of p53 are key mechanisms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Med Hypotheses. 2012;79(5):607–613. doi: 10.1016/j.mehy.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 93.Morgan MJ, Z-g L. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86–86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31(1):24. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hofmann C, Dunger N, Scholmerich J, Falk W, Obermeier F. Glycogen synthase kinase 3-beta: a master regulator of toll-like receptor-mediated chronic intestinal inflammation. Inflamm Bowel Dis. 2010;16(11):1850–1858. doi: 10.1002/ibd.21294. [DOI] [PubMed] [Google Scholar]
- 97.Taylor A, Harker JA, Chanthong K, Stevenson PG, Zuniga EI, Rudd CE Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of Co-receptor PD-1 to enhance CD8<sup>+</sup> cytolytic T cell responses. Immunity 44(2):274–286. 10.1016/j.immuni.2016.01.018 [DOI] [PMC free article] [PubMed]
- 98.Cortés-Vieyra R, Bravo-Patiño A, Valdez-Alarcón JJ, Juárez MC, Finlay BB, Baizabal-Aguirre VM. Role of glycogen synthase kinase-3 beta in the inflammatory response caused by bacterial pathogens. J Inflammation. 2012;9(1):23. doi: 10.1186/1476-9255-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang H, Brown J, Gu Z, Garcia CA, Liang R, Alard P, Beurel E, Jope RS, Greenway T, Martin M. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-beta-signaling pathways regulates the innate inflammatory response. J Immunol. 2011;186(9):5217–5226. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxidative Med Cell Longev. 2015;2015:18. doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Habib N, Goswami G, Mungre S. Inflammatory cytokines induce oxidative stress and apoptosis in PC12 cells. FASEB J. 2010;24(1 Supplement):485.488. [Google Scholar]
- 104.Morris G, Maes M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) Curr Neuropharmacol. 2014;12:168–185. doi: 10.2174/1570159X11666131120224653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21(3):396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morgan M, Liu Z. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakajima S, Kitamura M. Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med. 2013;65:162–174. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 108.Liu T, Wu L, Wang D, Wang H, Chen J, Yang C, Bao J, Wu C. Role of reactive oxygen species-mediated MAPK and NF-κB activation in polygonatum cyrtonema lectin-induced apoptosis and autophagy in human lung adenocarcinoma A549 cells. J Biochem. 2016;160(6):315–324. doi: 10.1093/jb/mvw040. [DOI] [PubMed] [Google Scholar]
- 109.Santos CX, Stolf BS, Takemoto PV, Amanso AM, Lopes LR, Souza EB, Goto H, Laurindo FR. Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J Leukoc Biol. 2009;86(4):989–998. doi: 10.1189/jlb.0608354. [DOI] [PubMed] [Google Scholar]
- 110.Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016;17(3):327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Pareja KA, Kaiser CA, Sevier CS. Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. eLife. 2014;3:e03496. doi: 10.7554/eLife.03496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Higa A, Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal. 2012;24(8):1548–1555. doi: 10.1016/j.cellsig.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 113.Perri ER, Thomas CJ, Parakh S, Spencer DM, Atkin JD (2016) The unfolded protein response and the role of protein disulfide isomerase in neurodegeneration. Front Cell Dev Biol 3 (80). doi:10.3389/fcell.2015.00080 [DOI] [PMC free article] [PubMed]
- 114.Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S, Lipton SA. Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron. 2013;78(4):596–614. doi: 10.1016/j.neuron.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo B, Lee AS (2013) The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anti-cancer therapies. Oncogene 32 (7). doi:10.1038/onc.2012.130 [DOI] [PMC free article] [PubMed]
- 116.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, Laurindo FR. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem. 2005;280(49):40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 118.Jaronen M, Goldsteins G, Koistinaho J. ER stress and unfolded protein response in amyotrophic lateral sclerosis—a controversial role of protein disulphide isomerase. Front Cell Neurosci. 2014;8:402. doi: 10.3389/fncel.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trevelin SC, Lopes LR. Protein disulfide isomerase and Nox: new partners in redox signaling. Curr Pharm Des. 2015;21(41):5951–5963. doi: 10.2174/1381612821666151029112523. [DOI] [PubMed] [Google Scholar]
- 120.Li G, Mongillo M, Chin K-T, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-α–mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186(6):783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katsuyama M, Matsuno K, Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin Biochem Nutr. 2012;50(1):9–22. doi: 10.3164/jcbn.11-06SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110(10):1364–1390. doi: 10.1161/circresaha.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30(4):653–661. doi: 10.1161/atvbaha.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 125.Fernandes DC, Manoel AH, Wosniak J, Jr, Laurindo FR. Protein disulfide isomerase overexpression in vascular smooth muscle cells induces spontaneous preemptive NADPH oxidase activation and Nox1 mRNA expression: effects of nitrosothiol exposure. Arch Biochem Biophys. 2009;484(2):197–204. doi: 10.1016/j.abb.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 126.Nakamura T, Lipton SA. S-nitrosylation of critical protein thiols mediates protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Antioxid Redox Signal. 2011;14(8):1479–1492. doi: 10.1089/ars.2010.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Benham AM, van Lith M, Sitia R. Braakman I (2013) Ero1-PDI interactions, the response to redox flux and the implications for disulfide bond formation in the mammalian endoplasmic reticulum. Philos Trans R Soc Lond Ser B Biol Sci. 1617;368:20110403. doi: 10.1098/rstb.2011.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tavender TJ, Bulleid NJ. Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum. Antioxid Redox Signal. 2010;13(8):1177–1187. doi: 10.1089/ars.2010.3230. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L, Niu Y, Zhu L, Fang J, Wang X, Wang L, Wang CC. Different interaction modes for protein-disulfide isomerase (PDI) as an efficient regulator and a specific substrate of endoplasmic reticulum oxidoreductin-1alpha (Ero1alpha) J Biol Chem. 2014;289(45):31188–31199. doi: 10.1074/jbc.M114.602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kozlov G, Maattanen P, Thomas DY, Gehring K. A structural overview of the PDI family of proteins. FEBS J. 2010;277(19):3924–3936. doi: 10.1111/j.1742-4658.2010.07793.x. [DOI] [PubMed] [Google Scholar]
- 131.Baker KM, Chakravarthi S, Langton KP, Sheppard AM, Lu H, Bulleid NJ. Low reduction potential of Ero1alpha regulatory disulphides ensures tight control of substrate oxidation. EMBO J. 2008;27(22):2988–2997. doi: 10.1038/emboj.2008.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chambers JE, Tavender TJ, Oka OBV, Warwood S, Knight D, Bulleid NJ. The reduction potential of the active site disulfides of human protein disulfide isomerase limits oxidation of the enzyme by Ero1α. J Biol Chem. 2010;285(38):29200–29207. doi: 10.1074/jbc.M110.156596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen VD, Wallis K, Howard MJ, Haapalainen AM, Salo KE, Saaranen MJ, Sidhu A, Wierenga RK, Freedman RB, Ruddock LW, Williamson RA. Alternative conformations of the x region of human protein disulphide-isomerase modulate exposure of the substrate binding b’ domain. J Mol Biol. 2008;383(5):1144–1155. doi: 10.1016/j.jmb.2008.08.085. [DOI] [PubMed] [Google Scholar]
- 134.Wang C, Chen S, Wang X, Wang L, Wallis AK, Freedman RB, Wang C-c. Plasticity of human protein disulfide isomerase: evidence for mobility around the x-linker region and its functional significance. J Biol Chem. 2010;285(35):26788–26797. doi: 10.1074/jbc.M110.107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zito E. ERO1: a protein disulfide oxidase and H2O2 producer. Free Radic Biol Med. 2015;83:299–304. doi: 10.1016/j.freeradbiomed.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 136.Ramming T, Okumura M, Kanemura S, Baday S, Birk J, Moes S, Spiess M, Jeno P, Berneche S, Inaba K, Appenzeller-Herzog C. A PDI-catalyzed thiol-disulfide switch regulates the production of hydrogen peroxide by human Ero1. Free Radic Biol Med. 2015;83:361–372. doi: 10.1016/j.freeradbiomed.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 137.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11(11):2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 138.Wang L, Zhang L, Niu Y, Sitia R, Wang CC. Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1alpha to promote oxidative protein folding. Antioxid Redox Signal. 2014;20(4):545–556. doi: 10.1089/ars.2013.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Halloran M, Parakh S, Atkin JD (2013) The role of S-nitrosylation and S-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. Int J Cell Biol. 10.1155/2013/797914 [DOI] [PMC free article] [PubMed]
- 140.Forrester MT, Benhar M, Stamler JS. Nitrosative stress in the ER: a new role for S-nitrosylation in neurodegenerative diseases. ACS Chem Biol. 2006;1(6):355–358. doi: 10.1021/cb600244c. [DOI] [PubMed] [Google Scholar]
- 141.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441(7092):513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 142.Andreu CI, Woehlbier U, Torres M, Hetz C. Protein disulfide isomerases in neurodegeneration: from disease mechanisms to biomedical applications. FEBS Lett. 2012;586(18):2826–2834. doi: 10.1016/j.febslet.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 143.Walker AK, Farg MA, Bye CR, McLean CA, Horne MK, Atkin JD. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain. 2010;133(Pt 1):105–116. doi: 10.1093/brain/awp267. [DOI] [PubMed] [Google Scholar]
- 144.Wu XF, Wang AF, Chen L, Huang EP, Xie WB, Liu C, Huang WY, Chen CX, Qiu PM, Wang HJ. S-Nitrosylating protein disulphide isomerase mediates alpha-synuclein aggregation caused by methamphetamine exposure in PC12 cells. Toxicol Lett. 2014;230(1):19–27. doi: 10.1016/j.toxlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 145.Qi H, Li L, Shuai J. Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Sci Rep. 2015;5:7984. doi: 10.1038/srep07984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20(12):1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41(10):1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bravo-Sagua R, Rodriguez AE, Kuzmicic J, Gutierrez T, Lopez-Crisosto C, Quiroga C, Díaz-Elizondo J, Chiong M, Gillette TG, Rothermel BA, Lavandero S. Cell death and survival through the endoplasmic reticulum-mitochondrial axis. Curr Mol Med. 2013;13(2):317–329. doi: 10.2174/156652413804810781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175(6):901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265(13):7248–7256. [PubMed] [Google Scholar]
- 151.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305(5685):858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 153.Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19(7):2777–2788. doi: 10.1091/mbc.e07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bui M, Gilady SY, Fitzsimmons RE, Benson MD, Lynes EM, Gesson K, Alto NM, Strack S, Scott JD, Simmen T. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285(41):31590–31602. doi: 10.1074/jbc.M110.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER–mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 2010;190(3):363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, Iglewski M, Chiong M, Simmen T, Zorzano A, Hill JA, Rothermel BA, Szabadkai G, Lavandero S. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124(13):2143–2152. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Margineantu DH, Gregory Cox W, Sundell L, Sherwood SW, Beechem JM, Capaldi RA. Cell cycle dependent morphology changes and associated mitochondrial DNA redistribution in mitochondria of human cell lines. Mitochondrion. 2002;1(5):425–435. doi: 10.1016/S1567-7249(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 158.Venkatesh S, Lee J, Singh K, Lee I, Suzuki CK. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim Biophys Acta. 2012;1823(1):56–66. doi: 10.1016/j.bbamcr.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adam-Vizi V, Starkov AA. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J Alzheimer’s Dis. 2010;20(Suppl 2):S413–S426. doi: 10.3233/JAD-2010-100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark edition) 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S-S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 162.Traaseth N, Elfering S, Solien J, Haynes V, Giulivi C. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim Biophys Acta. 2004;1658(1–2):64–71. doi: 10.1016/j.bbabio.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 163.Giulivi C, Kato K, Cooper CE. Nitric oxide regulation of mitochondrial oxygen consumption I: cellular physiology. Am J Physiol Cell Physiol. 2006;291(6):C1225–C1231. doi: 10.1152/ajpcell.00307.2006. [DOI] [PubMed] [Google Scholar]
- 164.Erusalimsky J, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 165.Morris G, Walder K, Carvalho AF, Tye SJ, Lucas K, Berk M, Maes M (2017) The role of hypernitrosylation in the pathogenesis and pathophysiology of neuroprogressive diseases. Neurosci Biobehav Rev. 10.1016/j.neubiorev.2017.07.017 [DOI] [PubMed]
- 166.Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal. 2013;6(256):rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions() Redox Biol. 2014;2:123–139. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Murray CI, Uhrigshardt H, O’Meally RN, Cole RN, Van Eyk JE. Identification and quantification of S-nitrosylation by cysteine reactive tandem mass tag switch assay. Mol Cell Proteomics. 2012;11(2):M111.013441. doi: 10.1074/mcp.M111.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Drose S, Brandt U, Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta. 2014;1844(8):1344–1354. doi: 10.1016/j.bbapap.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 170.Piantadosi CA. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim Biophys Acta. 2012;1820(6):712–721. doi: 10.1016/j.bbagen.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morris G, Berk M, Klein H, Walder K, Galecki P, Maes M (2016) Nitrosative stress, hypernitrosylation, and autoimmune responses to nitrosylated proteins: new pathways in neuroprogressive disorders including depression and chronic fatigue syndrome. Mol Neurobiol. 10.1007/s12035-016-9975-2 [DOI] [PubMed]
- 172.Sarti P, Arese M, Forte E, Giuffre A, Mastronicola D. Mitochondria and nitric oxide: chemistry and pathophysiology. Adv Exp Med Biol. 2012;942:75–92. doi: 10.1007/978-94-007-2869-1_4. [DOI] [PubMed] [Google Scholar]
- 173.Zhang J, Jin B, Li L, Block E, Patel J. Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Physiol Cell Physiol. 2005;288:C840–C849. doi: 10.1152/ajpcell.00325.2004. [DOI] [PubMed] [Google Scholar]
- 174.Sarti P, Forte E, Giuffre A, Mastronicola D, Magnifico M, Arese M. The chemical interplay between nitric oxide and mitochondrial cytochrome c oxidase: reactions, effectors and pathophysiology. Int J Cell Biol. 2012;2012:571067. doi: 10.1155/2012/571067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sarti P, Giuffre A, Barone M, Forte E, Mastronicola D, Brunori M. Nitric oxide and cytochrome oxidase: reaction mechanisms from the enzyme to the cell. Free Radic Biol Med. 2003;34:509–520. doi: 10.1016/S0891-5849(02)01326-6. [DOI] [PubMed] [Google Scholar]
- 176.Bolanos J, Almeida A, Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35:145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 177.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204(9):2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101(11):1155–1163. doi: 10.1161/circresaha.107.155879. [DOI] [PubMed] [Google Scholar]
- 179.Xu W, Liu L, Charles IG, Moncada S. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat Cell Biol. 2004;6(11):1129–1134. doi: 10.1038/ncb1188. [DOI] [PubMed] [Google Scholar]
- 180.Murphy Michael P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/bj20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baumgartner HK, Gerasimenko JV, Thorne C, Ferdek P, Pozzan T, Tepikin AV, Petersen OH, Sutton R, Watson AJM, Gerasimenko OV. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem. 2009;284(31):20796–20803. doi: 10.1074/jbc.m109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gerasimenko JV, Gerasimenko OV, Palejwala A, Tepikin AV, Petersen OH, Watson AJ. Menadione-induced apoptosis: roles of cytosolic Ca(2+) elevations and the mitochondrial permeability transition pore. J Cell Sci. 2002;115(Pt 3):485–497. doi: 10.1242/jcs.115.3.485. [DOI] [PubMed] [Google Scholar]
- 183.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186(6):783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and sustained opening of the permeability transition pore. J Cell Sci. 2002;115(Pt 6):1175–1188. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- 185.Runkel ED, Liu S, Baumeister R, Schulze E. Surveillance-activated defenses block the ROS-induced mitochondrial unfolded protein response. PLoS Genet. 2013;9(3):e1003346. doi: 10.1371/journal.pgen.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004;117(Pt 18):4055–4066. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- 187.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337(6094):587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu Y, Samuel BS, Breen PC, Ruvkun G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature. 2014;508(7496):406–410. doi: 10.1038/nature13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huang L-J, Ho J-J, Yang R-C. Mitochondrial unfolded protein response (mtUPR) participates in hepatic dysfunction in sepsis. FASEB J. 2013;27(1 Supplement):872.875. [Google Scholar]
- 190.Lin Y-F, Haynes Cole M. Metabolism and the UPRmt. Mol Cell. 2016;61(5):677–682. doi: 10.1016/j.molcel.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta. 2013;1833(2):410–416. doi: 10.1016/j.bbamcr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mottis A, Jovaisaite V, Auwerx J. The mitochondrial unfolded protein response in mammalian physiology. Mamm Genome. 2014;25(9):424–433. doi: 10.1007/s00335-014-9525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34(4):699–710. doi: 10.1128/mcb.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Papa L, Germain D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J Cell Sci. 2011;124(Pt 9):1396–1402. doi: 10.1242/jcs.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Radke S, Chander H, Schäfer P, Meiss G, Krüger R, Schulz JB, Germain D. Mitochondrial protein quality control by the proteasome involves ubiquitination and the protease Omi. J Biol Chem. 2008;283(19):12681–12685. doi: 10.1074/jbc.C800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Arnould T, Michel S, Renard P. Mitochondria retrograde signaling and the UPR mt: where are we in mammals? Int J Mol Sci. 2015;16(8):18224–18251. doi: 10.3390/ijms160818224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Baker MJ, Tatsuta T, Langer T (2011) Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol 3(7). 10.1101/cshperspect.a007559 [DOI] [PMC free article] [PubMed]
- 198.Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS One. 2007;2(9):e835. doi: 10.1371/journal.pone.0000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.van der Laan M, Hutu DP, Rehling P. On the mechanism of preprotein import by the mitochondrial presequence translocase. Biochim Biophys Acta. 2010;1803(6):732–739. doi: 10.1016/j.bbamcr.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 200.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138(4):628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, Meisinger C. The protein import machinery of mitochondria—a regulatory hub in metabolism, stress, and disease. Cell Metab. 2014;19(3):357–372. doi: 10.1016/j.cmet.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 202.Opalinska M, Meisinger C. Metabolic control via the mitochondrial protein import machinery. Curr Opin Cell Biol. 2015;33:42–48. doi: 10.1016/j.ceb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 203.Pellegrino MW, Haynes CM. Mitophagy and the mitochondrial unfolded protein response in neurodegeneration and bacterial infection. BMC Biol. 2015;13(1):22. doi: 10.1186/s12915-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wrobel L, Topf U, Bragoszewski P, Wiese S, Sztolsztener ME, Oeljeklaus S, Varabyova A, Lirski M, Chroscicki P, Mroczek S, Januszewicz E, Dziembowski A, Koblowska M, Warscheid B, Chacinska A. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature. 2015;524(7566):485–488. doi: 10.1038/nature14951. [DOI] [PubMed] [Google Scholar]
- 205.Wang X, Chen XJ. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature. 2015;524(7566):481–484. doi: 10.1038/nature14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol. 2016;26(15):2037–2043. doi: 10.1016/j.cub.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Hoj PB, Hoogenraad NJ. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240(1):98–103. doi: 10.1111/j.1432-1033.1996.0098h.x. [DOI] [PubMed] [Google Scholar]
- 208.Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, Jovaisaite V, Frochaux MV et al (2015) Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep. 10.1016/j.celrep.2015.02.034 [DOI] [PMC free article] [PubMed]
- 209.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tauffenberger A, Vaccaro A, Parker JA. Fragile lifespan expansion by dietary mitohormesis in C. elegans. Aging (Albany NY) 2016;8(1):50–61. doi: 10.18632/aging.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21(17):4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt) Mol Cell. 2015;58(1):123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Germain D. Sirtuins and the estrogen receptor as regulators of the mammalian mitochondrial UPR in cancer and aging. Adv Cancer Res. 2016;130:211–256. doi: 10.1016/bs.acr.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 214.Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, Berk M. A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev. 2017;74(Pt A):1–20. doi: 10.1016/j.neubiorev.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 215.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 216.Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39(2):87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kotiadis VN, Duchen MR, Osellame LD. Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim Biophys Acta. 2014;1840(4):1254–1265. doi: 10.1016/j.bbagen.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Quiros PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016;17(4):213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 219.Palikaras K, Lionaki E, Tavernarakis N. Coupling mitogenesis and mitophagy for longevity. Autophagy. 2015;11(8):1428–1430. doi: 10.1080/15548627.2015.1061172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Melser S, Lavie J, Benard G. Mitochondrial degradation and energy metabolism. Biochim Biophys Acta. 2015;1853(10 Pt B):2812–2821. doi: 10.1016/j.bbamcr.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 221.Jeyaraju DV, Cisbani G, Pellegrini L. Calcium regulation of mitochondria motility and morphology. Biochim Biophys Acta. 2009;1787(11):1363–1373. doi: 10.1016/j.bbabio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 222.Distelmaier F, Valsecchi F, Forkink M, van Emst-de Vries S, Swarts HG, Rodenburg RJ, Verwiel ET, Smeitink JA, Willems PH, Koopman WJ. Trolox-sensitive reactive oxygen species regulate mitochondrial morphology, oxidative phosphorylation and cytosolic calcium handling in healthy cells. Antioxid Redox Signal. 2012;17(12):1657–1669. doi: 10.1089/ars.2011.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40(3):141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Andres AM, Stotland A, Queliconi BB, Gottlieb RA. A time to reap, a time to sow: mitophagy and biogenesis in cardiac pathophysiology. J Mol Cell Cardiol. 2015;78:62–72. doi: 10.1016/j.yjmcc.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol. 2014;56:182–188. doi: 10.1016/j.exger.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 226.Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta. 2012;1817(10):1833–1838. doi: 10.1016/j.bbabio.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 227.Galloway CA, Lee H, Yoon Y. Mitochondrial morphology-emerging role in bioenergetics. Free Radic Biol Med. 2012;53(12):2218–2228. doi: 10.1016/j.freeradbiomed.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61(5):683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 229.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Narendra DP, Jin SM, Tanaka A, Suen D-F, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and parkin recruitment. EMBO Rep. 2012;13(4):378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lazarou M, Jin Seok M, Kane Lesley A, Youle Richard J. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22(2):320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Okatsu K, Oka T, Iguchi M, Imamura K, Kosako H, Tani N, Kimura M, Go E, Koyano F, Funayama M, Shiba-Fukushima K, Sato S, Shimizu H, Fukunaga Y, Taniguchi H, Komatsu M, Hattori N, Mihara K, Tanaka K, Matsuda N. PINK1 autophosphorylation upon membrane potential dissipation is essential for parkin recruitment to damaged mitochondria. Nat Commun. 2012;3:1016. doi: 10.1038/ncomms2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MM. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460(1):127–139. doi: 10.1042/bj20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Koyano F, Matsuda N. Molecular mechanisms underlying PINK1 and parkin catalyzed ubiquitylation of substrates on damaged mitochondria. Biochim Biophys Acta. 2015;1853(10 Pt B):2791–2796. doi: 10.1016/j.bbamcr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 236.Ordureau A, Heo JM, Duda DM, Paulo JA, Olszewski JL, Yanishevski D, Rinehart J, Schulman BA, Harper JW. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc Natl Acad Sci U S A. 2015;112(21):6637–6642. doi: 10.1073/pnas.1506593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Durcan TM, Fon EA. The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015;29(10):989–999. doi: 10.1101/gad.262758.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RLJ, Hess S, Chan DC. Broad activation of the ubiquitin–proteasome system by parkin is critical for mitophagy. Hum Mol Genet. 2011;20(9):1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191(7):1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 2010;5(4):e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ni H-M, Williams JA, Ding W-X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bernhardt D, Muller M, Reichert AS, Osiewacz HD. Simultaneous impairment of mitochondrial fission and fusion reduces mitophagy and shortens replicative lifespan. Sci Rep. 2015;5:7885. doi: 10.1038/srep07885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shirihai OS, Song M, Dorn GW., 2nd How mitochondrial dynamism orchestrates mitophagy. Circ Res. 2015;116(11):1835–1849. doi: 10.1161/circresaha.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Figge MT, Reichert AS, Meyer-Hermann M, Osiewacz HD. Deceleration of fusion-fission cycles improves mitochondrial quality control during aging. PLoS Comput Biol. 2012;8(6):e1002576. doi: 10.1371/journal.pcbi.1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sin J, Andres AM, Taylor DJ, Weston T, Hiraumi Y, Stotland A, Kim BJ, Huang C, Doran KS, Gottlieb RA. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2016;12(2):369–380. doi: 10.1080/15548627.2015.1115172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Suliman HB, Sweeney TE, Withers CM, Piantadosi CA. Co-regulation of nuclear respiratory factor-1 by NFkappaB and CREB links LPS-induced inflammation to mitochondrial biogenesis. J Cell Sci. 2010;123(Pt 15):2565–2575. doi: 10.1242/jcs.064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cherry AD, Piantadosi CA. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid Redox Signal. 2015;22(12):965–976. doi: 10.1089/ars.2014.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Peng K, Yang L, Wang J, Ye F, Dan G, Zhao Y, Cai Y, Cui Z, Ao L, Liu J, Zou Z, Sai Y, Cao J (2016) The Interaction of mitochondrial biogenesis and fission/fusion mediated by PGC-1α regulates rotenone-induced dopaminergic neurotoxicity. Mol Neurobiol:1–15. doi:10.1007/s12035-016-9944-9 [DOI] [PubMed]
- 251.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22(1):79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 252.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13(2):75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen H. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280(28):26185–26192. doi: 10.1074/jbc.m503062200. [DOI] [PubMed] [Google Scholar]
- 254.Cipolat S, de Brito OM, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci. 2004;101(45):15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.van der Bliek AM, Shen Q, Kawajiri S (2013) Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol 5(6) [DOI] [PMC free article] [PubMed]
- 256.Wei Y, Chiang WC, Sumpter R, Jr, Mishra P, Levine B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell. 2017;168(1-2):224–238.e210. doi: 10.1016/j.cell.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zorzano A, Hernandez-Alvarez MI, Sebastian D, Munoz JP. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal. 2015;22(12):1020–1031. doi: 10.1089/ars.2014.6208. [DOI] [PubMed] [Google Scholar]
- 258.Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Oresic M, Pich S, Burcelin R, Palacin M, Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109(14):5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dorn GW, 2nd, Song M, Walsh K. Functional implications of mitofusin 2-mediated mitochondrial-SR tethering. J Mol Cell Cardiol. 2015;78:123–128. doi: 10.1016/j.yjmcc.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ainbinder A, Boncompagni S, Protasi F, Dirksen RT. Role of mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle. Cell Calcium. 2015;57(1):14–24. doi: 10.1016/j.ceca.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lu ZQ, Tang LM, Zhao GJ, Yao YM, Zhu XM, Dong N, Yu Y. Overactivation of mitogen-activated protein kinase and suppression of mitofusin-2 expression are two independent events in high mobility group box 1 protein-mediated T cell immune dysfunction. J Interf Cytokine Res. 2013;33(9):529–541. doi: 10.1089/jir.2012.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Olichon A, Landes T, Arnaune-Pelloquin L, Emorine LJ, Mils V, Guichet A, Delettre C, Hamel C, Amati-Bonneau P, Bonneau D, Reynier P, Lenaers G, Belenguer P. Effects of OPA1 mutations on mitochondrial morphology and apoptosis: relevance to ADOA pathogenesis. J Cell Physiol. 2007;211(2):423–430. doi: 10.1002/jcp.20950. [DOI] [PubMed] [Google Scholar]
- 264.Amati-Bonneau P, Milea D, Bonneau D, Chevrollier A, Ferre M, Guillet V, Gueguen N, Loiseau D, de Crescenzo MA, Verny C, Procaccio V, Lenaers G, Reynier P. OPA1-associated disorders: phenotypes and pathophysiology. Int J Biochem Cell Biol. 2009;41(10):1855–1865. doi: 10.1016/j.biocel.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 265.Kao SH, Yen MY, Wang AG, Yeh YL, Lin AL. Changes in mitochondrial morphology and bioenergetics in human lymphoblastoid cells with four novel OPA1 mutations. Invest Ophthalmol Vis Sci. 2015;56(4):2269–2278. doi: 10.1167/iovs.14-16288. [DOI] [PubMed] [Google Scholar]
- 266.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21(6):834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Patten DA, Wong J, Khacho M, Soubannier V, Mailloux RJ, Pilon-Larose K, MacLaurin JG, Park DS, McBride HM, Trinkle-Mulcahy L, Harper ME, Germain M, Slack RS. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014;33(22):2676–2691. doi: 10.15252/embj.201488349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278(10):7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 269.Cogliati S, Frezza C, Soriano Maria E, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes Ligia C, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez Jose A, Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155(1):160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sanjuan Szklarz LK, Scorrano L. The antiapoptotic OPA1/Parl couple participates in mitochondrial adaptation to heat shock. Biochim Biophys Acta. 2012;1817(10):1886–1893. doi: 10.1016/j.bbabio.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sanderson TH, Raghunayakula S, Kumar R. Release of mitochondrial Opa1 following oxidative stress in HT22 cells. Mol Cell Neurosci. 2015;64:116–122. doi: 10.1016/j.mcn.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Laforge M, Rodrigues V, Silvestre R, Gautier C, Weil R, Corti O, Estaquier J. NF-kappaB pathway controls mitochondrial dynamics. Cell Death Differ. 2016;23(1):89–98. doi: 10.1038/cdd.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, Jana F, Ferreira J, Noguera E, Chiong M, Bernlohr DA, Klip A, Hill JA, Rothermel BA, Abel ED, Zorzano A, Lavandero S. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFkappaB-Opa-1 signaling pathway. Diabetes. 2014;63(1):75–88. doi: 10.2337/db13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Dabrowska A, Venero JL, Iwasawa R, M-k H, Rahman S, Boobis A, Hajji N. PGC-1α controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging (Albany NY) 2015;7(9):629–643. doi: 10.18632/aging.100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wu M, Katta A, Gadde MK, Liu H, Kakarla SK, Fannin J, Paturi S, Arvapalli RK, Rice KM, Wang Y, Blough ER. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLoS One. 2009;4(7):e6430. doi: 10.1371/journal.pone.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280(9):7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 277.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40(6):1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 278.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem. 2007;282(42):30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 279.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11(12):872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 280.Zhao GJ, Lu ZQ, Yao YM. Advances in mitochondrial fusion-fission and Ca2+ signaling in mammals. Sheng li ke xue jin zhan [Progress in Physiology] 2010;41(3):171–176. [PubMed] [Google Scholar]
- 281.Kaddour-Djebbar I, Choudhary V, Brooks C, Ghazaly T, Lakshmikanthan V, Dong Z, Kumar MV. Specific mitochondrial calcium overload induces mitochondrial fission in prostate cancer cells. Int J Oncol. 2010;36(6):1437–1444. doi: 10.3892/ijo_00000629. [DOI] [PubMed] [Google Scholar]
- 282.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833(5):1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 283.Li J, Wang Y, Wang Y, Wen X, Ma XN, Chen W, Huang F, Kou J, Qi LW, Liu B, Liu K. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J Mol Cell Cardiol. 2015;86:62–74. doi: 10.1016/j.yjmcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 284.Wikstrom JD, Israeli T, Bachar-Wikstrom E, Swisa A, Ariav Y, Waiss M, Kaganovich D, Dor Y, Cerasi E, Leibowitz G. AMPK regulates ER morphology and function in stressed pancreatic beta-cells via phosphorylation of DRP1. Mol Endocrinol. 2013;27(10):1706–1723. doi: 10.1210/me.2013-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mopert K, Hajek P, Frank S, Chen C, Kaufmann J, Santel A. Loss of Drp1 function alters OPA1 processing and changes mitochondrial membrane organization. Exp Cell Res. 2009;315(13):2165–2180. doi: 10.1016/j.yexcr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 286.Nakamura T, Cieplak P, Cho DH, Godzik A, Lipton SA. S-Nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion. 2010;10(5):573–578. doi: 10.1016/j.mito.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15(6):706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 289.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9(7):505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bossy B, Petrilli A, Klinglmayr E, Chen J, Lutz-Meindl U, Knott AB, Masliah E, Schwarzenbacher R, Bossy-Wetzel E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 2):S513–S526. doi: 10.3233/jad-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Höke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33(23):2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kang J-W, Hong J-M, Lee S-M. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res. 2016;60(4):383–393. doi: 10.1111/jpi.12319. [DOI] [PubMed] [Google Scholar]
- 293.Lin C, Chao H, Li Z, Xu X, Liu Y, Hou L, Liu N, Ji J. Melatonin attenuates traumatic brain injury-induced inflammation: a possible role for mitophagy. J Pineal Res. 2016;61(2):177–186. doi: 10.1111/jpi.12337. [DOI] [PubMed] [Google Scholar]
- 294.Coto-Montes A, Boga JA, Rosales-Corral S, Fuentes-Broto L, Tan D-X, Reiter RJ. Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol Cell Endocrinol. 2012;361(1–2):12–23. doi: 10.1016/j.mce.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 295.Parameyong A, Govitrapong P, Chetsawang B. Melatonin attenuates the mitochondrial translocation of mitochondrial fission proteins and Bax, cytosolic calcium overload and cell death in methamphetamine-induced toxicity in neuroblastoma SH-SY5Y cells. Mitochondrion. 2015;24:1–8. doi: 10.1016/j.mito.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 296.Parameyong A, Charngkaew K, Govitrapong P, Chetsawang B. Melatonin attenuates methamphetamine-induced disturbances in mitochondrial dynamics and degeneration in neuroblastoma SH-SY5Y cells. J Pineal Res. 2013;55(3):313–323. doi: 10.1111/jpi.12078. [DOI] [PubMed] [Google Scholar]
- 297.Suwanjang W, Abramov AY, Charngkaew K, Govitrapong P, Chetsawang B. Melatonin prevents cytosolic calcium overload, mitochondrial damage and cell death due to toxically high doses of dexamethasone-induced oxidative stress in human neuroblastoma SH-SY5Y cells. Neurochem Int. 2016;97:34–41. doi: 10.1016/j.neuint.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 298.Xu S, Pi H, Zhang L, Zhang N, Li Y, Zhang H, Tang J, Li H, Feng M, Deng P, Guo P, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Wang W, Reiter RJ, Yu Z, Zhou Z. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J Pineal Res. 2016;60(3):291–302. doi: 10.1111/jpi.12310. [DOI] [PubMed] [Google Scholar]
- 299.Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, Miar A, Rodriguez-Garcia A, Tan DX, Reiter RJ, Mayo JC, Sainz RM. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res. 2015;58(2):234–250. doi: 10.1111/jpi.12210. [DOI] [PubMed] [Google Scholar]
- 300.Tan D-X, Manchester LC, Qin L, Reiter RJ. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci. 2016;17(12):2124. doi: 10.3390/ijms17122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hsiao C-W, Peng T-I, Peng AC, Reiter RJ, Tanaka M, Lai Y-K, Jou M-J. Long-term Aβ exposure augments mCa2+-independent mROS-mediated depletion of cardiolipin for the shift of a lethal transient mitochondrial permeability transition to its permanent mode in NARP cybrids: a protective targeting of melatonin. J Pineal Res. 2013;54(1):107–125. doi: 10.1111/jpi.12004. [DOI] [PubMed] [Google Scholar]
- 302.Chuang J-I, Pan IL, Hsieh C-Y, Huang C-Y, Chen P-C, Shin JW. Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J Pineal Res. 2016;61(2):230–240. doi: 10.1111/jpi.12343. [DOI] [PubMed] [Google Scholar]
- 303.Pei H, Du J, Song X, He L, Zhang Y, Li X, Qiu C, Zhang Y, Hou J, Feng J, Gao E, Li D, Yang Y. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic Biol Med. 2016;97:408–417. doi: 10.1016/j.freeradbiomed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 304.Ganie SA, Dar TA, Bhat AH, Dar KB, Anees S, Zargar MA, Masood A. Melatonin: a potential anti-oxidant therapeutic agent for mitochondrial dysfunctions and related disorders. Rejuvenation Res. 2016;19(1):21–40. doi: 10.1089/rej.2015.1704. [DOI] [PubMed] [Google Scholar]
- 305.Acuna Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11(2):221–240. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- 306.Agil A, El-Hammadi M, Jimenez-Aranda A, Tassi M, Abdo W, Fernandez-Vazquez G, Reiter RJ. Melatonin reduces hepatic mitochondrial dysfunction in diabetic obese rats. J Pineal Res. 2015;59(1):70–79. doi: 10.1111/jpi.12241. [DOI] [PubMed] [Google Scholar]
- 307.Jimenéz-Aranda A, Fernández-Vázquez G, Mohammad A-Serrano M, Reiter RJ, Agil A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zücker diabetic fatty rats. J Pineal Res. 2014;57(1):103–109. doi: 10.1111/jpi.12147. [DOI] [PubMed] [Google Scholar]
- 308.Escames G, Lopez LC, Tapias V, Utrilla P, Reiter RJ, Hitos AB, Leon J, Rodriguez MI, Acuna-Castroviejo D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J Pineal Res. 2006;40(1):71–78. doi: 10.1111/j.1600-079X.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 309.Escames G (2003) Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 10.1096/fj.02-0692fje [DOI] [PubMed]
- 310.Yeung HM, Hung MW, Fung ML. Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J Pineal Res. 2008;45(4):373–382. doi: 10.1111/j.1600-079X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 311.Hakanson DO, Bergstrom WH. Pineal and adrenal effects on calcium homeostasis in the rat. Pediatr Res. 1990;27(6):571–573. doi: 10.1203/00006450-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 312.Waseem M, Tabassum H, Parvez S. Melatonin modulates permeability transition pore and 5-hydroxydecanoate induced KATP channel inhibition in isolated brain mitochondria. Mitochondrion. 2016;31:1–8. doi: 10.1016/j.mito.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 313.Jumnongprakhon P, Govitrapong P, Tocharus C, Tungkum W, Tocharus J. Protective effect of melatonin on methamphetamine-induced apoptosis in glioma cell line. Neurotox Res. 2013;25(3):286–294. doi: 10.1007/s12640-013-9419-y. [DOI] [PubMed] [Google Scholar]
- 314.Doerrier C, García JA, Volt H, Díaz-Casado ME, Lima-Cabello E, Ortiz F, Luna-Sánchez M, Escames G, López LC, Acuña-Castroviejo D. Identification of mitochondrial deficits and melatonin targets in liver of septic mice by high-resolution respirometry. Life Sci. 2015;121:158–165. doi: 10.1016/j.lfs.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 315.Ortiz F, García JA, Acuña-Castroviejo D, Doerrier C, López A, Venegas C, Volt H, Luna-Sánchez M, López LC, Escames G. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res. 2013;56(1):71–81. doi: 10.1111/jpi.12099. [DOI] [PubMed] [Google Scholar]
- 316.Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, Wang N, Liang Z, Li Y, Chen W, Yi D, Yu S. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res. 2013;55(3):275–286. doi: 10.1111/jpi.12070. [DOI] [PubMed] [Google Scholar]
- 317.Huang W-Y, Jou M-J, Tsung IP. mtDNA T8993G mutation-induced F1F0-ATP synthase defect augments mitochondrial dysfunction associated with hypoxia/reoxygenation: the protective role of melatonin. PLoS One. 2013;1(11):e81546. doi: 10.1371/journal.pone.0081546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Dragicevic N, Copes N, O’Neal-Moffitt G, Jin J, Buzzeo R, Mamcarz M, Tan J, Cao C, Olcese JM, Arendash GW, Bradshaw PC. Melatonin treatment restores mitochondrial function in Alzheimer’s mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res. 2011;51(1):75–86. doi: 10.1111/j.1600-079x.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 319.Ionov M, Burchell V, Klajnert B, Bryszewska M, Abramov AY. Mechanism of neuroprotection of melatonin against beta-amyloid neurotoxicity. Neuroscience. 2011;180:229–237. doi: 10.1016/j.neuroscience.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 320.Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol. 2008;226(2):161–168. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 321.Garcia JA, Volt H, Venegas C, Doerrier C, Escames G, Lopez LC, Acuna-Castroviejo D. Disruption of the NF-kappaB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-alpha and blocks the septic response in mice. FASEB J. 2015;29(9):3863–3875. doi: 10.1096/fj.15-273656. [DOI] [PubMed] [Google Scholar]
- 322.Tripathi DN, Jena GB. Effect of melatonin on the expression of Nrf2 and NF-kappaB during cyclophosphamide-induced urinary bladder injury in rat. J Pineal Res. 2010;48(4):324–331. doi: 10.1111/j.1600-079X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 323.Farez Mauricio F, Mascanfroni Ivan D, Méndez-Huergo Santiago P, Yeste A, Murugaiyan G, Garo Lucien P, Balbuena Aguirre María E, Patel B et al Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162(6):1338–1352. 10.1016/j.cell.2015.08.025 [DOI] [PMC free article] [PubMed]
- 324.Cardinali DP, Pagano ES, Scacchi Bernasconi PA, Reynoso R, Scacchi P. Melatonin and mitochondrial dysfunction in the central nervous system. Horm Behav. 2013;63(2):322–330. doi: 10.1016/j.yhbeh.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 325.Bortolato B, Miskowiak KW, Kohler CA, Maes M, Fernandes BS, Berk M, Carvalho AF. Cognitive remission: a novel objective for the treatment of major depression? BMC Med. 2016;14:9. doi: 10.1186/s12916-016-0560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.De Crescenzo F, Lennox A, Gibson JC, Cordey JH, Stockton S, Cowen PJ, Quested DJ. Melatonin as a treatment for mood disorders: a systematic review. Acta Psychiatr Scand. 2017;136(6):549–558. doi: 10.1111/acps.12755. [DOI] [PubMed] [Google Scholar]
- 327.Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuniga O, Delgado-Lista J, Gutierrez-Mariscal FM, Perez-Martinez P, Delgado-Casado N, Cruz-Teno C, Tinahones FJ, Villalba JM, Perez-Jimenez F, Lopez-Miranda J. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol Ser A Biol Med Sci. 2011;67A(1):3–10. doi: 10.1093/gerona/glr167. [DOI] [PubMed] [Google Scholar]
- 328.Mourier A, Motori E, Brandt T, Lagouge M, Atanassov I, Galinier A, Rappl G, Brodesser S, Hultenby K, Dieterich C, Larsson N-G. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J Cell Biol. 2015;208(4):429–442. doi: 10.1083/jcb.201411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Noh YH, Kim KY, Shim MS, Choi SH, Choi S, Ellisman MH, Weinreb RN, Perkins GA, Ju WK. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death and Disease. 2013;4(10):e820. doi: 10.1038/cddis.2013.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Genova ML, Lenaz G. New developments on the functions of coenzyme Q in mitochondria. Biofactors. 2011;37(5):330–354. doi: 10.1002/biof.168. [DOI] [PubMed] [Google Scholar]
- 331.Cotan D, Cordero MD, Garrido-Maraver J, Oropesa-Avila M, Rodriguez-Hernandez A, Gomez Izquierdo L, De la Mata M, De Miguel M, Bautista Lorite J, Rivas Infante E, Jackson S, Navas P, Sanchez-Alcazar JA. Secondary coenzyme Q10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB J. 2011;25(8):2669–2687. doi: 10.1096/fj.10-165340. [DOI] [PubMed] [Google Scholar]
- 332.Morris G, Anderson G, Berk M, Maes M. Coenzyme Q10 depletion in medical and neuropsychiatric disorders: potential repercussions and therapeutic implications. Mol Neurobiol. 2013;48(3):883–903. doi: 10.1007/s12035-013-8477-8. [DOI] [PubMed] [Google Scholar]
- 333.Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Khodadadi B, Jazayeri S, Gohari MR, Aryaeian N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutr Neurosci. 2015;18(4):169–176. doi: 10.1179/1476830513y.0000000106. [DOI] [PubMed] [Google Scholar]
- 334.Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Jazayeri S, Reza Gohari M. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int J Neurosci. 2013;123(11):776–782. doi: 10.3109/00207454.2013.801844. [DOI] [PubMed] [Google Scholar]
- 335.Lee BJ, Huang YC, Chen SJ, Lin PT. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition. 2012;28(3):250–255. doi: 10.1016/j.nut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 336.Alehagen U, Aaseth J, Johansson P. Reduced cardiovascular mortality 10 years after supplementation with selenium and coenzyme Q10 for four years: follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly citizens. PLoS One. 2015;10(12):e0141641. doi: 10.1371/journal.pone.0141641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC: Heart Failure. 2014;2(6):641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 338.Smith RAJ, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201(1):96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 339.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RAJ, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2000;276(7):4588–4596. doi: 10.1074/jbc.m009093200. [DOI] [PubMed] [Google Scholar]
- 340.Lukashev AN, Skulachev MV, Ostapenko V, Savchenko AY, Pavshintsev VV, Skulachev VP (2014) Advances in development of rechargeable mitochondrial antioxidants. Progress Mol Biol Transl Sci Elsevier BV. 10.1016/b978-0-12-394625-6.00010-6 [DOI] [PubMed]
- 341.Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2014;1842(8):1282–1294. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Carvalho AF, Miskowiak KK, Hyphantis TN, Kohler CA, Alves GS, Bortolato B, GS PM, Machado-Vieira R, Berk M, RS MI. Cognitive dysfunction in depression—pathophysiology and novel targets. CNS Neurol Disord Drug Targets. 2014;13(10):1819–1835. doi: 10.2174/1871527313666141130203627. [DOI] [PubMed] [Google Scholar]
- 343.Forester BP, Harper DG, Georgakas J, Ravichandran C, Madurai N, Cohen BM. Antidepressant effects of open label treatment with coenzyme Q10 in geriatric bipolar depression. J Clin Psychopharmacol. 2015;35(3):338–340. doi: 10.1097/jcp.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lin H, Liu XB, Yu JJ, Hua F, Hu ZW. Antioxidant N-acetylcysteine attenuates hepatocarcinogenesis by inhibiting ROS/ER stress in TLR2 deficient mouse. PLoS One. 2013;8(10):e74130. doi: 10.1371/journal.pone.0074130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Machado JT, Iborra RT, Fusco FB, Castilho G, Pinto RS, Machado-Lima A, Nakandakare ER, Seguro AC, Shimizu MH, Catanozi S, Passarelli M. N-Acetylcysteine prevents endoplasmic reticulum stress elicited in macrophages by serum albumin drawn from chronic kidney disease rats and selectively affects lipid transporters, ABCA-1 and ABCG-1. Atherosclerosis. 2014;237(1):343–352. doi: 10.1016/j.atherosclerosis.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 346.Sun Y, Pu L-Y, Lu L, Wang X-H, Zhang F, Rao J-H. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World J Gastroenterol. 2014;20(41):15289–15298. doi: 10.3748/wjg.v20.i41.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Lee ES, Kim HM, Kang JS, Lee EY, Yadav D, Kwon MH, Kim YM, Kim HS, Chung CH. Oleanolic acid and N-acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol Dial Transplant. 2016;31(3):391–400. doi: 10.1093/ndt/gfv377. [DOI] [PubMed] [Google Scholar]
- 348.Liu YY, Xie Q, Wang H, Lin LY, Jiang S, Zhou XQ, Yu H, Guo Q. The effect of N-acetyl-L-cysteine on endoplasmic reticulum stress mediated apoptosis of HepG2 cells. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi. 2008;16(7):524–527. [PubMed] [Google Scholar]
- 349.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 350.Deepmala SJ, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 351.Skvarc DR, Dean OM, Byrne LK, Gray L, Lane S, Lewis M, Fernandes BS, Berk M, Marriott A. The effect of N-acetylcysteine (NAC) on human cognition—a systematic review. Neurosci Biobehav Rev. 2017;78:44–56. doi: 10.1016/j.neubiorev.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 352.Morris G, Walker AJ, Berk M, Maes M, Puri BK (2017) Cell death pathways: a novel therapeutic approach for neuroscientists. Mol Neurobiol. 10.1007/s12035-017-0793-y [DOI] [PMC free article] [PubMed]
- 353.Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, Berk M. The new field of ‘precision psychiatry’. BMC Med. 2017;15(1):80. doi: 10.1186/s12916-017-0849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]