Abstract
The climbing fiber–Purkinje cell circuit is one of the most powerful and highly conserved in the central nervous system. Climbing fibers exert a powerful excitatory action that results in a complex spike in Purkinje cells and normal functioning of the cerebellum depends on the integrity of climbing fiber–Purkinje cell synapse. Over the last 50 years, multiple hypotheses have been put forward on the role of the climbing fibers and complex spikes in cerebellar information processing and motor control. Central to these theories is the nature of the interaction between the low-frequency complex spike discharge and the high-frequency simple spike firing of Purkinje cells. This review examines the major hypotheses surrounding the action of the climbing fiber–Purkinje cell projection, discussing both supporting and conflicting findings. The review describes newer findings establishing that climbing fibers and complex spikes provide predictive signals about movement parameters and that climbing fiber input controls the encoding of behavioral information in the simple spike firing of Purkinje cells. Finally, we propose the dynamic encoding hypothesis for complex spike function that strives to integrate established and newer findings.
Keywords: Purkinje cell, Complex spike, Climbing fibers, Simple spike, Motor error, Cerebellar cortex
Introduction
The distinctive morphology, cellular actions, and physiological properties of the climbing fiber–Purkinje cell synapse suggest a unique role in the cerebellum and behavior [1–3]. As one of the strongest synapses in the mammalian central nervous system, the action of climbing fibers on Purkinje cells sparked great interest among neuroscientists and continues to be at the center of efforts to understand cerebellar function. Climbing fiber input is essential to the cerebellum’s role in controlling movements, and malfunction of the climbing fiber–Purkinje cell synapse is a central component in the movement disorder characterizing several of the spinocerebellar ataxias [4]. Several major hypotheses have been proposed on the function of the climbing fiber–Purkinje cell synapse in both cerebellar information processing and behavior. This review examines each of these established hypotheses. The review also describes recent results that offer new insights into the action of the climbing fibers in the cerebellum.
Properties of Climbing Fibers and Synaptic Action on Purkinje Cells
Climbing fiber afferents originate solely from the inferior olive, a group of nuclei in the lower medulla, and form the olivocerebellar projection. As one of the most conserved pathways in the vertebrate nervous system, this highlights the importance of the olivocerebellar projection [5]. The axons from inferior olivary neurons cross in the midline and travel through the inferior cerebellar peduncle to enter the cerebellum. Climbing fibers provide one of the two main inputs to the cerebellar cortex, the other source being mossy fibers (Fig. 1a). Unique to the climbing fibers is that they monosynaptically innervate Purkinje cells, the sole output neuron of the cerebellar cortex. Therefore, climbing fibers have direct control over the output of the cerebellar cortex.
Fig. 1.
Circuitry of the cerebellum and synaptic action of the climbing fibers. a Canonical circuitry of the cerebellum including climbing fiber–Purkinje cell circuit, mossy fiber-granule cell-parallel fiber–Purkinje cell circuit and Purkinje cell projection to the cerebellar nuclei. “+” and “-” denote excitatory and inhibitory synapses, respectively (modified with permission from [31]). b Top panel: intracellular recording from a Purkinje cell of both a complex spike (CS) with its initial Na+ spike and prolonged depolarization and a spontaneous simple spike SS (modified with permission from [12]). Bottom panel: extracellular recording from a Purkinje cell shows the high-frequency SS firing and low-frequency CS discharge (red dots) (modified with permission from [61]). c Spatial profile of Ca2+ influx evoked by climbing fiber input displaying Ca2+ transients at multiple locations along the dendritic tree (modified with permission from [61])
Through hundreds of glutamatergic synapses along two thirds of the proximal dendritic tree, a Purkinje cell receives input from a single climbing fiber in the adult animal (Fig. 1a). Each climbing fiber synapses on 5–10 Purkinje cells that tend to be located in a parasagittal plane, a prominent feature of the olivocerebellar projection. This parasagittal architecture matches the overall longitudinal zonation of the cerebellum. In this organization, a parasagittal zone or strip of Purkinje cells, as defined by the presence of zebrin II ± bands [6], receives climbing fiber input from a circumscribed region of the inferior olive, and the same zone of Purkinje cells project to a specific region of the cerebellar nuclei (for review, see [7–9]). The differential expression of numerous molecules on Purkinje cells within these zonal compartments complements the parasagittal anatomy (for review, see [6]).
The climbing fiber–Purkinje cell synapse is one of the most powerful in the CNS and its distinctive properties have stimulated intense interest [10–12]. Firing at low rates (~ 0.5–2.0/s), a climbing fiber produces a massive depolarization of the entire Purkinje cell resulting in a complex spike (CS) (Fig. 1b). A CS consists of a large Na+ somatic spike and burst of smaller spikelets generated in the initial axon segment. The strong depolarization also opens voltage-gated Ca2+ channels that result in Ca2+ spikes throughout the entire dendritic tree (Fig. 1c) [13, 14]. Therefore, while separate mechanisms underlie the CS and dendritic Ca2+ spikes, both have important roles in understanding climbing fiber action on Purkinje cells. Although traditionally considered an all-or-none response [1], recent work demonstrates that both CSs and dendritic Ca2+ responses can be graded via pre- and post-synaptic modulation (for review, see [15]).
In sharp contrast to the monosynaptic climbing fiber–Purkinje cell circuit, mossy fibers act through the highly divergent granule cell-parallel fiber network (Fig. 1a). Mossy fibers originate from a large number of sites including the spinal cord and brainstem nuclei, with a large projection from the cerebral cortex via the pons [1, 2]. A Purkinje cell receives input from over 100,000 parallel fibers that modulate the intrinsically driven high-frequency simple spike (SS) discharge [1, 16]. Running transversely along a folium, an individual parallel fiber synapses on several hundred Purkinje neurons but makes only a few en-passant synapses on an individual Purkinje cell [1, 2]. In comparison with climbing fiber input, a parallel fiber produces only a small excitatory response in a Purkinje cell [17].
To complete the circuitry, Purkinje cells project to and inhibit the cerebellar and vestibular nuclei. In turn, a population of excitatory neurons in the cerebellar and vestibular nuclei project to the spinal cord, brainstem, and thalamic nuclei, modulating downstream structures including the cerebral cortex via the cerebello-thalamo-cortical pathway (Fig. 1a) [18]. A separate population of inhibitory neurons in the cerebellar nuclei project to the inferior olive, completing a closed-loop circuit of the cerebellar cortex, cerebellar nuclei, and inferior olive [19–21]. Using this nucleo-olivary circuit, the cerebellar cortex can modulate climbing fiber input to Purkinje cells [22–25].
Event Detection Hypothesis
The low firing frequency of climbing fibers and associated CSs evoked in Purkinje cells prompted the early suggestion that the olivocerebellar system is not capable of encoding information using a conventional rate code. Combining the “phasic” nature of the CS discharge with observations that climbing fibers are highly responsive to small perturbations led to the “event detector” hypothesis [26, 27]. Most of these early experiments were performed in anesthetized or decerebrate preparations. However, during voluntary movements, CS responses to somatosensory stimuli are greatly diminished and instead are evoked when a stimulus is not anticipated, leading to the “unexpected event” hypothesis (for reviews, see [11, 28]). Subsequent work in a variety of preparations and behaviors demonstrated that CSs provide considerable information about reflex and voluntary behaviors and do not just signal events (see Beyond Error Signaling: Parametric and Predictive Encoding). Therefore, the event detector hypothesis failed to capture the complete properties of climbing fibers and their action on Purkinje cells.
Error Hypothesis
One of the most accepted hypotheses is that CSs signal errors. Initially proposed in the framework of a comparator, Oscarsson postulated that the inferior olive compares command signals from higher centers with feedback from the spinal cord, thereby generating a type of error signal [29]. In support of the comparator hypothesis, the inferior olive integrates both feedforward and feedback information as it receives a variety of excitatory and inhibitory inputs from the spinal cord, nuclei at the mesodiencephalic junction, cerebellar nuclei, and cerebral cortex (for reviews, see [29–31]). However, individual inferior olive neurons generally do not receive both descending and ascending inputs, suggesting that these neurons do not perform the comparison necessary to generate an error signal [30].
The comparator hypothesis quickly evolved into the error hypothesis and was coupled to synaptic plasticity and motor learning (for reviews [32–34]). In the Marr-Albus-Ito hypothesis, motor learning is mediated by long-term depression (LTD) of parallel fiber–Purkinje cell synapses resulting from co-activation of parallel fiber and climbing fiber inputs [35–37]. In this view, CSs are evoked by errors and CSs provide a teaching signal that modifies subsequent SS activity to correct the behavior [37–41]. Although controversial (for reviews, see [34, 42, 43]), for nearly a half century, the error signaling/motor learning hypothesis has dominated the field’s view of climbing fiber function.
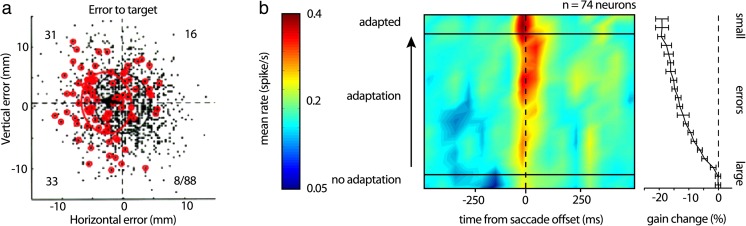
Many studies observed CS firing in relation to motor errors. In the floccular complex, CSs are driven by retinal-slip during smooth pursuit and VOR adaptation [44–46]. In the ventral paraflocculus, CSs modulate with the retinal slip during the ocular following response [47], and in the oculomotor vermis, CSs modulate with induced saccade errors [48]. The error hypothesis received further support with the observation of CS modulation in relation to reach end point errors in the monkey (Fig. 2a) [41]. In agreement, several arm movement studies documented that CSs modulate with unexpected loads [38], redirection of a reach [49], and during adaptation to visuomotor transformations [50]. In addition, CS discharge increases with perturbations applied during locomotion [51–53].
Fig. 2.
Complex spike firing in relation to errors. a Scatterplot of end point position relative to target center for all trials (black dots), CS occurrence trials marked by small red circles, during a reaching task to a target presented on a screen. Total of 88 CS occurred out of 1381 reaches, with the numbers of CSs in each quadrant as indicated. The large ellipses denote the equidistance points (Mahalanobis distance = 1) for each population (red–CS occurring trials, black–all trials). Black arrow illustrates the shift between the centers of the red and black ellipses that quantifies the CSs preferred error direction and defines the CSs error modulation (modified with permission from [41]). b Heat map of CS population response from 74 Purkinje cells during an inward saccade adaptation task. Right panel depicts the degree of saccade adaptation, which is inversely related to error magnitude. CS modulation is minimal at the start of the adaptation when errors are maximal and increases as adaptation develops and error magnitude decreases. Maximum CS firing occurs when errors are small (modified with permission from [54])
However, other studies found limited support for the classical error encoding view. As described above, CS discharge is associated with end point errors during saccades. One method to induce saccade end point errors is by changing the instructed target location while the eyes are in flight (Fig. 2b) [54]. Over time, the subject learns to predict the change in target location and alters the motor command so that the eyes successfully reach the desired end point. During this type of saccadic adaptation, the error encoding hypothesis predicts that CSs would be highly modulated early in adaptation, when errors are maximal. As the animal learns to predict the change in target position and errors are reduced, the CS modulation should also decrease. In the oculomotor vermis, however, the opposite relationship is observed, with CS discharge increasing late in adaptation when errors have decreased greatly (Fig. 2b) [54]. A similar build-up of CS discharge occurs as performance errors decrease during smooth pursuit adaptation [55, 56]. These observations demonstrate that CS error signals are conditional upon the behavioral and experimental context and challenge the assumption that climbing fibers predominately respond to motor errors.
Similarly, perturbations and performance errors during reaching in cats do not evoke responses in inferior olive neurons [57]. During reaching movements in the monkey, CS modulation could not be related to direction or speed errors [58, 59] nor were CSs associated with learning a mechanical perturbation [60]. During pseudo-random tracking, CSs are not associated with error events [61]. Smooth pursuit learning does not drive CS modulation in the oculomotor vermis [62]. Also, in the oculomotor vermis, CS error modulation with saccades may be limited to direction errors, and whether they encode error magnitude is unclear [63, 64]. Even when associated with errors, CSs occur with low probability [41, 50, 61]. Therefore, the precision, specificity, and extent to which CSs encode error information remain unknown.
Motor learning does not depend exclusively on climbing fiber input [60, 65–67] nor does it depend solely on LTD at the parallel fiber–Purkinje cell synapse [34]. Also, SS discharge carries robust error signals, both for eye and arm movements [68–72]. Therefore, error processing in the cerebellum is more multi-faceted than originally proposed, and to paraphrase a wise old man from in galaxy far away, “these are not the error signals you are looking for.”
Rhythmicity and Timing Hypothesis
One of the intriguing properties of the inferior olive neurons is the presence of gap junctions [73, 74]. When combined with a set of voltage-gated calcium and potassium conductances, the electronic coupling supports the synchronous [75–78] and rhythmic activity of most inferior olive neurons (with the exception of neurons in the dorsal cap of Kooy). The 1–10-Hz oscillations occur in both the subthreshold membrane potential [79, 80] and discharge of olivary neurons [81–84]. The timing of the inferior olive neuronal firing is tightly linked to membrane potential phase [85] and the firing waveform reflects the amplitude [86] and synchrony of the subthreshold oscillations [87]. Disruption of the electronic coupling in the inferior olive decreases the coherence of muscle activation [88].
The rhythmicity and synchronicity of climbing fibers suggest a role in movement timing independent of their action on SS firing [12, 84, 89]. Rhythmic CS discharge has been argued to underlie physiological tremor [90]. Elegant studies using simultaneous recordings from multiple Purkinje cells documented these oscillatory and coupled discharge properties of CSs in the intact animal [77, 83, 84, 91], including during rhythmic tongue movements [89]. However, the behavioral studies did not disambiguate the source of the rhythmicity, whether driven by the movements or by the inferior olive.
In the awake, behaving animal, evidence for strong CS rhythmicity remains controversial [11, 92, 93]. The lack of rhythmic CS firing has been particularly striking in the non-human primate [60, 69, 94, 95]. However, CS synchronicity is likely an important element of climbing fiber action in the cerebellar cortex and behavior. For example, the synchronous activation of CF microzones could reliably encode sensory inputs [96] or impose encoding changes on a functionally related group of Purkinje cells [69].
Control of Purkinje Cell Excitability, Gain Change, and Bistability Hypotheses
The error and motor learning hypotheses do not readily account for spontaneous CS firing and the observation that removal or stimulation of climbing fiber input results in a dramatic change in the SS firing pattern [97–100]. Lesions or blockade of the inferior olive produces large increases in the SS firing rate and stimulation largely decreases. Climbing fiber stimulation suppresses conditioned eye blink responses, suggesting that on-going CSs directly influence motor behavior [100]. Furthermore, lesions of the inferior olive result in the rapid emergence of a striking cerebellar-like motor disorder [101, 102]. Therefore, climbing fiber input must play a role in on-line cerebellar function and motor control.
Several earlier hypotheses on the CS contribution to real-time motor control emphasize short-term changes in Purkinje cell excitability. The “gain change” hypothesis postulated climbing fiber input controls the responses of Purkinje cell inputs, either increasing or decreasing the responsiveness [103, 104]. In the decerebrate preparation, the magnitude of the SS responses to peripheral inputs depends on the timing relative to CS occurrence. A similar gain was observed in the decerebrate ferret during locomotion and led to the “dynamic selection hypothesis” proposing that climbing fibers act to spatially focus a set of Purkinje cell by emphasizing their responsiveness to parallel fiber input [53, 105]. However, in the awake rabbit, SS responses to vestibular and optokinetic stimulation do not appear to be controlled by climbing fiber input [11]. Therefore, while the gain change hypothesis remains interesting, the concept lacks adequate support in intact, behaving animals.
Subsequently, the “bistability” hypothesis stated that CSs control the responses of a Purkinje cell to parallel fiber inputs by switching between “up” and “down” SS firing states [106–108]. In the context of error signaling, the bistability allows for toggling a Purkinje cell between states for instantaneous correction following an error [106]. Other possible functions include providing a short-term memory capabilities or generation of temporal pattern (for review, see [92]). However, CS-coupled changes in SS firing rates are only prominent in reduced or anesthetized preparations. In the awake mouse, togging between high and low SS firing was not observed during optokinetic and vestibular reflexes [92, 93]. In the monkey, short-term changes in SS firing following a CS are limited, with the exception of a brief inactivation period [50, 69, 109]. Several explanations have been offered to account for discrepancies across studies, including Purkinje cell heterogeneity, neuromodulators, and level of network activity [92]. However, in the awake animal, climbing fiber input appears to have a very restricted effect on SS firing rate.
Complex Spike-Simple Spike Discharge Reciprocity
The discharge of CSs and SSs is often modulated reciprocally in which an increase in CS firing is accompanied by a decrease in SS firing and vice versa. Reciprocal CS–SS modulation is thought to be a fundamental feature of Purkinje cell physiology and its role in controlling movements [98, 110–112]. This reciprocity has been described in several species, stimulus paradigms, and cerebellar cortical regions. Examples include during optokinetic stimulation in the flocculus and paraflocculus of the rabbit [44, 11] and mouse [112, 113] and during smooth pursuit in the monkey [114]. In the cat, CS–SS modulation reciprocity occurs in the vermis during background firing [110, 115], in lobules V and VI following electrical stimulation of the radial and vagus nerve [116], and during passive wrist movements [117]. However, it should be stressed that many of the above reports of CS–SS reciprocity were in reduced or anesthetized preparations.
Whether the reciprocity is due to climbing fiber input was difficult to test due to the tonic action of CSs on SS firing reviewed above [97–100]. A recent study provided clarification using Ptf1a::cre;Robo3lox/lox mice in which the climbing fibers are selectively re-routed from a contralateral to an ipsilateral projection [112]. Surprisingly, the CS modulation during optokinetic stimulation was reversed as was the SS firing. In addition, climbing fibers acting on inhibitory interneurons likely contribute to the antiphase CS–SS firing pattern, as the interneuron modulation was also inverted.
Reciprocal CS–SS modulation may be less prominent than previously thought. Reciprocity is not present in the dark or during three-dimensional vestibular stimulation [118, 119]. In the oculomotor vermis, reciprocity was not observed for either saccades or smooth pursuit [62]. During pseudorandom tracking, the differences in CS and SS directional tuning are distributed uniformly [61]. A similar diversity in CS and SS tuning occurs in reaching tasks [41, 50, 59]. Therefore, CS and SS discharge reciprocity appears to be conditional rather than deterministic.
Beyond Classical Concepts: Parametric and Predictive Encoding
During various vestibular and oculomotor behaviors, CSs carry parametric information about movements. For example, CS modulation occurs in the flocculus during VOR rotation in the dark when retinal slip is absent [119], carries kinematic information, in addition to retinal slip signals, in the ventral paraflocculus during ocular pursuit [47], and is directionally tuned in the nodulus during three-dimensional vestibular stimulation (Fig. 3a) [118]. Also, CS firing modulates with both reach direction and amplitude [58]. A relationship to motor commands has been suggested based on the increased CS discharge around limb movement onset [58, 109] and climbing fiber modulation with vibrissae and tongue movements [58, 109].
Fig. 3.
Complex spike modulation with movement parameters. a Example of CS and SS firing relative to head acceleration (Hacc) during lateral translation at 0.16 Hz (modified with permission from [118]). b Feedforward and feedback CS probability maps (CS prob) with hand position (X, Y), velocity (VX, VY), and acceleration (AX, AY) during pseudo-random tracking, each map obtained from a different cell (modified with permission from [61]). The modulation significance of each firing plot is indicated by the directional tuning vectors (black arrows). c Top panel shows average eyelid responses to periocular airpuffs (unexpected–black, paired with conditioning LED cue–red, conditioning LED cue only–blue, green vertical bar indicates conditioning LED cue). Bottom panel demonstrates CS modulation preceding the airpuff in the conditioning stimulus period (modified with permission from [123])
Previous studies typically examined CS modulation during low dimensional, stereotypic movements, including saccades, VOR, and reaching. Therefore, we evaluated CS firing during a pseudo-random, manual tracking task in the monkey [61]. Pseudo-random tracking allows for an examination of the interactions among CS discharge and behavior in which the correlations between parameters are reduced and provides extensive coverage of the work space [68, 120]. The result is a robust data set to assess the behavioral parameters that modulate climbing fiber input. Using reverse correlation, we constructed both feedforward and feedback two-dimensional probability maps of CS firing with kinematics (hand position, velocity, and acceleration) and with position error, a measure of tracking performance (Fig. 3b).
For Purkinje cells in lobules V and VI, CSs significantly encode all three kinematic parameters and position error in this task (Fig. 3b). The CSs are spatially tuned and provide a linear representation of each parameter. Modulation with acceleration is particularly common. The paradigm also provides a definition of salient events, for example an error event defined as crossing out of the target, as this triggers the need for a timely corrective action. During pseudo-random tracking, climbing fiber modulation was not related to “events”, either for position error or kinematics. Therefore, CSs carry an array of continuous parametric motor signals and support the hypothesis that climbing fiber input has a prominent role in online motor control.
In addition, increasing evidence challenges the concept that climbing fiber input is solely feedback driven. For example, CSs modulate in response to inferred errors related to eye movements [119, 121, 122]. During pseudo-random tracking, feedforward CS modulation is almost three times more common than feedback modulation (Fig. 3b) [61]. Highlighting the predictive nature of climbing fiber input, CSs precede but rarely respond to errors. Feedforward CS responses occur during eye blink conditioning, with CS increases prior to and predicting the conditioned response (Fig. 3c) [123, 124].
The mechanism underlying predictive CS encoding remains to be determined. As noted above, the inferior olive receives a variety of excitatory and inhibitory inputs from multiple structures that provide both feedforward and feedback information. For the development of predictive CS signals during classical conditioning, recurrent activity within the olivocerebellar network was postulated to play a major role [124]. Other potential sources of feedforward signals are the premotor and motor cortices. Intriguingly, the responses of inferior olivary neurons to glutamatergic inputs from the motor cortex are bi-phasic in a manner that appears to penalize late inputs [125]. This pattern could create a bias toward feedforward motor signals and a mechanism for predictive CS modulation. Taken together, these observations demonstrate that CS discharge contains predictive motor signals about multiple aspects of the upcoming behavior, instead of only reporting errors or providing only sensory feedback.
Dynamic Encoding Hypothesis
Error and motor learning roles for climbing fiber action in the cerebellum have dominated the literature since their introduction. This review has highlighted several problems with these views. Clearly, CSs do not simply signal errors, as they fail to respond with errors in many behaviors and instead modulate predictively with multiple parameters of movement. Movements with higher dimensionality reveal the predictive nature of CS discharge. Therefore, CS modulation with behavior is highly dependent on the experimental paradigm, further raising the need to consider other functions for climbing fiber input.
Two additional features of climbing fiber input and CS discharge lead us to consider other possible functions. First, CSs fire spontaneously and the importance of that background firing to SS firing and cerebellar function implies an ongoing role in cerebellar computations, irrespective of errors or learning. Second, the massive depolarization of the Purkinje cell due to climbing fiber input likely resets the residual effects of prior inputs as well as change how subsequent inputs act on the Purkinje cell (Fig. 1c). Therefore, we hypothesized that climbing fibers change the encoding of the information in the SS firing.
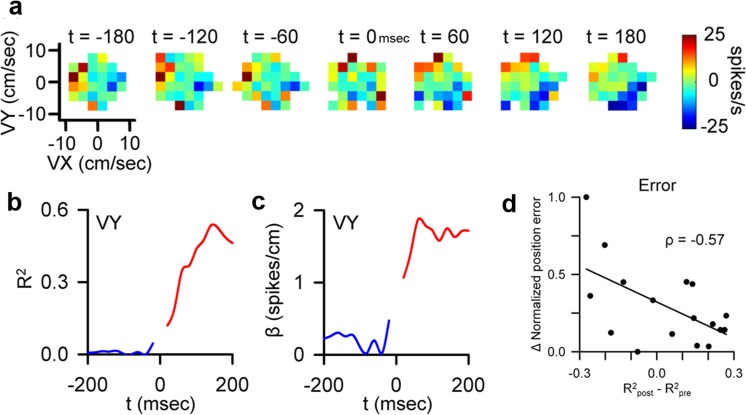
We evaluated this hypothesis during pseudo-random tracking, revealing that climbing fiber discharge dynamically controls the information present in the SS firing, triggering robust and rapid changes in SS encoding of motor signals in Purkinje cells [69]. The changes in encoding consist of increases or decreases in the SS sensitivity to kinematics or position errors. An example of the change in SS encoding is shown in Fig. 4 for a Purkinje cell with SS modulation with hand velocity. Prior to CS occurrence, the encoding of velocity in the SS discharge is weak and markedly increases following CS occurrence (Fig. 4a–c). Furthermore, the changes in encoding are tightly coupled to CS occurrence. In the light of the gain change, bistability, and rhythmicity hypotheses, we investigated and showed that encoding changes are not due to differences in SS firing rates or variability. Nor are the changes in sensitivity due to CS rhythmicity, as there was no evidence for rhythmicity of CS firing during pseudorandom tracking.
Fig. 4.
Complex spike-coupled changes in SS encoding. a Purkinje cell SS firing maps (mean-subtracted) with hand velocity (VX and VY) reveal an increased modulation with VY after CS occurrence (t = 0 msec). The SS modulation with each parameter before and after the CS discharge was quantified with a regression analysis in which the coefficient of determination, R2, provides a measure of SS encoding strength and the regression coefficient, β, a measure of SS sensitivity (see Methods in [69]). b Plotted for this example Purkinje neuron is the pre- (blue trace) and post-CS (red trace) R2 plot that highlights the increase in SS modulation with VY following CS occurrence. Note the step changes between blue and red traces. c Plot of pre- (blue trace) and post-CS (red trace) SS sensitivity relative to VY demonstrates a corresponding increase after CS occurrence. Plots in b and c from the same cell shown in a. d Plot of change in position error relative to CS occurrence (post-pre) with the strength (defined by R2) of CS-coupled changes in SS encoding for the population of Purkinje cells. Magnitude of position error decreases as the SS encoding of error increases (modified with permission from [69])
In addition, the CS-coupled changes in encoding are not evoked by changes in kinematics or position errors. Instead, CS discharge most often leads alterations in behavior, consistent with our recent report [61]. Nearly all of the CS-coupled changes in SS encoding changes are associated with predictive CS modulation with behavior. Furthermore, at the population level, the changes in sensitivity are consistent with optimizing behavior. Increases in SS encoding of position error are followed by and scale with decreases in error (Fig. 4d). Also, increases in SS encoding of a kinematic parameter are associated with larger changes in that parameter than are decreases in SS encoding. Intriguingly, the CS-coupled changes in encoding for a given Purkinje cell tend to oppose the encoding drift occurring independent of CS firing. For example, a Purkinje cell with a CS-coupled increase in SS encoding of velocity will tend to show a decrease in velocity encoding in the absence of CSs.
An outstanding question is the potential mechanism(s) by which these alterations in SS encoding occur. A number of candidates could explain the changes. First, the number of spikes in a given climbing fiber discharge affects the CS burst pattern, dendritic Ca2+ spikes, and plasticity [86, 126]. Just as the degree of parallel fiber–Purkinje cell synaptic plasticity varies with the duration of the CS discharge [39, 127], short-term changes in firing have been observed. In anesthetized rats, increased SS firing preceding climbing fiber discharge is followed by a higher number of CS spikelets that in turn associated with a subsequent reduction in SS activity [128]. An additional mechanism for CS-coupled changes in SS encoding is via local inhibition by GABAergic interneurons that modifies the conductance changes and Ca2+ fluxes evoked by climbing fiber input [129, 130]. Low amplitude, extended Ca2+ responses inhibit the parallel fiber–Purkinje cell synapse, while high amplitude Ca2+ fluxes could increase the gain, potentially facilitating bidirectional changes in SS encoding [131, 132]. Also, the timing of climbing fiber discharge may differentially modulate parallel fiber input and contribute to the direction of synaptic potentiation [133, 134].
Integrating these new observations with previous results argues for a new hypothesis in which climbing fiber discharge dynamically controls the information present in the SS discharge (Fig. 5). Purkinje cells have the capacity for a large theoretical bandwidth [135], and an array of movement signals is encoded in the SS firing (for review, see [136]). Both climbing fiber input and Purkinje cell output are crucial for continuous, online control of movement as well as motor learning and adaptation. The dynamic encoding hypothesis provides a framework for both spontaneous and evoked climbing fiber discharge. In this view, spontaneous climbing fiber input resets the encoding state of the Purkinje cell to maintain an optimal computational state of the SS firing. In other words, the CS corrects for continuous “drifts” in SS encoding, stabilizing the information conveyed by the Purkinje cell output (Fig. 5). Conversely, behaviorally evoked climbing fiber discharge, either predictive or feedback driven, reallocates the bandwidth of the Purkinje cell to optimize the SS information to the most salient aspects of the task to control behavior (Fig. 5).
Fig. 5.
Illustration of the dynamic encoding hypothesis. Inferior olive integrates behavioral information from the cerebral cortex, sensory system, and computational state of the cerebellar cortex from the cerebellar nuclei. Cerebellar cortex also receives cortical, brainstem (not shown), and sensory information and transforms these inputs into representations of movement kinematics (red arrow) and motor errors (blue arrow) in the simple spike firing of Purkinje cells. The dynamic encoding hypothesis states that climbing fibers, the output of the inferior olive, provide an encoding control signal (red arrow) that resets the sensitivity of the Purkinje cell simple spike firing. Cerebellar cortex output is integrated at level of the cerebellar nuclei. In this example, inferior olive activity drives an increase in simple spike encoding of kinematics as indicated by increased thickness of red lines (similar to Fig. 4 example). Climbing fiber can also drive decreases in kinematic encoding, as well as bidirectional changes in error encoding
A major challenge in determining the function of climbing fiber discharge is interpreting the spectrum of experimental observations regarding spontaneous and evoked CS firing. Importantly, the dynamic encoding hypothesis is compatible with most previous results. The same behavioral parameters linearly modulate both CSs and SSs in the same reference frames [61, 68, 70, 137]. This suggests that these two discharge modes of Purkinje cells function in concert during movements, as opposed to acting independently. In agreement, Purkinje cells are organized according to CS directional tuning such that the SS population response, when based on climbing fiber directional responsiveness, improves the decoding the speed and direction of saccades [138]. Also, the parasagittal organization of the olivocerebellar projection and tendency for synchronous climbing fiber activation will work to reset the SS representations within a microzone and effect a coordinated change of the information to downstream targets.
Recent studies emphasize that the heterogeneity in the cerebellar circuity is associated with differential Purkinje cell excitability and firing statistics. For example, the spontaneous SS and CS firing rates are higher in zebrin II − zones than in zebrin II + zones [139, 139–141]. Zebrin II + zones also have higher SS variability and longer duration SS suppression following a CS [139]. In addition, Purkinje cells in zebrin II ± bands respond differently to PF input [142, 143] and show different SS modulation patterns preceding CS occurrence [144]. In our study, the CS-coupled encoding changes occur in the vast majority of Purkinje cells recorded, arguing that the encoding is not necessarily restricted to specific subpopulations defined by the local heterogeneity of the cerebellar cortex. However, it remains to be determined to what degree local differences in the circuitry modulate the dynamic encoding processes.
For CS signaling events or errors, the evoked climbing fiber discharge reflects the need to change the encoding state of Purkinje cells due to errors. The changes in SS sensitivity evoked by CS are arguably a form of bistability. Given that bistability occurs primarily in reduced or anesthetized preparations in which the physiology of both Purkinje cells and the cerebellar cortical circuitry is altered (for review, see [92]), the information present in SS firing is limited. Therefore, the effect of a CS manifests as an overall change in firing rate as opposed to a change in encoding. Finally, the dynamic encoding hypothesis accounts for the suppressive action of climbing fibers on SS firing. As uncorrected drifts in SS encoding accumulate and firing rates increase, cerebellar representations of motor behavior are corrupted and ataxia develops. Thus, this novel framework provides a unifying view of climbing fiber function, capable of incorporating previous experimental observations.
Importantly, the dynamic encoding concept provides a unifying framework for both spontaneous and evoked CSs. The hypothesis is also compatible with climbing fiber involvement in learning and adaptation [32–34]. For example, spontaneous climbing fiber input has been proposed to perturb movements as a probe for initiating plasticity [145] and CS-evoked changes in SS encoding are consistent with this view. Behaviorally evoked CSs are likely to engage plasticity mechanisms at the parallel fiber–Purkinje cell synapses within a microzone. Together with previous descriptions of bi-directional plasticity mechanisms [133, 134, 146, 147], SS resetting may contribute to the rules governing the direction of plasticity.
Conclusions
This review examined the various hypotheses on the function of the climbing fiber–Purkinje cell synapse. Each of the traditional hypotheses has merit and has problems. Newer results show that the field needs to move beyond CSs serving as an error feedback signal. Climbing fiber input has spatially rich information about kinematics and performance errors present. We postulate that climbing fiber discharge controls the signals encoded in the SS firing. This dynamic encoding hypothesis is consistent with many of the observations in the literature.
Acknowledgements
We wish to thank Kathleen Beterams for help with manuscript preparation.
Funding
This study was supported in part by NIH grants: R01 NS18338, T32 GM008471, and F31 NS095408, and NSF grant IGERT DGE-1069104.
Compliance with Ethical Standards
Conflict of Interest Statement
There are no current or potential conflicts of interest for the three authors, Martha L. Streng, Laurentiu S. Popa and Timothy J. Ebner.
References
- 1.Eccles JC, Ito M, Szentagothai J. The cerebellum as a neuronal machine. Berlin: Springer-Verlag; 1967. [Google Scholar]
- 2.Ito M. The cerebellum and neural control. New York: Raven Press; 1984. [Google Scholar]
- 3.Ito M: The cerebellum: brain for an implicit self. FT Press, 2011.
- 4.Smeets CJ, Verbeek DS. Climbing fibers in spinocerebellar ataxia: a mechanism for the loss of motor control. Neurobiol Dis. 2016;88:96–106. doi: 10.1016/j.nbd.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Ariens-Kappers CU, Huber GC, Crosby EC. The comparative anatomy of the nervous system of vertebrates including man. New York: Macmillan; 1936. [Google Scholar]
- 6.Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–681. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- 7.Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci. 2004;24:8771–8785. doi: 10.1523/JNEUROSCI.1961-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voogd J, Ruigrok TJ. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol. 2004;33:5–21. doi: 10.1023/B:NEUR.0000029645.72074.2b. [DOI] [PubMed] [Google Scholar]
- 9.Najac M, Raman IM. Integration of Purkinje cell inhibition by cerebellar nucleo-olivary neurons. J Neurosci. 2015;35:544–549. doi: 10.1523/JNEUROSCI.3583-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmolesky MT, Weber JT, De Zeeuw CI, Hansel C. The making of a complex spike: ionic composition and plasticity. Ann N Y Acad Sci. 2002;978:359–390. doi: 10.1111/j.1749-6632.2002.tb07581.x. [DOI] [PubMed] [Google Scholar]
- 11.Simpson JI, Wylie DR, DeZeeuw CI. On climbing fiber signals and their consequence(s) Behav Brain Sci. 1995;19:385–398. [Google Scholar]
- 12.Llinas RR. The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. 2013;7:96. doi: 10.3389/fncir.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davie JT, Clark BA, Hausser M. The origin of the complex spike in cerebellar Purkinje cells. J Neurosci. 2008;28:7599–7609. doi: 10.1523/JNEUROSCI.0559-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najafi F, Medina JF. Beyond "all-or-nothing" climbing fibers: graded representation of teaching signals in Purkinje cells. Front Neural Circuits. 2013;7:115. doi: 10.3389/fncir.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbour B. Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron. 1993;11:759–769. doi: 10.1016/0896-6273(93)90085-6. [DOI] [PubMed] [Google Scholar]
- 18.Allen GI, Tsukahara N. Cerebro-cerebellar communication systems. Physiol Rev. 1974;54:957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- 19.Chan-Palay V. Cerebellar Dentate Nucleus. New York: Springer; 1977. [Google Scholar]
- 20.Teune TM, Van d BJ, De Zeeuw CI, Voogd J, Ruigrok TJ. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.De Zeeuw CI, Holstege JC, Ruigrok TJ, Voogd J. Ultrastructural study of the GABAergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: anterograde tracing combined with immunocytochemistry. J Comp Neurol. 1989;284:12–35. doi: 10.1002/cne.902840103. [DOI] [PubMed] [Google Scholar]
- 22.Chaumont J, Guyon N, Valera AM, Dugue GP, Popa D, Marcaggi P, Gautheron V, Reibel-Foisset S, Dieudonne S, Stephan A, Barrot M, Cassel JC, Dupont JL, Doussau F, Poulain B, Selimi F, Lena C, Isope P. Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. Proc Natl Acad Sci U S A. 2013;110:16223–16228. doi: 10.1073/pnas.1302310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witter L, Canto CB, Hoogland TM, de G, Jr, De Zeeuw CI. Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits. 2013;7:133. doi: 10.3389/fncir.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall SP, Lang EJ. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci. 2009;29:14352–14362. doi: 10.1523/JNEUROSCI.3498-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Lisberger SG. Interaction of plasticity and circuit organization during the acquisition of cerebellum-dependent motor learning. elife. 2013;2:e01574. doi: 10.7554/eLife.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rushmer DS, Roberts WJ, Augter GK. Climbing fiber responses of cerebellar Purkinje cells to passive movement of the cat forepaw. Brain Res. 1976;106:1–20. doi: 10.1016/0006-8993(76)90069-x. [DOI] [PubMed] [Google Scholar]
- 27.Gellman R, Gibson AR, Houk JC. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985;54:40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- 28.Apps R. Movement-related gating of climbing fibre input to cerebellar cortical zones. Prog Neurobiol. 1999;57:537–562. doi: 10.1016/s0301-0082(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 29.Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, editor. The inferior Olivary nucleus: anatomy and physiology. New York: Raven; 1980. pp. 279–290. [Google Scholar]
- 30.De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- 31.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- 32.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 33.Ito M. Error detection and representation in the olivo-cerebellar system. Front Neural Circuits. 2013;7:1–8. doi: 10.3389/fncir.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–635. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- 35.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 37.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–532. doi: 10.1038/nature13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitazawa S, Kimura T, Yin PB. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature. 1998;392:494–497. doi: 10.1038/33141. [DOI] [PubMed] [Google Scholar]
- 42.Llinas R, Lang EJ, Welsh JP. The cerebellum, LTD, and memory: alternative views. Learn Mem. 1997;3:445–455. doi: 10.1101/lm.3.6.445. [DOI] [PubMed] [Google Scholar]
- 43.Popa LS, Streng ML, Hewitt AL, Ebner TJ. The errors of our ways: understanding error representations in cerebellar-dependent motor learning. Cerebellum. 2016;15:93–103. doi: 10.1007/s12311-015-0685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit's cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol. 1988;60:2091–2121. doi: 10.1152/jn.1988.60.6.2091. [DOI] [PubMed] [Google Scholar]
- 45.Barmack NH, Shojaku H. Vestibular and visual climbing fiber signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74:2573–2589. doi: 10.1152/jn.1995.74.6.2573. [DOI] [PubMed] [Google Scholar]
- 46.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. II. Complex spikes. J Neurophysiol. 1990;63:1262–1275. doi: 10.1152/jn.1990.63.5.1262. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi Y, Kawano K, Takemura A, Inoue Y, Kitama T, Gomi H, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys II. Complex spikes. J Neurophysiol. 1998;80:832–848. doi: 10.1152/jn.1998.80.2.832. [DOI] [PubMed] [Google Scholar]
- 48.Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol. 2008;100:1949–1966. doi: 10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang JJ, Kim JH, Ebner TJ. Climbing fiber afferent modulation during a visually guided, multi-joint arm movement in the monkey. Brain Res. 1987;410:323–329. doi: 10.1016/0006-8993(87)90331-3. [DOI] [PubMed] [Google Scholar]
- 50.Ojakangas CL, Ebner TJ. Purkinje cell complex spike activity during voluntary motor learning: relationship to kinematics. J Neurophysiol. 1994;72:2617–2630. doi: 10.1152/jn.1994.72.6.2617. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Wang JJ, Ebner TJ. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol. 1987;57:787–802. doi: 10.1152/jn.1987.57.3.787. [DOI] [PubMed] [Google Scholar]
- 52.Andersson G, Armstrong DM. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol. 1987;385:107–134. doi: 10.1113/jphysiol.1987.sp016487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lou JS, Bloedel JR. Responses of sagittally aligned Purkinje cells during perturbed locomotion: synchronous activation of climbing fiber inputs. J Neurophysiol. 1992;68:570–580. doi: 10.1152/jn.1992.68.2.570. [DOI] [PubMed] [Google Scholar]
- 54.Catz N, Dicke PW, Thier P. Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Curr Biol. 2005;15:2179–2189. doi: 10.1016/j.cub.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 55.Dash S, Catz N, Dicke PW, Thier P. Specific vermal complex spike responses build up during the course of smooth-pursuit adaptation, paralleling the decrease of performance error. Exp Brain Res. 2010;205:41–55. doi: 10.1007/s00221-010-2331-2. [DOI] [PubMed] [Google Scholar]
- 56.Prsa M, Thier P. The role of the cerebellum in saccadic adaptation as a window into neural mechanisms of motor learning. Eur J Neurosci. 2011;33:2114–2128. doi: 10.1111/j.1460-9568.2011.07693.x. [DOI] [PubMed] [Google Scholar]
- 57.Horn KM, van Kan PL, Gibson AR. Reduction of rostral dorsal accessory olive responses during reaching. J Neurophysiol. 1996;76:4140–4151. doi: 10.1152/jn.1996.76.6.4140. [DOI] [PubMed] [Google Scholar]
- 58.Fu QG, Mason CR, Flament D, Coltz JD, Ebner TJ. Movement kinematics encoded in complex spike discharge of primate cerebellar Purkinje cells. Neuroreport. 1997;8:523–529. doi: 10.1097/00001756-199701200-00029. [DOI] [PubMed] [Google Scholar]
- 59.Ebner TJ, Johnson MT, Roitman A, Fu Q. What do complex spikes signal about limb movements? Ann N Y Acad Sci. 2002;978:205–218. doi: 10.1111/j.1749-6632.2002.tb07568.x. [DOI] [PubMed] [Google Scholar]
- 60.Hewitt AL, Popa LS, Ebner TJ. Changes in Purkinje cell simple spike encoding of reach kinematics during adaptation to a mechanical perturbation. J Neurosci. 2015;35:1106–1124. doi: 10.1523/JNEUROSCI.2579-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Streng ML, Popa LS, Ebner TJ. Climbing fibers predict movement kinematics and performance errors. J Neurophysiol. 2017;118:1888–1902. doi: 10.1152/jn.00266.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raghavan RT, Lisberger SG. Responses of Purkinje cells in the oculomotor vermis of monkeys during smooth pursuit eye movements and saccades: comparison with floccular complex. J Neurophysiol. 2017;118:986–1001. doi: 10.1152/jn.00209.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soetedjo R, Fuchs AF. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci. 2006;26:7741–7755. doi: 10.1523/JNEUROSCI.4658-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soetedjo R, Kojima Y, Fuchs A. Complex spike activity signals the direction and size of dysmetric saccade errors. Prog Brain Res. 2008;171:153–159. doi: 10.1016/S0079-6123(08)00620-1. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen-Vu TD, Kimpo RR, Rinaldi JM, et al. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. 2013;16:1734–1736. doi: 10.1038/nn.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahlon M, Lisberger SG. Changes in the responses of Purkinje cells in the floccular complex of monkeys after motor learning in smooth pursuit eye movements. J Neurophysiol. 2000;84:2945–2960. doi: 10.1152/jn.2000.84.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popa LS, Hewitt AL, Ebner TJ. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J Neurosci. 2012;32:15345–15358. doi: 10.1523/JNEUROSCI.2151-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Streng ML, Popa LS, Ebner TJ. Climbing fibers control Purkinje cell representations of behavior. J Neurosci. 2017;37:1997–2009. doi: 10.1523/JNEUROSCI.3163-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Popa LS, Streng ML, Ebner TJ: Long-term predictive and feedback encoding of motor signals in the simple spike discharge of Purkinje cells. eNeuro 2017;4, ENEURO.0036, ENEU17.2017. [DOI] [PMC free article] [PubMed]
- 71.Streng ML, Popa LS, Ebner TJ. Modulation of sensory prediction error in Purkinje cells during visual feedback manipulations. Nat Commun. 2018;9:1099. doi: 10.1038/s41467-018-03541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomi H, Shidara M, Takemura A, Inoue Y, Kawano K, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys I. Simple spikes. J Neurophysiol. 1998;80:818–831. doi: 10.1152/jn.1998.80.2.818. [DOI] [PubMed] [Google Scholar]
- 73.Sotelo C, Llinas R, Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974;37:541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- 74.King JS. The synaptic cluster (glomerulus) in the inferior olivary nucleus. J Comp Neurol. 1976;165:387–400. doi: 10.1002/cne.901650307. [DOI] [PubMed] [Google Scholar]
- 75.Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 76.Leznik E, Llinas R. Role of gap junctions in synchronized neuronal oscillations in the inferior olive. J Neurophysiol. 2005;94:2447–2456. doi: 10.1152/jn.00353.2005. [DOI] [PubMed] [Google Scholar]
- 77.Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26:1739–1748. doi: 10.1523/JNEUROSCI.3677-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turecek J, Han VZ, Cuzon CV, Grant KA, Welsh JP. Electrical coupling and synchronized subthreshold oscillations in the inferior olive of the rhesus macaque. J Neurosci. 2016;36:6497–6502. doi: 10.1523/JNEUROSCI.4495-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bleasel AF, Pettigrew AG. Development and properties of spontaneous oscillations of the membrane potential in inferior olivary neurons in the rat. Brain Res Dev Brain Res. 1992;65:43–50. doi: 10.1016/0165-3806(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 80.Chorev E, Yarom Y, Lampl I. Rhythmic episodes of subthreshold membrane potential oscillations in the rat inferior olive nuclei in vivo. J Neurosci. 2007;27:5043–5052. doi: 10.1523/JNEUROSCI.5187-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bloedel JR, Ebner TJ. Rhythmic discharge of climbing fibre afferents in response to natural peripheral stimuli in the cat. J Physiol. 1984;352:129–146. doi: 10.1113/jphysiol.1984.sp015282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sugihara I, Lang EJ, Llinas R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–534. doi: 10.1111/j.1460-9568.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 83.Lang EJ, Sugihara I, Llinas R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–275. doi: 10.1152/jn.1996.76.1.255. [DOI] [PubMed] [Google Scholar]
- 84.Lang EJ, Sugihara I, Welsh JP, Llinas R. Patterns of spontaneous purkinje cell complex spike activity in the awake rat. J Neurosci. 1999;19:2728–2739. doi: 10.1523/JNEUROSCI.19-07-02728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Giessen RS, Koekkoek SK, van DS, et al. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612. doi: 10.1016/j.neuron.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Bazzigaluppi P, de G, Jr, van der Giessen RS, Khosrovani S, De Zeeuw CI, De Jeu MT. Olivary subthreshold oscillations and burst activity revisited. Front Neural Circuits. 2012;6:91. doi: 10.3389/fncir.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lang EJ, Tang T, Suh CY, et al. Modulation of Purkinje cell complex spike waveform by synchrony levels in the olivocerebellar system. Front Syst Neurosci. 2014;8:210. doi: 10.3389/fnsys.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Placantonakis DG, Bukovsky AA, Zeng XH, Kiem HP, Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc Natl Acad Sci U S A. 2004;101:7164–7169. doi: 10.1073/pnas.0400322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welsh JP, Lang EJ, Suglhara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 90.Llinas R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- 91.Khosrovani S, van der Giessen RS, De Zeeuw CI, De Jeu MT. In vivo mouse inferior olive neurons exhibit heterogeneous subthreshold oscillations and spiking patterns. Proc Natl Acad Sci U S A. 2007;104:15911–15916. doi: 10.1073/pnas.0702727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engbers JD, Fernandez FR, Turner RW. Bistability in Purkinje neurons: ups and downs in cerebellar research. Neural Netw. 2013;47:18–31. doi: 10.1016/j.neunet.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 93.Schonewille M, Khosrovani S, Winkelman BH, et al. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci. 2006;9:459–461. doi: 10.1038/nn0406-459. [DOI] [PubMed] [Google Scholar]
- 94.Keating JG, Thach WT. Nonclock behavior of inferior olive neurons: interspike interval of Purkinje cell complex spike discharge in the awake behaving monkey is random. J Neurophysiol. 1995;73:1329–1340. doi: 10.1152/jn.1995.73.4.1329. [DOI] [PubMed] [Google Scholar]
- 95.Hakimian S, Norris SA, Greger B, Keating JG, Anderson CH, Thach WT. Time and frequency characteristics of Purkinje cell complex spikes in the awake monkey performing a nonperiodic task. J Neurophysiol. 2008;100:1032–1040. doi: 10.1152/jn.90277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozden I, Sullivan MR, Lee HM, Wang SS. Reliable coding emerges from coactivation of climbing fibers in microbands of cerebellar Purkinje neurons. J Neurosci. 2009;29:10463–10473. doi: 10.1523/JNEUROSCI.0967-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colin F, Manil J, Desclin JC. The olivocerebellar system. I. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res. 1980;187:3–27. doi: 10.1016/0006-8993(80)90491-6. [DOI] [PubMed] [Google Scholar]
- 98.Montarolo PG, Palestini M, Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol. 1982;332:187–202. doi: 10.1113/jphysiol.1982.sp014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cerminara NL, Rawson JA. Evidence that climbing fibers control an intrinsic spike generator in cerebellar Purkinje cells. J Neurosci. 2004;24:4510–4517. doi: 10.1523/JNEUROSCI.4530-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zucca R, Rasmussen A, Bengtsson F. Climbing fiber regulation of spontaneous Purkinje cell activity and cerebellum-dependent blink responses. eNeuro. 2016;3 [DOI] [PMC free article] [PubMed]
- 101.Llinas R, Walton K, Hillman DE, Sotelo C. Inferior olive: its role in motor learing. Science. 1975;190:1230–1231. doi: 10.1126/science.128123. [DOI] [PubMed] [Google Scholar]
- 102.Horn KM, Deep A, Gibson AR. Progressive limb ataxia following inferior olive lesions. J Physiol. 2013;591:5475–5489. doi: 10.1113/jphysiol.2012.234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ebner TJ, Yu QX, Bloedel JR. Increase in Purkinje cell gain associated with naturally activated climbing fiber input. J Neurophysiol. 1983;50:205–219. doi: 10.1152/jn.1983.50.1.205. [DOI] [PubMed] [Google Scholar]
- 104.McDevitt CJ, Ebner TJ, Bloedel JR. The changes in Purkinje cell simple spike activity following spontaneous climbing fiber inputs. Brain Res. 1982;237:484–491. doi: 10.1016/0006-8993(82)90460-7. [DOI] [PubMed] [Google Scholar]
- 105.Lou JS, Bloedel JR. Responses of sagittally aligned Purkinje cells during perturbed locomotion: relation of climbing fiber activation to simple spike modulation. J Neurophysiol. 1992;68:1820–1833. doi: 10.1152/jn.1992.68.5.1820. [DOI] [PubMed] [Google Scholar]
- 106.Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Häusser M. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci. 2005;8:202–211. doi: 10.1038/nn1393. [DOI] [PubMed] [Google Scholar]
- 107.Yartsev MM, Givon-Mayo R, Maller M, Donchin O. Pausing purkinje cells in the cerebellum of the awake cat. Front Syst Neurosci. 2009;3:2. doi: 10.3389/neuro.06.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McKay BE, Engbers JD, Mehaffey WH, et al. Climbing fiber discharge regulates cerebellar functions by controlling the intrinsic characteristics of Purkinje cell output. J Neurophysiol. 2007;97:2590–2604. doi: 10.1152/jn.00627.2006. [DOI] [PubMed] [Google Scholar]
- 109.Mano N, Kanazawa I, Yamamoto K. Complex-spike activity of cerebellar Purkinje cells related to wrist tracking movement in monkey. J Neurophysiol. 1986;56:137–158. doi: 10.1152/jn.1986.56.1.137. [DOI] [PubMed] [Google Scholar]
- 110.Bloedel JR, Roberts WJ. Action of climbing fibers in cerebellar cortex of the cat. J Neurophysiol. 1971;34:17–31. doi: 10.1152/jn.1971.34.1.17. [DOI] [PubMed] [Google Scholar]
- 111.Rawson JA, Tilokskulchai K. Suppression of simple spike discharges of cerebellar Purkinje cells by impulses in climbing fibre afferents. Neurosci Lett. 1981;25:125–130. doi: 10.1016/0304-3940(81)90319-0. [DOI] [PubMed] [Google Scholar]
- 112.Badura A, Schonewille M, Voges K, Galliano E, Renier N, Gao Z, Witter L, Hoebeek FE, Chédotal A, de Zeeuw CI. Climbing fiber input shapes reciprocity of Purkinje cell firing. Neuron. 2013;78:700–713. doi: 10.1016/j.neuron.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 113.Yakhnitsa V, Barmack NH. Antiphasic Purkinje cell responses in mouse uvula-nodulus are sensitive to static roll-tilt and topographically organized. Neuroscience. 2006;143:615–626. doi: 10.1016/j.neuroscience.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 114.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- 115.Murphy JT, Sabah NH. The inhibitory effect of climbing fiber activation on cerebellar purkinje cells. Brain Res. 1970;19:486–490. doi: 10.1016/0006-8993(70)90391-4. [DOI] [PubMed] [Google Scholar]
- 116.Rubia FJ, Kolb FP. Responses of cerebellar units to a passive movement in the decerebrate cat. Exp Brain Res. 1978;31:387–401. doi: 10.1007/BF00237297. [DOI] [PubMed] [Google Scholar]
- 117.Rubia FJ, Hennemann HE. Discharge patterns of Purkinje cells activated through the climbing fiber system by stimulation of somatic and visceral afferents. Pflugers Arch. 1978;375:125–129. doi: 10.1007/BF00584234. [DOI] [PubMed] [Google Scholar]
- 118.Yakusheva T, Blazquez PM, Angelaki DE. Relationship between complex and simple spike activity in macaque caudal vermis during three-dimensional vestibular stimulation. J Neurosci. 2010;30:8111–8126. doi: 10.1523/JNEUROSCI.5779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winkelman BH, Belton T, Suh M, Coesmans M, Morpurgo MM, Simpson JI. Nonvisual complex spike signals in the rabbit cerebellar flocculus. J Neurosci. 2014;34:3218–3230. doi: 10.1523/JNEUROSCI.3080-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hewitt A, Popa LS, Pasalar S, Hendrix CM, Ebner TJ. Representation of limb kinematics in Purkinje cell simple spike discharge is conserved across multiple tasks. J Neurophysiol. 2011;106:2232–2247. doi: 10.1152/jn.00886.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frens MA, Mathoera AL, van der SJ. Floccular complex spike response to transparent retinal slip. Neuron. 2001;30:795–801. doi: 10.1016/s0896-6273(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 122.Winkelman B, Frens M. Motor coding in floccular climbing fibers. J Neurophysiol. 2006;95:2342–2351. doi: 10.1152/jn.01191.2005. [DOI] [PubMed] [Google Scholar]
- 123.Ohmae S, Medina JF. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci. 2015;18:1798–1803. doi: 10.1038/nn.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ten Brinke MM, Boele HJ, Spanke JK, et al. Evolving models of Pavlovian conditioning: cerebellar cortical dynamics in awake behaving mice. Cell Rep. 2015;13:1977–1988. doi: 10.1016/j.celrep.2015.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garden DL, Rinaldi A, Nolan MF. Active integration of glutamatergic input to the inferior olive generates bidirectional postsynaptic potentials. J Physiol. 2017;595:1239–1251. doi: 10.1113/JP273424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mathy A, Ho SS, Davie JT, Duguid IC, Clark BA, Hausser M. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–399. doi: 10.1016/j.neuron.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rasmussen A, Jirenhed DA, Zucca R, Johansson F, Svensson P, Hesslow G. Number of spikes in climbing fibers determines the direction of cerebellar learning. J Neurosci. 2013;33:13436–13440. doi: 10.1523/JNEUROSCI.1527-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burroughs A, Wise AK, Xiao J, Houghton C, Tang T, Suh CY, Lang EJ, Apps R, Cerminara NL. The dynamic relationship between cerebellar Purkinje cell simple spikes and the spikelet number of complex spikes. J Physiol. 2017;595:283–299. doi: 10.1113/JP272259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+] increases in the dendrites of cerebellar Purkinje neurons. J Neurosci. 1995;15:2777–2787. doi: 10.1523/JNEUROSCI.15-04-02777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kitamura K, Hausser M. Dendritic calcium signaling triggered by spontaneous and sensory-evoked climbing fiber input to cerebellar Purkinje cells in vivo. J Neurosci. 2011;31:10847–10858. doi: 10.1523/JNEUROSCI.2525-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Forrest MD. Intracellular calcium dynamics permit a Purkinje neuron model to perform toggle and gain computations upon its inputs. Front Comput Neurosci. 2014;8:86. doi: 10.3389/fncom.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piochon C, Kruskal P, Maclean J, Hansel C. Non-Hebbian spike-timing-dependent plasticity in cerebellar circuits. Front Neural Circuits. 2012;6:124. doi: 10.3389/fncir.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suvrathan A, Payne HL, Raymond JL. Timing rules for synaptic plasticity matched to behavioral function. Neuron. 2016;92:959–967. doi: 10.1016/j.neuron.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Isope P, Barbour B. Properties of unitary granule cell-->Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22:9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ebner TJ, Hewitt AL, Popa LS. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum. 2011;10:683–693. doi: 10.1007/s12311-010-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mothersill O, Knee-Zaska C, Donohoe G. Emotion and theory of mind in schizophrenia-investigating the role of the cerebellum. Cerebellum. 2016;15:357–368. doi: 10.1007/s12311-015-0696-2. [DOI] [PubMed] [Google Scholar]
- 138.Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. Encoding of action by the Purkinje cells of the cerebellum. Nature. 2015;526:439–442. doi: 10.1038/nature15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou H, Lin Z, Voges K, et al. Cerebellar modules operate at different frequencies. elife. 2014;3:e02536. doi: 10.7554/eLife.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiao J, Cerminara NL, Kotsurovskyy Y, Aoki H, Burroughs A, Wise AK, Luo Y, Marshall SP, Sugihara I, Apps R, Lang EJ. Systematic regional variations in Purkinje cell spiking patterns. PLoS One. 2014;9:e105633. doi: 10.1371/journal.pone.0105633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16:79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gao W, Chen G, Reinert KC, Ebner TJ. Cerebellar cortical molecular layer inhibition is organized in parasagittal zones. J Neurosci. 2006;26:8377–8387. doi: 10.1523/JNEUROSCI.2434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X, Chen G, Gao W, Ebner TJ. Parasagittally aligned, mGluR1-dependent patches are evoked at long latencies by parallel fiber stimulation in the mouse cerebellar cortex in vivo. J Neurophysiol. 2011;105:1732–1746. doi: 10.1152/jn.00717.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang T, Xiao J, Suh CY, Burroughs A, Cerminara NL, Jia L, Marshall SP, Wise AK, Apps R, Sugihara I, Lang EJ. Heterogeneity of Purkinje cell simple spike-complex spike interactions: zebrin- and non-zebrin-related variations. J Physiol. 2017;595:5341–5357. doi: 10.1113/JP274252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bouvier G, Aljadeff J, Clopath C et al: Cerebellar learning using perturbations. bioRxiv. 2017. [DOI] [PMC free article] [PubMed]
- 146.Bouvier G, Higgins D, Spolidoro M, Carrel D, Mathieu B, Léna C, Dieudonné S, Barbour B, Brunel N, Casado M. Burst-dependent bidirectional plasticity in the cerebellum is driven by presynaptic NMDA receptors. Cell Rep. 2016;15:104–116. doi: 10.1016/j.celrep.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 147.Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]