Abstract
Complicated skin and soft tissue infections (cSSTIs) represent the severe form of infectious disease that involves deeper soft tissues. Involvement of methicillin-resistant Staphylococcus aureus (MRSA) further complicates cSSTI with increased hospitalization, health care costs, and overall mortality. Various international guidelines provide recommendations on the management of cSSTIs, with the inclusion of newer antibiotics. This literature-based review discusses the overall management of cSSTI, including appropriate use of antibiotics in clinical practice. Successful treatment of cSSTIs starts with early and precise diagnosis, including identification of causative pathogen and its load, determination of infection severity, associated complications, and risk factors. The current standard-of-care for cSSTIs involves incision, drainage, surgical debridement, broad-spectrum antibiotic therapy, and supportive care. In recent years, the emergence of newer antibiotics (eg, ceftaroline, tigecycline, daptomycin, linezolid, etc) has provided clinicians wider options of antimicrobial therapy. Selection of antibiotics should be based on the drug characteristics, effectiveness, safety, and treatment costs, alongside other aspects such as host factors and local multidrug resistance rates. However, larger studies on newer antibiotics are warranted to refine the decision making on the appropriate antimicrobial therapy. Local Antimicrobial Stewardship Program strategies in health care settings could guide clinicians for early initiation of specific treatments to combat region-specific antimicrobial resistance, minimize adverse effects, and to improve outcomes such as reduction in Clostridium difficile infections. These strategies involving iv-to-oral switch, de-escalation to narrow-spectrum antibiotics, and dose optimization have an impact on the overall improvement of cSSTI therapy outcomes, especially in countries like Singapore that has a high disease burden.
Keywords: antibiotics, complicated skin and soft tissue infections, methicillin-resistant Staphylococcus aureus, Singapore
Introduction
Skin and soft tissue infections (SSTIs) encompass a wide clinical spectrum of common infectious diseases that often require acute treatment and inpatient hospital admission. These infections have a heterogeneous manifestation and involve microbial infection of the epidermis, dermis, superficial fascia, subcutaneous tissues, and muscle in an increasing order of severity.1 Complicated SSTIs (cSSTIs) are the most severe, involving deeper soft tissues and include infective cellulitis, ulcer or wound site infections, surgical site infections, major abscesses, infected burns, skin ulcers, and diabetic foot ulcers.2 The US FDA in 2013 grouped all SSTIs under a unified term, Acute Bacterial Skin and Skin Structure Infection (ABSSSI), which includes cellulitis/erysipelas, wound infection, and major cutaneous abscesses. It is defined as a bacterial infection of the skin with a lesion size area of at least 75 cm2 (lesion size measured by the area of redness, edema, or induration).3
Staphylococcus aureus, an aerobic Gram-positive coccus, is the most dominant causative pathogen and has paramount epidemiological significance in cSSTI. Pseudomonas aeruginosa, Escherichia coli, and Enterococcus spp. have also been identified as causes of cSSTI; however, these are not the predominant causative pathogens.4 The rampant emergence of methicillin-resistant S. aureus (MRSA) infections, endemic to several countries worldwide, has confounded the treatment of cSSTIs.5 Initially, as MRSA emerged (from 1961 to 1990), hospital-associated MRSA (HA-MRSA) predominated, but in recent years, preponderance of community-associated MRSA (CA-MRSA) in SSTIs is noted in the US and Europe.6,7 Dense population and relatively greater indiscriminate use of antibiotics predisposes Asian countries to high prevalence of endemic MRSA with estimated proportions of up to 70%.8 A multinational surveillance study conducted across eight Asian countries, namely, Korea, Taiwan, Hong Kong, Thailand, the Philippines, Vietnam, India, and Sri Lanka, from September 2004 to August 2006 showed increase in the emergence of CA-MRSA, with incidence ranging from 2.5% to 39%.9
The epidemiology of MRSA infections reflects the overall increasing burden of SSTIs worldwide.10,11 In Asia, variable rates of MRSA (7.3%–74%) are reported in patients with SSTIs tested positive for S. aureus.12–17 In Singapore, high rates of MRSA (35.3%) were reported among S. aureus isolates, generally comparable with Western countries (23.6%–73.8%).18,19 Also, the SSTI-associated nosocomial infections account for 7.2% of all hospital-associated (HA) infections, with S. aureus identified as the predominant pathogen (27%).20 MRSA is a common cause of nosocomial infections in Singapore with incidences of serious outbreaks reported in general hospitals and intensive care units.21–23 Additionally, elderly patients and patients with skin lesions or dermatological conditions were noted with a higher risk of harboring MRSA.23 The looming danger of severe infectious diseases like cSSTIs and the associated microbial resistance are persuasive, underscoring the need for implementation of systematic surveillance of cSSTI in Singapore. Several international working groups provide guidance on the surgical and pharmacological management of cSSTIs recommending inclusion of recently approved newer antibiotics. However, local data from Singapore for the prudent use of antibiotics in cSSTIs are scanty. The review, therefore, aims to provide an overview of the microbiology, drug resistance issues, diagnosis, and the overall management of cSSTI. In particular, the review explores the current medical evidence for appropriate use of antimicrobials and current management strategies for cSSTIs to achieve the best clinical outcomes, with special focus on newer antibiotics, which are approved by US FDA after 2000.
Literature search methodology
An electronic search of PubMed was conducted to source relevant articles using a combination of MeSH terms “complicated skin and skin structure infections”, “skin and soft tissue infections”, “Gram-positive pathogens”, “Staphylococcus aureus”, “methicillin-resistant Staphylococcus aureus”, “MRSA”, “multi drug resistance”, “nosocomial infections”, and “community-acquired infections”. Relevant articles included randomized controlled trials, surveillance, outcome studies, and expert opinions. Bibliographies of relevant articles were manually screened to broaden the literature search. All articles were restricted to English language and no restrictions were set with respect to year or type of publication.
Microbiology of cSSTI
Invasion of pathogens through disruptions of skin or soft tissue structure is the fundamental etiology of SSTIs and several local and systemic risk factors further increase vulnerability to cSSTIs (Table 1).24
Table 1.
Skin and soft tissue infections: risk factors and common causative pathogens
| Risk factors | |
|---|---|
| Local | Systemic |
| • Soft tissue trauma • Animal or human bites • Burns • Operative or highly contaminated wounds • Diminished perfusion due to peripheral vascular disease, obesity • Poor hygiene • Exposure to contaminated water • Presence of foreign body (eg, piercing, tattoos) • Venous insufficiency and stasis |
• Poorly controlled diabetes mellitus • Immunocompromised comorbidities (eg, HIV infection, cellular or humoral immune deficiencies) • Concomitant drugs (ie, corticosteroids, cyclosporine) • Sensory neuropathies • Chronic systemic illness (eg, cirrhosis) |
|
| |
| Infection or risk factor | Causative pathogen |
|
| |
| Abscess | Staphylococcus aureus |
| Polymicrobial | |
| Cellulitis | S. aureus |
| GABHS | |
| Associated with injection use | S. aureus |
| Associated with water exposure | Aeromonas hydrophila, Vibrio vulnificus, Mycobacterium marinum |
| Associated with animal bite | Pasteurella multocida, Capnocytophaga canimorsus |
| Associated with human bite | Human oral flora |
| Surgical site infections | Anaerobes |
| Enterococcus spp. | |
| Enterobacter spp. | |
| Gram-negative bacilli | |
| Staphylococcus spp. | |
| Streptococcus spp. | |
| Escherichia coli | |
| Necrotizing fasciitis | Monomicrobial: |
| S. pyogenes, S. aureus, Enterobacteriaceae, Bacteroides, and Peptostreptococcus species | |
| Polymicrobial: | |
| Clostridium perfringens, S. aureus, Pseudomonas aeruginosa | |
| Myonecrosis | C. perfringens |
| Recurrent hospital admissions | MRSA |
| Diabetes | S. aureus (MRSA and MSSA), GABHS, anaerobes, Gram-negative bacilli |
| Diabetic foot ulcers | S. aureus, Enterococcus spp., Streptococcus spp. |
| P. aeruginosa, Enterobacter spp., Acinetobacter spp. | |
| Bacteroides spp | |
| Cirrhosis | Campylobacter fetus, Klebsiella pneumoniae, E. coli, Capnocytophaga canimorsus, other |
| Gram-negative bacilli, Vibrio vulnificus | |
| Neutropenia | Gram-negative bacilli, P. aeruginosa |
Abbreviations: GABHS, group A β-hemolytic streptococci; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S. aureus.
S. aureus, P. aeruginosa, E. coli, Enterococcus spp., Enterobacter spp., Klebsiella spp., and b-Streptococcus are the frequently encountered pathogens causing SSTIs in hospitalized patients.19 cSSTIs may be monomicrobial or polymicrobial in nature with increased systemic inflammatory responses. Management of polymicrobial or mixed infections requires a multidrug approach that is effective against aerobic, anaerobic, and facultative bacteria.25 Diabetic patients are at twice the risk vs non-diabetics, for cSSTI-related hospitalizations.26 In cases where vascular circulation is compromised such as diabetic foot infection, or infection of ischemic or venous ulcers, the risk of poly-microbial infections is high. These complications are more common in patients who had previously received antibiotics for chronic infections.27
Issues related to the emergence of multidrug-resistant cSSTI and pertinent clinical management issues
The empirical approach for the treatment of cSSTI uses a combination of surgical, supportive, and antimicrobial therapies. However, a rise in antibiotic-resistant microorganisms,27 particularly multidrug-resistant organisms, has complicated the treatment of cSSTI. Among multidrug-resistant organisms, MRSA, vancomycin-resistant Enterococcus spp. (VRE), and extended-spectrum β-lactamase (ESBL)–producing isolates of E.coli and Klebsiella spp. have the highest occurrence.19 Factors contributing to the development of MRSA SSTIs are intravenous drug use, HIV seropositivity, MRSA colonization or previous infection, African-American race, indwelling devices, and previous antibiotics or hospi-talizations.11,28 The strains of CA-MRSA are genetically and phenotypically distinct from HA-MRSA, and hence possess a risk of infections having greater severity and easier transmission of resistance.29 The presence of Panton-Valentine leucocidin, a cytolytic toxin, encoding genes in MRSA isolated from CA-SSTIs are postulated to play a significant role in the increased virulence of these strains and are associated with tissue necrosis, and greater severity of local and systemic manifestations.5,29 The CA-MRSA strains also carry the staphylococcal cassette chromosome mec (SCCmec) genes (types IV and V) that confer resistance to methicillin and currently available β-lactam antimicrobial agents and aid easy transfer of resistance between organisms.30 Although MRSA was considered to be chiefly an HA infection, recent evidence suggests rapid emergence of CA-MRSA even in hospital settings.31–33 This complex infection epidemiology trend has further complicated the selection of antibiotics for treatment of cSSTI.
Diagnosis of complicated skin and soft tissue infections
Accurate diagnosis involving assessment of complications, severity, and risk factors, followed by identification of causative microorganism is essential for the management of cSSTI.
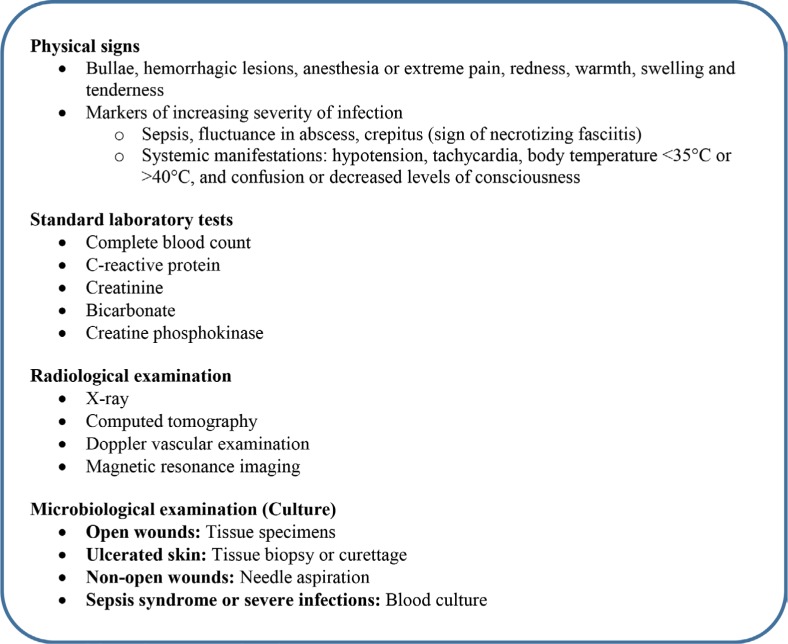
Comprehensive diagnosis of cSSTIs often starts with a clinical history and findings from physical examination that help assess the severity of an infection followed by investigation of causative microorganism and its load. The precise diagnosis subsequently guides the decision on prompt and appropriate treatments34,35 (Figure 1).
Figure 1.
Diagnosis of complicated skin and soft tissue infections.
Standard laboratory investigations are performed to augment clinical assessment, especially for inpatients. In addition to the patient history, relevant risk factors such as recurrent hospital admissions, diabetes, neutropenia, bite wounds, and animal contacts should be taken into consideration, which may indicate the possible microorganism responsible for the infection.27 Complications likely related to cSSTIs such as lymphadenitis, myositis/necrotizing fasciitis, gangrene, osteomyelitis, bacteremia, endocarditis, septicemia, or sepsis must also be factored in during diagnosis.36 The presence of significant leucocytosis (or leucopenia) may potentially indicate sepsis syndrome, while elevated creatine kinase (CK) levels may be suggestive of myonecrosis caused by necrotizing fasciitis or a compartment syndrome.27,34 Radiological examination and imaging aid investigations of deep tissue infections to assess site and size of infection and any vascular involvement that can guide surgical drainage procedures. Microbiological culture tests should be performed in all cases to differentiate MRSA cSSTIs from non-MRSA infections and thus refine the decision on the definitive antibiotics administration to minimize the risk of potential treatment failure.35,37
Accurate diagnosis of cSSTIs can be an indicator of appropriate referrals in addition to immediate hospitalization. Observation of limb-threatening infections in patients with diabetes should prompt urgent referral to a multidisciplinary team, including infectious disease experts, podiatry, and/or surgical (eg, orthopedic, vascular) specialists for effective surgical consultation and appropriate antimicrobial use.34 Surgical consultation might also be required in cases of infections such as necrotizing fasciitis, synergic gangrene, and osteomyelitis or septic arthritis.
Management of complicated skin and soft tissue infections
The three fundamental pillars of cSSTI management are surgical drainage with debridement where necessary, broad-spectrum antibiotic therapy, and physiological supportive care (Table 2).27,31 These interventions together are necessary to ensure effective cSSTIs management, especially for a disease with diverse clinical presentation and dynamic interplay of multiple microbial, host, and local factors. The goals of cSSTI treatment are source control, antimicrobial therapy, and organ supportive care. Source control achieved by debridement only facilitates wound healing process that may be managed further by pharmacological interventions.38
Table 2.
Recommendations for antibiotic treatment of methicillin-resistant Staphylococcus aureus complicated skin and soft tissue infections
| Guidelines | Infection | Recommended therapy |
|---|---|---|
| Infectious Diseases Society of America31 | cSSTI (deeper soft-tissue infections, surgical/traumatic wound infection, major abscesses, cellulitis, and infected ulcers and burns) Non-purulent cellulitis |
• Surgical debridement • Vancomycin, linezolid, daptomycin, telavancin, clindamycin • β-lactam antibiotic active against MRSA |
| Surgical Infection Society118 | Complex abscesses with cellulitis and polymicrobial cSSTIs | • Incision and drainage (in case of abscess) • Vancomycin, clindamycin, linezolid, or erythromycin |
| Gruppo Italiano di Studio sulle Infezioni Gravi41 | MRSA-related cSSTIs and severe surgical infections | • Use of TNP/VAC (for deep surgical infections) • Vancomycin, teicoplanin, linezolid, tigecycline, daptomycin |
| Italian Society of Infectious Diseases and international Society of Chemotherapy119 | MRSA-related cSSTIs | • Early surgical treatment wherever feasible • Vancomycin, teicoplanin, linezolid, tigecycline, daptomycin |
|
Spanish Society of Chemotherapy35,120 British Society of Antimicrobial Chemotherapy121 |
MRSA-related cSSTIs Severe cSSTI with MRSA | Linezolid, daptomycin, vancomycin, teicoplanin Linezolid, daptomycin, vancomycin, teicoplanin |
Abbreviations: cSSTIs, complicated skin and soft tissue infections; MRSA, methicillin-resistant Staphylococcus aureus; TNP/VAC, topical negative pressure/vacuum-assisted closure.
Surgical methods and supportive care
Exudates, fluid collections of abscess, and ulcerations are common features of cSSTIs. Therefore, aggressive surgical debridement of the necrotic/infected tissue using chemical or mechanical methods is preferred whenever feasible to arrest the spread of infection and promote wound healing (Table 2). Delay in definitive debridement of necrotizing soft tissue infections is known as the single most significant risk factor for death.39 Incision and drainage are performed for abscess and purulent infections.40 Other approaches include topical negative pressure (TNP) dressing for chronic infections or large wounds with excessive exudate,35,41 vacuum-assisted closure (as alternative for wound healing) especially for surgical wounds or post-surgical deep infections,41 thrombec-tomy for infections involving septic venous thrombosis, and reconstructive revascularization for cases involving arterial vessel injuries.42 Supportive cares involving fluid resuscitation, organs support, and nutritional management to maintain oxygenation and tissue perfusion are critical interventions in the clinical outcomes of these patients.35,39
Pharmacological management
Systemic antibiotic therapy against the causative pathogen remains the mainstay for treatment of cSSTIs. Antibiotics against S. aureus are recommended for all cases involving systemic presentations such as temperature >38°C or <36°C, tachypnea (>24 breaths per minute), tachycardia (>90 beats per minute), or white blood cell count (WBC) >12,000 or <400 cells/µL.40 Most eminent international guidelines recommend initial management with empirical antibiotic therapy against locally prevalent MRSA strains. The Infectious Diseases Society of America recommends bacterial culture assessment to aid the selection of antibiotics against the causative pathogens and initiate definitive therapy for severe infection, non-responsiveness to the current line of antibiotics, or recurrent infections.40 The UK’s National Institute for Clinical Excellence also recommends monitoring of clinical progress and reassessment of treatment based on culture findings.43
The timing of initiation and choice of appropriate antimicrobial therapy against the causative organism are critical determinants of treatment outcome. Delay in initiating treatment within 8 hours of presentation of cSSTI is associated with longer hospital stays44 and lack of active antibiotic therapy within 48 hours of admission with treatment failure.45 The US FDA suggests evaluation of clinical response to antibiotics in ABSSSI within 48–72 hours after initiation of therapy to identify potential treatment failure.3 In a large study, treatment failure in 22.8% patients with cSSTIs was associated with an increased risk of mortality (OR: 2.91; 95% CI: 2.34–3.62).46 Prolonged hospital stay or readmission and associated increase in health care cost due to inadequate initial antimicrobial therapy for cSSTI are also reported.46,47
Successful therapy requires clinical acumen of empiric and definitive treatment substantiated with knowledge of pharmacokinetic and pharmacodynamic properties of the antibiotics. Minimum inhibitory concentration (MIC) helps interpret susceptibility of the particular pathogen to the antimicrobial agent and rising MICs is indicative of emerging resistance of the pathogen to antibiotics (Table 3). Hence, the antibiotic should achieve a concentration equal to or greater than MIC at the site of infection.35 Poor tissue penetration adversely impacts the clinical and microbiological treatment outcomes and increases the risk of resistance among pathogens.48 Several factors such as vascular insufficiency or presence of comorbid diabetes or vascular diseases predominant in cSSTIs are known to interfere with antibiotic tissue penetration, thus restricting their efficacy.49,50 Hence, for antibiotic selection, a careful evaluation of the key determinants of a drug’s tissue penetration like the molecular weight, lipophilicity, tissue to plasma penetration ratio, protein binding, and volume of distribution is recommended.35,51,52
Table 3.
Minimum inhibitory concentration of antibiotics used for cSSTIs (European Committee on Antimicrobial Susceptibility Testing)
| Drug | Pathogens | Minimum inhibitory concentrations (µg/mL)
|
|
|---|---|---|---|
| Susceptible ≤ | Resistant > | ||
| Older Antibiotics | |||
|
| |||
| Vancomycin | Staphylococcus aureus | 2 | 2 |
| Coagulase-negative staphylococci | 4 | 4 | |
| Enterococcus spp. | 4 | 4 | |
| Streptococcus groups A, B, C, and G | 2 | 2 | |
| Streptococcus pneumoniae | 2 | 2 | |
| Viridans group streptococci | 2 | 2 | |
| Gram-positive anaerobes except Clostridium difficile | 2 | 2 | |
| C. difficile | 2 | 2 | |
| Corynebacterium spp. | 2 | 2 | |
| Aerococcus sanguinicola and urinae | 1 | 1 | |
|
| |||
| Amoxicillin/ clavulanic acid | Enterococcus spp. | 4 | 8 |
| Viridans group streptococci | 0.5 | 2 | |
| Haemophilus influenzae | 2 | 2 | |
| Neisseria meningitidis | 0.125 | 1 | |
| Gram-positive anaerobes except C. difficile | 4 | 8 | |
| Gram-negative anaerobes | 0.5 | 2 | |
| Helicobacter pylori | 0.125 | 0.125 | |
| Pasteurella multocida | 1 | 1 | |
| Kingella kingae | 0.125 | 0.125 | |
| Enterobacteriaceae | 8 | 8 | |
| Enterococcus spp. | 4 | 8 | |
|
| |||
| Clindamycin | Staphylococcus spp. | 0.25 | 0.5 |
| Streptococcus groups A, B, C, and G | 0.5 | 0.5 | |
| S. pneumoniae | 0.5 | 0.5 | |
| Viridans group streptococci | 0.5 | 0.5 | |
| Gram-positive anaerobes except C. difficile | 4 | 4 | |
| Gram-negative anaerobes | 4 | 4 | |
| Corynebacterium spp. | 0.5 | 0.5 | |
|
| |||
| Teicoplanin | Enterococcus spp. | 2 | 2 |
| Streptococcus groups A, B, C, and G | 2 | 2 | |
| S. pneumoniae | 2 | 2 | |
| Viridans group streptococci | 2 | 2 | |
| Staphylococcus spp. | 2 | 2 | |
| Coagulase-negative staphylococci | 4 | 4 | |
|
| |||
| Newer antibiotics** | |||
|
| |||
| Linezolid | Staphylococcus spp. | 4 | 4 |
| Enterococcus spp. | 4 | 4 | |
| Streptococcus groups A, B, C, and G | 2 | 4 | |
| S. pneumoniae | 2 | 4 | |
| Corynebacterium spp. | 2 | 2 | |
|
| |||
| Daptomycin | Streptococcus groups A, B, C, and G | 1 | 1 |
| Staphylococcus spp. | 1 | 1 | |
|
| |||
| Tigecycline | Enterobacteriaceae | 1 | 2 |
| Staphylococcus spp. | 0.5 | 0.5 | |
| Enterococcus spp. | 0.25 | 0.5 | |
| Streptococcus groups A, B, C, and G | 0.25 | 0.5 | |
|
| |||
| Ceftaroline | S. pneumoniae | 0.25 | 0.25 |
| Haemophilus influenzae | 0.03 | 0.03 | |
| Enterobacteriaceae | 0.5 | 0.5 | |
| Staphylococcus spp., S. aureus | 1 | 1 | |
|
| |||
| Tedizolid | Staphylococcus spp. | 0.5 | 0.5 |
| Streptococcus groups A, B, C, and G | |||
| Viridans group streptococci (S. anginosus group) | 0.25 | 0.25 | |
|
| |||
| Dalbavancin* | Viridans group streptococci (S. anginosus group) | 0.125 | 0.125 |
| Staphylococcus spp. | 0.125 | 0.125 | |
| Streptococcus groups A, B, C, and G | 0.125 | 0.125 | |
|
| |||
| Oritavancin* | Viridans group streptococci (S. anginosus group) | 0.25 | 0.25 |
| Staphylococcus spp. (S. aureus) | 0.125 | 0.125 | |
| Streptococcus groups A, B, C, and G | 0.25 | 0.25 | |
Notes:
Dalbavancin and oritavancin are not approved in Singapore.
“Newer antibiotics” refers to antibiotics which are approved by US FDA from 2000 onward. These data have been produced in part under ECDC service contracts and made available by EUCAST at no cost to the user and can be accessed on the EUCAST website www.eucast.org. EUCAST recommendations are frequently updated and the latest versions are available at www.eucast.org.
Older antibiotics
Vancomycin is effective against susceptible Gram-positive bacteria including MRSA,53 and has shown comparable efficacy to newer agents (linezolid, daptomycin, tigecycline); however, poor tissue penetration (8%–10%) lowers its efficacy in severe cases of cSSTIs53 (Table 4). The risk of nephrotoxicity and complications necessitates the need for dose titration in the special population with impaired renal function.54 Clindamycin, active against MRSA, has good tissue penetration (97%) and tolerability profile, and the ability to inhibit production of bacterial toxins common in CA-MRSA infection.27 However, development of Clostridium difficile-associated colitis requires monitoring during therapy.55 The use of penicillin derivatives and β-lactamase inhibitors (cloxacillin, amoxicillin/clavulanic acid) is discouraged in most cSSTIs cases due to lack of efficacy against MRSA infections. Also, fosfomycin and quinupristin/dalfopristin for cSSTIs use are challenged due to safety concerns of hepatic dysfunction, severe myalgia, and rapid emergence of resistance.53,56 Inadequate evidence from controlled studies demonstrating efficacy and safety of older antibiotics for cSSTI also limit their clinical use.53 With the advent of life-threatening MRSA and CA-cSSTIs, streamlining the use of available and effective antimicrobi-als becomes obligatory. Several national and international treatment guidelines have revisited the evidence to provide recommendations for antimicrobial therapy (Table 2).35
Table 4.
General features of older and newer antibiotics with activity against MRSA
| Antibiotic | Dosage | Route of administration | Mode of action | Tissue penetration | Use in special population | Strengths | Limitations |
|---|---|---|---|---|---|---|---|
| Older antibiotics | |||||||
|
| |||||||
| Vancomycin53 | 500 mg iv infusion for 60 minutes every 6 hours or 1 g iv infusion for 100 minutes every 12 hours | Intravenous | Bactericidal glycopeptide | 8%–10% | Dose titration in patients with renal impairment | Gold standard for MRSA infections. Established efficacy and tolerability |
Emerging resistance. Increasing MIC Poor tissue penetration |
| Clindamycin53 | IV: 0.6–2.7 g infusion for 10–60 minute daily in 2–4 divided doses Oral: 150–300 mg every 6 hours, up to 450 mg in severe infections |
Intravenous/oral | Bacteriostatic | 95% | No dose adjustment in patients with renal impairment | Preferred agent for necrotizing fasciitis as it blocks bacterial toxin production | Emerging resistance Clostridium difficile-associated colitis |
| Cloxacillin122 | Oral: 0.5 g every 6 hours IV: 1–2 g every 6 hours 2 g every 4 hours in case of severe infections | Oral/intravenous | Bactericidal | NA | No dose adjustment in patients with renal impairment | Main stream agent of treating methicillin-susceptible Staphylococcus aureus (MSSA) | Lack of efficacy against MRSA infections |
|
| |||||||
| Newer antibiotics | |||||||
|
| |||||||
| Linezolid123 | 600 mg IV or orally every 12 hours | Intravenous/oral | Bacteriostatic | 105% | No dose adjustment in patients with renal impairment | Bioavailable as oral formulation Preferred agent for necrotizing fasciitis as it inhibits bacterial toxin production | Risk of toxicity with prolonged use Oral switch |
| Daptomycin124 | 4–6 mg/kg every 24 or 48 hours | Intravenous | Bactericidal | 68% | No dose adjustment in patients with renal impairment | Once-daily iv regimen suitable for outpatient use | Emerging resistance |
| Tigecycline125 | 50 or 100 mg every 12 hours | Intravenous | Bacteriostatic | 91% | No dose adjustment in patients with renal impairment | Broad spectrum activity against multidrug resistant Gram- positive and Gram-negative pathogens | Low serum levels |
| Ceftaroline126 | 600 mg iv infusion for 60 minutes every 12 hours | Intravenous | Bactericidal | NA | Dose titration in patients with renal impairment. No dose adjustments in obese patients | Good clinical efficacy and tolerability. Low propensity for C. difficile- related diarrhea | Low activity against Gram negative pathogens. Bid or tid dosing |
| Tedizolid127 | 200 mg iv infusion for 60 minutes, once daily Oral: 200 mg once daily |
Intravenous/ oral | Bacteriostatic | NA | No dose adjustment in patients with renal impairment | Bioavailable as oral formulation Preferred agents for necrotizing fasciitis as it inhibits bacterial toxin production |
Lower risk of myelotoxicity and drug–drug interactions. Oral switch |
| Dalbavancin68* | 1,000 mg iv infusion for 30 minutes | Intravenous | Bactericidal | NA | Dose titration in patients with renal impairment | Long half-life allowing once-daily iv administration suitable for outpatient use | Low activity against Gram negative pathogens. Cannot be cleared by hemodialysis in case of toxicity Early discharge |
| Oritavancin68* | 1,200 mg single dose iv infusion for over 3 hours | Intravenous | Bactericidal | NA | Dose titration in patients with renal impairment | Long half-life allowing once-daily iv administration | Low activity against Gram negative pathogens |
Notes: Dosage approved in Singapore mentioned in the table, except for dalbavancin and oritavancin.
Dalbavancin and oritavancin are not approved in Singapore.
Abbreviations: MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; NA, not available.
Newer antibiotics
Newer antibiotics (refers to antibiotics which are approved by US FDA from 2000 onward) provide clinicians good opportunities to overcome current challenges in the management and treatment of MRSA infections (Table 4).
Linezolid, an oxazolidinone, is a newer alternative to glycopeptide antimicrobials, approved for use in serious cSSTIs caused by MRSA and VRE (US FDA approval: April 2000). It also inhibits bacterial exotoxins production that aggravates the severity of CA-MRSA infections.27 Linezolid has demonstrated high clinical cure rates and non-inferiority to oxacillin/dicloxacillin in the treatment of cSSTIs, including cellulitis, skin abscesses, erysipelas, and surgical infections.57 Linezolid also showed higher cure rates than vancomycin (67% vs 62%) against suspected MRSA-associated cSSTIs, and more recently, has demonstrated superior clinical and microbiological outcomes vs vancomycin.58–60 In addition, equivalent oral bioavailability of linezolid offers a pragmatic advantage that facilitates shorter iv therapy and early hospital discharge.60 Several studies have shown linezolid to be safe; however, in post-marketing studies, prolonged use of linezolid was associated with neuropathy (peripheral or optical), hematological abnormalities (particularly thrombocytopenia or anemia), and hyperlactatemia.61
Daptomycin, a cyclic lipopeptide approved for use in cSSTIs has shown adequate clinical activity against MRSA and VRE (US FDA approval: December 2003). Real-world data collected across wide geographical regions (US, Europe, Latin America, and Asia) demonstrated high clinical success rates (81%) with daptomycin for cSSTIs.62 Daptomycin showed non-inferiority to vancomycin and semi-synthetic penicillins in the treatment of cSSTIs.63 In an open-label study, 77% of 53 cSSTIs patients treated with daptomycin achieved complete resolution of infection (vs 42% of 221 patients on vancomycin) with rapid symptom resolution in a shorter inpatient treatment course.64 The overall efficacy and safety, with the practical advantage of a once-daily intravenous (iv) regimen, supports the use of daptomycin in cSSTIs.65,66 Overall, daptomycin has a good safety profile; however, clinical studies have reported elevations in creatine phosphokinase and associated muscle toxicity. Therefore, monitoring of laboratory abnormalities is recommended during treatment.67
Tigecycline, a tetracycline-related antimicrobial, is an advantageous empiric treatment option for cSSTIs (US FDA approval: June 2005). In 2013, the US FDA issued a black box warning of increased risk of mortality of tigecycline vs comparator drugs. However, tigecycline may likely be considered instead of combination therapy, where this is deemed necessary, due to its broad-spectrum activity (Table 4).68
Ceftaroline, a new generation cephalosporin with clinically meaningful activity against MRSA, is approved for the treatment of cSSTIs and community-acquired pneumonia (US FDA approval: October 2010). Over 90% of MRSA isolates were found to be susceptible to ceftaroline in US, Europe, Middle East, and Africa with MICs ranging from 0.25 to 4 mg/L. Ceftaroline has also shown potent antimicrobial activity against a large number of SSTI-associated bacterial isolates including MRSA (80.6% susceptibility) from South Africa and Asia-Pacific region.69 The pharmacodynamic profile of ceftaroline 600 mg every 8 hours (q8h) and every 12 hours (q12h) infusion was generally comparable and is adequate for the effective eradication of MRSA. The q8h infusion treatment had a higher probability of attaining MIC of 4 mg/L; however, this treatment approach has limited evidence from clinical studies and is yet to receive approval in this region. The safety profile of ceftaroline was consistent with cephalosporin class. The most common adverse events observed were diarrhea, nausea, and rash.70 Administration of ceftaroline demonstrated no significant effect on the intestinal flora in healthy participants, and thus, risk of C. difficile-related diarrhea is low.71 Ceftaroline (q12 dosing schedule) is a viable option in the armamentarium of antibiotics for treating cSSTIs due to Gram-negative and Gram-positive (with MRSA) infections or following failure of vancomycin therapy (Table 4).72 Studies with a q8h dosing of ceftaroline are ongoing; if the findings are conducive, this dosing may become a better alternative than the standard dose, especially in areas of high MRSA endemicity.
Tedizolid, another novel oxazolidinone is approved for use in ABSSSI due to MRSA (US FDA approval: June 2014).73 In two Phase III studies of ABSSSI, a 6-day course with tedizolid was significantly non-inferior to a 10-day linezolid course for an early clinical response (48–72 hour after treatment initiation).74 A better tolerability profile (few gastrointestinal adverse events, lower myelotoxicity risk) and an option of iv-to-oral switch make tedizolid a valuable treatment choice for management of cSSTIs.74 The oxazolidinones are particularly helpful for the treatment of necrotizing fasciitis since they inhibit the production of bacterial pyrogenic endotoxins.75
Oritavancin and dalbavancin are lipoglycopeptides (approved by US FDA in 2014) with the pharmacokinetic advantage of extended plasma half-life, enabling single-dose regimens.68 These antibiotics are yet to receive approval in Singapore. Oritavancin, a derivative of vancomycin, has extended bactericidal activity against MRSA and daptomycin non-susceptible VRE. Single iv dose, oritavancin, was non-inferior to twice-daily vancomycin (7-to-10 day course) in two Phase III studies of ABSSSI caused by Gram-positive pathogens.76,77 Dalbavancin, a derivative of a teicoplanin-like natural antibiotic has antimicrobial activity against almost all clinical MRSA isolates. Once-weekly iv dalbavancin was non-inferior to twice-daily vancomycin followed by oral linezolid with comparable success rates in ABSSSI as demonstrated in a pooled analysis.78 Dalbavancin and oritavancin has a similar safety profile as vancomycin. In clinical studies, the most commonly reported adverse events were headache, nausea, diarrhea, and vomiting.79 The elimination of multidose and multiday regimen and lower propensity for resistance development are potential benefits of dalbavancin and oritavancin treatments. However, the absence of de-escalation, high-cost acquisition, and limited clinical evidence for these agents is noteworthy.68
Intravenous-to-oral switch
For the rapid attainment of desired serum therapeutic levels, parenteral antibacterial therapy is recommended for cSSTIs in hospitalized or ambulatory care settings.34 Oral formulations with convenient dosing and administration at home offer effective outpatient management, allow a seamless transition of continued care, and recovery. Potent antibiotics with high oral bioavailability offer an additional advantage without compromising clinical efficacy (eg, linezolid, tedizolid). The key advantage of iv-to-oral switch is early hospital discharge, which from a physician’s perspective lowers risks of HA complications and infections.35,80 From a patient’s perspective it results in reduced isolation, early recovery, and improved quality-of-life.35,81 The critical decision of switching a patient from iv-to-oral therapy depends on the patient’s ability to tolerate the switch and on the microbiological etiology.82 Afebrile (>24 hours) patients with clinically stable infection, normalized WBC count (<4×109/L or >12×109/L), no cardiovascular abnormality (no tachycardia and systolic blood pressure ≥100 mgHg) and who have received iv antibiotic therapy for >24 hours can be switched to oral therapy. Patients should be able to tolerate oral fluids or diet to allow administration of oral medications with no gastrointestinal complications.35,83 The switch to oral therapy is contraindicated in patients with severe vomiting and diarrhea, hematological malignancies or neutropenia, impaired gastrointestinal absorption, dementia, and severe infections of the musculoskeletal, central nervous system, or vascular systems.35,83 Presence of comorbidities, unavailability of caregiver, and an advancing age may commonly delay oral switch and hospital discharge.35
De-escalation strategy
Antibiotic de-escalation is a scheme of utilizing a discreet antibiotic regimen to avoid indiscriminate antibiotic use that increases the risk of resistance.84 The de-escalation strategy can be implemented by switching from empirical broad-spectrum antimicrobials to a narrower-spectrum or targeted treatment after a systematic reassessment.85 Currently, the acceptable approach is the timely initiation of antibiotic therapy that provides appropriate coverage for key pathogens including resistant strains, followed by a de-escalation approach on the availability of susceptibility results to avoid prolonged exposure to a broader-spectrum antibiotic therapy.84 Available evidence indicates that antibiotic deescalation has no safety concerns, showed improved clinical outcomes (based on microbiological data, novel inflammatory markers), lesser antibiotic resistance profiles, and lowered chances of recurrent infections along with substantial reductions in adverse effects of non-judicious use of antibiotics.86,87
The de-escalation strategy aims to reduce costs and duration of hospitalization by switching from empiric antibiotics to definitive culture-directed agents, curbing unnecessary or redundant treatment, and switching from iv to oral therapy. This switch to oral therapy also results in direct cost savings (supplies, nursing time, cost-effective drug), lowers risk of line infections (via the catheters), and increases the patient’s mobility, thereby reducing the duration of hospital stay.88 Protocols that guide transitioning to more effective anti-microbials with reduced toxicity (eg, β-lactams instead of vancomycin for confirmed MSSA) may ease selection pressures and help achieve appropriate and safe de-escalations for critical infections.89 The duration of antibiotic use (less by 2 days) and the length of stay were significantly reduced in patients with ABSSSIs subjected to Antimicrobial Stewardship Program (ASP)-based intervention. The reduction in length of hospital stay culminated into a substantial cost reduction.90
Development of C. difficile-associated diarrhea following exposure to most antibiotics is a more predominant public health problem as compared with antimicrobial resistance.91 Therefore, another critical target for ASP should be preventing infection or restricting nosocomial infections with C difficile in susceptible individuals. The incidence of C. difficile infections can be considerably lowered by optimizing the selection of antibiotics (eg, possibly substitution with newer cephalosporins like ceftaroline), dosing, de-escalation, and duration of therapy.88,91
Antimicrobial stewardship program
ASP is a strategy to improve the current antibiotic prescription practice through education of prescribers, development of antimicrobial formulary, and review-feedback process of the prescription pattern for prescribers.92,93 Excessive or indiscriminate use of antibiotics, particularly the broad-spectrum antibiotics, is associated with the emergence of antibiotic-resistant bacteria such as ESBL-producing Gram-negative bacteria, MRSA, and development of C. difficile infections. A multifaceted strategy is required to combat the antimicrobial resistance. ASP, a multidisciplinary approach, encourages various measures, including optimization of prescribing practice. It mainly aims to improve the clinical outcomes of antibiotic use, minimize the adverse effects, and impede the development of antimicrobial resistance and C. difficile infections.94 Several countries have successfully adopted ASP in hospital settings based on the WHO Global Action Plan (mandated in May 2015) to tackle antimicrobial resistance.95 ASPs implemented by the National Health Service (NHS) across the UK utilizes “Start Smart-Then Focus” approach to improve prudent use of antibiotics.94,96 It comprises several strategies, including implementation of evidence-based local prescribing guidelines relevant to local health care setting and antibiotic resistance pattern, iv-to-oral switch, de-escalation, dose optimization, quality assurance audits, education, and training.96 These strategies have substantially supported the improvement in discreet antibiotic usage, reduced length of hospital stay, and improved clinical outcomes, such as reductions in C. difficile infections.97,98 Hospital settings in Singapore employ a similar ASP model of iv-to-oral switch and de-escalation. A 3-year retrospective review from Singapore General Hospital revealed that patients with ABSSSI had an acceptance rate of 66% for the interventions recommended by the ASP and these interventions caused no safety concerns.99 Nevertheless, to improve the overall success in hospital stays and for patient safety, regular review and upgrading of ASPs with a holistic health care approach is advisable.
Nursing and postoperative wound management
Optimal management of wounds is critical to prevent potential complications in postoperative surgical sites or dehiscence of the surgical wounds and improve recovery with appropriate functional and esthetic results in patients with cSSTIs.100 Wound dressings aid wound healing by maintaining a moist environment (accentuates wound re-epithelization and healing), provide a barrier against bacterial or fluid contamination, and help in the removal of excessive exudate (by absorbing, gelling, and transfer of fluid away from the wound bed), preventing likely wound maceration (Figure 2).100,101
Figure 2.
Nursing and postoperative care in complicated skin and soft tissue infections.
Advanced therapies in the wound management such as silver compound dressing and negative wound pressure treatment may improve the wound care by decreasing the chances of reinfection and help in the removal of exudate, reduction of lateral incision tension, and reduction of hematoma or seroma formation.43,102–104 Silver and silver compounds have broad-spectrum bactericidal properties and have been used effectively as wound dressings.105 Sustained-release silver dressings, silver-donating nanocrystalline dressings, and antimicrobial negative-pressure dressing (NPD) with silver have proven to be effective in promoting wound healings.106–108 Use of silver-based dressing in packing abscess cavities after incision and drainage treatment may cause faster healing along with improvements in pain intensity.109 Silver dressings have proven to be safe and effective as compared to normal gauze dressings in preventing postoperative surgical site infections.106,110
Physical methods of exudate control in infected post-surgical wounds such as negative wound pressure therapy or a combination of a silver antimicrobial NPD have demonstrated to improve healing outcomes along with decreased nursing time and reduced cost.41,111,112 In cases of minor dehiscence, secondary closure of wounds following removal of necrotic tissue must be opted. For severe dehiscence, TNP therapy following local debridement is recommended.100 Selection of appropriate methods of cleansing and dressing along with prompt intervention of wound complications holds the key to effective wound management.
Future directions
A myriad of antibiotics is already available and a number of novel ones have been or are being developed for the management of cSSTIs. Clear differentiation, in terms of therapy outcomes, among these newer agents is hampered by the lack of robust clinical data on head-to-head comparison between these antibiotics. Although newer antibiotics may confer several advantages over older agents, they are in general expensive and less accessible. Furthermore, data regarding their usage in special populations, eg, diabetic foot infections, peripheral vascular disease, etc, is inadequate.113 Special indications for these newer agents such as oxazolidinones can be regarded as preferred agents for necrotizing fasciitis because they inhibit bacterial toxin production.75 Ceftaroline offers additional advantages with possible lower risk for C. difficile infection. Additionally, there are emerging data on combination with older antibiotics to achieve synergistic efficacy and cost-effectiveness in therapy. Studies exploring vancomycin and beta-lactam combinations for MRSA have demonstrated shortening of the duration of MRSA bacteremia.114,115 Nonetheless, larger sample size, double-blind, adequately powered future trials with clinically relevant end points for newer antibiotics and newer combinations of antibiotics for cSSTIs are required to fill the information gaps for clinicians to augment decision making.116 Real-world evidence would further add to the information on safety, efficacy, and cost-effectiveness of these therapies for cSSTI management.117 In short, a prudent clinical decision on the selection of the most appropriate pharmacological agent or agents would be based on their efficacy, safety profile, tolerability, cost-effectiveness, accessibility, and practicability.
Conclusion
In the light of epidemiological shift in global prevalence of MRSA and associated cSSTIs, clinicians need to constantly challenge and review the existing clinical guidelines and practices. With the emergence of newer antibiotics and current medical evidence in clinical practice, optimal clinical usage needs to be determined. Decision on the choice of antimicrobial therapy for cSSTI is guided by both scientific data and clinical experience. Multiple factors, including host factors, local multidrug resistance data, cost consideration, and drug characteristics, have to be considered holistically in the management of cSSTIs. Often, the outcomes, despite prudent clinical judgment, remain unpredictable. Simply, other factors at play that may not be within the clinician’s scope of knowledge could also impact treatment outcomes. Local ASP strategies in health care institutions would guide clinicians in early initiation of specific treatments to combat region-specific CA-MRSA infections. Hence, adopting well-tested approaches such as iv-to-oral switch, regular review of clinical response, de-escalation to narrow-spectrum antibiotics to reduce the length of hospital stay, hospital-related complications, and overall treatment expenses can modify the paradigm and the clinical outcomes for management of cSSTIs in countries like Singapore.
Acknowledgments
This paper was compiled based on discussions during an expert meeting that convened in Singapore on 18th July 2017 and was attended by the authors (sponsored by Pfizer). The content reflects the opinion of the authors with editorial support from Ramji Narayanan from SIRO Clinpharm Pvt Ltd and See Mee Yen from Medica Comms Pte Ltd and funded by Pfizer, Singapore. None of the authors received any honorarium for the preparation of the manuscript.
Footnotes
Disclosure
HW has received consulting fees from Abbott Laboratories, Actelion, Antabio, AiCuris, Astellas, Astra-Zeneca, Bayer, Biomèrieux, Cambimune, Cerexa, Da Volterra, The European Tissue Symposium, Ferring, The Medicines Company, MedImmune, Menarini, Merck, Meridian, Motif Biosciences, Nabriva, Paratek, Pfizer, Qiagen, Roche, Surface Skins, Sanofi-Pasteur, Seres, Summit, Synthetic Biologics and Valneva; lecture fees from Abbott, Alere, Allergan, Astellas, Astra-Zeneca, Merck, Pfizer, Roche & Seres; grant support from Abbott, Actelion, Astellas, Biomèrieux, Cubist, Da Volterra, MicroPharm, Morphochem AG, Sanofi-Pasteur, Seres, Spero, Summit and The European Tissue Symposium, Merck. The authors report no other conflicts of interest in this work.
References
- 1.Rajan S. Skin and soft-tissue infections: classifying and treating a spectrum. Cleve Clin J Med. 2012;79(1):57–66. doi: 10.3949/ccjm.79a.11044. [DOI] [PubMed] [Google Scholar]
- 2.Merlino JI, Malangoni MA. Complicated skin and soft-tissue infections: diagnostic approach and empiric treatment options. Cleve Clin J Med. 2007;74(Suppl_4):S21–28. doi: 10.3949/ccjm.74.suppl_4.s21. [DOI] [PubMed] [Google Scholar]
- 3.Food US, Administration Drug. Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. US Food and Drug Administration; Washington, DC: [Accessed September 20, 2017]. http://www.fda.gov/downloads/Drugs/./Guidances/ucm071185.pdf. [Google Scholar]
- 4.Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):109–115. doi: 10.1097/QCO.0000000000000239. Accessed September 20, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Loewen K, Schreiber Y, Kirlew M, et al. Community-associated methicillin-resistant Staphylococcus aureus infection: literature review and clinical update. Can Fam Physician. 2017;63(7):512–520. [PMC free article] [PubMed] [Google Scholar]
- 6.King MD, Humphrey BJ, Wang YF, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144(5):309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Dryden M, Andrasevic AT, Bassetti M, et al. Managing skin and soft-tissue infection and nosocomial pneumonia caused by MRSA: a 2014 follow-up survey. Int J Antimicrob Agents. 2015;45(Suppl 1):S1–S14. doi: 10.1016/S0924-8579(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20(7):605–623. doi: 10.1111/1469-0691.12705. [DOI] [PubMed] [Google Scholar]
- 9.Song J-H, Hsueh P-R, Chung DR, et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011;66(5):1061–1069. doi: 10.1093/jac/dkr024. [DOI] [PubMed] [Google Scholar]
- 10.Hersh AL, et al. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168(14):1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 11.Ray GT, Suaya JA, Incidence BR. microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis. 2013;13:252. doi: 10.1186/1471-2334-13-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou Y-H, Lee M-S, Lin R-Y, Wu C-Y, et al. Risk factors for methicillin-resistant Staphylococcus aureus skin and soft-tissue infections in outpatients in Taiwan. Epidemiol Infect. 2015;143(04):749–753. doi: 10.1017/S0950268814001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Okamura S, Miura Y, Koyama S, Yanagisawa H, Matsu-moto T. Molecular characterization of community-associated methicillin-resistant Staphylococcus aureus isolated from skin and pus samples of outpatients in Japan. Microb Drug Resist. 2015;21(4):441–447. doi: 10.1089/mdr.2014.0153. [DOI] [PubMed] [Google Scholar]
- 14.Pl H, Chuang SK, Choi YF, et al. Community-associated methicillin-resistant and methicillin-sensitive Staphylococcus aureus: skin and soft tissue infections in Hong Kong. Diagn Microbiol Infect Dis. 2008;61(3):245–250. doi: 10.1016/j.diagmicrobio.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Noh JY, Cheong HJ, Song JY, et al. Skin and soft tissue infections: experience over a five-year period and clinical usefulness of ultrasonography-guided gun biopsy-based culture. Scand J Infect Dis. 2011;43(11–12):870–876. doi: 10.3109/00365548.2011.600324. [DOI] [PubMed] [Google Scholar]
- 16.Phakade RS, Nataraj G, Kuyare SS, et al. Is methicillin-resistant Staphylococcus aureus involved in community acquired skin and soft tissue infections? Experience from a tertiary care centre in Mumbai. J Postgrad Med. Jan. 2012;58(1):3–7. doi: 10.4103/0022-3859.93245. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Chen Y, Gao W, et al. Epidemiology and outcomes of complicated skin and soft tissue infections among inpatients in Southern China from 2008 to 2013. PLoS One. 2016;11(2):e0149960. doi: 10.1371/journal.pone.0149960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu L-Y, Tan T-Y, Jureen R, et al. Antimicrobial drug resistance in Singapore hospitals. Emerg Infect Dis. 2007;13(12):1944–1947. doi: 10.3201/eid1312.070299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moet GJ, Jones RN, Biedenbach DJ, et al. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004) Diagn Microbiol Infect Dis. 2007;57(1):7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Venkatachalam I, Tee NW, et al. Prevalence of healthcare-associated infections and antimicrobial use among adult inpatients in Singapore acute-care hospitals: results from the first national point prevalence survey. Clin Infect Dis. 2017;64(Suppl_2):S61–S67. doi: 10.1093/cid/cix103. [DOI] [PubMed] [Google Scholar]
- 21.Chan KS, Ling ML, Hsu LY, Tan AL. Methicillin-resistant Staphylococcus aureus throat colonization among healthcare workers during an outbreak in Singapore General Hospital. Infect Control Hosp Epidemiol. 2009;30(1):95–97. doi: 10.1086/593123. [DOI] [PubMed] [Google Scholar]
- 22.Hsu L-Y, Loomba-Chlebicka N, Koh Y-L, et al. Evolving EMRSA-15 epidemic in Singapore hospitals. J Med Microbiol. 2007;56(3):376–379. doi: 10.1099/jmm.0.46950-0. [DOI] [PubMed] [Google Scholar]
- 23.Win M-K, Soliman TAA, Lee LK, et al. Review of a two-year methicillin-resistant Staphylococcus aureus screening program and cost-effectiveness analysis in Singapore. BMC Infect Dis. 2015;15(1):391. doi: 10.1186/s12879-015-1131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turina M, Cheadle WG. Clinical challenges and unmet needs in the management of complicated skin and skin structure, and soft tissue infections. Surg Infect. 2005;6(s2):s-23–20. [PubMed] [Google Scholar]
- 25.Peterson LR. A review of tigecycline – the first glycylcycline. Int J Antimicrob Agents. 2008;32(Suppl 4):S215–S222. doi: 10.1016/S0924-8579(09)70005-6. [DOI] [PubMed] [Google Scholar]
- 26.Lipsky BA, Tabak YP, Johannes RS, et al. Skin and soft tissue infections in hospitalised patients with diabetes: culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia. 2010;53(5):914–923. doi: 10.1007/s00125-010-1672-5. [DOI] [PubMed] [Google Scholar]
- 27.Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother. 2010;65(Suppl 3):iii35–iii44. doi: 10.1093/jac/dkq302. [DOI] [PubMed] [Google Scholar]
- 28.Stenstrom R, Grafstein E, Romney M, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus skin and soft tissue infection in a Canadian emergency department. CJEM. 2009;11(05):430–438. doi: 10.1017/s1481803500011623. [DOI] [PubMed] [Google Scholar]
- 29.Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27(1):1–7. doi: 10.1097/INF.0b013e31815819bb. [DOI] [PubMed] [Google Scholar]
- 30.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44(6):1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 32.Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007;44(4):471–482. doi: 10.1086/511033. [DOI] [PubMed] [Google Scholar]
- 33.D’Agata EM, Webb GF, Horn MA, Moellering RC, Ruan S. Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals. Clin Infect Dis. 2009;48(3):274–284. doi: 10.1086/595844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eron LJ, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(90001):i3–i17. doi: 10.1093/jac/dkg466. [DOI] [PubMed] [Google Scholar]
- 35.Bassetti M, Baguneid M, Bouza E, Dryden M, Nathwani D, Wilcox M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20(Suppl 4):3–18. doi: 10.1111/1469-0691.12463. [DOI] [PubMed] [Google Scholar]
- 36.Suaya JA, Eisenberg DF, Fang C, Miller LG, et al. Skin and soft tissue infections and associated complications among commercially insured patients aged 0–64 years with and without diabetes in the U.S. PLoS One. 2013;8(4):e60057. doi: 10.1371/journal.pone.0060057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zilberberg MD, Chaudhari P, Nathanson BH, et al. Development and validation of a bedside risk score for MRSA among patients hospitalized with complicated skin and skin structure infections. BMC Infect Dis. 2012;12(1):154. doi: 10.1186/1471-2334-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enoch S, Price P. Should alternative endpoints be considered to evaluate outcomes in chronic recalcitrant wounds. World Wide Wounds. 2004. [Accessed September 20, 2017]. Available from: http://www.worldwidewounds.com/2004/october/Enoch-Part2/Alternative-Enpoints-To-Healing.html.
- 39.Napolitano LM. Severe soft tissue ifections. Infect Dis Clin North Am. 2009;23(3):571–591. doi: 10.1016/j.idc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 41.Pan A, Cauda R, Concia E, et al. Consensus document on controversial issues in the treatment of complicated skin and skin-structure infections. Int J Infect Dis. 2010;14(Suppl 4):S39–S53. doi: 10.1016/j.ijid.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Kujath P, Kujath C. Complicated skin structure and soft tissue infections – are we threatened by multi-resistant pathogens? Eur J Med Res. 2010;15(12):544–553. doi: 10.1186/2047-783X-15-12-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Institute for Health and Clinical Excellence Surgical site infection prevention and treatment of surgical site infection. 2013. [Accessed September 20, 2017]. Available from: https://www.nice.org.uk/guidance/qs49/resources/surgical-site-infection-2098675107781.
- 44.Figtree M, Konecny P, Jennings Z, et al. Risk stratification and outcome of cellulitis admitted to hospital. J Infect. 2010;60(6):431–439. doi: 10.1016/j.jinf.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Ruhe JJ, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777–784. doi: 10.1086/511872. [DOI] [PubMed] [Google Scholar]
- 46.Edelsberg J, Berger A, Weber DJ, Mallick R, Kuznik A, Oster G. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160–169. doi: 10.1086/526444. [DOI] [PubMed] [Google Scholar]
- 47.Zervos MJ, Freeman K, Vo L, et al. Epidemiology and outcomes of complicated skin and soft tissue infections in hospitalized patients. J Clin Microbiol. 2012;50(2):238–245. doi: 10.1128/JCM.05817-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fish DN. Meropenem in the treatment of complicated skin and soft tissue infections. Ther Clin Risk Manag. 2006;2(4):401–415. doi: 10.2147/tcrm.2006.2.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frewin DB, Bartholomeusz RCA, Gaffney RD, et al. A comparison of the effect of lisinopril and hydrochlorothiazide on electrolyte balance in essential hypertension. Eur J Clin Pharmacol. 1992;42(5):487–490. doi: 10.1007/BF00314855. [DOI] [PubMed] [Google Scholar]
- 50.Joukhadar C, Klein N, Frossard M, et al. Angioplasty increases target site concentrations of ciprofloxacin in patients with peripheral arterial occlusive disease. Clin Pharmacol Ther. 2001;70(6):532–539. doi: 10.1067/mcp.2001.120762. [DOI] [PubMed] [Google Scholar]
- 51.Gee T, Ellis R, Marshall G, et al. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob Agents Chemother. 2001;45(6):1843–1846. doi: 10.1128/AAC.45.6.1843-1846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skhirtladze K, Hutschala D, Fleck T, et al. Impaired target site penetration of vancomycin in diabetic patients following cardiac surgery. Antimicrob Agents Chemother. 2006;50(4):1372–1375. doi: 10.1128/AAC.50.4.1372-1375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckmann C, Dryden M. Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res. 2010;15(12):554–563. doi: 10.1186/2047-783X-15-12-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hermsen ED, Hanson M, Sankaranarayanan J, et al. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9–14. doi: 10.1517/14740330903413514. [DOI] [PubMed] [Google Scholar]
- 55.Hyun DY, Mason EO, Forbes A, et al. Trimethoprim-sulfamethoxazole or clindamycin for treatment of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2009;28(1):57–59. doi: 10.1097/INF.0b013e3181826e5e. [DOI] [PubMed] [Google Scholar]
- 56.Falagas ME, Roussos N, Gkegkes ID, et al. Fosfomycin for the treatment of infections caused by Gram-positive cocci with advanced antimicrobial drug resistance: a review of microbiological, animal and clinical studies. Expert Opin Investig Drugs. 2009;18(7):921–944. doi: 10.1517/13543780902967624. [DOI] [PubMed] [Google Scholar]
- 57.Stevens DL, Smith LG, Bruss JB, et al. Randomized comparison of linezolid (PNU-100766) versus oxacillin-dicloxacillin for treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2000;44(12):3408–3413. doi: 10.1128/aac.44.12.3408-3413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481–1490. doi: 10.1086/340353. [DOI] [PubMed] [Google Scholar]
- 59.Bounthavong M, Hsu DI. Efficacy and safety of linezolid in methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infection (cSSTI): a meta-analysis. Curr Med Res Opin. 2010;26(2):407–421. doi: 10.1185/03007990903454912. [DOI] [PubMed] [Google Scholar]
- 60.Itani KM, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2010;199(6):804–816. doi: 10.1016/j.amjsurg.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 61.Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59(Suppl 1):S59–S74. doi: 10.1016/S0163-4453(09)60009-8. [DOI] [PubMed] [Google Scholar]
- 62.Seaton RA, Gonzalez-Ruiz A, Cleveland KO, et al. Real-world daptomycin use across wide geographical regions: results from a pooled analysis of CORE and EU-CORE. Ann Clin Microbiol Antimicrob. 2016;15(1):18. doi: 10.1186/s12941-016-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nathwani D. New antibiotics for the management of complicated skin and soft tissue infections: are they any better? Int J Antimicrob Agents. 2009;34(Suppl 1):S24–S29. doi: 10.1016/S0924-8579(09)70546-1. [DOI] [PubMed] [Google Scholar]
- 64.Davis SL, Mckinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611–1618. doi: 10.1592/phco.27.12.1611. [DOI] [PubMed] [Google Scholar]
- 65.Owens RC, Lamp KC, Friedrich LV, Russo R, et al. Postmarketing clinical experience in patients with skin and skin-structure infections treated with daptomycin. Am J Med. 2007;120(10):S6–S12. doi: 10.1016/j.amjmed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15–iii23. doi: 10.1093/jac/dkn368. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of Gram-positive infections. Infect Drug Resist. 2016;9:47–58. doi: 10.2147/IDR.S99046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.David MZ, Dryden M, Gottlieb T, et al. Recently approved antibacterials for methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive pathogens: the shock of the new. Int J Antimicrob Agents. 2017;50(3):303–307. doi: 10.1016/j.ijantimicag.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Sader HS, Flamm RK, Jones RN. Antimicrobial activity of ceftaroline and comparator agents tested against bacterial isolates causing skin and soft tissue infections and community-acquired respiratory tract infections isolated from the Asia-Pacific region and South Africa (2010) Diagn Microbiol Infect Dis. 2013;76(1):61–68. doi: 10.1016/j.diagmicrobio.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Corey GR, Wilcox M, Talbot GH, et al. Integrated analysis of CANVAS 1 and 2: phase 3, multicenter, randomized, double-blind studies to evaluate the safety and efficacy of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin-structure infection. Clin Infect Dis. 2010;51(6):641–650. doi: 10.1086/655827. [DOI] [PubMed] [Google Scholar]
- 71.Panagiotidis G, Backstrom T, Asker-Hagelberg C, et al. Effect of ceftaroline on normal human intestinal microflora. Antimicrob Agents Chemother. 2010;54(5):1811–1814. doi: 10.1128/AAC.01716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen HH, Hon PY, Hsu LY. Ceftaroline –an anti-MRSA cephalosporin and its implications for Singapore. Ann Acad Med Singapore. 2014;43(3):177–186. [PubMed] [Google Scholar]
- 73.Esposito S, Bassetti M, Concia E, et al. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: an update. J Chemother. 2017;29(4):197–214. doi: 10.1080/1120009X.2017.1311398. [DOI] [PubMed] [Google Scholar]
- 74.Shorr AF, Lodise TP, Corey GR, et al. Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2015;59(2):864–871. doi: 10.1128/AAC.03688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coyle EA, Cha R, Rybak MJ. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on Streptococcal pyrogenic exotoxin A release. Antimicrob Agents Chemother. 2003;47(5):1752–1755. doi: 10.1128/AAC.47.5.1752-1755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of Gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254–262. doi: 10.1093/cid/ciu778. [DOI] [PubMed] [Google Scholar]
- 77.Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 78.Boucher HW, Wilcox M, Talbot GH, et al. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 79.Roberts KD, Sulaiman RM, Rybak MJ. Dalbavancin and oritavancin: an innovative approach to the treatment of Gram-positive infections. Pharmacotherapy. 2015;35(10):935–948. doi: 10.1002/phar.1641. [DOI] [PubMed] [Google Scholar]
- 80.Dryden M, Saeed K, Townsend R, et al. Antibiotic stewardship and early discharge from hospital: impact of a structured approach to antimicrobial management. J Antimicrob Chemother. 2012;67(9):2289–2296. doi: 10.1093/jac/dks193. [DOI] [PubMed] [Google Scholar]
- 81.Bamford KB, Desai M, Aruede MJ, et al. Patients’ views and experience of intravenous and oral antimicrobial therapy: room for change. Injury. 2011;42(Suppl 5):S24–S27. doi: 10.1016/S0020-1383(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 82.Lee SL, Azmi S, Wong PS. Clinicians’ knowledge, beliefs and acceptance of intravenous-to-oral antibiotic switching, Hospital Pulau Pinang. Med J Malaysia. 2012;67(2):190–198. [PubMed] [Google Scholar]
- 83.Mertz D, Koller M, Haller P, et al. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J Antimicrob Chemother. 2009;64(1):188–199. doi: 10.1093/jac/dkp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149–162. doi: 10.1016/j.ccc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Ohji G, Doi A, Yamamoto S, Iwata K. Is de-escalation of antimicrobials effective? A systematic review and meta-analysis. Int J Infect Dis. 2016;49:71–79. doi: 10.1016/j.ijid.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 86.Shime N, Satake S, Fujita N. De-escalation of antimicrobials in the treatment of bacteraemia due to antibiotic-sensitive pathogens in immunocompetent patients. Infection. 2011;39(4):319–325. doi: 10.1007/s15010-011-0116-6. [DOI] [PubMed] [Google Scholar]
- 87.Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi: 10.1186/cc9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de With K, Allerberger F, Amann S, et al. Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases. Infection. 2016;44(3):395–439. doi: 10.1007/s15010-016-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang SY, Kumar A. Empiric antimicrobial therapy in severe sepsis and septic shock: optimizing pathogen clearance. Curr Infect Dis Rep. 2015;17(7):493–493. doi: 10.1007/s11908-015-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loo LW, Liew YX, Lee W, et al. Impact of Antimicrobial Stewardship Program (ASP) on outcomes in patients with acute bacterial skin and skin structure infections (ABSSSIs) in an acute-tertiary care hospital. Infect Dis Ther. 2015;4(S1):15–25. doi: 10.1007/s40121-015-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piacenti FJ, Leuthner KD. Antimicrobial stewardship and Clostridium difficile – associated diarrhea. J Pharm Pract. 2013;26(5):506–513. doi: 10.1177/0897190013499528. [DOI] [PubMed] [Google Scholar]
- 92.Macdougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doron S, Davidson LE. Antimicrobial sStewardship. Mayo Clin Proc. 2011;86(11):1113–1123. doi: 10.4065/mcp.2011.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ashiru-Oredope D, Sharland M, Charani E, et al. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart – Then Focus. J Antimicrob Chemother. 2012;67(Suppl 1):i51–i63. doi: 10.1093/jac/dks202. [DOI] [PubMed] [Google Scholar]
- 95.World Health Organization . World Health Organization Sixty-Eighth World Health Assembly. Geneva, Switzerland: May 25, 2015. Global action plan on antimicrobial resistance. Draft resolution with amendments resulting from informal consultations. [Google Scholar]
- 96.Public Health Wales Antimicrobial stewardship: “Start Smart– Then Focus”: Guidance for antimicrobial stewardship for hospitals in Wales. 2011. [Accessed September 20, 2017]. Available from: http://www.wales.nhs.uk/sitesplus/documents/888/Public%20Health%20Wales%20Antimicrobial%20Stewardship%20Guidance.pdf.
- 97.Gilchrist M, Wade P, Ashiru-Oredope D, et al. Antimicrobial stewardship from policy to practice: experiences from UK antimicrobial pharmacists. Infect Dis Ther. 2015;4(Suppl 1):51–64. doi: 10.1007/s40121-015-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Talpaert MJ, Gopal Rao G, Cooper BS, et al. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J Antimicrob Chemother. 2011;66(9):2168–2174. doi: 10.1093/jac/dkr253. [DOI] [PubMed] [Google Scholar]
- 99.Loo LW, Liew YX, Lee W, Chlebicki P, Kwa AL. Impact of Antimicrobial Stewardship Program (ASP) on outcomes in patients with acute bacterial skin and skin structure infections (ABSSSIs) in an acute-tertiary care hospital. Infect Dis Ther. 2015;4(Suppl 1):15–25. doi: 10.1007/s40121-015-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao K, Bae L, Yew WP. Post-operative wound management. Aust Fam Physician. 2013;42(12):867–870. [PubMed] [Google Scholar]
- 101.Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care. 2014;3(8):511–529. doi: 10.1089/wound.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sibbald RG, Mahoney J. A consensus report on the use of vacuum-assisted closure in chronic, difficult-to-heal wounds. Ostomy Wound Manage. 2003;49(11):52–66. [PubMed] [Google Scholar]
- 103.Expert Working Group Vacuum assisted closure: recommendations for use. A consensus document. Int Wound J. 2008;5(Suppl 4):iii–19. doi: 10.1111/j.1742-481X.2008.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henderson V, Timmons J, Hurd T. NPWT in everyday practice made easy. Wounds International. 2010;1(5):1–6. [Google Scholar]
- 105.White RJ, Cooper R, Kingsley A. Wound colonization and infection: the role of topical antimicrobials. Br J Nurs. 2001;10(9):563–578. doi: 10.12968/bjon.2001.10.9.9387. [DOI] [PubMed] [Google Scholar]
- 106.Abboud EC, Settle JC, Legare TB, et al. Silver-based dressings for the reduction of surgical site infection: review of current experience and recommendation for future studies. Burns. 2014;40(Suppl 1):S30–S39. doi: 10.1016/j.burns.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 107.Edwards-Jones V. Antimicrobial and barrier effects of silver against methicillin-resistant Staphylococcus aureus. J Wound Care. 2006;15(7):285–290. doi: 10.12968/jowc.2006.15.7.26951. [DOI] [PubMed] [Google Scholar]
- 108.Halim AS, Khoo TL, Saad AZM. Wound bed preparation from a clinical perspective. Indian J Plast Sug. 2012;45(2):193–202. doi: 10.4103/0970-0358.101277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alimov V, Lovecchio F, Sinha M, Foster KN, Drachman D. Use of a silver-containing hydrofiber dressing for filling abscess cavity following incision and drainage in the emergency department: a randomized controlled trial. Adv Skin Wound Care. 2013;26(1):20–25. doi: 10.1097/01.ASW.0000425936.94874.9a. [DOI] [PubMed] [Google Scholar]
- 110.Krieger BR, Davis DM, Sanchez JE, et al. The use of silver nylon in preventing surgical site infections following colon and rectal surgery. Dis Colon Rectum. 2011;54(8):1014–1019. doi: 10.1097/DCR.0b013e31821c495d. [DOI] [PubMed] [Google Scholar]
- 111.White R, Cutting F. Modern exudate management: a review of wound treatments. World Wide Wounds. 2006. [Accessed September 20, 2017]. Available form: http://www.worldwidewounds.com/2006/september/White/Modern-Exudate-Mgt.
- 112.Karr JC, de Mola FL, Pham T, Tooke L. Wound healing and cost-saving benefits of combining negative-pressure wound therapy with silver. Adv Skin Wound Care. 2013;26(12):562–565. doi: 10.1097/01.ASW.0000434518.86905.8a. [DOI] [PubMed] [Google Scholar]
- 113.Dryden MS. Novel antibiotic treatment for skin and soft tissue infection. Curr Opin Infect Dis. 2014;27(2):116–124. doi: 10.1097/QCO.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 114.Davis JS, Sud A, O’Sullivan MVN, et al. Combination of vancomycin and β-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis. 2016;62(2):173–180. doi: 10.1093/cid/civ808. [DOI] [PubMed] [Google Scholar]
- 115.Mcconeghy KW, Bleasdale SC, Rodvold KA. The empirical combination of vancomycin and a β-lactam for Staphylococcal bacteremia. Clin Infect Dis. 2013;57(12):1760–1765. doi: 10.1093/cid/cit560. [DOI] [PubMed] [Google Scholar]
- 116.Russo A, Concia E, Cristini F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect. 2016;22(Suppl 2):S27–S36. doi: 10.1016/S1198-743X(16)30095-7. [DOI] [PubMed] [Google Scholar]
- 117.Bassetti M, Righi E, Carnelutti A. New therapeutic options for skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):99–108. doi: 10.1097/QCO.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 118.May AK. Skin and soft tissue infections: the new surgical infection society guidelines. Surg Infect (Larchmt) 2011;12(3):179–184. doi: 10.1089/sur.2011.034. [DOI] [PubMed] [Google Scholar]
- 119.Esposito S, Bassetti M, Borre S, et al. Diagnosis and management of skin and soft-tissue infections (SSTI): a literature review and consensus statement on behalf of the Italian Society of Infectious Diseases and International Society of Chemotherapy. J Chemother. 2011 Oct;23(5):251–262. doi: 10.1179/joc.2011.23.5.251. [DOI] [PubMed] [Google Scholar]
- 120.Mensa J, Soriano A, Llinares P, et al. Guidelines for antimicrobial treatment of the infection by Staphylococcus aureus. Rev Esp Quimioter. 2013;26(Suppl 1):1–84. [PubMed] [Google Scholar]
- 121.Gould FK, Brindle R, Chadwick PR, et al. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63(5):849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 122.White B, Seaton RA. Complicated skin and soft tissue infections: literature review of evidence for and experience with daptomycin. Infect Drug Resist. 2011;4:115–127. doi: 10.2147/IDR.S13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zyvox (linezolid, Pfizer Inc., USA) [Singapore product information] 2008 [Google Scholar]
- 124.Cubicin (daptomycin, MSD Pharma Pte Ltd, Singapore) [Singapore product information] 2017 [Google Scholar]
- 125.Tygacil (tigecycline, Wyeth, USA). [Singapore product information] 2016 [Google Scholar]
- 126.Zinforo (ceftaroline, AstraZeneca AB, Sweden.) [Singapore product information] 2016 [Google Scholar]
- 127.Sivextro (tedizolid), Bayer (South East Asia) Pte Ltd, Singapore [Singapore product information] 2016 [Google Scholar]