Abstract
Objectives
We investigated the in vitro antimicrobial susceptibilities of clinically important Gram-negative bacteria (GNB) from 16 major teaching hospitals in Taiwan in 2017.
Materials and methods
Escherichia coli (n=686) and Klebsiella pneumoniae bloodstream isolates (n=673), non-typhoid Salmonella (NTS; n=221) from various sources, Shigella species (n=21) from fecal samples, and Neisseria gonorrhoeae (n=129) from the genitourinary tract were collected. Antibiotic minimum inhibitory concentrations (MICs) were determined using the broth microdilution method. Alleles encoding K. pneumoniae carbapenemases (KPCs), New Delhi metallo-β-lactamases (NDMs), Verona integron-encoded metallo-β-lactamase, imipenemase, OXA-48-like, and mcr-1-5 genes were detected by molecular methods in Enterobacteriaceae isolates.
Results
Five (0.7%) E. coli isolates harbored mcr-1 alleles. Twenty-four (3.6%), seven (1.0%), four (0.6%), and one (0.15%) K. pneumoniae isolates contained bla KPC, bla OXA-48-like, mcr-1, and bla NDM, respectively. Three (1.4%) NTS and no Shigella isolates harbored mcr-1 genes. Seventy-one (10.5%) K. pneumoniae isolates displayed non-susceptibility (NS) to carbapenem agent(s). Phenotypically extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae isolates showed significantly higher rates of ertapenem, tigecycline, and ceftolozane–tazobactam (CLZ– TAZ) NS (40.2%, 16.3%, and 71%–80%, respectively) than E. coli isolates exhibiting ESBL phenotypes (5.4%, 0.7%, and 18%–28%, respectively). All phenotypically ESBL-producing E. coli isolates were ceftazidime–avibactam (CAZ–AVB) susceptible. Two (8.3%) KPC-producing K. pneumoniae isolates showed CAZ–AVB NS. Hospital-acquired K. pneumoniae isolates were significantly less susceptible to ertapenem and CLZ–TAZ than hospital-acquired E. coli isolates.
Conclusion
Third-generation cephalosporins remain the optimal choice for treating NTS, Shigella, and gonococcal infections in Taiwan. Hospital-acquired and phenotypically ESBL-producing K. pneumoniae are a heavy resistance burden in Taiwan.
Keywords: Enterobacteriaceae, Neisseria gonorrhoeae, extended-spectrum β-lactamases, carbapenemase, ceftolozane-tazobactam, ceftazidime, avibactam
Introduction
Infections that become septicemic conditions, regardless of whether acquired from the community or hospitals, are typically associated with major fatalities and prolonged hospital stays,1,2 particularly in the immunosuppressed2 and elderly populations (≥60 years).3,4 Over the last decade, infections caused by multidrug-resistant (MDR) Gram-negative enteric bacteria, including extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae isolates, and carbapenemase-producing Enterobacteriaceae (CPE), have shown significantly higher case fatality rates than infections caused by susceptible bacteria.5–8 Given the present antibiotic pipeline, it is necessary to prescribe the appropriate antimicrobials to combat difficult-to-treat pathogens and continuously monitor country-specific susceptibility profiles to determine the optimal treatment option, including antibiotics that will become available in a region in the future.
Ceftazidime–avibactam (CAZ–AVB) and ceftolozane– tazobactam (CLZ–TAZ), which are new second-generation β-lactam–β-lactamase inhibitors combinations, show good in vitro efficacy against K. pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae and carbapenem non-susceptible (NS) Pseudomonas aeruginosa isolates, respectively.9 These two novel drugs will become available in Taiwan in 2018. In addition, data regarding differences in the susceptibility of community- and hospital-acquired Gram-negative bacteria (GNB) to CAZ–AVB and CLZ–TAZ are lacking.
The Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) Program was initiated in 2002 to investigate the evolution of antibiotic susceptibility of a variety of clinically important pathogens collected from hospitals throughout Taiwan. Although the data related to CPE are important for providing real-time revision of infection control policies, little information was available regarding the prevalence and susceptibilities (particularly to new β-lactam combination agents) of diverse carbapenemases on clinical isolates of several important Enterobacteriaceae species in Taiwan until 2018.10,11 This nationwide study was conducted, and isolates were collected in 2017 to survey susceptibility, including that of bloodstream isolates of E. coli and K. pneumoniae, isolates of non-typhoid Salmonella (NTS) regardless of sources, fecal Shigella species isolates, and genitourinary Neisseria gonorrhoeae isolates.
Materials and methods
Isolate collection
From January 2017 through December 2017, 16 major teaching hospitals in Taiwan, including eight, two, five, and one in the northern, central, southern, and eastern regions, respectively, participated in this nationwide resistance surveillance plan to examine clinically important pathogens. This survey was conducted by the Centers for Disease Control of Taiwan. In this survey, no less than four non-duplicate bloodstream isolates of E. coli and K. pneumoniae were required to be submitted by each medical center (n=11) every month, whereas at least two isolates were submitted by every district teaching hospital (n=5) each month. In addition, regardless of the source of the cultured isolate, at least one non-duplicate NTS and N. gonorrhoeae isolates and any available isolate of Shigella species were collected every month from all participating hospitals.
Compared to community-acquired isolates (defined as being collected ≤48 hours after admission to the hospital), isolates collected at >48 hours after admission as well as those collected from patients not displaying signs and symptoms of infections initially on admission were considered as hospital acquired. Moreover, hospital settings, such as the emergency department, outpatient clinic, general ward, and intensive care unit (ICU), and the outcomes of hospitalized patients from whom GNB isolates were collected were also recorded. The defined daily dose per 1,000 inhabitants per day in every hospital participating in this surveillance plan was also collected in 2017 to detect any gross changes in each antibiotic category. The institutional review board of the National Taiwan University Hospital (201609066RINB) approved this study and waived the written informed consent. Patient consent was waived because this in vitro antimicrobial susceptibility surveillance of research on bacterial isolates involved no more than a minimal risk to the subjects and the links to limited clinical information required for the subjects in this study were removed.
Antimicrobial susceptibility testing
In this survey, the broth microdilution method with Sensititre Gram-negative minimum inhibitory concentration (MIC) plates panels (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the MICs of the evaluated antibiotics for all included Enterobacteriaceae isolates. In addition, 1% GC agar with IsoVitaleX™ (Becton-Dickinson Microbiology Systems, Sparks, MD, USA) was used to determine the MICs of the evaluated antibiotics for the enrolled N. gonorrhoeae isolates. Previous studies12,13 have described the phenotypic categories of Enterobacteriaceae isolates, and thus, the resistance mechanisms of the enrolled Enterobacteriaceae isolates were classified based on the results of susceptibility tests. The MIC breakpoints recommended by the Clinical and Laboratory Standards Institute (CLSI) in 2018,14 European Committee on Antimicrobial Susceptibility Testing (EUCAST) in 2018,15 and US Food and Drug Administration (FDA) were adopted to compare the differences in susceptibility rates between the defined groups of enrolled GNB isolates. In addition, random amplification of polymorphic DNA was used to delineate the clonal relatedness of CPE isolates if necessary.
Determinations of key carbapenemase-encoding and mcr genes
The Xpert® Carba-R assay (Cepheid, Sunnyvale, CA, USA) was used to detect the carbapenemase-encoding alleles, including blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48-like, in Enterobacteriaceae isolates displaying in vitro NS to any carbapenem agents.16 CPE isolates were defined as those harboring genes encoding any carbapenemase. PCR amplification of whole-cell DNA of the isolates showing colistin MICs of >2 mg/L was performed using previously described primers specific for mcr-1, mcr-2, mcr-3, mcr-4, and mcr-5, and the PCR products were sequenced17 (Table S1).
Statistical analyses
Differences in group percentages were assessed using Pearson’s chi-squared test or Fisher’s exact test as appropriate. Two-tailed P-values of <0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Science Version 17 (SPSS Inc., Chicago, IL, USA).
Results
Profiles of overall susceptibility of all GNB isolates
The important MIC ranges and significant antibiotic susceptibility percentages of the clinical isolates of five different GNB species are illustrated in Table 1. There were notable differences in the susceptibility of E. coli and K. pneumoniae isolates to two anti-pseudomonal fluoroquinolones; NTS to levofloxacin; and N. gonorrhoeae to cefotaxime, cefixime, and azithromycin. In addition, no significant fluctuations were observed in daily dose per 1,000 inhabitants per day for all classes of antibiotics prescribed in the participating Taiwanese hospitals (data not shown). The complete MIC results, ranges, and susceptibility data for five GNB species in this study are shown in Table S2.
Table 1.
In vitro susceptibilities (evaluated based on the criteria of the Clinical and Laboratory Standards Institute 2018 and European Committee on Antimicrobial Susceptibility Testing 2018a) of bloodstream isolates of Escherichia coli, Klebsiella pneumoniae, and other Gram-negative bacteria, including isolates of non-typhoid Salmonella species from any site, fecal Shigella species, and Neisseria gonorrhoeae from the genitourinary system, collected from patients treated at 16 major teaching hospitals across Taiwan in 2017 to key antimicrobial agents tested
| Bacterial species (isolate no.) and antimicrobial agent tested | MIC (mg/L) | % of indicated susceptibility | ||||
|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | S | I | R | |
| Escherichia coli (686) | ||||||
| Ceftolozane–tazobactam | 0.12–>64 | 0.25 | 1 | 94.3 | 2.2 | 3.5 |
| Ceftazidime–avibactam | ≤0.06–>64 | 0.12 | 0.25 | 99.9a | NA | 0.1a |
| Ceftazidime | ≤0.12–>256 | 0.25 | 32 | 74.5 | 5.1 | 20.4 |
| Cefepime | ≤0.12–>64 | ≤0.12 | 64 | 78.0 | 7.0 | 15.0 |
| Ertapenem | ≤0.06–>64 | ≤0.06 | 0.12 | 98.0 | 0.7 | 1.3 |
| Ciprofloxacin | ≤0.06–>64 | 0.25 | 64 | 68.2/59.9a | 1.3/6.7a | 30.5/33.4a |
| Levofloxacin | ≤0.06–>64 | 0.25 | 16 | 69.2/63.8a | 0.6/4.4a | 30.2/31.8a |
| Colistin | ≤0.12–8 | 0.25 | 0.25 | 98.8 | NA | 1.2 |
| Klebsiella pneumoniae (673) | ||||||
| Ceftolozane–tazobactam | ≤0.06–>64 | 0.5 | 64 | 84.1/80.7a | 1.2/NA | 14.7/19.1a |
| Ceftazidime–avibactam | ≤0.06–>64 | 0.12 | 1 | 99.1a | NA | 0.9a |
| Ceftazidime | ≤0.12–>256 | 0.25 | 256 | 73.7 | 3.4 | 22.9 |
| Cefepime | ≤0.12–>64 | ≤0.12 | 32 | 82.8 | 2.5 | 14.7 |
| Ertapenem | ≤0.06–>64 | ≤0.06 | 1 | 89.5 | 3.6 | 7.0 |
| Meropenem | ≤0.06–>64 | ≤0.06 | 0.12 | 94.5 | 0.6 | 4.9 |
| Ciprofloxacin | ≤0.06–>64 | ≤0.06 | 64 | 79.5/66.4a | 1.8/6.7a | 18.7/26.9a |
| Levofloxacin | ≤0.06–>64 | ≤0.06 | 32 | 81.0/69.7a | 2.2/9.5a | 16.8/20.8a |
| Colistin | ≤0.12–>64 | 0.25 | 0.5 | 97.6 | NA | 2.4 |
| Non-typhoid Salmonella spp. (221) | ||||||
| Ampicillin | 0.5–>64 | 4 | >64 | 51.6 | 1.8 | 46.6 |
| Cefixime | ≤0.12–>64 | ≤0.12 | >64 | 87.3 | 0.5 | 12.2 |
| Ceftriaxone | ≤0.12–64 | ≤0.12 | 1 | 92.3 | 1.8 | 5.9 |
| Ertapenem | ≤0.06–0.25 | ≤0.06 | ≤0.06 | 100 | 0 | 0 |
| Ciprofloxacin | ≤0.06–16 | ≤0.06 | 0.5 | 78.7/78.7a | 18.1/NAa | 3.2/21.3a |
| Levofloxacin | ≤0.06–16 | ≤0.06 | 1 | 78.7/89.6a | 10.9/8.6a | 10.4/1.8a |
| Moxifloxacin | ≤0.06–16 | 0.12 | 2 | 79.2a | NAa | 20.8a |
| Gentamicin | ≤0.12–>64 | 0.5 | 1 | 96.4 | 0.5 | 3.2 |
| Trimethoprim–sulfamethoxazole | ≤0.12–>32 | ≤0.12 | >32 | 74.2 | NA | 25.8 |
| Colistin | 0.25–16 | 2 | 4 | 53.8 | NA | 46.2 |
| Shigella spp. (21) | ||||||
| Ampicillin | 1–>64 | >64 | >64 | 38.1 | 0 | 61.9 |
| Ceftriaxone | ≤0.12 | ≤0.12 | ≤0.12 | 100 | 0 | 0 |
| Ertapenem | ≤0.06 | ≤0.06 | ≤0.06 | 100 | 0 | 0 |
| Ciprofloxacin | ≤0.06–16 | ≤0.06 | 16 | 61.9 | 0 | 38.1 |
| Levofloxacin | ≤0.06–8 | ≤0.06 | 8 | 61.9 | 19.0 | 19.0 |
| Trimethoprim–sulfamethoxazole | ≤0.12–>32 | 1 | >32 | 61.9 | NA | 38.1 |
| Azithromycin–Shigella flexneri (17) | 1–>64 | 4 | >64 | 70.6 | NA | 29.4 |
| Azithromycin–Shigella sonnei (4) | 8–128 | 8 | 128 | 75 | NA | 25 |
| N. gonorrhoeae (129) | ||||||
| Cefotaxime | ≤0.03–0.5 | 0.06 | 0.25 | 100/89.1a | NA/NAa | NA/10.9a |
| Cefixime | ≤0.03–0.5 | 0.06 | 0.12 | 96.1/89.1a | NA/NAa | NA/10.9a |
| Cefpodoxime | ≤0.03–2 | 0.25 | 0.5 | 89.9 | NA | NA |
| Ciprofloxacin | ≤0.008–128 | 4 | 16 | 2.3 | 0 | 97.7 |
| Doxycycline | 0.25–32 | 8 | 16 | NA | NA | NA |
| Azithromycin | ≤0.03–>32 | 0.25 | 0.5 | 96.9/60.5a | NA/31.0a | 3.1/8.5a |
Note:
Denotes a significantly statistical difference (P<0.05) between different susceptibility subgroups.
Abbreviations: I, intermediate; MIC, minimum inhibitory concentration; NA, non-applicable; R, resistant; S, susceptible.
Susceptibility of bloodstream E. coli isolates
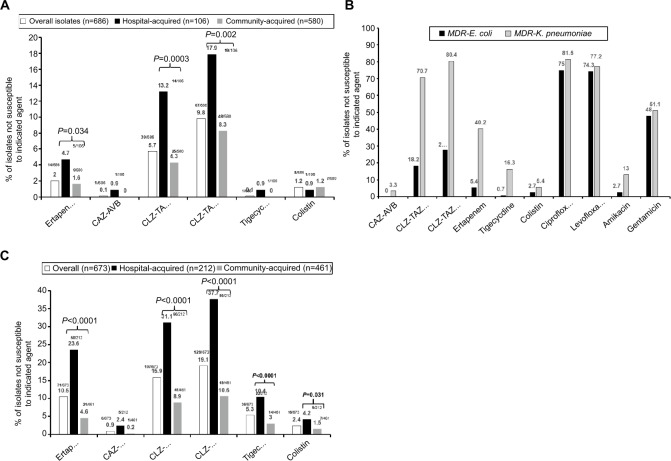
Among the 686 bloodstream E. coli isolates, 14 (2.0%) isolates showed NS to ertapenem. A total of 148 (21.6%) isolates exhibited ESBL phenotypes not associated with KPC or New Delhi metallo-β-lactamase (NDM) production (NS rates for piperacillin–tazobactam, ceftazidime, and cefepime were 18.2%, 75.0%, and 99.3%, respectively). Among these 148 isolates, 43 (29.1%) isolates were hospital acquired. Figure 1A illustrates the NS to some important antibiotics of hospital-acquired (n=106) and community-acquired (n=580) E. coli isolates. Significantly higher NS rates (P<0.05) to ertapenem (4.7%) and CLZ–TAZ (13.2%–17.9%) were found among hospital-acquired K. pneumoniae isolates compared to community-acquired isolates (1.6% vs 4.3%–8.3%, respectively). All phenotypically ESBL-producing E. coli isolates were susceptible to CAZ–AVB. In contrast, a trend toward a significant difference in the NS rate (P=0.053) to CLZ–TAZ was observed when comparing the MIC breakpoints of the CLSI 2018 (18.2%) and EUCAST 2018 (27.7%). In addition, high NS rates (up to 75%) to ciprofloxacin and levofloxacin were found among phenotypically ESBL-producing E. coli isolates. When in vitro susceptibility of the phenotypically ESBL-producing E. coli isolates was further assessed, 78 (11.4%) isolates were judged to be likely producers of ESBL alone. All 78 isolates were sensitive in vitro to CAZ–AVB and tigecycline, while less than 10% (5.1% based on the CLSI 2018 criteria and 9.0% based on the EUCAST 2018 criteria) were NS to CLZ–TAZ.
Figure 1.
Comparison of (A) in vitro non-susceptibility to important antibiotics among overall, hospital-acquired, and community-acquired bloodstream Escherichia coli isolates, (B) Klebsiella pneumoniae isolates collected from 16 major teaching hospitals across Taiwan in 2017, and (C) in vitro non-susceptibility to important antibiotics between bloodstream Escherichia coli (n=148) and K. pneumoniae isolates (n=92) exhibiting the phenotype of extended-spectrum β-lactamase (unrelated to carbapenemase) production (ie, MDR phenotype).
Abbreviations: CAZ–AVB, ceftazidime–avibactam; CLSI, Clinical and Laboratory Standards Institute 2018; CLZ–TAZ, ceftolozane–tazobactam; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MDR, multidrug resistance.
Moreover, five (0.7%) E. coli isolates harbored the mcr-1 allele, while the blaKPC and blaNDM-1 alleles were each detected in one E. coli isolate. All five mcr-1-harboring E. coli isolates were susceptible to all carbapenem agents, tigecycline, CLZ–TAZ, and CAZ–AVB, while three displayed ESBL phenotypes.
Susceptibility of bloodstream K. pneumoniae isolates
Figure 1B illustrates the NS to important antibiotics of hospital-acquired (n=212) and community-acquired (n=461) K. pneumoniae isolates. There were significantly higher NS rates (P<0.05) to ertapenem (23.6%), CLZ–TAZ (31%–38%), tigecycline (10.4%), and colistin (4.2%) among hospital-acquired K. pneumoniae isolates compared to those in community-acquired (n=461) K. pneumoniae isolates (4.6%, 8.9%–10.6%, 10.4%, and 1.5%, respectively).
Of the 673 bloodstream K. pneumoniae isolates, 71 (10.5%) displayed NS to at least one carbapenem agent. A total of 92 (13.8%) K. pneumoniae isolates exhibited ESBL phenotypes not associated with KPC and/or NDM production (NS rates of piperacillin–tazobactam, ceftazidime, and cefepime were 92.4%, 100%, and 73.9%, respectively). Among the 92 phenotypically ESBL-producing K. pneumoniae isolates, 57 (61.0%) isolates were hospital acquired, three (3.3%) isolates were NS to CAZ–AVB, and five (5.4%) were NS to colistin. Furthermore, compared to the 148 phenotypically ESBL-producing E. coli isolates, these isolates showed significantly higher NS rates to ertapenem (40.2%), tigecycline (7.6% based on the US FDA criteria and 16.3% based on the EUCAST 2018 criteria), CLZ–TAZ (70.7% based on the CLSI 2018 criteria and 80.4% based on the EUCAST 2018 criteria), and amikacin (13.0%), as shown in Figure 1C. Careful evaluation of antibiotic susceptibility of 92 phenotypically ESBL-producing K. pneumoniae isolates revealed that the in vitro phenotypes of 31 (4.6%) K. pneumoniae isolates were consistent with those producing ESBL alone. Evaluation based on either the CLSI 2018 or the EUCAST 2018 criteria showed that these ESBL producers still had a high rate of NS to CLZ–TAZ (38.7% based on the CLSI 2018 criteria and 54.8% based on the EUCAST 2018 criteria), but fewer isolates were NS to tigecycline (9.7% based on the US FDA criteria and 16.1% based on the EUCAST 2018 criteria) and only one isolate was NS to colistin.
Notably, among the bloodstream K. pneumoniae isolates verified as CPE, 24 (3.6%) harbored blaKPC alleles, with 16 (66.7%) acquired in hospital settings (one of which was susceptible to all carbapenems), seven (1.0%) harbored blaOXA-48-like alleles (six exhibited ESBL phenotypes), and one (0.15%) and four (0.6%) isolates harbored blaNDM and mcr-1 alleles, respectively. Moreover, two (8.3%) KPC-producing isolates were NS to CAZ–AVB in contrast to 22 (91.7%) isolates, which were NS to CLZ–TAZ (P<0.001). In addition, five (20.8%) KPC-producing K. pneumoniae isolates were NS to colistin and four (16.7%) were NS to tigecycline based on the EUCAST 2018 criteria. Notably, the MICs for CAZ–AVB/CLZ–TAZ/tigecycline/colistin for one E. coli and one K. pneumoniae isolates harboring the blaNDM allele were >64/>64/0.06/0.25 and >64/>64/0.25/0.25 mg/L, respectively.
Susceptibility of E. coli and K. pneumoniae isolates carrying mcr-1 genes
Table 2 shows the MIC levels against important antibiotics for five E. coli and four K. pneumoniae isolates harboring the mcr-1 gene. Compared to mcr-1-harboring E. coli isolates, grossly lower susceptibility rates to cefoxitin (0%), ceftazidime (25%), piperacillin–tazobactam (0%), and all carbapenem agents (25%, 50%, and 50% to ertapenem, imipenem, and meropenem, respectively) were observed for mcr-1-harboring K. pneumoniae isolates. Among the four mcr-1-harboring K. pneumoniae isolates, one isolate concomitantly carried the blaKPC and blaOXA-48-like alleles, while the other co-harbored the blaKPC allele.
Table 2.
MIC values of some key antibiotics against isolates of mcr-1-harboring Escherichia coli (n=5) and Klebsiella pneumoniae (n=4) collected from 16 major teaching hospitals throughout Taiwan in 2017
| Bacterial species and isolates | MIC (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | ||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2a | 3 | 4b | |
| Amoxicillin–clavulanate | 32 | 8 | 8 | 64 | 8 | 32 | 128 | 64 | 128 |
| Cefoxitin | 128 | 8 | 4 | 128 | 2 | 32 | 128 | 128 | 128 |
| Ceftazidime | 16 | 0.5 | 0.5 | 32 | 0.0625 | 1 | 512 | 512 | 512 |
| Ceftriaxone | 16 | 128 | 128 | 128 | 0.0625 | 0.25 | 128 | 16 | 128 |
| Cefepime | 0.25 | 4 | 4 | 8 | 0.0625 | 0.25 | 128 | 0.25 | 128 |
| Piperacillin–tazobactam | 4 | 2 | 2 | 8 | 2 | 256 | 256 | 256 | 256 |
| Ceftazidime–avibactam | 0.12 | 0.12 | 0.03 | 0.25 | 0.03 | 0.50 | 4 | 2 | 128 |
| Ceftolozane–tazobactam | 1 | 0.25 | 0.25 | 2 | 0.50 | 1 | 64 | 32 | 128 |
| Ertapenem | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 128 | 1 | 128 |
| Imipenem | 0.25 | 0.12 | 0.12 | 0.25 | 0.12 | 0.03 | 64 | 0.50 | 64 |
| Meropenem | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 128 | 0.12 | 128 |
| Ciprofloxacin | 128 | 0.03 | 0.03 | 128 | 8 | 0.25 | 128 | 8 | 64 |
| Amikacin | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 128 |
| Tigecycline | 0.25 | 0.50 | 0.25 | 0.50 | 0.25 | 1 | 2 | 1 | 0.50 |
Notes:
This isolate also harbors blaKPC- and blaOXA-48-like alleles.
This isolate also harbors the blaKPC allele.
Abbreviation: MIC, minimum inhibitory concentration.
Susceptibility of NTS, Shigella, and N. gonorrhoeae isolates
A total of 221 NTS isolates were collected in 2017; none displayed an ESBL phenotype, while six (2.7%) were considered as likely AmpC producers. Modest susceptibilities to ciprofloxacin, levofloxacin (78.7%), trimethoprim–sulfamethoxazole (TMP–SMX; 74.2%), and amoxicillin–clavulanate (81.0%) were observed. Approximately 24.0% of NTS isolates were NS to ampicillin plus TMP–SMX. Notably, 46.2% of these NTS isolates were NS to colistin. Based on the PCR results, however, only three (1.4%) NTS isolates harbored the mcr-1 alleles, while all NTS isolates were susceptible to ceftazidime and ceftriaxone. In addition, among the 26 (11.8%) hospital-acquired NTS isolates, half belonged to serogroup D. Among the 26 patients suffering from hospital-acquired NTS infections (case fatality rate, 27.2%), most (88.5%) NTS isolates were collected at general wards. Of 21 Shigella isolates (17 S. flexneri and 4 S. sonnei isolates) collected from fecal specimens, modest susceptibilities to two anti-pseudomonal fluoroquinolones (61.9%), azithromycin (71.4%), and TMP–SMX (61.9%) were detected.
Among the 129 urinary N. gonorrhoeae isolates collected in 2017, most (≥89%) were susceptible in vitro to spectinomycin and third-generation cephalosporins, including ceftriaxone, cefotaxime, cefixime, and cefpodoxime, based on the CLSI 2018 and EUCAST 2018 criteria. However, a significant discrepancy in the susceptibilities to azithromycin was found in evaluations based on the CLSI 2018 and EUCAST 2018 criteria (96.9% vs 60.5%, P<0.001). In addition, an extremely low susceptibility rate (2.3%) against ciprofloxacin was found for these gonococcal isolates in Taiwan.
Susceptibility of hospital-acquired E. coli and K. pneumoniae isolates and fatality rates of infected hospitalized patients
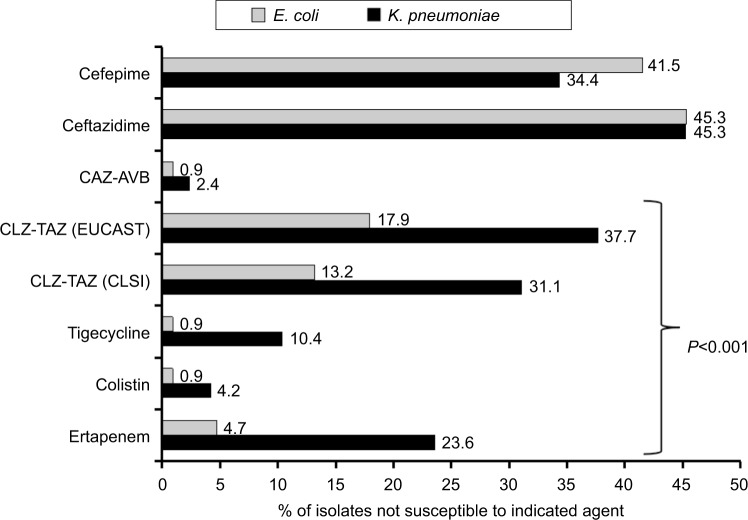
The case fatality rates for the patient subsets who experienced hospital-acquired bloodstream infections with E. coli (n=106) and K. pneumoniae (n=212) were 31.0% and 36.5%, respectively. As shown in Figure 2, significantly higher NS rates (P<0.001) were observed for hospital-acquired K. pneumoniae than for E. coli isolates against ertapenem (23.6% vs 4.7%), colistin (4.2% vs 0.9%), tigecycline (10.4% vs 0.9% based on the EUCAST 2018 criteria), and CLZ–TAZ (31.1% vs 13.2% based on the CLSI 2018 criteria and 37.7% vs 17.9% based on the EUCAST 2018 criteria) but not against CAZ–AVB (2.4% vs 0.9%, P=0.38), ceftazidime (45.3% vs 45.3%), and cefepime (34.4% vs 41.5%) by chi-squared analysis. Furthermore, 10 E. coli (none were CPE) and 44 K. pneumoniae (nine KPC producers and two harbored blaOXA-48-like alleles) isolates were collected from patients hospitalized in the ICU.
Figure 2.
Comparison of in vitro non-susceptibility to important antibiotics of hospital-acquired Escherichia coli (n=106) and Klebsiella pneumoniae bloodstream isolates (n=212) collected from 16 major teaching hospitals across Taiwan in 2017.
Abbreviations: CAZ–AVB, ceftazidime–avibactam; CLSI, Clinical and Laboratory Standards Institute 2018; CLZ–TAZ, ceftolozane–tazobactam; EUCAST, European Committee on Antimicrobial Susceptibility Testing 2018.
Discussion
No comparative studies have addressed the distinctions in NS rates against CAZ–AVB and CLZ–TAZ between hospital-acquired and community-acquired bloodstream E. coli and K. pneumoniae isolates. As expected, significantly higher NS rates of hospital-acquired isolates than those of community-acquired bacteremic E. coli and K. pneumoniae isolates to these two new β-lactam combination agents were found, although these two agents have not been launched in Taiwan. Previously, one Taiwanese multicenter survey detected four K. pneumoniae isolates harboring blaOXA-48-like allele among the 760 carbapenem-NS K. pneumoniae isolates collected between 2012 and 2014.18 In contrast, seven K. pneumoniae isolates harbored plasmidic blaOXA-48-like (three of which were community acquired), and a significantly higher ertapenem NS rate (40.2%) was observed among phenotypically ESBL-producing K. pneumoniae isolates than that (5.4%) of ESBL-producing E. coli isolates in 2017, raising concerns about K. pneumoniae displaying NS to carbapenem agent(s) in Taiwan. In addition, although the blaNDM-harboring Enterobacteriaceae isolates have high potential for dissemination (from the Indian subcontinent to Vietnam and the Philippines, identified among the abdominal isolates since 2011),19 the prevalence rates of bloodstream blaNDM-harboring E. coli and K. pneumoniae isolates were low (both were 0.15%) in Taiwan in 2017.
A review of the PubMed database on ESBL rates of clinical Taiwanese E. coli and K. pneumoniae isolates revealed that one survey focusing on ICU Enterobacteriaceae isolates in 2005 found that 14% of E. coli and 26% of K. pneumoniae were ESBL producers.20 Another survey on bloodstream E. coli and K. pneumoniae isolates cultured from patients with hematological malignancies in 2008–2013 showed that 33.2% of E. coli and 12.6% of K. pneumoniae isolates were ESBL producers.4 Therefore, the ESBL rates in our study (21.6% of E. coli and 13.8% of K. pneumoniae) were more similar to the results of the latter survey. Moreover, 45.3% of Taiwanese hospital-acquired K. pneumoniae isolates had a ceftazidime-NS phenotype, which was only slightly lower than those (58.1%) collected from patients with hematologic malignancies in Italy.3 This result raised concerns regarding the future role of ceftazidime for treating hospital-acquired K. pneumoniae septicemia in Taiwan.
The prevalence rate of KPC positivity (3.6%) among Taiwanese bloodstream K. pneumoniae isolates in 2017 was much lower than that in Italy (42%).21 In addition, among the ertapenem-NS E. coli (n=14) and K. pneumoniae (n=71) isolates in this study, two E coli isolates, of which one (7.1%) was a KPC producer, and 27 K. pneumoniae isolates, of which 23 (32.4%) were KPC producers, were determined as CPE. The KPC-producing Enterobacteriaceae isolates also have a high potential for spreading in clinical setting,5 an important monitor focus in infection control. Regardless of the isolate source, one Taiwanese multicenter study investigating 247 K. pneumoniae isolates with NS to imipenem or meropenem in 2012 showed that the KPC production rate was 16.6%.10 In contrast, another Taiwanese multicenter study (2010–2012) focusing on 1,135 Enterobacteriaceae isolates with NS to any carbapenem agent revealed that 2.8% of K. pneumoniae isolates produced KPC.11 Therefore, among the ertapenem-NS bloodstream K. pneumoniae isolates collected in 2017, a strikingly higher rate of KPC production was observed than that previously observed in Taiwan (P<0.001). However, in contrast to carbapenemase production, a Taiwanese survey investigating the main resistance mechanisms of carbapenem-NS E. coli isolates found that plasmidic AmpC (mainly CMY-2) in combination with changes in outer membrane porins (OmpC/F) predominantly conferred resistance to carbapenems.22 This result agrees with our findings of the low carbapenemase rate among ertapenem-NS E. coli isolates. Notably, five (0.7%) E. coli and four (0.6%) K. pneumoniae bloodstream isolates collected in 2017 harbored plasmidic mcr-1 alleles (all of which were clonally unrelated, data not shown). The low mcr-1 prevalence was similar to those in China from 2013 through 2014.23 In addition, of the Taiwan-ese isolates of mcr-1-harboring E. coli and K. pneumoniae, the former species were less likely to co-harbor other carbapenemase(s)-encoding alleles than the latter, resulting in more significant drug-resistant phenotypes. Furthermore, all four mcr-1-harboring E. coli isolates showed susceptibility to all carbapenem agents, which agrees with previous results.24
One review article addressed that KPC producers are likely to co-harbor ESBL alleles to a considerable degree.5 As stated by van Duin et al9 and Tato et al,25 our survey also revealed that CAZ–AVB had much better in vitro efficacy against ESBL-producing K. pneumoniae isolates (regardless of whether co-producing carbapenemase) than CLZ–TAZ.
Based on the susceptibilities observed in this study, third-generation cephalosporins are considered the mainstay for treating NTS, shigellosis, and gonococcal urinary tract infections (UTIs). Compared to the NS rates (1999–2003) of Taiwanese NTS isolates, in which Salmonella enterica serovar Choleraesuis accounted for most MDR-NTS isolates according to the study by Su et al,26 an increasing trend in the ciprofloxacin NS rate (5.0% vs 21.3%) was found among the Taiwanese NTS isolates in 2017. An alarmingly high rate (>45%) of NS to colistin was detected in Taiwanese NTS isolates in 2017, contrasting the only three NTS isolates harboring mcr-1 alleles. The high colistin-NS rate was notably different from the rate of 17% calculated in an India-Arabian study investigating human NTS isolates.27 However, a high mcr-1-independent colistin-NS rate among NTS isolates was found to be related to intrinsic PmrD overexpression.28,29 In contrast, nanoarchitectural changes in capsular polysaccharides that increase capsule thickness and hardness following colistin exposure were observed for only a few K. pneumoniae isolates also displaying mcr-1-independent colistin NS.29,30
Apart from the modest (28.6%) NS rate to azithromycin, approximately 40% of Taiwanese Shigella isolates showed NS to fluoroquinolones, a rate similar to the norfloxacin resistance rate (36.8%) of clinical S. flexneri isolates collected from China.31 Because >20% of NTS and fecal Shigella isolates among Taiwanese isolates were NS to two anti-pseudomonal fluoroquinolones in 2017, these agents should be cautiously prescribed when treating foodborne enteropathogens.
Ison et al32 suggested that azithromycin (<5% with MIC ≥1 mg/L) can be combined with cefixime to treat gonococcal infections in the UK. In accordance with pharmacokinetic data on azithromycin determined by Ballow et al,33 its urinary excretion was low (7.0%). A high percentage (40%) of N. gonorrhoeae isolates in this study had an azithromycin MIC of >0.25 mg/L, which is the NS MIC breakpoint of EUCAST 2018. Furthermore, among the 21 gonococcal isolates exhibiting NS to any third-generation cephalosporin agent, 13 (61.9%) had azithromycin MICs of >0.25 mg/L. Therefore, azithromycin is possibly positioned as a supplement to enhance the success of treating gonococcal UTI in Taiwan. By contrast, as suggested by Cunha34 and Agwuh and MacGowan,35 the pharmacokinetic data on doxycycline revealed a urinary excretion rate of 35%–60%, with a high urinary concentration (>150 mg/L). The use of doxycycline to treat gonococcal UTI in Taiwan is likely plausible.
There were some limitations to this survey. First, information on the clinical sources of bloodstream E. coli and K. pneumoniae isolates was lacking, precluding further analysis of differences in NSs between isolates of various origins. Second, the presence of the sequence type 131 E. coli clone among the collected E. coli isolates was not excluded. Third, we did not delineate the existence of membrane impermeability on carbapenem-resistant Enterobacteriaceae isolates.
Conclusion
Significantly higher ertapenem- and CLZ–TAZ-NS rates were found among the phenotypically ESBL-producing K. pneumoniae bloodstream isolates in Taiwan. In contrast, CAZ–AVB showed excellent in vitro efficacy against all GNB under survey, regardless of where the infection was acquired. Third-generation cephalosporins remain a reliable mainstay for treating NTS infection, fecal shigellosis, and gonococcal infections. Regular monitoring of local antimicrobial resistance profiles of clinically important pathogens is crucial for guiding the prescription of effective antibiotics and for early initiation of control measures to stop the spread of GNB with high resistance levels.
Acknowledgments
We thank all investigators of the participating hospitals for their cooperation and support in the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) Program.
Investigators from the SMART Program 2017: Shio-Shin Jean (Wan Fang Hospital, Taipei), Wen-Sen Lee (Wan Fang Hospital, Taipei), Min-Chi Lu (China Medical University Hospital, Taichung), Zhi-Yuan Shi (Taichung Veterans General Hospital, Taichung), Yao-Shen Chen (Kaohsiung Veterans General Hospital, Kaohsiung), Lih-Shinn Wang (Bud-dhist Tzu Chi General Hospital, Hualien), Shu-Hui Tseng (Ministry of Health and Welfare, Taipei), Chao-Nan Lin (National Pingtung University of Science and Technology, Pingtung), Yin-Ching Chuang (Chi Mei Hospital, Tainan), Yu-Hui Chen (Chi Mei Hospital, Tainan), Wang-Huei Sheng (National Taiwan University Hospital, Taipei), Chang-Pan Liu (MacKay Memorial Hospital, Taipei), Ting-Shu Wu (Chang Gung Memorial Hospital, Taoyuan), Chun-Ming Lee (St. Joseph’s Hospital, Yunlin), Po-Liang Lu (Kaohsiung Medical University Hospital, Kaohsiung), Muh-Yong Yen (Taipei City Hospital, Taipei), Pei-Lan Shao (National Taiwan University Hospital, Hsin-Chu), Shu-Hsing Cheng (Taoyuan General Hospital, Taoyuan), Chi-Ying Lin (National Taiwan University Hospital, Yun-Lin), Ming-Huei Liao (National Pingtung University of Science and Technology, Pingtung), Yen-Hsu Chen (Kaohsiung Medical University, Kaohsiung), Wen-Chien Ko (National Cheng Kung University Hospital, Tainan), Fu-Der Wang (Taipei Veterans General Hospital, Taipei), Po-Ren Hsueh (National Taiwan University Hospital, Taipei), and Infection Control Society of Taiwan.
This work was supported by grants from the Centers for Disease Control and Prevention, Minister of Health and Welfare, Executive Yuan, Taiwan (MOHW106-CDC-C-114-114701).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.Chiu CC, Lin TC, Wu RX, et al. Etiologies of community-onset urinary tract infections requiring hospitalization and antimicrobial susceptibilities of causative microorganisms. J Microbiol Immunol Infect. 2017;50(6):879–885. doi: 10.1016/j.jmii.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Trecarichi EM, Pagano L, Candoni A, et al. HeMABIS Registry— SEIFEM Group, Italy Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect. 2015;21(4):337–343. doi: 10.1016/j.cmi.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Chen CY, Tien FM, Sheng WH, et al. Clinical and microbiological characteristics of bloodstream infections among patients with haematological malignancies with and without neutropenia at a medical centre in northern Taiwan, 2008-2013. Int J Antimicrob Agents. 2017;49(3):272–281. doi: 10.1016/j.ijantimicag.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Hu YF, Hou CJ, Kuo CF, et al. Emergence of carbapenem-resistant Acinetobacter baumannii ST787 in clinical isolates from blood in a tertiary teaching hospital in Northern Taiwan. J Microbiol Immunol Infect. 2017;50(5):640–645. doi: 10.1016/j.jmii.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Lee CM, Lai CC, Chiang HT, et al. Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan. J Microbiol Immunol Infect. 2017;50(2):133–144. doi: 10.1016/j.jmii.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Jean SS, Lee WS, Lam C, Hsu CW, Chen RJ, Hsueh PR. Carbapenemase-producing Gram-negative bacteria: current epidemics, antimicrobial susceptibility and treatment options. Future Microbiol. 2015;10(3):407–425. doi: 10.2217/fmb.14.135. [DOI] [PubMed] [Google Scholar]
- 8.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 9.van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: Second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis. 2016;63(2):234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu SK, Wu TL, Chuang YC, et al. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One. 2013;8(7):e69428. doi: 10.1371/journal.pone.0069428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JT, Wu UI, Lauderdale TL, et al. Carbapenem-nonsusceptible Enterobacteriaceae in Taiwan. PLoS One. 2015;10(3):e0121668. doi: 10.1371/journal.pone.0121668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruppé É, Woerther PL, Barbier F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care. 2015;5(1):61. doi: 10.1186/s13613-015-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livermore DM. Bacterial resistance: origins, epidemiology, and impact. Clin Infect Dis. 2003;36(Suppl 1):S11–S23. doi: 10.1086/344654. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Eighth Informational Supplement M100-S28. Wayne (PA): CLSI; 2018. [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing [webpage on the Internet] Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 5.0. [Accessed March 26, 2018]. Available from: http://www.eucast.org/clinical_breakpoints/
- 16.Rebelo AR, Bortolaia V, Kjeldgaard JS, et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018;23(6) doi: 10.2807/1560-7917.ES.2018.23.6.17-00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SA, Hindler JA, Chengcuenca A, Humphries RM. Use of ancillary carbapenemase tests to improve specificity of phenotypic definitions for carbapenemase-producing Enterobacteriaceae. J Clin Microbiol. 2017;55(6):1827–1836. doi: 10.1128/JCM.00157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma L, Wang JT, Wu TL, et al. Emergence of OXA-48-Producing Klebsiella pneumoniae in Taiwan. PLoS One. 2015;10(9):e0139152. doi: 10.1371/journal.pone.0139152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jean SS, Hsueh PR, SMART Asia-Pacific Group Distribution of ESBLs, AmpC β-lactamases and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal and urinary tract infections in the Asia-Pacific region during 2008-14: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) J Antimicrob Chemother. 2017;72(1):166–171. doi: 10.1093/jac/dkw398. [DOI] [PubMed] [Google Scholar]
- 20.Jean SS, Hsueh PR, Lee WS, et al. Nationwide surveillance of antimicrobial resistance among Enterobacteriaceae in intensive care units in Taiwan. Eur J Clin Microbiol Infect Dis. 2009;28(2):215–220. doi: 10.1007/s10096-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 21.Girometti N, Lewis RE, Giannella M, et al. Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore) 2014;93(17):298–309. doi: 10.1097/MD.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Siu LK, Lin JC, et al. Updated molecular epidemiology of carbapenem-non-susceptible Escherichia coli in Taiwan: first identification of KPC-2 or NDM-1-producing E. coli in Taiwan. BMC Infect Dis. 2013;13:599. doi: 10.1186/1471-2334-13-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan J, Li X, Chen Y, et al. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis. 2017;17(4):400–410. doi: 10.1016/S1473-3099(16)30528-X. [DOI] [PubMed] [Google Scholar]
- 24.Hasman H, Hammerum AM, Hansen F, et al. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill. 2015;20(49) doi: 10.2807/1560-7917.ES.2015.20.49.30085. [DOI] [PubMed] [Google Scholar]
- 25.Tato M, García-Castillo M, Bofarull AM, Cantón R, CENIT Study Group In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacteriaceae recovered in Spanish medical centres: Results of the CENIT study. Int J Antimicrob Agents. 2015;46(5):502–510. doi: 10.1016/j.ijantimicag.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Su LH, Wu TL, Chia JH, Chu C, Kuo AJ, Chiu CH. Increasing ceftriaxone resistance in Salmonella isolates from a university hospital in Taiwan. J Antimicrob Chemother. 2005;55(6):846–852. doi: 10.1093/jac/dki116. [DOI] [PubMed] [Google Scholar]
- 27.Damjanovic V, Furtado M, Patmore M. Antibiotic sensitivity of enteropathogenic bacteria isolated from patients in a Sharjah hospital. J Hyg (Lond) 1984;92(2):205–208. doi: 10.1017/s0022172400064226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hjort K, Nicoloff H, Andersson DI. Unstable tandem gene amplification generates heteroresistance (variation in resistance within a population) to colistin in Salmonella enterica. Mol Microbiol. 2016;102(2):274–289. doi: 10.1111/mmi.13459. [DOI] [PubMed] [Google Scholar]
- 29.Zhou K, Cattoir V, Xiao Y. Intrinsic colistin resistance. Lancet Infect Dis. 2016;16(11):1227–1228. doi: 10.1016/S1473-3099(16)30394-2. [DOI] [PubMed] [Google Scholar]
- 30.Formosa C, Herold M, Vidaillac C, Duval RE, Dague E. Unravelling of a mechanism of resistance to colistin in Klebsiella pneumoniae using atomic force microscopy. J Antimicrob Chemother. 2015;70(8):2261–2270. doi: 10.1093/jac/dkv118. [DOI] [PubMed] [Google Scholar]
- 31.Qin T, Bi R, Fan W, Kang H, Ma P, Gu B. Novel mutations in quinolone resistance-determining regions of gyrA, gyrB, parC and parE in Shigella flexneri clinical isolates from eastern Chinese populations between 2001 and 2011. Eur J Clin Microbiol Infect Dis. 2016;35(12):2037–2045. doi: 10.1007/s10096-016-2761-2. [DOI] [PubMed] [Google Scholar]
- 32.Ison CA, Town K, Obi C, et al. GRASP Collaborative Group Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007-2011. Lancet Infect Dis. 2013;13(9):762–768. doi: 10.1016/S1473-3099(13)70143-9. [DOI] [PubMed] [Google Scholar]
- 33.Ballow CH, Amsden GW, Highet VS, Forrest A. Pharmacokinetics of oral azithromycin in serum, urine, polymorphonuclear leucocytes and inflammatory vs non-inflammatory skin blisters in healthy volunteers. Clin Drug Investig. 1998;15(2):159–167. doi: 10.2165/00044011-199815020-00009. [DOI] [PubMed] [Google Scholar]
- 34.Cunha BA. Oral doxycycline for non-systemic urinary tract infections (UTIs) due to P. aeruginosa and other Gram negative uropathogens. Eur J Clin Microbiol Infect Dis. 2012;31(11):2865–2868. doi: 10.1007/s10096-012-1680-0. [DOI] [PubMed] [Google Scholar]
- 35.Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother. 2006;58(2):256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]