Abstract
Background
Noonan syndrome (NS) is an autosomal dominant genetic condition that has a number of clinical features, including bleeding diathesis and a number of hematological abnormalities including clotting factor deficiencies, von Willebrand disease and abnormal platelet count/function.
Methods
We evaluated the frequency/types of bleeding disorders, and associated hematological laboratory findings, in patients with NS, using published data from 1965 to 2014.
Results
Of 45 studies identified, 31 included data for 428 patients with NS. Of these patients, 43% had reported bleeding, 26% had no reported bleeding and no bleed data was reported for 31%. Most patients (90%) had bleeding-related laboratory test abnormalities, but only 194 (45%) had a confirmed diagnosis of a specific bleeding disorder. Abnormal laboratory tests included: prolonged prothrombin time, activated partial thromboplastin time, and other platelet-related disorders. Of the 194 patients with a confirmed diagnosis of a specific bleeding disorder, 153 (79%) had single clotting factor deficiencies, von Willebrand disease or platelet-related disorders, and 41 (21%) had multiple deficiencies including platelet-related disorders.
Conclusion
As patients with NS can experience multiple bleeding disorders, including abnormal platelet function, clinical evaluations should be performed at diagnosis, after diagnosis, before any surgery is undertaken, and if patients become symptomatic.
Keywords: bleeding disorders, children, laboratory test abnormalities, Noonan syndrome, screening, surgical procedures
Introduction
Noonan syndrome (NS) is an autosomal dominant genetic condition that affects one in 1,000–2,500 individuals. Typical signs of NS include characteristic facial features, short stature, congenital heart defect, skeletal and thoracic anomalies, developmental delay, and bleeding problems; these are seen in 30%–72% of patients with the condition.1 Until recently, the molecular etiology of NS was unknown; this, coupled with the highly variable phenotype observed in these patients, made diagnosis difficult. It is now recognized, however, that ~50% of patients with NS demonstrate pathological variants of the PTPN11 gene. This results in the development of NS, or another disorder involving PTPN11 (such as LEOPARD syndrome), where cardiac defects also frequently manifest as pulmonary valve stenosis and hypertrophic cardiomyopathy.2 A number of genes are known to play a role in NS; molecular testing of the four best-recognized genes in NS is now generally available and has identified mutations in PTPN11 in ~50%, KRAS in <5%, SOS1 in ~15%, and RAF1 in 3%–17% of patients with the disorder.3
NS can be associated with an increased risk of bleeding and bruising, and a variety of bleeding abnormalities, with factor XI deficiency and platelet abnormalities being described most frequently.4–6 However, it is not clear whether there is any direct correlation between bleeding risk and results of coagulation tests so there is currently increased focus on evaluating bleeding risk in patients with NS. As a consequence of their condition, many patients undergo multiple surgeries, often starting in early childhood, so it is important to establish bleeding risk prior to any intervention. For example, pulmonary valve stenosis, which is often reported in patients with NS, is typically unsuitable for interventional balloon dilation due to bleeding risk, and usually requires surgery for correction. Also, the presence of a partial atrioventricular canal with outflow obstruction can result in a demanding surgical procedure to correct the malformation.7 The aim of this systematic review was to identify the frequency and types of bleeding disorders, and to evaluate any links with associated laboratory findings, in patients with NS, with a view to providing physicians with guidelines on how to evaluate bleeding complications in patients with NS.
Methods and results
Publications from 1965 to 2014 were reviewed. They included trials, case reports/series, and reviews identified via MEDLINE®, EMBASE®, and Scopus®. Key search terms included: Noonan; bleed*3; hemorrhag*3; thrombocytop*enia; h*emostatic; h*emostasis; bleed* diathesis; platelet* disorder*.
Studies of patients with NS were included in the analysis only if the bleeding phenotype was described. Studies were excluded if NS was not present or not confirmed in all cases; the publication was a secondary analysis (review of other case reports with no new information); there was no patient-level bleeding phenotype information reported for any patients. All available patient data were abstracted, and included demographics, bleeding symptoms, laboratory abnormalities, bleeding score, and specific disorders reported. The numbers of patients in each study are shown in Table S1.
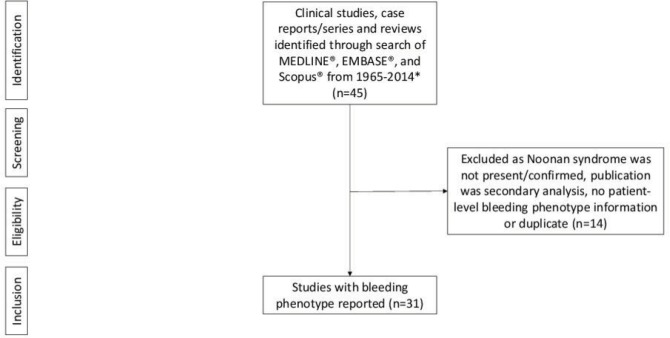
Of 45 studies identified,1,4–6,8–48 314–6,8–35 had relevant data from 428 patients with NS (Figure 1). Of the 31 studies included, 13 were single case studies, five included <10 patients, and 13 included >10 patients. The largest cumulative study, including 151 patients,18 had previously been reported with 725 and 3133 patients; however, different sets of information are presented in each publication. Nearly half (49%) of the patients were male; 43% (183 patients) had reported bleeding, 26% (112 patients) had not reported bleeding, and for 31% (133 patients) there was no data on bleeding. Most patients (384; 90%) had some reported laboratory test abnormalities (platelet function and/or coagulation abnormalities). Of the patients with reported bleeding, abnormal laboratory tests included: prolonged prothrombin time (PT) (29 patients; 7%); activated partial thromboplastin time (aPTT) (71 patients; 17%); PT/aPTT (23 patients; 5%); and platelet-related disorders (42 patients; 10%). There were also reports of normal PT (104 patients; 24%) and aPTT (157 patients; 37%).
Figure 1.
Prisma figure of studies for inclusion.
Note: *Key search terms included: Noonan; bleed*3; hemorrhag*3; thrombocytop*enia; h*emostatic; h*emostasis; bleed* diathesis; platelet* disorder*.
In one of the studies of 39 patients (and 28 controls), nearly 40% of patients with NS had a bleeding diathesis but >90% had platelet function and/or coagulation abnormalities.9 Another study of 13 patients suggested that bleeding signs do not appear to be due to coagulation disorders.22
Of the 428 patients evaluated, only 46% (195 patients) had a specific diagnosis of factor deficiency, von Willebrand disease, or platelet-related disorder. Of these patients, 154 (78%) had single-factor deficiencies or von Willebrand disease, and 42 (22%) had multiple factor deficiencies (Table 1). Of the factor deficiencies, factor XI (FXI) deficiency was most common (81 patients), followed by factor XII (FXII) (34 patients), and factor VIII (FVIII) (28 patients) (Table 2). Platelet-related disorders were reported in 46 patients, with thrombocytopenia and platelet aggregation abnormality being most commonly reported. In the 42 patients reporting multiple factor deficiencies, FXI+FXII combined (11 patients), and FVIII+ FXI combined (seven patients) were the most common (Table 2).
Table 1.
Factor deficiencies and other bleeding disorders reported in patients with NS
| Type of deficiency | Single factor deficiency | Multiple factor deficiency | Total |
|---|---|---|---|
| FI | 2 | 1 | 3 |
| FII | 0 | 1 | 1 |
| FV | 0 | 5 | 5 |
| FVII | 6 | 6 | 12 |
| FVIII | 18 | 12 | 30 |
| FIX | 4 | 7 | 11 |
| FX | 0 | 5 | 5 |
| FXI | 57 | 30 | 87 |
| FXII | 19 | 16 | 35 |
| FXIII | 1 | 0 | 1 |
| vWD | 4 | 6 | 10 |
| Platelet-relateda | 42 | 5 | 47 |
| Totalb | 153 | 42 | 195 |
Notes:
Thrombocytopenia: 20 (two were transient after birth); ITP + cyclooxygenase deficiency: 1; Bernard–Soulier syndrome: 1; platelet aggregation abnormality: 19; platelet storage pool deficiency: 1.
Total is the number of patients with factor deficiencies, and for multiple factor deficiencies patients could have more than one deficiency type.
Abbreviations: ITP, idiopathic thrombocytopenia; FI, factor I; FII, factor II; FV, factor V; FVII, factor VII; FVIII, factor VIII; FIX, factor IX; FX, factor X; FXI, factor XI, FXII, factor XII; FXIII, factor XIII; NS, Noonan syndrome; vWD, von Willebrand disease.
Table 2.
Multiple factor deficiencies and platelet-related disorders reported in patients with NS
| Multiple factor deficiency | Number of patients |
|---|---|
| FI + FXI + FXII | 1 |
| FII + FXI | 1 |
| FV + FVII + FX | 1 |
| FV + FVIII | 3 |
| FV + FIX + FX | 1 |
| FVII + FIX + FXI + vWD | 2 |
| FVII + FX | 2 |
| FVII + FXII + platelet-related disorder | 1 |
| FVIII + FXI + FXII | 1 |
| FVIII + vWD | 1 |
| FVIII + FXI | 7 |
| FIX + FX | 1 |
| FIX + FXII + platelet-related disorder | 1 |
| FIX + FXI | 2 |
| FXI + FXII | 11 |
| FXI + vWD | 2 |
| FXI + platelet-related disorder | 3 |
| FXII + vWD | 1 |
| Total | 42 |
Notes:
Platelet-related disorders included: thrombocytopenia; idiopathic thrombocytopenic purpura + cyclooxygenase deficiency; Bernard–Soulier syndrome, platelet aggregation abnormality; platelet storage pool deficiency.
Abbreviations: FI, factor I; FII, factor II; FV, factor V; FVII, factor VII; FVIII, factor VIII; FIX, factor IX; FX, factor X; FXI, factor XI, FXII, factor XII; FXIII, factor XIII; NS, Noonan syndrome; vWD, von Willebrand disease.
Discussion
Physicians are diagnosing NS more readily; however, this literature review identifies a clear gap in the evaluation and diagnosis of bleeding diathesis and specific bleeding disorders in these patients. Indeed, several studies have highlighted that there is no correlation between coagulation study results and bleeding risk. In one study of 39 patients (and 28 controls), whilst only 40% of patients with NS had a bleeding diathesis, more than 90% had platelet function and/or coagulation abnormalities.9 Another study of 13 patients suggested that bleeding signs do not appear to be due to coagulation disorders.22 These findings suggest that screening needs to include tests beyond PT, aPTT and platelet count. Therefore, guidelines for clinical evaluation in NS should highlight the importance of comprehensive testing, as well as the need for specialist involvement by a pediatric hematologist in differential diagnosis, both at diagnosis of NS and pre-operatively, whenever screening test abnormalities are identified.49
Although there is currently no consensus on the optimum strategy for diagnosis of bleeding in patients with NS, a review by Roberts et al in 2013 suggested that at diagnosis of NS a complete blood cell count (CBC) with differential and PT/aPTT should be undertaken. After diagnosis, repeat CBC with differential and PT/aPTT if aged 6–12 months at initial screen, and pre-operatively, CBC with differential and PT/aPTT, then in consultation with hematologist FIX, FXI and FXII concentrations, von Willebrand factor, and platelet aggregation. Furthermore, if symptomatic, PT/aPTT if bleeding is abnormal or persistent, then refer to a hematologist.50
Due to the inconsistent reporting of platelet test results and platelet disorders in the studies included in this analysis, it does not appear that appropriate tests are being undertaken in clinical practice. Platelet aggregation, for example, needs to be conducted on a fresh specimen and is more likely to be offered at regional centers. It is therefore important to distinguish between abnormalities in platelet function suspected by screening tests (eg, platelet function analysis-100) and confirmed platelet function disorders based upon aggregometry patterns and confirmed with flow cytometry, electron microscopy, or genetic testing.
The surprisingly high incidence of multiple coagulation disorders suggests that work up in patients with NS needs to be more comprehensive. While FXI deficiency was most common individually and in combination, there were several multiple defects (both factor deficiencies and platelet function disorders) reported in the same patient.
Given that several germline mutations including PTPN11 and SOS1 are reported in patients with NS, there may be a possible correlation with bleeding phenotype. However, of the studies included in our analysis, only a few evaluated genetic mutations in relation to the reported bleeding disorder and results were not consistent. An evaluation of patients with NS reported PTPN11 gene mutations in 21 of 27 patients, and hematological disorders in nine of 27, with authors suggesting a near significant correlation.12 Another study of 19 patients with NS showed that coagulation abnormalities were reported in patients with PTPN11, SOS1 and SOS1/RAF1 mutations and without a gene mutation, but they were not correlated with a specific gene mutation.14 In addition, a study of 13 patients with NS (six with PTPN11 mutations) found that 12/13 had normal hematological assessment and only one had a platelet function disorder (storage pool disease).22 One of the studies of 15 patients (14 with PTPN11 mutation and one with SOS1 mutation), showed that nine had a bleeding diathesis and complained of easy bruising, despite having normal platelet count, basic coagulation parameters, fibrinogen and antithrombin, and without a relevant reduction of coagulation factor activities. Furthermore, three of them had potentially acquired von Willebrand disease, which the authors suggested may explain the bleeding in those with pulmonary stenosis.47 Further analysis of a large cohort of individuals with NS has suggested that PTPN11 gene mutations are more likely to be found in those with pulmonary stenosis, whereas hypertrophic cardiomyopathy is less prevalent among those with PTPN11 mutations.2 Thus, bleeding disorders in NS do not appear to correlate with a particular genotype.
There are limitations to this analysis, which included mostly spontaneous case reports/series, because the reporting of symptoms, laboratory evaluations, and diagnosis may have been incomplete. Furthermore, while attempts were made to remove duplicate reporting of the same case in both an individual case report and prior compiled series/review, there may have been cases that were not explicitly referenced. When limited coagulation evaluation does not confirm to one or more specific diagnoses, it underscores the importance of referral to a pediatric hematologist so that the patient’s parents can give a detailed bleeding history. This should then be followed through with a differential diagnosis, using appropriate laboratory assays. In future case reports/series, there is an important need to ensure that symptoms, laboratory results, and ultimate diagnoses are tracked.
Conclusion
Patients with NS can experience multiple bleeding disorders, including platelet-related disorders. Comprehensive clinical evaluations should be carried out both at diagnosis, after diagnosis if patients are symptomatic, and prior to any surgical procedures, even if tests are normal, the risk for bleeding events should be carefully considered. Furthermore, as there is no current consensus on management of bleeding complications in patients with NS, it is important that physicians closely monitor these patients.
Supplementary materials
Table S1.
Case reports/series on bleeding disorders in patients with NS
| Reference | Number of patients |
|---|---|
| Argyrou A, et al. Arch Hell Med, 20101 | 1 |
| Artoni A, et al. Pediatrics, 20142 | 39 |
| Bertola DR, et al. Rev Hosp Clin Fac Med Sao Paulo, 20033 | 30 |
| González Casado I, et al. Horm Res Paediatr. 20114 | 27 |
| de Haan M, et al. Am J Med Genet. 19885 | 12 |
| Flick JT, et al. Am J Clin Pathol. 19916 | 1 |
| Gamba G, et al. Horm Res Paediatr. 20117 | 19 |
| Kitchens CS, et al. J Pediatr. 19838 | 4 |
| Koc A, et al. Turk J Haematol. 20019 | 1 |
| Massarano AA, et al. Acta Paediatr. 199610 | 18 |
| Nunes P, et al. BMJ Case Rep. 201211 | 1 |
| Patrick K, et al. Br J Haematol. 201012 | 1 |
| Sharland M, et al. Arch Dis Child. 199213 | 151a |
| Sharland M, et al. Lancet. 199214 | 72a |
| Staudt JM, et al. Scand J Plast Reconstr Surg Hand Surg. 200515 | 1 |
| Stoffman JM, et al. Blood. 200416 | 28 |
| Tanaka Y, et al. Am J Med Genet. 199917 | 2 |
| Troiano M, et al. Horm Res Paediatr. 201118 | 13 |
| Vortia E, et al. Am J Gastroenterol. 201119 | 2 |
| Waespe N, et al. Hämostaseologie. 201320 | 15 |
| Witt DR, et al. Am J Med Genet. 198821 | 19 |
| Caralis DG, et al. Johns Hopkins Med J. 197422 | 1 |
| Evans DG, et al. Clin Genet. 199123 | 1 |
| Grange CS, et al. Can J Anaesth. 199824 | 1 |
| Humbert JA, et al. Lancet. 197025 | 1 |
| Komp DM, et al. Pediatr Res. 197526 | 2 |
| Phillips WG, et al. Br J Dermatol. 199327 | 1 |
| Sgouros SN, et al. J Gastroenterol Hepatol. 200428 | 1 |
| Sharland M, et al. J Med Genet. 199029 | 31a |
| Singer ST, et al. J Pediatr Hematol Oncol. 199730 | 3 |
| Sugar AW, et al. J Oral Maxillofac Surg. 199431 | 1 |
Note:
Substudies (31 and 72 of 151 patients from Sharland et al. Arch Dis Child. 1992).63
Abbreviation: NS, Noonan syndrome.
References
- 1.Argyrou A, Marinakis T, Kalofolias N, Papazoglou S. Thrombotic thrombocytopenic purpura in a young patient with Noonan syndrome and systemic lupus erythematosus. Arch Hell Med. 2010;27(3):545–548. [Google Scholar]
- 2.Artoni A, Selicorni A, Passamonti SM, et al. Hemostatic abnormalities in Noonan syndrome. Pediatrics. 2014;133(5):e1299–e1304. doi: 10.1542/peds.2013-3251. [DOI] [PubMed] [Google Scholar]
- 3.Bertola DR, Carneiro JD, D’Amico EA, et al. Hematological findings in Noonan syndrome. Rev Hosp Clin Fac Med Sao Paulo. 2003;58(1):5–8. doi: 10.1590/s0041-87812003000100002. [DOI] [PubMed] [Google Scholar]
- 4.González-Casado I, Barreda Bonis A, Salamanca Fresno L, et al. Noonan syndrome and hemato-oncological anomalies. Horm Res Paediatr. 2011;76(Suppl 2):167–168. [Google Scholar]
- 5.de Haan M, Vd Kamp JJ, Briët E, Dubbeldam J. Noonan syndrome: partial factor XI deficiency. Am J Med Genet. 1988;29(2):277–282. doi: 10.1002/ajmg.1320290205. [DOI] [PubMed] [Google Scholar]
- 6.Flick JT, Singh AK, Kizer J, Lazarchick J. Platelet dysfunction in Noonan’s syndrome. A case with a platelet cyclooxygenase-like deficiency and chronic idiopathic thrombocytopenic purpura. Am J Clin Pathol. 1991;95(5):739–742. doi: 10.1093/ajcp/95.5.739. [DOI] [PubMed] [Google Scholar]
- 7.Gamba G, Marabotto F, Losa L, et al. Co-agulation factor deficiencies and abnormal bleeding in Noonan’s syndrome. Horm Res Paediatr. 2011;76(Suppl 2):321. [Google Scholar]
- 8.Kitchens CS, Alexander JA. Partial deficiency of coagulation factor XI as a newly recognized feature of Noonan syndrome. J Pediatr. 1983;102(2):224–227. doi: 10.1016/s0022-3476(83)80525-3. [DOI] [PubMed] [Google Scholar]
- 9.Koç A, Kösecik M, Tatlı MM, Atas A, Emiroğlu HH. Bernard-Soulier Syndrome like platelet defect in a patient with noonan syndrome; a case report. Turk J Haematol. 2001;18(3):191–193. [PubMed] [Google Scholar]
- 10.Massarano AA, Wood A, Tait RC, Stevens R, Super M. Noonan syndrome: coagulation and clinical aspects. Acta Paediatr. 1996;85(10):1181–1185. doi: 10.1111/j.1651-2227.1996.tb18225.x. [DOI] [PubMed] [Google Scholar]
- 11.Nunes P, Aguilar S, Prado SN, Palaré MJ, Ferrão A, Morais A. Severe congenital thrombocytopaenia − first clinical manifestation of Noonan syndrome. BMJ Case Rep. 2012;2012:bcr1020114940. doi: 10.1136/bcr.10.2011.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick K, Makris M. Images in haematology. Noonan syndrome associated with bleeding disorders. Br J Haematol. 2010;151(2):117. doi: 10.1111/j.1365-2141.2010.08310.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharland M, Burch M, McKenna WM, Paton MA. A clinical study of Noonan syndrome. Arch Dis Child. 1992;67(2):178–183. doi: 10.1136/adc.67.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharland M, Patton MA, Talbot S, Chitolie A, Bevan DH. Coagulation-factor deficiencies and abnormal bleeding in Noonan’s syndrome. Lancet. 1992;339(8784):19–21. doi: 10.1016/0140-6736(92)90141-o. [DOI] [PubMed] [Google Scholar]
- 15.Staudt JM, van der Horst CM, Peters M, Melis P. Bleeding diathesis in Noonan syndrome. Scand J Plast Reconstr Surg Hand Surg. 2005;39(4):247–248. doi: 10.1080/02844310510006231. [DOI] [PubMed] [Google Scholar]
- 16.Stoffman JM, Chodirker BN, Israels SJ. Coagulation abnormalities in patients with Noonan Syndrome − a single centre case series. Blood. 2004;104(11):1035–1035. [Google Scholar]
- 17.Tanaka Y, Masuno M, Iwamoto H, et al. Noonan syndrome and cavernous hemangioma of the brain. Am J Med Genet. 1999;82(3):212–214. doi: 10.1002/(sici)1096-8628(19990129)82:3<212::aid-ajmg3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Troiano M, Gottlieb S, Rey R, et al. Noonan Syndrome: assessment of bleeding disorders. Horm Res Paediatr. 2011;76(Suppl 2):21. [Google Scholar]
- 19.Vortia E, Mahajan L, Kaplan B. Duodenal hematoma complicating upper endoscopy with biopsy in two pediatric patients with Noonan’s syndrome: what pediatric gastroenterologists need to know. Am J Gastroenterol. 2011;106(Suppl 2):S400. [Google Scholar]
- 20.Waespe N, Prader S, Kroiss S, Knirsch W, Speer O, Schmuge M. Clinical and laboratory manifestations of bleeding diathesis in Noonan syndrome. Hämostaseologie. 2013;33(1):A74. [Google Scholar]
- 21.Witt DR, Mcgillivray BC, Allanson JE, et al. Bleeding diathesis in Noonan syndrome: a common association. Am J Med Genet. 1988;31(2):305–317. doi: 10.1002/ajmg.1320310208. [DOI] [PubMed] [Google Scholar]
- 22.Caralis DG, Char F, Graber JD, Voigt GC. Delineation of multiple cardiac anomalies associated with the Noonan syndrome in an adult and review of the literature. Johns Hopkins Med J. 1974;134(6):346–355. [PubMed] [Google Scholar]
- 23.Evans DG, Lonsdale RN, Patton MA. Cutaneous lymphangioma and amegakaryocytic thrombocytopenia in Noonan syndrome. Clin Genet. 1991;39(3):228–232. doi: 10.1111/j.1399-0004.1991.tb03017.x. [DOI] [PubMed] [Google Scholar]
- 24.Grange CS, Heid R, Lucas SB, Ross PL, Douglas MJ. Anaesthesia in a parturient with Noonan’s syndrome. Can J Anaesth. 1998;45(4):332–336. doi: 10.1007/BF03012024. [DOI] [PubMed] [Google Scholar]
- 25.Humbert JA, Hammond KB, Hathaway WE. Trimethylaminuria: the fish-odour syndrome. Lancet. 1970;2(7676):770–771. doi: 10.1016/s0140-6736(70)90241-2. [DOI] [PubMed] [Google Scholar]
- 26.Komp DM. “Car. factor” deficiency revisited”. Pediatric Res. 1975;9(4):184–189. doi: 10.1203/00006450-197504000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Phillips WG, Dunnill MG, Kurwa AR, Black MM. Orbital oedema: an unusual presentation of Noonan’s syndrome. Br J Dermatol. 1993;129(2):190–192. doi: 10.1111/j.1365-2133.1993.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 28.Sgouros SN, Karamanolis G, Papadopoulou E, et al. Postbiopsy intramural hematoma of the duodenum in an adult with Noonan’s syndrome. J Gastroenterol Hepatol. 2004;19(10):1217–1219. doi: 10.1111/j.1440-1746.2004.02931.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharland M, Patton MA, Chitolie A, Talbot S, Bevan D. Coagulation factor abnormalities in Noonan syndrome. J Med Genet. 1990;27(10):646. [Google Scholar]
- 30.Singer ST, Hurst D, Addiego JE. Bleeding disorders in Noonan syndrome: three case reports and review of the literature. J Pediatr Hematol Oncol. 1997;19(2):130–134. doi: 10.1097/00043426-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Sugar AW, Ezsias A, Bloom AL, Morcos WE. Orthognathic surgery in a patient with Noonan’s syndrome. J Oral Maxillofac Surg. 1994;52(4):421–425. doi: 10.1016/0278-2391(94)90454-5. [DOI] [PubMed] [Google Scholar]
Acknowledgments
Some study information presented in this manuscript was previously presented as a poster at The American Society of Pediatric Hematology/Oncology (ASPHO) 2016 congress. Poster#549: “Evaluation of bleeding disorders in patients with Noonan syndrome: a systematic review”. Nugent D, Romano A, Sabharwal S, Germak J, Cooper DL. Editorial assistance for this manuscript was provided by PAREXEL, and funded by Novo Nordisk A/S.
Footnotes
Disclosure
Alicia A Romano is a consultant for Novo Nordisk and Genentech, and is on the speaker bureau for Novo Nordisk, Genentech, and Genzyme Corporation. Shreya Sabharwal and David L Cooper are employees of Novo Nordisk. The authors report no other conflicts of interest in this work.
References
- 1.Briggs BJ, Dickerman JD. Bleeding disorders in Noonan syndrome. Pediatr Blood Cancer. 2012;58(2):167–172. doi: 10.1002/pbc.23358. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia M, Kalidas K, Shaw A, et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70(6):1555–1563. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner AM. Noonan syndrome. J Paediatr Child Health. 2014;50(10):E14–E20. doi: 10.1111/j.1440-1754.2010.01970.x. [DOI] [PubMed] [Google Scholar]
- 4.Massarano AA, Wood A, Tait RC, Stevens R, Super M. Noonan syndrome: coagulation and clinical aspects. Acta Paediatr. 1996;85(10):1181–1185. doi: 10.1111/j.1651-2227.1996.tb18225.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharland M, Patton MA, Talbot S, Chitolie A, Bevan DH. Coagulation-factor deficiencies and abnormal bleeding in Noonan’s syndrome. Lancet. 1992;339(8784):19–21. doi: 10.1016/0140-6736(92)90141-o. [DOI] [PubMed] [Google Scholar]
- 6.Kitchens CS, Alexander JA. Partial deficiency of coagulation factor XI as a newly recognized feature of Noonan syndrome. J Pediatr. 1983;102(2):224–227. doi: 10.1016/s0022-3476(83)80525-3. [DOI] [PubMed] [Google Scholar]
- 7.Formigari R, Michielon G, Digilio MC, et al. Genetic syndromes and congenital heart defects: how is surgical management affected? Eur J Cardiothorac Surg. 2009;35(4):606–614. doi: 10.1016/j.ejcts.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Argyrou A, Marinakis T, Kalofolias N, Papazoglou S. Thrombotic thrombocytopenic purpura in a young patient with Noonan syndrome and systemic lupus erythematosus. Arch Hell Med. 2010;27(3):545–548. [Google Scholar]
- 9.Artoni A, Selicorni A, Passamonti SM, et al. Hemostatic abnormalities in Noonan syndrome. Pediatrics. 2014;133(5):e1299–e1304. doi: 10.1542/peds.2013-3251. [DOI] [PubMed] [Google Scholar]
- 10.Bertola DR, Carneiro JD, D’Amico EA, et al. Hematological findings in Noonan syndrome. Rev Hosp Clin Fac Med Sao Paulo. 2003;58(1):5–8. doi: 10.1590/s0041-87812003000100002. [DOI] [PubMed] [Google Scholar]
- 11.de Haan M, Vd Kamp JJ, Briët E, Dubbeldam J. Noonan syndrome: partial factor XI deficiency. Am J Med Genet. 1988;29(2):277–282. doi: 10.1002/ajmg.1320290205. [DOI] [PubMed] [Google Scholar]
- 12.González-Casado I, Barreda Bonis A, Salamanca Fresno L, et al. Noonan syndrome and hemato-oncological anomalies. Horm Res Paediatr. 2011;76(Suppl 2):167–168. [Google Scholar]
- 13.Flick JT, Singh AK, Kizer J, Lazarchick J. Platelet dysfunction in Noonan’s syndrome. A case with a platelet cyclooxygenase-like deficiency and chronic idiopathic thrombocytopenic purpura. Am J Clin Pathol. 1991;95(5):739–742. doi: 10.1093/ajcp/95.5.739. [DOI] [PubMed] [Google Scholar]
- 14.Gamba G, Marabotto F, Losa L, et al. Co-agulation factor deficiencies and abnormal bleeding in Noonan’s syndrome. Horm Res Paediatr. 2011;76(Suppl 2):321. [Google Scholar]
- 15.Koç A, Kösecik M, Tatlı MM, Atas A, Emiroğlu HH. Bernard-Soulier syndrome like platelet defect in a patient with Noonan Syndrome; a case report. Turk J Haematol. 2001;18(3):191–193. [PubMed] [Google Scholar]
- 16.Nunes P, Aguilar S, Prado SN, Palaré MJ, Ferrão A, Morais A. Severe congenital thrombocytopaenia−first clinical manifestation of Noonan syndrome. BMJ Case Rep. 2012;2012:bcr1020114940. doi: 10.1136/bcr.10.2011.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrick K, Makris M. Images in haematology. Noonan syndrome associated with bleeding disorders. Br J Haematol. 2010;151(2):117. doi: 10.1111/j.1365-2141.2010.08310.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharland M, Burch M, McKenna WM, Paton MA. A clinical study of Noonan syndrome. Arch Dis Child. 1992;67(2):178–183. doi: 10.1136/adc.67.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staudt JM, van der Horst CM, Peters M, Melis P. Bleeding diathesis in Noonan syndrome. Scand J Plast Reconstr Surg Hand Surg. 2005;39(4):247–248. doi: 10.1080/02844310510006231. [DOI] [PubMed] [Google Scholar]
- 20.Stoffman JM, Chodirker BN, Israels SJ. Coagulation abnormalities in patients with Noonan Syndrome − a single centre case series. Blood. 2004;104(11):1035–1035. [Google Scholar]
- 21.Tanaka Y, Masuno M, Iwamoto H, et al. Noonan syndrome and cavernous hemangioma of the brain. Am J Med Genet. 1999;82(3):212–214. doi: 10.1002/(sici)1096-8628(19990129)82:3<212::aid-ajmg3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Troiano M, Gottlieb S, Rey R, et al. Noonan Syndrome: assessment of bleeding disorders. Horm Res Paediatr. 2011;76(Suppl 2):21. [Google Scholar]
- 23.Vortia E, Mahajan L, Kaplan B. Duodenal hematoma complicating upper endoscopy with biopsy in two pediatric patients with Noonan’s syndrome: what pediatric gastroenterologists need to know. Am J Gastroenterol. 2011;106(Suppl 2):S400. [Google Scholar]
- 24.Waespe N, Prader S, Kroiss S, Knirsch W, Speer O, Schmuge M. Clinical and laboratory manifestations of bleeding diathesis in Noonan syndrome. Hämostaseologie. 2013;33(1):A74. [Google Scholar]
- 25.Witt DR, McGillivray BC, Allanson JE, et al. Bleeding diathesis in Noonan syndrome: a common association. Am J Med Genet. 1988;31(2):305–317. doi: 10.1002/ajmg.1320310208. [DOI] [PubMed] [Google Scholar]
- 26.Caralis DG, Char F, Graber JD, Voigt GC. Delineation of multiple cardiac anomalies associated with the Noonan syndrome in an adult and review of the literature. Johns Hopkins Med J. 1974;134(6):346–355. [PubMed] [Google Scholar]
- 27.Evans DG, Lonsdale RN, Patton MA. Cutaneous lymphangioma and amegakaryocytic thrombocytopenia in Noonan syndrome. Clin Genet. 1991;39(3):228–232. doi: 10.1111/j.1399-0004.1991.tb03017.x. [DOI] [PubMed] [Google Scholar]
- 28.Grange CS, Heid R, Lucas SB, Ross PL, Douglas MJ. Anaesthesia in a parturient with Noonan’s syndrome. Can J Anaesth. 1998;45(4):332–336. doi: 10.1007/BF03012024. [DOI] [PubMed] [Google Scholar]
- 29.Humbert JA, Hammond KB, Hathaway WE. Trimethylaminuria: the fish-odour syndrome. Lancet. 1970;2(7676):770–771. doi: 10.1016/s0140-6736(70)90241-2. [DOI] [PubMed] [Google Scholar]
- 30.Komp DM. “Car. factor” deficiency revisited”. Pediatric Res. 1975;9(4):184–189. doi: 10.1203/00006450-197504000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Phillips WG, Dunnill MG, Kurwa AR, Black MM. Orbital oedema: an unusual presentation of Noonan’s syndrome. Br J Dermatol. 1993;129(2):190–192. doi: 10.1111/j.1365-2133.1993.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 32.Sgouros SN, Karamanolis G, Papadopoulou E, et al. Postbiopsy intramural hematoma of the duodenum in an adult with Noonan’s syndrome. J Gastroenterol Hepatol. 2004;19(10):1217–1219. doi: 10.1111/j.1440-1746.2004.02931.x. [DOI] [PubMed] [Google Scholar]
- 33.Sharland M, Patton MA, Chitolie A, Talbot S, Bevan D. Coagulation factor abnormalities in Noonan syndrome. J Med Genet. 1990;27(10):646. [Google Scholar]
- 34.Singer ST, Hurst D, Addiego JE. Bleeding disorders in Noonan syndrome: three case reports and review of the literature. J Pediatr Hematol Oncol. 1997;19(2):130–134. doi: 10.1097/00043426-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Sugar AW, Ezsias A, Bloom AL, Morcos WE. Orthognathic surgery in a patient with Noonan’s syndrome. J Oral Maxillofac Surg. 1994;52(4):421–425. doi: 10.1016/0278-2391(94)90454-5. [DOI] [PubMed] [Google Scholar]
- 36.Char F, Rodriguez-Fernandez H, Scott C, Borgaonkar D, Bell D, Rowe D. The Noonan Syndrome − a clinical study forty-five cases. Birth Defects: Original Article Series. 1972;VIII(5):110–118. [Google Scholar]
- 37.Collins E, Turner G. The Noonan syndrome − a review of the clinical and genetic features of 27 cases. J Pediatr. 1973;83(6):941–950. doi: 10.1016/s0022-3476(73)80527-x. [DOI] [PubMed] [Google Scholar]
- 38.Derbent M, Öncel Y, Tokel K, et al. Clinical and hematologic findings in Noonan syndrome patients with PTPN11 gene mutations. Am J Med Genet A. 2010;152A(11):2768–2774. doi: 10.1002/ajmg.a.33713. [DOI] [PubMed] [Google Scholar]
- 39.Hathaway WE. Bleeding disorders due to platelet dysfunction. Am J Dis Child. 1971;121(2):127–134. doi: 10.1001/archpedi.1971.02100130081009. 1960. [DOI] [PubMed] [Google Scholar]
- 40.Hilgartner M, Engle AM, Redo SF. Cardiac surgery in patients with plasma thromboplastin antecedent (P.T.A.) deficiency. J Thorac Cardiovasc Surg. 1965;49:974–981. [PubMed] [Google Scholar]
- 41.Calvert GD. Trimethylaminuria and inherited Noonan’s syndrome. Lancet. 1973;1(7798):320–321. doi: 10.1016/s0140-6736(73)91566-3. [DOI] [PubMed] [Google Scholar]
- 42.Noonan JA. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am J Dis Child. 1968;116(4):373–380. doi: 10.1001/archpedi.1968.02100020377005. 1960. [DOI] [PubMed] [Google Scholar]
- 43.Sharathkumar AA. Bleeding disorders and Noonan syndrome. [Accessed September 18, 2018];Pediatric Blood Cancer. 2012 59(3):592. doi: 10.1002/pbc.24151. author reply 593. Available from: https://onlinelibrary.wiley.com/doi/pdf/10.1002/pbc.24151. [DOI] [PubMed] [Google Scholar]
- 44.Smpokou P, Tworog-Dube E, Kucherlapati RS, Roberts AE. Medical complications, clinical findings, and educational outcomes in adults with Noonan syndrome. Am J Med Genet A. 2012;158A(12):3106–3111. doi: 10.1002/ajmg.a.35639. [DOI] [PubMed] [Google Scholar]
- 45.Tofil NM, Winkler MK, Watts RG, Noonan J. The use of recombinant factor VIIa in a patient with Noonan syndrome and life-threatening bleeding. Pediatr Crit Care Med. 2005;6(3):352–354. doi: 10.1097/01.PCC.0000160656.71424.D1. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida R, Hasegawa T, Hasegawa Y, et al. Protein-tyrosine phosphatase, nonreceptor type 11 mutation analysis and clinical assessment in 45 patients with Noonan syndrome. J Clin Endocrinol Metab. 2004;89(7):3359–3364. doi: 10.1210/jc.2003-032091. [DOI] [PubMed] [Google Scholar]
- 47.Wiegand G, Hofbeck M, Zenker M, Budde U, Rauch R. Bleeding diathesis in Noonan syndrome: is acquired von Willebrand syndrome the clue? Thromb Res. 2012;130(5):e251–e254. doi: 10.1016/j.thromres.2012.08.314. [DOI] [PubMed] [Google Scholar]
- 48.Biss TT, Blanchette VS, Clark DS, et al. Evaluation of bleeding symptoms in children with an inherited mucocutaneous bleeding disorder. Blood. 2009;114(22):1292–1292. [Google Scholar]
- 49.Romano AA, Allanson JE, Dahlgren J, et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2010;126(4):746–759. doi: 10.1542/peds.2009-3207. [DOI] [PubMed] [Google Scholar]
- 50.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381(9863):333–342. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Case reports/series on bleeding disorders in patients with NS
| Reference | Number of patients |
|---|---|
| Argyrou A, et al. Arch Hell Med, 20101 | 1 |
| Artoni A, et al. Pediatrics, 20142 | 39 |
| Bertola DR, et al. Rev Hosp Clin Fac Med Sao Paulo, 20033 | 30 |
| González Casado I, et al. Horm Res Paediatr. 20114 | 27 |
| de Haan M, et al. Am J Med Genet. 19885 | 12 |
| Flick JT, et al. Am J Clin Pathol. 19916 | 1 |
| Gamba G, et al. Horm Res Paediatr. 20117 | 19 |
| Kitchens CS, et al. J Pediatr. 19838 | 4 |
| Koc A, et al. Turk J Haematol. 20019 | 1 |
| Massarano AA, et al. Acta Paediatr. 199610 | 18 |
| Nunes P, et al. BMJ Case Rep. 201211 | 1 |
| Patrick K, et al. Br J Haematol. 201012 | 1 |
| Sharland M, et al. Arch Dis Child. 199213 | 151a |
| Sharland M, et al. Lancet. 199214 | 72a |
| Staudt JM, et al. Scand J Plast Reconstr Surg Hand Surg. 200515 | 1 |
| Stoffman JM, et al. Blood. 200416 | 28 |
| Tanaka Y, et al. Am J Med Genet. 199917 | 2 |
| Troiano M, et al. Horm Res Paediatr. 201118 | 13 |
| Vortia E, et al. Am J Gastroenterol. 201119 | 2 |
| Waespe N, et al. Hämostaseologie. 201320 | 15 |
| Witt DR, et al. Am J Med Genet. 198821 | 19 |
| Caralis DG, et al. Johns Hopkins Med J. 197422 | 1 |
| Evans DG, et al. Clin Genet. 199123 | 1 |
| Grange CS, et al. Can J Anaesth. 199824 | 1 |
| Humbert JA, et al. Lancet. 197025 | 1 |
| Komp DM, et al. Pediatr Res. 197526 | 2 |
| Phillips WG, et al. Br J Dermatol. 199327 | 1 |
| Sgouros SN, et al. J Gastroenterol Hepatol. 200428 | 1 |
| Sharland M, et al. J Med Genet. 199029 | 31a |
| Singer ST, et al. J Pediatr Hematol Oncol. 199730 | 3 |
| Sugar AW, et al. J Oral Maxillofac Surg. 199431 | 1 |
Note:
Substudies (31 and 72 of 151 patients from Sharland et al. Arch Dis Child. 1992).63
Abbreviation: NS, Noonan syndrome.