Abstract
Multiple sclerosis (MS) is a chronic and often progressive, demyelinating disease of the central nervous system (CNS) white and gray matter and the single most common cause of disability in young adults. Age is one of the factors most strongly influencing the course of progression in MS. One of the hallmarks of aging is cellular senescence. The elimination of senescent cells with senolytics has very recently been shown to delay age-related dysfunction in animal models for other neurological diseases. In this review, the possible link between cellular senescence and the progression of MS is discussed, and the potential use of senolytics as a treatment for progressive MS is explored. Currently, there is no cure for MS and there are limited treatment options to slow the progression of MS. Current treatment is based on immunomodulatory approaches. Various cell types present in the CNS can become senescent and thus potentially contribute to MS disease progression. We propose that, after cellular senescence has indeed been shown to be directly implicated in disease progression, administration of senolytics should be tested as a potential therapeutic approach for the treatment of progressive MS.
Keywords: Aging, Senolytics, Glia, Neurodegeneration, Inflammation, Autoimmunity
Introduction
Age is one of the most influential factors in MS progression [1, 2]. Several studies have shown that age affects disease progression of MS independently of initial disease pattern, disease duration, and gender [2, 3]. Aging can be defined as the time-dependent decline of functional capacity, which affects most living organisms [4]. The nine hallmarks that are generally considered to contribute to the aging process are genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. These hallmarks are interconnected and contribute to aging and the development of age-related diseases [4]. Cellular senescence, one of the major hallmarks of the aging process, is a phenomenon by which cells go into irreversible growth arrest and become resistant to apoptosis. The number of senescent cells present in the human body increases with aging, which can have deleterious effects on the tissue microenvironment [5].
Several drugs have been approved for the treatment of relapsing-remitting phase of MS. Unfortunately, these drugs show little or no therapeutic effect in progressive MS. The first drug which was approved for the treatment of relapsing-remitting MS (RR-MS) was interferon-β1 (IFN-β1). To date, there are 13 FDA-approved drugs available for treatment of RR-MS. In general, these drugs act mainly by suppressing or altering the immune system. Also, these drugs have side effects, do not halt or reverse the disease, and most have limited long-term effectiveness [6]. The exception may be alemtuzumab for which durable efficiency was reported in people with RR-MS, including confirmed disability improvement [7]. However, it is unknown how long the drug effectiveness may last. Recently, ocrelizumab was approved as the first drug for treatment of primary progressive MS (PP-MS). Ocrelizumab is a humanized monoclonal antibody designed to selectively target CD20-positive B cells [8]. In this trial, a subset of people with PP-MS receiving ocrelizumab showed a moderate degree of slowing of disability accumulation compared to the placebo group [8]. In addition to suppression of ongoing inflammation, remyelination is essential in MS to restore saltatory conduction and axonal protection. Promotion of remyelination and/or inhibition of demyelination is critical to prevent further neuronal loss and cognitive decline observed in (progressive) MS [9]. Failure of remyelination is one of the pathologic hallmarks of progressive MS.
The relation of aging with disease progression in MS lends strong support to the hypothesis that progression could potentially be induced by increased cellular senescence in the CNS. Eliminating senescent cells delays age-related dysfunction in mouse models [10, 11]. Therefore, the aim of this review is to explore whether elimination of senescent cells could be a potential therapeutic strategy for delaying progression of MS. When cellular senescence is involved in MS disease progression, one could consider senolytics as a therapeutic treatment to delay progression. First, cellular senescence and its links with the progression of MS are discussed. Second, the concept of senolytics and the potential use of these drugs which specifically target senescent cells as a treatment for progressive MS will be discussed.
Cellular senescence
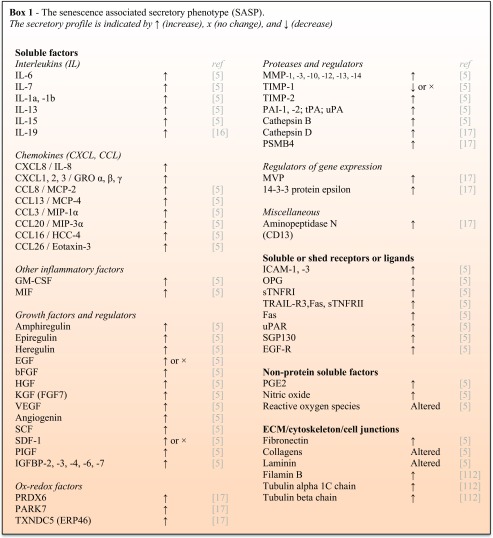
Cellular senescence can be defined as an irreversible arrest of the cell cycle coupled to stereotyped phenotypic changes to decrease the risk for malignant transformation of the cell [5]. The term senescence was first introduced by Hayflick and Moorhead to describe the phenomenon of irreversible growth arrest in serially passaged human fibroblast culture, also known as replicative senescence [12]. Now, it is known that the senescence observed here was caused by telomere attrition [12, 13]. Cellular senescence can also be induced by many other stressors, including mitochondrial deterioration, oxidative stress, the expression of certain oncogenes, DNA damage, chromatin disruption, spindle stress, low expression of the mitotic spindle checkpoint protein budding uninhibited by benzimidazole-related 1 (BubR1), and other insults [14]. Senescence can be characterized by various markers, none of which is specific to senescent cells only and senescent cells may express only some of the markers used to characterize senescence. Major examples of these markers are G1 arrest (high expression of cell-cycle inhibitors p16Ink4a and p21), high senescence-associated β-galactosidase (SA-β-gal) activity, altered epigenome, oxidative stress, DNA damage, and most importantly the senescence-associated secretory phenotype (SASP) as detailed in Box 1 [5, 15].
The secretory phenotype of senescent cells
One of the key characteristics that distinguish senescent cells from other non-proliferating cells is the SASP (Box 1). The SASP refers to the release of a wide array of pro-inflammatory cytokines and chemokines, tissue-damaging proteases, factors influencing stem- and progenitor cell function, and haemostatic factors and growth factors among other factors. The SASP factors can lead to the development of local and systemic pathogenic effects [5]. However, the influence of the SASP on the microenvironment can also be beneficial during embryonic development or in an acute setting of wound healing [18, 19].
The SASP factors can be subdivided into soluble signaling factors, soluble shed receptors or ligands, non-protein soluble factors, and insoluble factors (extracellular matrix (ECM)/cytoskeleton/cell junctions). Soluble signaling factors are the major components of the SASP and can be subdivided into interleukins, chemokines, other inflammatory factors, growth factors, ox-redox factors, proteases and their regulators, regulators of gene expression, and miscellaneous.
The most prominent SASP cytokine is IL-6, which is associated with senescence in various cell types such as mouse and human keratinocytes, melanocytes, monocytes, fibroblasts, and epithelial cells [20–22]. Senescent cells can also influence the tissue microenvironment by the secretion of non-protein soluble factors, such as reactive oxygen species (ROS) [23–25]. Moreover, senescent cells can have increased fibronectin expression as shown in prematurely aging fibroblasts in Werner Syndrome [26, 27]. Fibronectin is a major ECM glycoprotein of connective tissue, on cell surfaces and in plasma and other body fluids. The glycoprotein can bind to integrins and ECM components, such as collagen, and plays major roles in cell adhesion, growth, migration, and differentiation.
Mechanisms of tissue deterioration by cellular senescence
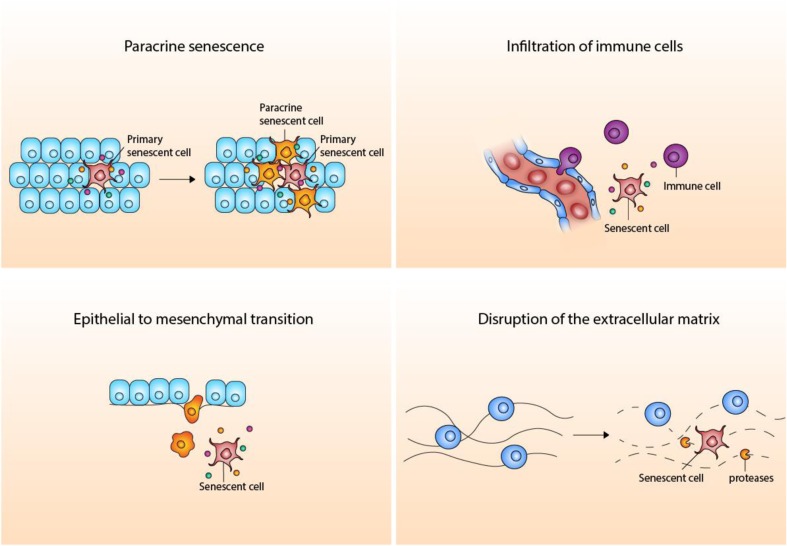
Senescent cells are found in the affected tissues of patients with age-related diseases and are thought to promote age-related tissue dysfunction. The age-related diseases osteoarthritis, pulmonary fibrosis, diabetes, atherosclerosis, and Alzheimer’s disease are already thought to be connected with cellular senescence, suggesting that cellular senescence could be associated with their genesis and progression [28]. The SASP can contribute to age-related inflammatory diseases by causing a paracrine spread of cell dysfunction and tissue damage [29]. This can be induced by disruption of the stem cell niche, and therefore disruption of the tissue regeneration, by SASP factors [30, 31]. Furthermore, SASP proteases might also cause disruption of the extracellular matrix by cleavage of components of the tissue microenvironment [32]. Other SASP components, including IL-6 and IL-8, may induce epithelial-mesenchymal transition, stimulating tissue fibrosis and tumor metastasis [33, 34]. Senescent cells may also play a role in chronic tissue inflammation associated with the development of age-related diseases. Senescent cells accumulate in tissues manifesting age-related inflammatory pathologies and promote this inflammation through pro-inflammatory SASP factors. SASP factors IL-1β, TGFβ, and certain chemokine ligands may induce senescence in neighboring cells, sustaining and also exacerbating the previously mentioned mechanisms of tissue deterioration by increasing the number of senescent cells (Fig. 1) [35].
Fig. 1.
Mechanisms of tissue deterioration by cellular senescence. Cellular senescence can contribute to age-related tissue dysfunction by at least the following general mechanisms: paracrine senescence, stimulation of the infiltration of immune cells, disruption of the extracellular matrix, and by induction of epithelial to mesenchymal transition
Aging and cellular senescence in MS
MS is a chronic, often progressive, demyelinating disease of the CNS white and gray matter. Despite the fact that the disease course and symptomatology of MS are very heterogeneous from person to person, several disease subtypes can be recognized. The most common subtype is RR-MS, characterized by acute episodes of neurological deficits followed by periods of recovery. Aging seems to be a significant factor in MS disease progression. Those diagnosed with RR-MS have a 50% chance to transit to secondary progressive MS (SP-MS) within 10 years, and a 90% chance within 25 years. SP-MS is characterized by progressive decline, with or without relapses. In approximately 10–20% of the individuals that develop MS, the disease from the start slowly progresses over time and there are no (or occasionally minor) signs of remission after onset of the initial symptoms. This subtype is referred to as PP-MS. The histopathological hallmarks of MS are multifocal lesions with demyelination, oligodendrocyte death, axonal loss, and accumulation of blood-borne immune cells. The RR-MS course is characterized by inflammation followed by demyelination, adaptive immunity, activated astrocytes, disturbed blood-brain barrier (BBB) function, and (incomplete) remyelination. Conversely, SP-MS course is characterized by axonal degeneration, innate immunity, reactive astrocytes (gliosis), closed BBB, and hardly any remyelination [36].
The age distribution of MS is shifting to older age groups, from a peak prevalence of 50–54 years in 1984 to 55–59 years in 2004. People over the age of 65 who have been diagnosed with MS are more likely to have PP-MS or have an earlier transition to a SP-MS course, compared to younger people (under 65) [3, 37, 38]. MS disease progression can occur at varying rates between individuals, possibly due to varying underlying biological mechanisms often related to genetic and/or environmental factors [39, 40]. Importantly, increasing age has been shown to be a strong predictor for progression in MS, independent of the subtype and age of onset [38].
Mechanisms of senescence-related MS disease progression
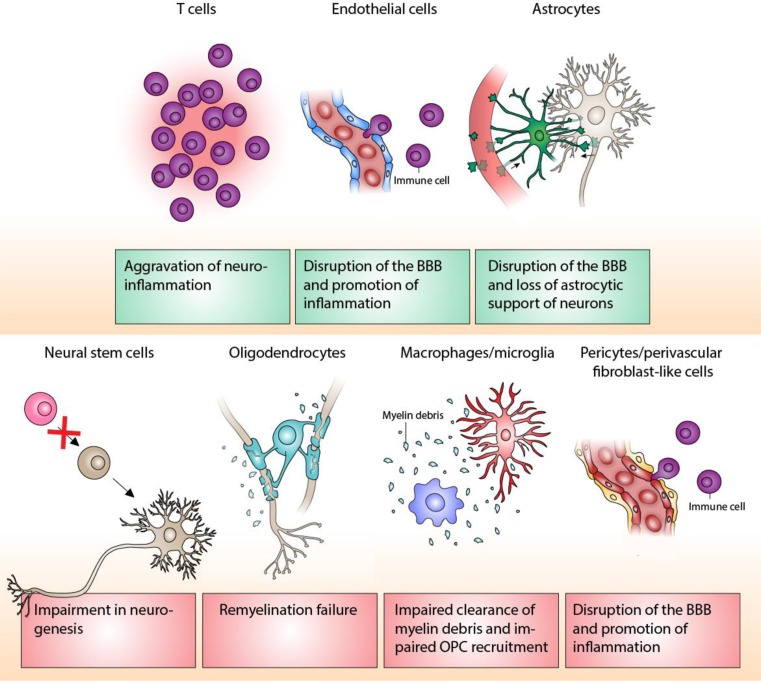
It was recently shown that chronic demyelination observed in a cuprizone mouse model is associated with accelerated glial cell senescence in demyelinated lesions [41]. Moreover, inflammation, ROS, and fibronectin accumulation, which are also part of the SASP, are thought to play a role in the pathogenesis of MS. This suggests that the age-related disease progression observed in MS could potentially be functionally linked to cellular senescence. Here, we describe the mechanisms by which different senescent cell types could influence MS disease progression. An overview of these mechanisms is shown in Fig. 2.
Fig. 2.
Potential mechanisms contributing to MS disease progression by senescence of different cell types. Different cell types could potentially become senescent and contribute to MS disease progression. The putative mechanisms are shown in the red and green boxes. Green boxes indicate that senescence of these cell types has been observed in vivo; red boxes indicate that senescence of these cell types has not yet been observed in vivo. Free-ware images of microglia, oligodendrocyte, astrocyte, and neuron are based on “cells of the CNS” [42]
Senescence of microglia and macrophages
The aging CNS shows decreased capacity for tissue repair, which could contribute to the progression of MS [43, 44]. One potential mechanism for this decreased repair response is immune-aging of microglia and macrophages [45]. Microglia are the resident immune cells of the CNS providing surveillance during homeostasis and against a wide variety of insults. This surveillance state is maintained by soluble molecules expressed in the CNS, such as transforming growth factor β, and ligands expressed on the surface of neurons, astrocytes, and oligodendrocytes [46]. Microglia are activated in MS, possibly promoting the infiltration of monocytes and lymphocytes into the CNS, changes in BBB integrity and secretion of inflammatory cytokines. Finally, this could promote to neuronal damage and death. Microglia also reactivate T cells, which enter the CNS from the periphery [47].
One of the main pathological differences between progressive and non-progressive MS is remyelination failure. Remyelination is essential for restoration of saltatory conduction and axonal protection [48]. Remyelination does occur in the early stages of the disease, but it declines as the disease progresses [44]. The expression of genes involved in the retinoid X receptor pathway is decreased in aging myelin-phagocytosing macrophages [49]. In addition, disruption of retinoid X receptor function in young macrophages results in aging-related decrease in myelin debris uptake [49]. Furthermore, retinoid X receptor agonists were able to partially restore myelin debris clearance. These results suggest that the retinoid X receptor pathway, which shows decreased activity in aging macrophages, plays a key role in remyelination due to its influence on myelin debris clearance [49, 50]. Moreover, aging microglia show decreased motility and cellular migration in response to tissue injury compared to young microglia [51]. Also, macrophages exhibit a decreased ability to produce a pro-inflammatory response, while microglia show an increased ability to produce a pro-inflammatory response [46, 52]. This increased microglial response is referred to as microglial priming and might lead to increased neuronal loss and accelerated progression in MS [53]. The age-associated delay in remyelination efficiency has been associated with reduction in macrophage/microglia recruitment in a toxin-induced demyelinating model [54]. The age-associated delay in remyelation can be explained by the decreased ability to resolve the inflammatory response initiated after myelin damage [55]. The breakdown of myelin results in the release of large amounts of cholesterol from the myelin [55]. Ingestion of myelin debris by macrophages induces an anti-inflammatory program [56]. Very recent mouse studies demonstrate large amounts of cholesterol overwhelm the efflux capacity of aged phagocytes, resulting in a phase transition of cholesterol into crystals and thereby inducing lysosomal rupture and NOD-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome stimulation [55]. Thus, age-related defective cholesterol clearance limits remyelination.
Microglia and macrophages play a major role in the clearance of myelin debris and the recruitment of oligodendrocyte precursor cells (OPC) to the lesion site [57]. Aged microglia and macrophages show decreased phagocytosis and chemotaxis [58]. Decreased chemotaxis could result in impairment of the recruitment of endogenous OPC. The impairment in phagocytosis could result in impaired clearance of myelin debris and subsequent arrest of the differentiation of OPC [50]. The exact role of senescent microglia and macrophages in these processes is not known. There is some evidence supporting the induction of cellular senescence in microglia. In vitro experiments with the microglia-like cell line BV2 showed that these cells go into senescence after multiple inflammatory challenges indicating that this might also be possible in vivo [59]. The actual existence of senescent microglia and macrophages in vivo is yet to be confirmed.
Senescence of T cells
Besides microglia and macrophages, senescent brain-infiltrating T cells are likely critical in the progression of MS. Unlike the previously mentioned cell types, it has been shown that T cells can become senescent in vivo, as reflected by increased expression of CD57 and killer cell lectin like receptor G1 (KLRG1) on CD8+ T cells from aged individuals [60]. The numbers of senescent CD8+ T cells are increased in the aging brain and their increased pro-inflammatory cytokine production could aggravate the neuro-inflammation. This could worsen the cognitive function and drive progression of MS, since cytotoxic CD8+ T cells can drive neuronal damage [61]. CD8+ T cells are found abundantly in MS lesions and the number of cells correlates with the axonal damage rate in the lesions [62, 63]. Furthermore, all CNS cell types show increased MHC class I expression in the lesions [64]. This suggests that senescent, dysfunctional CD8+ T cells can target glial cells and neurons by direct recognition of the cell and/or myelin sheath via MHC class I or by excessive cytokine production, and therefore they likely play a role in the progression of MS.
Senescence of astrocytes
Aging can also influence the function of astrocytes, the most abundant cell type in the brain. Senescence of astrocytes may lead to changes in many astrocyte-regulated processes among which synaptic plasticity, metabolic balance, and BBB permeability. Senescent astrocytes have an increased expression of glial fibrillary acidic protein (GFAP) and vimentin filaments, increased expression of pro-inflammatory cytokines, and increased accumulation of proteotoxic aggregates [65]. GFAP levels in CSF of rodents and humans correlate with age and disease progression in MS, suggesting that senescent astrocytes might also contribute to disease progression [66, 67]. Senescent astrocytes could have a decreased capacity to support neurogenesis and to provide neuronal protection. GFAP/vimentin knockout mice have an increased cellular proliferation and neurogenesis [68]. Moreover, astrocytes without GFAP have an increased capacity to provide neuronal survival and neurite outgrowth compared to wild-type astrocytes [69]. Furthermore, senescent astrocytes have an increased expression of pro-inflammatory cytokines, such as IL-6, TNF-α, IL-1β, and prostaglandins, which negatively affect BBB function [70].
Astrocytes also have an important role in the neuron-glial crosstalk; they maintain metabolic and ion homeostasis of neurons, modulate synaptic transmission via glutamate, and they modulate neuronal activity [71, 72]. Therefore, senescence of astrocytes might promote neuronal dysfunction and degeneration, contributing to the progression of MS.
Glial scar formation, also referred to as gliosis, is effected by reactive astrocytes and it develops as the disease progresses [73]. Therefore, this process could be related to cellular senescence of astrocytes and other CNS cell types. Gliosis can have both beneficial and detrimental effects. The supposed beneficial effect is to physically isolate the damaged CNS areas to prevent spread of tissue destruction. Nonetheless, this process also has detrimental effects since it inhibits remyelination and axonal regeneration. The overproduction of FGF-2 and hyaluronan by astrocytes inhibits OPC differentiation and therefore remyelination. Also, the release of chondroitin sulphate proteoglycans (CSGP), ephrins (EPH), and their receptors, as well as myelin-associated inhibitors (MAI), inhibits remyelination and suppresses axonal growth [73].
Nonetheless, the role of senescent astrocytes in MS is not well-studied and the previously mentioned mechanisms by which senescent astrocytes could influence MS disease progression are not yet confirmed. Interestingly, it has been shown very recently that post-mortem Parkinson’s disease (PD) brain samples show increased astrocytic senescence and that astrocytic senescence could be induced by the herbicide paraquat both in vitro and in vivo [74]. Moreover, clearance of senescent cells by using a special PD mouse model that allows selective depletion without apparent off-target effects mitigates paraquat-induced neuropathology. Therefore, accumulation of senescent astrocytes might contribute to development of sporadic PD [74].
Senescence of endothelial cells
The senescence of endothelial cells lining the surface of blood vessels in the CNS is thought to contribute to disruption of BBB function. Impaired barrier integrity was observed in an in vitro BBB model, composed of senescent endothelial cells, pericytes, and astrocytes [75]. In vivo experiments with BubR1H/H progeria mice showed impaired BBB integrity and increased senescence of endothelial cells and pericytes. Moreover, oxidative stress can induce endothelial senescence possibly by downregulation of sirtuin 6 (Sirt6), a regulator of endothelial cell senescence [76]. Radiation-induced senescence of endothelial cells results in the downregulation of a disintegrin and metalloprotease (ADAM) 10 [77]. This protein is the alpha-secretase that cleaves amyloid precursor protein (APP). This mechanism is considered important in preventing the formation of amyloid beta in Alzheimer’s disease (AD). In addition, ADAM10 can cleave many more proteins, including TNF-α and E-cadherin, and thereby promoting inflammation and affecting epithelial cell-cell adhesion [78, 79]. ADAM10 can also cleave the extracellular domain of the B cell-activating factor (BAFF)—a proliferation-inducing ligand (APRIL)—receptor transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), releasing soluble TACI (sTACI) [80]. The BAFF-APRIL system is involved in the regulation of B cell homeostasis. sTACI levels are increased in CSF of MS patients and are thought to induce B cell accumulation and activation [80].
Senescence of pericytes and perivascular fibroblast-like cells
In addition to endothelial cells and astrocytes, pericytes contribute to the BBB. However, the physiological role of these cells is not well established [81]. Pericytes are essential in maintaining the BBB during brain aging and loss of pericytes leads to reduction in brain microcirculation and BBB breakdown [82]. Senescence of these cells might lead to impairment of their normal function and therefore contribution to neuro-inflammation and neurodegeneration. Recently, perivascular fibroblast-like cells were identified. These cells are located between the vessel wall and the astrocytic end-feet, and show resemblance to lung fibroblasts combined with epithelial (Lama1), endothelial (Cdh5), and mesothelial (Efemp1) markers [83]. Perivascular fibroblast-like cells could be the origin of pathological fibroblasts [84]. These pathological fibroblast cells are activated in experimental models of neuro-inflammation such as EAE and infection with the neurotropic hepatitis virus, as evidenced by rapid production of chemokine receptor 7 ligands [85, 86]. Fibroblast activation during chronic CNS inflammation contributes to the inflammatory response by recruitment of immune cells at sites of inflammation and secretion pro-inflammatory cytokines and survival factors to retain activated immune cells [87]. Senescence of vascular cells contributes to BBB disruption, as shown by impaired barrier integrity and tight junction structure in an in vitro BBB model constructed with senescent endothelial cells and pericytes [75].
Senescence of oligodendrocytes
OPC migrate toward the injured axon site after signaling by microglia or astrocytes. At the site, they must differentiate into mature oligodendrocytes (OLG) to be able to remyelinate the axon. Most OPC, which have a crucial role in remyelination, can avoid replicative senescence [88]. Nonetheless, there is evidence that OPC can become senescent [89]. Senescence of OLG might decrease remyelination capacity of demyelinated axons, which could lead to decrease in signaling ability and loss of protection of neurons and eventually neuronal cell death in MS.
Senescence of neurons
Senescence of neural stem cells could reduce neuronal neurogenesis, which can also contribute to the aging-associated progression of MS [90]. The expression of transcriptional regulator Hmga2 (high-mobility group AT-hook 2) in neural stem cells declines with age, resulting in an increased expression of p16Ink4a and p19Arf which can both induce cellular senescence [91].
SA-β-gal is commonly used biomarker of cell senescence and is found to be increased in the hippocampus of 24-month-old mice [92]. However, relatively high expression of SA-β-gal activity was also found in the hippocampus of 3-month-old mice, suggesting that SA-β-gal in neurons is not a unique marker of neuronal senescence [92]. DNA damage does not increase the number of SA-β-gal-positive neurons [92]. Moreover, sustained DNA damage of post-mitotic neurons can lead to the development of a p21-dependent senescent phenotype [93]. However, p21 expression did not show days-in-culture-dependent changes in cortical neurons [92]. These findings suggest that cell-cycle regulators associated with cellular senescence may not be relevant markers of senescence in post-mitotic neurons. Alternatively, repressor element 1-silencing transcription factor (REST) could possibly be used as a specific marker of neuronal aging in vitro [92]. Despite the controversy about senescence markers for neurons, overall, these results suggest that neurons indeed can become senescent. Senescence then can have direct effects on neuronal function and possibly MS disease progression.
In AD models, neuronal senescence is thought to be triggered by amyloid β and tau hyper-phosphorylation/accumulation. Neuronal senescence eventually might cause chronic neurodegeneration and cognitive impairment [94, 95].
Role of the SASP
Increased levels of pro-inflammatory molecules, secreted by senescent cells, can promote inflammation and might promote progression of MS. SASP factors secreted by senescent cells are also able to influence the extracellular matrix. The extracellular matrix is one of the factors regulating migration and proliferation of oligodendrocyte progenitor cells and their differentiation. For example, fibronectin promotes proliferation and reduces myelin-like membrane formation [96]. Fibronectin is upregulated in MS lesions and CNS parenchyma, affecting remyelination [97, 98]. Astrocytes generate fibronectin aggregates upon engagement with inflammatory mediators [99]. The pro-inflammatory factors of the SASP could therefore induce the generation of fibronectin aggregates. Senescent endothelial cells and fibroblasts have increased fibronectin expression [27]. The age-related increase of senescent cells in MS could therefore contribute to the increased fibronectin expression and progression of MS.
Targeting senescent cells with senolytic drugs
Potential therapeutic strategies to prevent the deleterious effects caused by senescent cells are based on preventing formation of senescent cells, removal of senescent cells, and targeting the effects of senescent cells. Preventing formation of senescent cells requires interference with pathways leading to senescence. In addition, cellular senescence is a defense mechanism against cancer and therefore long-term interference with these pathways is likely to promote cancer [100]. Preventing or ameliorating the effects of the SASP could also be a potential therapeutic approach to decrease inflammation and cancer risk. However, this also inhibits the beneficial arm of the SASP. Elimination of senescent cells, on the other hand, has a larger potential to delay age-related degenerative pathologies [10]. Cancer risk would be reduced by activation of the tumor-suppressive pathway, leading to cellular senescence and, by removal of senescent cells, prevent malignant transformation of neighboring cells [10, 101]. Furthermore, elimination of senescent cells can be done intermittently which does not affect the formation of new senescent cells for purposes such as wound healing [19].
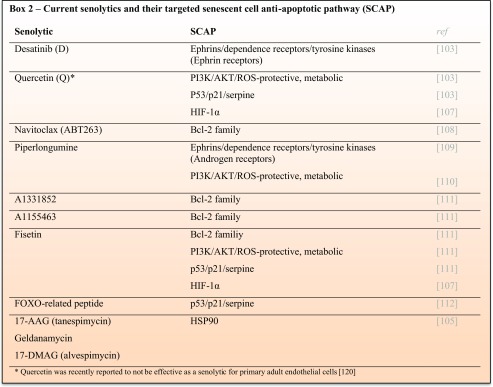
Different agents, including small molecules, peptides, and antibodies, called senolytics, are being developed to specifically remove senescent cells [102]. Senescent cells are resistant to apoptosis despite their own pro-apoptotic SASP factor release. Senolytics are directed against the pro-survival pathways of senescent cells, also referred to as senescent cell anti-apoptotic pathways (SCAP). These SCAP, responsible for the survival of senescent cells, were identified as the Achilles’ heel of senescent cells [103]. Both cellular senescence and mitochondrial dysfunction are the hallmarks of aging and are closely interlinked; upregulation of the SCAP is related to senescence-associated mitochondrial dysfunction (SAMD) and, at the same time, SAMD also drives and maintains cellular senescence [4, 104].
Thus far, six different SCAP are known: Bcl-2/Bcl-XL family, PI3K/Akt/ROS protective/metabolic, p53/p21/serpine, ephrins/dependence receptors/tyrosine kinases, HIF-1α, and heat shock protein 90 (HSP-90) [103, 105]. A number of senolytic drugs are developed based on interference with these SCAP. Current senolytics are listed in Box 2. Senolytics can selectively induce apoptosis in senescent cells and can potentially be used in multiple age-related phenotypes. Recently, it has been shown that intermittent oral administration of the senolytic cocktail of dasatinib and quercetin decreased the number of naturally occurring senescent cells and alleviated physical dysfunction and increased survival in old mice [106].
Effects of cellular senescence in MS
There is initial evidence from animal models supporting the role of cellular senescence in the processes underlying disease progression in MS, for instance the increased cellular senescence observed in the cuprizone model [41]. This is a model in which young adult mice are fed with the copper chelator cuprizone, resulting in demyelination. This demyelination is caused by apoptotic cell death of oligodendrocytes, due to a disturbance in energy metabolism. This model has been associated with microglia and macrophage responses, but not with T cell activation and their recruitment into to the CNS [114]. In this model, they observed a 2.9-fold increase in senescent glial cell load in the corpus callosum as evidenced by SA-β-gal histochemistry at week 16.
Also in MS, there is some evidence for premature immunosenescence. One of the characteristics of immunosenescence is the expansion of CD4+ CD28− T cells. These cells accumulate in MS lesions [115–117]. It has also been suggested that senescent CD8+ T cells could contribute to disease progression but the evidence is less clear [118].
The occurrence of other effects of cellular senescence in MS has only been hypothesized and has not been confirmed yet. For example, the chronic secretion of ROS generated by senescent cells and inflammatory cells as they attack myelin might cause a spread of demyelination. This can at least partly explain why cortical demyelination is found in patients suffering from progressive MS, but not in acute MS [119]. Another SASP factor that could contribute to MS disease progression is the ECM glycoprotein fibronectin. Fibronectin contributes to remyelination failure and increased fibronectin expression by senescent cells and can therefore enhance this phenomenon [97, 98]. Furthermore, the pro-inflammatory cytokines secreted by senescent cells can directly influence the surrounding brain tissue, which might drive neurodegeneration. Also, inflammatory mediators are thought to induce increased fibronectin expression by astrocytes [99]. Moreover, the age-related iron accumulation observed in progressive MS patients is likely to be a consequence of cellular senescence [36, 120]. In conclusion, senescence of cells present in the CNS in MS could contribute to MS disease progression, but more mechanistic research is necessary to support this hypothesis.
Conclusions and future outlook
From our review we conclude that there is ample evidence to warrant further studies into the possible link between cellular senescence and progression of MS. Future in vitro studies on the different cell types present in the CNS can elucidate the mechanisms through which these cells become senescent and if the senescent phenotype alters their role in important processes such as myelin debris clearance, remyelination, and axonal protection. The results of these studies can be used to guide a focused search for senescent cells in MS lesions in post-mortem brain tissue from different MS subtypes. This will further strengthen the link between cellular senescence and disease progression in MS. The final preclinical step would be to test senolytic treatment protocols using in vitro (e.g., brain-on-a-chip) and in vivo (EAE, cuprizone) models of demyelination and MS.
Adding senolytic treatment to effective immunomodulatory and remyelination promoting therapy could result in a treatment strategy which can limit further disability accrual in patients with MS and thus have great impact on the prognosis for people with MS.
Abbreviations
- AD
Alzheimer’s disease
- ADAM
A disintegrin and metalloprotease
- APP
Amyloid precursor protein
- APRIL
A proliferation-inducing ligand
- BAFF
B cell-activating factor
- BBB
Blood-brain barrier
- BubR1
Budding uninhibited by benzimidazole-related 1
- CCL
CC chemokine ligand
- CD
Cluster of differentiation
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CSGP
Chondroitin sulphate proteoglycans
- CXCL
Chemokine CXC motif ligand
- EAE
Experimental autoimmune encephalomyelitis
- ECM
Extracellular matrix
- EGF
Endothelial growth factor
- EPH
Ephrin
- ERP
Endoplasmic reticulum protein
- FGF
Fibroblast growth factor
- GFAP
Glial fibrillary acidic protein
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GRO
Growth regulated oncogene
- HCC
Hepatocellular carcinoma-associated protein
- HGF
Hepatocyte growth factor
- Hmga2
High-mobility group AT-hook 2
- HSP
Heat shock protein
- ICAM
Intercellular adhesion molecule
- IGF
Insulin-like growth factor
- IGFBP
IGF binding protein
- IL
Interleukin
- KGF
Keratinocyte growth factor
- KLRG1
Killer cell lectin like receptor G1
- MAI
Myelin-associated inhibitors
- MCP
Membrane cofactor protein
- MHC
Major histocompatibility complex
- MIF
Macrophage migration inhibitory factor
- MIP
Macrophage inflammatory protein
- MMP
Matrix metalloproteinases
- MVP
Major vault protein
- MS
Multiple sclerosis
- NLRP3
NOD-like receptor family pyrin domain-containing protein 3
- OLG
Oligodendrocyte
- OPC
Oligodendrocyte precursor cells
- OPG
Osteoprotegerin
- PAI
Plasminogen activator inhibitor
- PARK
Protein deglycase DJ-1
- PD
Parkinson’s disease
- PDH
Pyruvate dehydroxygenase
- PDK1
Pyruvate dehydrogenase kinase 1
- PDP2
Pyruvate dehyrogenase phosphatase catalytic subunit 2
- PGE2
Prostaglandin E2
- PI3K
Phosphatidylinositol 3-kinase
- PIGF
Placental growth factor
- PP-MS
Primary progressive MS
- PRDX
Peroxiredoxin
- PSMB
Proteasome subunit beta
- REST
Repressor element 1-silencing transcription factor
- ROS
Reactive oxygen species
- RR-MS
Relapsing-remitting MS
- SA-β-gal
Senescence-associated β-galactosidase
- SAMD
Senescence-associated mitochondrial dysfunction
- SASP
Senescence-associated secretory phenotype
- SCAP
Senescent cell anti-apoptotic pathway
- SCF
Stem cell factor
- SDF
Stromal cell-derived factor
- SGP
Soluble glycoprotein
- Sirt6
Sirtuin 6
- SP-MS
Secondary progressive MS
- sTNFR
Soluble tumor necrosis factor receptor
- TACI
Transmembrane activator and calcium modulator and cyclophilin ligand interactor
- TGFβ
Transforming growth factor β
- TIMP
Tissue inhibitor of metalloproteinases
- TNF-α
Tumor necrosis factor α
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- TXNDC
Thioredoxin domain containing
- tPA
Tissue-plasminogen activator
- uPA
Urokinase-type plasminogen activator
- uPAR
uPA receptor
- VEGF
Vascular endothelial growth factor
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Sanai SA, Saini V, Benedict RH, et al. Aging and multiple sclerosis. Mult Scler J. 2016;22:717–725. doi: 10.1177/1352458516634871. [DOI] [PubMed] [Google Scholar]
- 2.Koch M, Mostert J, Heersema D, De Keyser J. Progression in multiple sclerosis: further evidence of an age dependent process. J Neurol Sci. 2007;255:35–41. doi: 10.1016/j.jns.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 3.Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77:1246–1252. doi: 10.1212/WNL.0b013e318230a17d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dargahi N, Katsara M, Tselios T, Androutsou ME, de Courten M, Matsoukas J, Apostolopoulos V. Multiple sclerosis: immunopathology and treatment update. Brain Sci. 2017;7:e78. doi: 10.3390/brainsci7070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannoni G, Cohen JA, Coles AJ, Hartung HP, Havrdova E, Selmaj KW, Margolin DH, Lake SL, Kaup SM, Panzara MA, Compston DA, CARE-MS II Investigators Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology. 2016;87:1985–1992. doi: 10.1212/WNL.0000000000003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, ORATORIO Clinical Investigators Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 9.Plemel JR, Liu W-Q, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov. 2017;16:617–634. doi: 10.1038/nrd.2017.115. [DOI] [PubMed] [Google Scholar]
- 10.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65:2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 14.Van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnero A. Markers of cellular senescence. Methods Mol Biol. 2013;965:63–81. doi: 10.1007/978-1-62703-239-1_4. [DOI] [PubMed] [Google Scholar]
- 16.Small SH, Ragland RL, Ruzankina Y, Schoppy DW, Johnson FB, Brown EJ. 169: IL-19, a novel SASP factor, is upregulated during senescence and in response to DSBs. Cytokine. 2014;70:69. [Google Scholar]
- 17.Özcan S, Alessio N, Acar MB, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 2016;8:1316–1329. doi: 10.18632/aging.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, van Steeg H, Dollé MET, Hoeijmakers JHJ, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Coppé J-P, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmieri D, Watson JM, Rinehart CA. Age-related expression of PEDF/EPC-1 in human endometrial stromal fibroblasts: implications for interactive senescence. Exp Cell Res. 1999;247:142–147. doi: 10.1006/excr.1998.4341. [DOI] [PubMed] [Google Scholar]
- 23.Macip S, Igarashi M, Fang L, Chen A, Pan ZQ, Lee SW, Aaronson SA. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 2002;21:2180–2188. doi: 10.1093/emboj/21.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin M-G, Zhang J, Block ER, Patel JM. Senescence-enhanced oxidative stress is associated with deficiency of mitochondrial cytochrome c oxidase in vascular endothelial cells. Mech Ageing Dev. 2003;124:911–919. doi: 10.1016/s0047-6374(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 25.Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduction. 2012;2012:646354–646313. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasoamanantena P, Thweatt R, Labat-Robert J, Goldstein S. Altered regulation of fibronectin gene expression in werner syndrome fibroblasts. Exp Cell Res. 1994;213:121–127. doi: 10.1006/excr.1994.1181. [DOI] [PubMed] [Google Scholar]
- 27.Kumazaki T, Kobayashi M, Mitsui Y. Enhanced expression of fibronectin during in vivo cellular aging of human vascular endothelial cells and skin fibroblasts. Exp Cell Res. 1993;205:396–402. doi: 10.1006/excr.1993.1103. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17:324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 29.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krtolica A, Larocque N, Genbacev O, Ilic D, Coppe JP, Patil CK, Zdravkovic T, McMaster M, Campisi J, Fisher SJ. GROα regulates human embryonic stem cell self-renewal or adoption of a neuronal fate. Differentiation. 2011;81:222–232. doi: 10.1016/j.diff.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrinello S, Coppe J-P, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laberge R-M, Awad P, Campisi J, Desprez P-Y. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012;5:39–44. doi: 10.1007/s12307-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11:345–349. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 37.Marrie RA, Yu N, Blanchard J, Leung S, Elliott L. The rising prevalence and changing age distribution of multiple sclerosis in Manitoba. Neurology. 2010;74:465–471. doi: 10.1212/WNL.0b013e3181cf6ec0. [DOI] [PubMed] [Google Scholar]
- 38.Minden SL, Frankel D, Hadden LS, Srinath KP, Perloff JN. Disability in elderly people with multiple sclerosis: an analysis of baseline data from the Sonya Slifka Longitudinal Multiple Sclerosis Study. NeuroRehabilitation. 2004;19:55–67. [PubMed] [Google Scholar]
- 39.Kister I, Chamot E, Cutter G, Bacon TE, Jokubaitis VG, Hughes SE, Gray OM, Trojano M, Izquierdo G, Grand’Maison F, Duquette P, Lugaresi A, Grammond P, Boz C, Hupperts R, Petersen T, Giuliani G, Oreja-Guevara C, Iuliano G, Lechner-Scott J, Bergamaschi R, Rio ME, Verheul F, Fiol M, van Pesch V, Slee M, Butzkueven H, Herbert J, MSBase Investigators Increasing age at disability milestones among MS patients in the MSBase Registry. J Neurol Sci. 2012;318:94–99. doi: 10.1016/j.jns.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Benedict RHB, Morrow SA, Weinstock Guttman B, et al. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc. 2010;16:829–835. doi: 10.1017/S1355617710000688. [DOI] [PubMed] [Google Scholar]
- 41.Papadopoulos D, Karamita M, Mitsikostas DD, et al (2017) Accelerated cellular senescence in a model of multiple sclerosis. Neurology 88:supplement S50.004
- 42.Neurons and glial cells of the CNS. https://www.dreamstime.com/royalty-free-stock-photos-neurons-glial-cells-cns-image 18808418. Accessed 26 Apr 2018
- 43.Marquez de la Plata CD, Hart T, Hammond FM, Frol AB, Hudak A, Harper CR, O’Neil-Pirozzi TM, Whyte J, Carlile M, Diaz-Arrastia R. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil. 2008;89:896–903. doi: 10.1016/j.apmr.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldschmidt T, Antel J, Konig FB, Bruck W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- 45.Grebenciucova E, Berger JR. Immunosenescence: the role of aging in the predisposition to neuro-infectious complications arising from the treatment of multiple sclerosis. Curr Neurol Neurosci Rep. 2017;17:61. doi: 10.1007/s11910-017-0771-9. [DOI] [PubMed] [Google Scholar]
- 46.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 47.Jack C, Ruffini F, Bar-Or A, Antel JP. Microglia and multiple sclerosis. J Neurosci Res. 2005;81:363–373. doi: 10.1002/jnr.20482. [DOI] [PubMed] [Google Scholar]
- 48.Franklin RJM, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 49.Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nuñez V, Johnson KR, Wu T, Fitzgerald DC, Ricote M, Bielekova B, Franklin RJM. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain. 2015;138:3581–3597. doi: 10.1093/brain/awv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotter MR, Li W-W, Zhao C, Franklin RJM. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno B, Jukes J-P, Vergara-Irigaray N, Errea O, Villoslada P, Perry VH, Newman TA. Systemic inflammation induces axon injury during brain inflammation. Ann Neurol. 2011;70:932–942. doi: 10.1002/ana.22550. [DOI] [PubMed] [Google Scholar]
- 54.Zhao C, Li W-W, Franklin RJM. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol Aging. 2006;27:1298–1307. doi: 10.1016/j.neurobiolaging.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, Möbius W, Simons M. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359:684–688. doi: 10.1126/science.aan4183. [DOI] [PubMed] [Google Scholar]
- 56.Boven LA, Van Meurs M, Van Zwam M, et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129:517–526. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- 57.Olah M, Amor S, Brouwer N, Vinet J, Eggen B, Biber K, Boddeke HWGM. Identification of a microglia phenotype supportive of remyelination. Glia. 2012;60:306–321. doi: 10.1002/glia.21266. [DOI] [PubMed] [Google Scholar]
- 58.Njie EG, Boelen E, Stassen FR, et al. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195.e1–195.e12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu H-M, Zhao Y-M, Luo X-G, Feng Y, Ren Y, Shang H, He ZY, Luo XM, Chen SD, Wang XY. Repeated lipopolysaccharide stimulation induces cellular senescence in BV2 cells. Neuroimmunomodulation. 2012;19:131–136. doi: 10.1159/000330254. [DOI] [PubMed] [Google Scholar]
- 60.Dolfi DV, Mansfield KD, Polley AM, Doyle SA, Freeman GJ, Pircher H, Schmader KE, Wherry EJ. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J Leukoc Biol. 2013;93:825–836. doi: 10.1189/jlb.0912438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, Schramm J, Elger CE, Lassmann H. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol. 2002;51:311–318. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- 62.Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain. 1997;120(Pt 8):1461–1483. doi: 10.1093/brain/120.8.1461. [DOI] [PubMed] [Google Scholar]
- 63.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 64.Höftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14:43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 66.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Axelsson M, Malmeström C, Nilsson S, Haghighi S, Rosengren L, Lycke J. Glial fibrillary acidic protein: a potential biomarker for progression in multiple sclerosis. J Neurol. 2011;258:882–888. doi: 10.1007/s00415-010-5863-2. [DOI] [PubMed] [Google Scholar]
- 68.Larsson Å, Wilhelmsson U, Pekna M, Pekny M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP-/- vim-/- mice. Neurochem Res. 2004;29:2069–2073. doi: 10.1007/s11064-004-6880-2. [DOI] [PubMed] [Google Scholar]
- 69.Menet V, Giménez Y, Ribotta M, Sandillon F, Privat A. GFAP null astrocytes are a favorable substrate for neuronal survival and neurite growth. Glia. 2000;31:267–272. doi: 10.1002/1098-1136(200009)31:3<267::aid-glia80>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 70.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 71.Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- 72.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 73.Correale J, Farez MF. The role of astrocytes in multiple sclerosis progression. Front Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J, Andersen JK. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep. 2018;22:930–940. doi: 10.1016/j.celrep.2017.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamazaki Y, Baker DJ, Tachibana M, Liu CC, van Deursen JM, Brott TG, Bu G, Kanekiyo T. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke. 2016;47:1068–1077. doi: 10.1161/STROKEAHA.115.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu R, Liu H, Ha Y, Tilton RG, Zhang W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res Int. 2014;2014:902842–902813. doi: 10.1155/2014/902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McRobb LS, McKay MJ, Gamble JR, et al. Ionizing radiation reduces ADAM10 expression in brain microvascular endothelial cells undergoing stress-induced senescence. Aging. 2017;9:1248–1268. doi: 10.18632/aging.101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kieseier BC, Pischel H, Neuen-Jacob E, Tourtellotte WW, Hartung HP. ADAM-10 and ADAM-17 in the inflamed human CNS. Glia. 2003;42:398–405. doi: 10.1002/glia.10226. [DOI] [PubMed] [Google Scholar]
- 79.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoffmann FS, Kuhn P-H, Laurent SA, Hauck SM, Berer K, Wendlinger SA, Krumbholz M, Khademi M, Olsson T, Dreyling M, Pfister HW, Alexander T, Hiepe F, Kümpfel T, Crawford HC, Wekerle H, Hohlfeld R, Lichtenthaler SF, Meinl E. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol. 2015;194:542–552. doi: 10.4049/jimmunol.1402070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36:451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 84.Di Carlo SE, Peduto L. The perivascular origin of pathological fibroblasts. J Clin Invest. 2018;128:54–63. doi: 10.1172/JCI93558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pikor NB, Astarita JL, Summers-Deluca L, Galicia G, Qu J, Ward LA, Armstrong S, Dominguez CX, Malhotra D, Heiden B, Kay R, Castanov V, Touil H, Boon L, O’Connor P, Bar-Or A, Prat A, Ramaglia V, Ludwin S, Turley SJ, Gommerman JL. Integration of Th17- and lymphotoxin-derived signals initiates meningeal-resident stromal cell remodeling to propagate neuroinflammation. Immunity. 2015;43:1160–1173. doi: 10.1016/j.immuni.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 86.Cupovic J, Onder L, Gil-Cruz C, et al. Central nervous system stromal cells control local CD8+ T cell responses during virus-induced neuroinflammation. Immunity. 2016;44:622–633. doi: 10.1016/j.immuni.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pikor NB, Cupovic J, Onder L, et al. Stromal cell niches in the inflamed central nervous system. J Immunol. 2017;198:1775–1781. doi: 10.4049/jimmunol.1601566. [DOI] [PubMed] [Google Scholar]
- 88.Tang DG, Tokumoto YM, Apperly JA, Lloyd AC, Raff MC. Lack of replicative senescence in cultured rat oligodendrocyte precursor cells. Science. 2001;291:868–871. doi: 10.1126/science.1056780. [DOI] [PubMed] [Google Scholar]
- 89.Kujuro Y, Suzuki N, Kondo T. Esophageal cancer-related gene 4 is a secreted inducer of cell senescence expressed by aged CNS precursor cells. Proc Natl Acad Sci U S A. 2010;107:8259–8264. doi: 10.1073/pnas.0911446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishino J, Kim I, Chada K, Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16ink4a and p19arf expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piechota M, Sunderland P, Wysocka A, et al. Is senescence-associated β-galactosidase a marker of neuronal senescence? Oncotarget. 2016;7:81099–81109. doi: 10.18632/oncotarget.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Jill Saffrey M, Cameron K, von Zglinicki T. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei Z, Chen X-C, Song Y, et al. Amyloid β protein aggravates neuronal senescence and cognitive deficits in 5XFAD mouse model of Alzheimer’s Disease. Chin Med J (Engl) 2016;129:1835. doi: 10.4103/0366-6999.186646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang J-Z, Wang Z-H. Senescence may mediate conversion of tau phosphorylation-induced apoptotic escape to neurodegeneration. Exp Gerontol. 2015;68:82–86. doi: 10.1016/j.exger.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Baron W, Colognato H, Ffrench-Constant C, Ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- 97.Sobel RA, Mitchell ME. Fibronectin in multiple sclerosis lesions. Am J Pathol. 1989;135:161–168. [PMC free article] [PubMed] [Google Scholar]
- 98.van Horssen J, Bö L, Dijkstra CD, de Vries HE. Extensive extracellular matrix depositions in active multiple sclerosis lesions. Neurobiol Dis. 2006;24:484–491. doi: 10.1016/j.nbd.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Stoffels JMJ, de Jonge JC, Stancic M, Nomden A, van Strien ME, Ma D, Šišková Z, Maier O, ffrench-Constant C, Franklin RJM, Hoekstra D, Zhao C, Baron W. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136:116–131. doi: 10.1093/brain/aws313. [DOI] [PubMed] [Google Scholar]
- 100.Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature. 2001;413:83–86. doi: 10.1038/35092584. [DOI] [PubMed] [Google Scholar]
- 101.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 102.Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol. 2015;68:19–25. doi: 10.1016/j.exger.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Passos JF, von Zglinicki T, Saretzki G. Mitochondrial dysfunction and cell senescence: cause or consequence? Rejuvenation Res. 2006;9:64–68. doi: 10.1089/rej.2006.9.64. [DOI] [PubMed] [Google Scholar]
- 105.Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL, Dorronsoro A, Corbo L, Tang P, Bukata C, Ring N, Giacca M, Li X, Tchkonia T, Kirkland JL, Niedernhofer LJ, Robbins PD. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8:422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;1:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Triantafyllou A, Mylonis I, Simos G, Bonanou S, Tsakalof A. Flavonoids induce HIF-1α but impair its nuclear accumulation and activity. Free Radic Biol Med. 2008;44:657–670. doi: 10.1016/j.freeradbiomed.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 108.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golovine KV, Makhov PB, Teper E, Kutikov A, Canter D, Uzzo RG, Kolenko VM. Piperlongumine induces rapid depletion of the androgen receptor in human prostate cancer cells. Prostate. 2013;73:23–30. doi: 10.1002/pros.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang F, Mao Y, You Q, Hua D, Cai D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int J Immunopathol Pharmacol. 2015;28:362–373. doi: 10.1177/0394632015598849. [DOI] [PubMed] [Google Scholar]
- 111.Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baar MP, Brandt RMC, Putavet DA, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hwang HV, Tran DT, Rebuffatti MN, Li CS, Knowlton AA. Investigation of quercetin and hyperoside as senolytics in adult human endothelial cells. PLoS One. 2018;13:e0190374. doi: 10.1371/journal.pone.0190374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Torkildsen Ø, Brunborg LA, Myhr K-M, Bø L. The cuprizone model for demyelination. Acta Neurol Scand. 2008;117:72–76. doi: 10.1111/j.1600-0404.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 115.Thewissen M, Somers V, Venken K, Linsen L, van Paassen P, Geusens P, Damoiseaux J, Stinissen P. Analyses of immunosenescent markers in patients with autoimmune disease. Clin Immunol. 2007;123:209–218. doi: 10.1016/j.clim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 116.Broux B, Pannemans K, Zhang X, Markovic-Plese S, Broekmans T, Eijnde BO, van Wijmeersch B, Somers V, Geusens P, van der Pol S, van Horssen J, Stinissen P, Hellings N. CX3CR1 drives cytotoxic CD4+CD28− T cells into the brain of multiple sclerosis patients. J Autoimmun. 2012;38:10–19. doi: 10.1016/j.jaut.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 117.Broux B, Mizee MR, Vanheusden M, van der Pol S, van Horssen J, van Wijmeersch B, Somers V, de Vries HE, Stinissen P, Hellings N. IL-15 amplifies the pathogenic properties of CD4 + CD28 − T cells in multiple sclerosis. J Immunol. 2015;194:2099–2109. doi: 10.4049/jimmunol.1401547. [DOI] [PubMed] [Google Scholar]
- 118.van Nierop GP, van Luijn MM, Michels SS, Melief MJ, Janssen M, Langerak AW, Ouwendijk WJD, Hintzen RQ, Verjans GMGM. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 2017;134:383–401. doi: 10.1007/s00401-017-1744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 120.Killilea DW, Wong SL, Cahaya HS, et al. Iron accumulation during cellular senescence. Ann N Y Acad Sci. 2004;1019:365–367. doi: 10.1196/annals.1297.063. [DOI] [PubMed] [Google Scholar]