Abstract
Succinate-driven reverse electron transport (RET) is one of the main sources of mitochondrial reactive oxygen species (mtROS) in ischemia-reperfusion injury. RET is dependent on mitochondrial membrane potential (Δψm) and transmembrane pH difference (ΔpH), components of the proton motive force (pmf); a decrease in Δψm and/or ΔpH inhibits RET. In this study we aimed to determine which component of the pmf displays the more dominant effect on RET-provoked ROS generation in isolated guinea pig brain and heart mitochondria respiring on succinate or α-glycerophosphate (α-GP). Δψm was detected via safranin fluorescence and a TPP+ electrode, the rate of H2O2 formation was measured by Amplex UltraRed, the intramitochondrial pH (pHin) was assessed via BCECF fluorescence. Ionophores were used to dissect the effects of the two components of pmf. The K+/H+ exchanger, nigericin lowered pHin and ΔpH, followed by a compensatory increase in Δψm that led to an augmented H2O2 production. Valinomycin, a K+ ionophore, at low [K+] increased ΔpH and pHin, decreased Δψm, which resulted in a decline in H2O2 formation. It was concluded that Δψm is dominant over ∆pH in modulating the succinate- and α-GP-evoked RET. The elevation of extramitochondrial pH was accompanied by an enhanced H2O2 release and a decreased ∆pH. This phenomenon reveals that from the pH component not ∆pH, but rather absolute value of pH has higher impact on the rate of mtROS formation. Minor decrease of Δψm might be applied as a therapeutic strategy to attenuate RET-driven ROS generation in ischemia-reperfusion injury.
Keywords: Reactive oxygen species, Mitochondria, Proton motive force, Membrane potential, Reverse electron transport, Nigericin, Valinomycin, Succinate, Alpha-glycerophosphate
Introduction
There is a large body of experimental evidence demonstrating pathologically enhanced mitochondrial reactive oxygen species (mtROS) production in several diseases such as diabetes, neurodegenerative conditions including Alzheimer’s and Parkinson’s diseases, diabetes, and ischemia-reperfusion injury; for review see (Beal 1996; Giacco and Brownlee 2010; Chouchani et al. 2014). Respiratory Complex I (CI) is a primary source of mtROS and its dysfunction is thought to be pathologically relevant (Cadenas et al. 1977, Grivennikova and Vinogradov 2006, Treberg et al. 2011). In isolated mitochondria CI-mediated mtROS generation can be initiated under the following conditions: 1) with NADH-linked substrates (such as glutamate and malate), which generate mtROS at a relatively low rate; 2) with NADH-linked substrates in the presence of a CI inhibitor, like rotenone producing high rate of ROS; 3) with FADH2-linked substrates like succinate or (Lambert and Brand 2004; Zoccarato et al. 2004; Treberg et al. 2011; Orr et al. 2012) alpha-glycerophosphate (α-GP) (Tretter et al. 2007b, c). In hyperpolarized non-phosphorylating mitochondria, FADH2-linked substrates generate mtROS at a higher rate by supporting a reverse electron transport (RET) which occurs from Complex II (CII) or alpha-glycerophosphate dehydrogenase (α-GPDH) to CI via the Q-junction (Treberg et al. 2011). According to prior reports, succinate-driven mtROS production appears to have the highest rate in isolated murine mitochondria in the absence of ADP compared to NADH-linked substrates initiated mtROS (Korshunov et al. 1997; Kwong and Sohal 1998; Votyakova and Reynolds 2001; Liu et al. 2002; Zoccarato et al. 2011); this is attributed primarily to RET towards CI and partially to the forward electron transport (FET) towards CIII (Grivennikova and Vinogradov 2006; Treberg et al. 2011; Zoccarato et al. 2011; Quinlan et al. 2013). Upon succinate oxidation, in the absence of ATP synthesis, FET secures the energy demands of RET.
Rate of RET-associated ROS production is thought to be dependent on pmf which comprises mitochondrial transmembrane potential (Δψm) and mitochondrial transmembrane pH gradient (∆pH) (Liu 1997; Votyakova and Reynolds 2001; Lambert and Brand 2004). It is a well-known phenomenon that high pmf, such as the one measured in the absence of ADP, is required for maintenance of RET. It has been shown that succinate- or α-GP-fuelled RET is very sensitive to minor changes in Δψm in isolated mammalian (Tretter and Adam-Vizi 2007) and Drosophila (Miwa and Brand 2003) mitochondria.
More specifically, a 10% decrease in Δψm (caused by an uncoupler agent) gave rise to a 90% decrease in succinate-driven ROS production in rat heart mitochondria (Korshunov et al. 1997). The other component of pmf, ∆pH, also appears to have a regulating effect on mtROS formation. Upon acidification of the matrix, mtROS generation is decelerated, which can be explained by the stabilisation of the semiquinone radicals (SQ.-) (Selivanov et al. 2008). The question arises as to which component of pmf plays the key role in the control of mtROS production. According to Lambert and Brand (Lambert and Brand 2004), succinate-driven ROS production is more dependent on ∆pH than on Δψm, as detected in mitochondria isolated from rat skeletal muscle. On the contrary, Selivanov and co-workers (Selivanov et al. 2008) revealed that mtROS generation is significantly affected by the actual value of pH itself (extramitochondrial pH; pHextra and intramitochondrial pH; pHin), and not much influenced by ∆pH or Δψm, as measured in rat brain mitochondria.
The aim of the present study was to clarify which of the two components of pmf has a predominant role in the control of mtROS formation and to assess whether absolute pH value modulates RET-dependent mtROS production. We also aimed to test whether the effect of Δψm, ΔpH, and absolute pH values on ROS formation is different in brain compared to heart muscle mitochondria. Δψm and ΔpH usually change in the same direction; for example, uncoupling depolarisation (decrease of Δψm) is generally followed by a decrease in ∆pH as well. With ionophores, like valinomycin and nigericin, it is possible to dissect the two components of pmf: Δψm and ΔpH can be varied in a different direction. Nigericin decreases pHin (Rottenberg and Lee 1975) and hyperpolarises Δψm (Selivanov et al. 2008), whilst valinomycin elevates pHin (Selivanov et al. 2008) and depolarizes Δψm (Selivanov et al. 2008) under specific conditions. In the present study, Δψm, pHin, and H2O2 production were measured systematically and ∆pH was calculated. To scrutinize Selivanov’s theory, pH dependence of the above-mentioned parameters was examined. In contrast to Lambert and co-workers (Lambert and Brand 2004), we concluded that the succinate-driven RET-evoked ROS production is more dependent on Δψm and less influenced by ∆pH in both guinea pig brain and heart mitochondria. Furthermore, we showed, in agreement with Selivanov and colleagues, that absolute pH rather than ∆pH itself modulates succinate- and α-GP-driven RET. Our results suggest that lowering Δψm might be an effective solution to reduce the RET-provoked mtROS load in conditions like ischemia-reperfusion where oxidative stress and high Δψm prevail.
Materials and methods
Chemicals
Standard laboratory reagents, except ADP, were obtained from Sigma (St. Louis, MO, USA). ADP was purchased from Merck (Darmstadt, Germany). BCECF/AM and Amplex UltraRed were obtained from TermoFisher Scientific (Waltham, MA, USA).
Preparation of mitochondria
Mitochondria were prepared from albino guinea pig brain cortex using a Percoll gradient (Rosenthal et al. 1987,Tretter and Adam-Vizi 2007) and from whole heart using differential centrifugation (Mela and Seitz 1979,Korshunov et al. 1997), as previously described. Animal experiments were performed in accordance with the Guidelines for Animal Experiments of Semmelweis University. A modified biuret method was used to determine mitochondrial protein concentration (Bradford 1976).
Brain mitochondria
The brain was rapidly homogenized in Buffer A (in mM: 225 mannitol, 75 sucrose, 5 HEPES, 1 EGTA, pH 7.4) and centrifuged for 3 min at 1300 g. The supernatant was centrifuged for 10 min at 20,000 g, and the resulting pellet was resuspended in 15% Percoll and layered on a discontinuous gradient consisting of 40 and 23% Percoll. This was centrifuged for 8 min at 30,700 g using no brake. After resuspension of the lower fraction in Buffer A, centrifugation was applied at 16,600 g for 10 min. Pellet was resuspended in Buffer A and centrifuged at 6300 g for 10 min. Subsequently, supernatant was discharged, and the pellet was resuspended in Buffer B (in mM: 225 mannitol, 75 sucrose, 5 HEPES, pH 7.4). All operations above were performed either on ice or at 4 °C (Komary et al. 2008).
Heart mitochondria
Mitochondria from heart were isolated following the modified protocol of Korshunov and co-workers (Korshunov et al. 1997). The heart was repeatedly washed in homogenisation buffer (in mM: 200 mannitol, 50 sucrose, 5 NaCl, 5 MOPS, 1 EGTA, 0.1% BSA, pH 7.15) to remove residual blood. Afterwards, it was cut into small pieces with scissors under 2.5 ml homogenisation buffer supplemented with 10 U protease (Protease from Bacillus licheniformis, Type VIII). After adding 17 ml of homogenisation buffer, the preparation was properly homogenised and centrifuged for 10 min at 10,500 g. The supernatant was discharged, the pellet was resuspended in 25 ml homogenisation buffer, and then centrifuged for 10 min at 3000 g. The supernatant was centrifuged for 10 min at 10,500 g and the formed pellet was resuspended in the homogenisation buffer. All operations above were performed either at 4 °C or on ice.
Buffers
Depending on the requirement of K+ of the applied ionophore (nigericin or valinomycin), one of the following media was applied in the relevant experiments:
Standard medium A (high K+ content for nigericin; in mM): 125 KCl, 20 HEPES, 2 KH2PO4, 0.1 EGTA, 1 MgCl2, and 0.025% BSA.
Standard medium B (low K+ content for valinomycin to avoid mitochondrial swelling; in mM): 240 sacharose, 10 Tris, 2 KH2PO4, 4 KCl, 0.1 EGTA, 1 MgCl2, and 0.025% BSA. pH of the respiratory media was adjusted prior to the measurements, in the absence of mitochondria, with HCl or NaOH to 6.4, 6.8, 7.0, 7.2, 7.4, 7.6 or 8.0. Addition of mitochondria suspended in buffered solution and addition of high concentrations of respiratory substrates (succinate or α-GP) could shift the pH of the incubation medium slightly. In order to calculate an accurate ∆pH, pHextra measured in the presence of mitochondria and respiratory substrate was applied in this study.
Measurement of mitochondrial H2O2 production
The assay is based on detection of H2O2 in the medium using the Amplex UltraRed fluorescent dye. In the presence of horseradish peroxidase (HRP), Amplex UltraRed reacts with H2O2 in a 1:1 stoichiometry producing fluorescent Amplex UltroxRed. HRP (5 U/2 ml) and Amplex UltraRed (3 μM) were added to standard medium A or B. Subsequently, mitochondria (0.05 mg/ml) and succinate (5 mM) or α-GP (20 mM) were added. Resorufin fluorescence was detected using a Photon Technology International (PTI; Lawrenceville, NJ, USA) Deltascan fluorescence spectrophotometer. The excitation wavelength was 550 nm, while the emission was detected at 585 nm. At the end of each experiment, the fluorescence signal was calibrated with 100 pmol H2O2. All the measurements were performed at 37 °C.
Measurement of the mitochondrial membrane potential (Δψm)
Measurement with safranine-O
Δψm was assessed using safranine-O, a lipophilic cationic fluorescent dye, which accumulates in the mitochondrial membrane upon hyperpolarisation resulting in fluorescence quenching (Akerman and Wikstrom 1976). Safranine (2 μM) fluorescence (495 nm for excitation, 585 nm for emission) was detected using a Hitachi F-4500 spectrofluorimeter (Hitachi High Technologies, Maidenhead, UK). All measurements were carried out at 37 °C in standard medium A or B, as previously described.
Measurement with TPP+ electrode
Δψm was estimated via the distribution of the tetraphenylphosphonium ion (TPP+). TPP+ was detected using a custom-made TPP+-selective electrode (Kamo et al. 1979), as described previously (Tretter et al. 2007a). Δψm was calculated using the Nernst equation and the reported binding correction factor for brain mitochondria, as previously described (Rottenberg 1984; Rolfe et al. 1994). The calculation was performed according to Rottenberg and co-workers (Rottenberg 1984) assuming that the matrix volume of the mitochondria is 1 μl/mg protein (D.G. Nicholls, personal communication). The sensitivity of the TPP+ electrode was found to be decreased at low Δψm (less than ~120 mV) (Starkov and Fiskum 2003).
Measurement of the intramitochondrial pH (pHin)
pHin of isolated mitochondria was measured with the acetoxymethyl ester form of 2,7-biscarboxyethyl-5(6)-carboxyfluorescein (BCECF/AM) (Jung et al. 1989), as described earlier (Sipos et al. 2005). Briefly; 100 μl mitochondria (35–40 mg/ml protein) were incubated with 50 μM BCECF/AM in Buffer C (in mM: 225 mannitol, 75 sucrose, 5 HEPES, 0.1 EGTA, pH 7.4) for 10 min at 25 °C. Ice-cold Buffer C (325 μl) was supplemented with 0.1 mM ADP (in order to prevent permeability transition pore opening). Loaded mitochondria were centrifuged for 2 min at 13000 g, the supernatant was removed, the pellet was resuspended in 450 μl Buffer C, and this was centrifuged for 2 min at 13000 g. The new pellet was resuspended in 450 μl Buffer C minus ADP, left standing for hydrolysis (10 min), and then centrifuged for 2 min at 13000 g. All centrifugation steps were performed at 4 °C. The supernatant was discharged. The pellet was supplemented with 13 μl Buffer C. BCECF-loaded mitochondria were used within 90 min. For fluorescence measurements, 3 μl aliquots of mitochondria were diluted in 2 ml of standard medium A or B. Fluorescence ratios were determined using the PTI Deltascan fluorescence spectrophotometer (440 or 505 nm for excitation, 540 nm for emission). Leaching of BCECF from mitochondria was determined by measuring the fluorescence of the supernatant of the centrifuged loaded mitochondria. Corrections were made by subtracting the fluorescence values of the supernatant from those of the experimental values. For calibration, the external and internal [H+] were equilibrated at varying pHextra values by the addition of a mixture of 8 μM nigericin (K+/H+ antiporter), 2.5 μM gramicidin (Na+/K+ ionophore), and 8 μM monensin (Na+/H+ antiporter), as previously described (Sipos et al. 2005).
Statistical analysis
The statistical differences in multiple comparisons were evaluated with ANOVA (SigmaPlot™, Version 11, Systat Software, Inc., San Jose, CA, USA). Values of p < 0.05 were considered to be statistically significant.
Results
In order to dissect Δψm and ΔpH, the two components of pmf, ionophores were introduced throughout the experiments. The standard media A contained 2 mM K2HPO4 and 125 mM KCl, whilst standard medium B was supplemented with 2 mM K2HPO4 and 4 mM K+. ADP was absent providing a high Δψm to support RET in succinate- or α-GP-energised mitochondria. At the end of each experiment the uncoupler FCCP was given to eliminate any Δψm and abolish the succinate- or α-GP-driven RET.
Effects of nigericin on pHin, ΔpH, Δψm, and mtROS production in brain mitochondria at medium pH 7.0
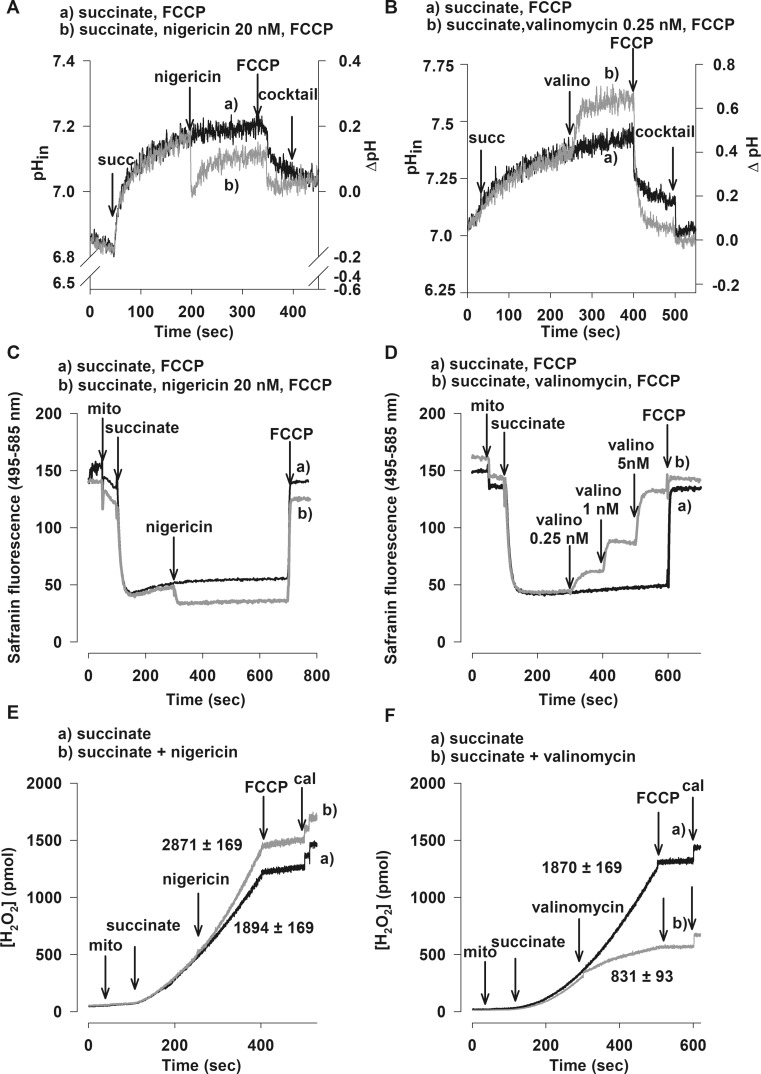
Nigericin, a K+/H+ antiporter, allows the electroneutral transport of these two ions in opposite directions across the mitochondrial inner membrane following the K+ concentration gradient (Henderson et al. 1969; Rottenberg and Lee 1975). As displayed in Fig. 1, nigericin (20 nM) decreased pHin (Fig. 1a) at pHextra = 6.84 ± 0.01 (medium pH = 7.0) by 0.13 ± 0.04 pH unit and ∆pH from 0.23 ± 0.06 to 0.089 ± 0.02 (Fig. 1c). In addition, nigericin increased Δψm by 7.78 ± 2.5 mV; Δψm could not be increased any further by subsequent additions of nigericin. In contrast to Lambert and co-workers (Lambert and Brand 2004), we found that nigericin increased the rate of H2O2 generation by 52 ± 11% (from 1894 ± 169 to 2871 ± 169 pmol/min/mg protein) in succinate-respiring brain mitochondria (Fig. 1e). We can conclude that in succinate-supported mitochondria, nigericin decreased ∆pH and induced mitochondrial hyperpolarization, simultaneously elevating H2O2 production.
Fig. 1.
Effect of nigericin (a, c, e) and valinomycin (b, d, f) on pHin and ∆pH (a, b), Δψm (c, d) and the rate of H2O2 production (e, f) in succinate-energised brain mitochondria. Mitochondria (0.05 or 0.1 mg/ml) were incubated in different standard media as described under Materials and Methods. Succinate (5 mM), FCCP (250 nM), valinomycin (0.25 nM), nigericin (20 nM) and cocktail (gramicidin, monensin, nigericin) were given as indicated. ∆pH (a, b) values were calculated from the difference between pHin and pHextra. In A and B each experiment was calibrated by KOH. In E and F results (slope) are expressed in pmol/min/mg protein and each experiment was calibrated by 100 pmol H2O2. For (a, b, c, d, e, f) traces are representative of at least three independent experiments
In order to gain a deeper insight into the effects of nigericin on RET, α-GP was also applied as a respiratory substrate. Unlike succinate, α-GP does not enter the mitochondria, it is oxidized by α-GPDH on the outer surface of the inner mitochondrial membrane and does not form NADH. Addition of rotenone diminished the H2O2 production both in succinate and α-GP energised mitochondria, which points to a CI-related ROS production, likely RET (Votyakova and Reynolds 2001). Both respiratory substrates upon their oxidation by succinate dehydrogenase (SDH) or α-GPDH reduce the coenzyme Q (Q; ubiquinone)-junction bypassing CI. Similarly to that observed with succinate, nigericin decreased pHin, increased Δψm (data not shown), and stimulated H2O2 production (Fig. 2c) in α-GP-energised mitochondria as well.
Fig. 2.
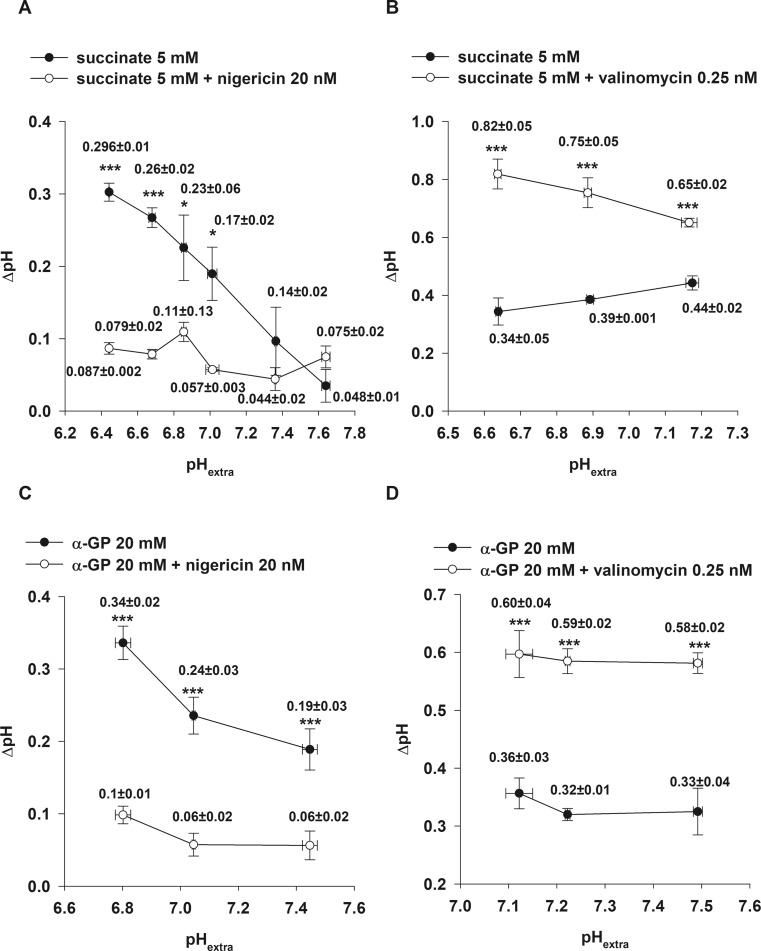
Effect of nigericin (a, c) and valinomycin (b, d) on the rate of succinate (a, b) and α-glycerophosphate (c, d)-driven H2O2 production as a function of pHextra in brain mitochondria. Mitochondria (0.05 mg/ml) were incubated in the standard media as described under Materials and Methods. Succinate (5 mM), α-glycerophosphate (α-GP; 20 mM), valinomycin (0.25 nM) and nigericin (20 nM) were added. The results are expressed as the rate of H2O2 production in pmol/min/mg protein mean ± SEM (n > 4) and pHextra given as mean ± SEM (n > 4) and written in the graphs; ***p < 0.001; **p < 0.01
Effects of valinomycin on pHin, ΔpH, Δψm, and mtROS production in brain mitochondria at pH 7.0
Valinomycin is a K+ ionophore transporting K+ along its electrochemical gradient across the mitochondrial inner membrane. In succinate-supported mitochondria, valinomycin (0.25 nM) increased pHin by 0.38 ± 0.04 pH unit (Fig. 1b), ∆pH from 0.39 ± 0.001 to 0.75 ± 0.04, and depolarized ∆ψm in a dose-dependent manner (Fig. 1d). We found that valinomycin decreased the rate of H2O2 generation by 44.5 ± 4% when mitochondria were supported by succinate (Fig. 1f, trace b). Valinomycin displayed similar effects on α-GP-respiring brain mitochondria. At pHextra = 7.22 ± 0.01 (Fig. 2d), valinomycin alkalized the mitochondrial matrix by 0.26 ± 0.02 pH unit, while ∆pH was increased from 0.32 ± 0.01 to 0.59 ± 0.02 (Fig. 3d) with α-GP. Simultaneously, a decreased rate of the α-GP-evoked H2O2 production (by 45 ± 14%) was measured, similarly to that observed in succinate-supported mitochondria.
Fig. 3.
Effect of nigericin (a, c) and valinomycin (b, d) on ∆pH in succinate (a, b) and α-glycerophosphate (c, d)-respiring brain mitochondria as a function of pHextra. Mitochondria were incubated in the standard media as described under Materials and Methods. Succinate (5 mM), α-glycerophosphate (α-GP; 20 mM), valinomycin (0.25 nM) and nigericin (20 nM) were used. The results are expressed as pH value mean ± SEM (n > 4) and written in the graphs; ***p < 0.001; *p < 0.05
Effects of pHextra on H2O2 production, pHin, ΔpH, and Δψm in succinate- and α-GP-respiring brain mitochondria
To examine the influence of changes in pHextra on ∆pH and H2O2 production, experiments were carried out in standard media A (nigericin) or B (valinomycin) varying pH from 6.4 to 8.0 (see Materials and Methods).
H2O2 production
As seen in Fig. 2, upon increasing pHextra a sharp increase of succinate- and α-GP-related H2O2 generation was observed both in the absence (Fig. 2a, c, black circles) and presence (Fig. 2a, c, white circles) of nigericin. The nigericin treatment of succinate-supported mitochondria elevated the rate of H2O2 production significantly between pHextra = 6.45 ± 0.004 and 7.03 ± 0.02 (Fig. 2a). Similarly, in α-GP-respiring mitochondria nigericin increased the rate of H2O2 formation by 48 ± 3% at pHextra = 6.81 ± 0.01, 42 ± 4% at pHextra = 7.05 ± 0.05, and 21 ± 11% at pHextra = 7.45 ± 0.02 (Fig. 2c). The addition of valinomycin to succinate- and α-GP-supported mitochondria significantly reduced the rate of H2O2 formation at all different pHextra values (Fig. 2b, d).
Δψm
Measuring Δψm by a TPP+ electrode, it was concluded that nigericin always increased the Δψm approximately to the same level (~ − 195 ˗ -200 mV), even at different pHextra values in brain mitochondria. At pHextra = 6.45 ± 0.004, nigericin hyperpolarized the membrane by 12.5 mV, at pHextra = 6.84 ± 0.013 by 19 mV, and at pHextra = 7.30 ± 0.047 by 8.5 mV. Taken together, these data show that Δψm and the rate of H2O2 production were the highest when nigericin was present and the medium was the most alkaline.
pHin and ΔpH
As shown in Fig. 3, upon elevation of pHextra, ΔpH was concomitantly decreased in both succinate- and α-GP-respiring mitochondria. The addition of nigericin was followed by acidification of the mitochondrial matrix, resulting in a drop of ∆pH (Fig. 3a, c). At the most alkaline pHextra (7.45 ± 0.02), in the presence of succinate, nigericin could neither decrease pHin nor ∆pH. However, in α-GP-respiring mitochondria, nigericin reduced both pHin and ∆pH at all measured pHextra values (Fig. 3c). Valinomycin treatment of both succinate- and α-GP-respiring brain mitochondria caused alkalinization of the mitochondrial matrix and a corresponding elevation of ΔpH (Fig. 3b, d).
Heart mitochondria. Effects of nigericin and valinomycin on mitochondrial parameters
Detecting RET is also relevant in organs other than the brain, like heart, regarding their exposure to oxidative stress under pathological conditions, like ischemia-reperfusion (Chouchani et al. 2014). To deepen our understanding on RET in heart mitochondria, effects of ∆pH and Δψm on succinate-supported H2O2 production were investigated applying the above-mentioned ionophores.
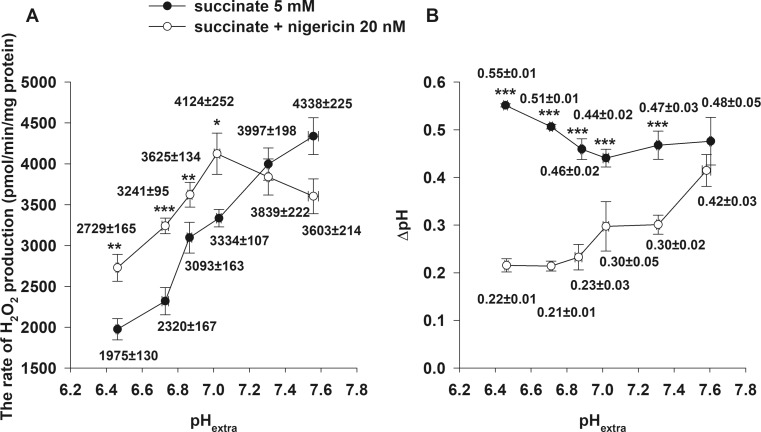
Similarly to brain, in heart mitochondria nigericin hyperpolarized the membrane at various pHextra values. In the absence of nigericin, Δψm of succinate-supported, non-phosphorylating mitochondria was similar at all pHextra values analogously to brain. In contrast to that observed in brain mitochondria, in heart, the addition of nigericin led to an increase of the rate of succinate-evoked H2O2 generation only between pHextra = 6.46 ± 0.005 and 7.03 ± 0.008 (Fig. 4a). At a more alkaline pH (pHextra = 7.54 ± 0.002), nigericin decreased the rate of H2O2 formation by 22 ± 8% (Fig. 4a, white circles). In the absence of nigericin, upon elevation of pHextra, the rate of the succinate-initiated H2O2 generation was steeply increasing (Fig. 4a, black circles). In the absence of nigericin, ∆pH decreased with incrementing pHextra (pHextra from 6.46 ± 0.005 to 7.03 ± 0.008) until pHextra 7.03 ± 0.008; at pHextra above such value, ∆pH increased (Fig. 4b, black circles). Contrary to this, in the presence of nigericin, ∆pH was slightly ascending upon pHextra elevation (Fig. 4b, white circles) and at pHextra = 7.54 ± 0.002 there was no statistically significant difference between ∆pH in the presence of nigericin compared to ∆pH in its absence.
Fig. 4.
Effect of nigericin on the rate of H2O2 production (a) and on ∆pH (b) at different pHextra in succinate-respiring isolated heart mitochondria. Mitochondria (0.05 or 0.1 mg/ml) were incubated in standard medium A as described under Materials and Methods. Succinate (5 mM) and nigericin (20 nM) were given. The results of A are expressed as the rate of H2O2 production in pmol/min/mg protein mean ± SEM (n > 4) and written in the graphs, pHextra given as mean ± SEM (n > 4). For B the results are expressed in pH values mean ± SEM and written in the graphs (n > 4); ***p < 0.001; **p < 0.01; *p < 0.05
Discussion
There is a lack of consensus regarding the role of ∆pH and Δψm on mtROS generation (Lambert and Brand 2004; Selivanov et al. 2008), therefore, in our study, we aimed to clarify the dependence of succinate- and α-GP-driven H2O2 production on components of pmf. The results presented above allow the conclusion that Δψm displays a stronger influence on the succinate- or α-GP-supported, RET-initiated H2O2 production than ΔpH. In this study, we did not only measure H2O2 production and Δψm, but we also detected matrix pH (pHin) with the fluorescent dye BCECF and calculated ΔpH. Under most physiological conditions depolarization of the inner membrane (decrease of the absolute value of Δψm) is associated with a decrease of ∆pH and an elevation of matrix [H+]. It is unfeasible to create conditions where one of the components of pmf is maintained constant whilst the other one is independently altered. With ionophores however, these two parameters can be changed in opposite directions. In order to increase Δψm, nigericin was applied, which decreased ∆pH and increased H2O2 production (Fig.1) suggesting that mtROS production is directly proportional to Δψm. If ∆pH was the dominant factor of the RET-initiated H2O2 formation, then H2O2 production should have been decreased. To increase ∆pH, valinomycin was added, which simultaneously depolarized the inner membrane and decreased the rate of H2O2 generation which changed in accordance with Δψm values. If ∆pH had been the major player in H2O2 production, then H2O2 production should have been higher in the presence of valinomycin than in its absence.
Our measurements were carried out not only in brain but also in heart mitochondria, both displaying similar effects. Based on these observations, we can exclude the tissue specific modification of RET– supported H2O2 generation in these tissues.
In summary, our studies with the two ionophores showed that RET-evoked H2O2 production always varied in accordance with changes of Δψm, which leads to the conclusion that Δψm has a greater influence on mitochondrial RET-initiated H2O2 formation than ΔpH. In addition, we also showed that elevation of pHextra resulted in increased H2O2 generation, a finding that suggests a clear correlation between absolute pH and H2O2 production.
Nigericin
Nigericin, as a K+/H+ antiporter, is responsible for the electroneutral exchange of K+ and H+ (Henderson et al. 1969; Bernardi 1999). In our preliminary experiments, the dose-dependent effects of nigericin on Δψm were studied, and the lowest possible concentration was used which created a maximal mitochondrial hyperpolarization measured by safranin fluorescence (data not shown). Contrary, Lambert and colleagues (Lambert and Brand 2004) as well as Selivanov’s group (Selivanov et al. 2008) applied 100 nM nigericin, which in our hands did neither increase Δψm further, nor dissipate ΔpH completely but established a new equilibrium with lower pHin. ΔpH, after administration of 100 nM nigericin, could be decreased further by addition of 250 nM FCCP and mixture of ionophores (see Materials and Methods). To eliminate confounding factors that could have influenced ROS production (e.g. succinate transport or further metabolism of succinate in the tricarboxylic acid cycle), not only succinate, but also α-GP was used to energize mitochondria and support RET-mediated ROS production. Results with α-GP were qualitatively equivalent to those obtained in succinate-supported mitochondria (Figs. 2 and 3). The stimulating effect of nigericin on H2O2 generation was more pronounced at acidic pHextra. Interestingly, in heart mitochondria, at alkaline pHextra, nigericin decreased the rate of H2O2 release (Fig. 4a). It appears that in heart mitochondria, the diminution in the rate of H2O2 production at alkaline pH cannot be explained by depolarisation of the mitochondrial membrane.
Valinomycin
In the presence of valinomycin, the mitochondrial membrane is permeable to K+; its effect is highly dependent on the K+ concentration of the medium and the applied valinomycin concentration. High K+ concentrations in the presence of 2 mM KH2PO4 and valinomycin lead to high amplitude mitochondrial swelling (Ligeti and Fonyo 1977; Bernardi 1999), therefore, 4 mM KCl was used in valinomycin experiments. It is well known that in isolated mitochondria the highest ΔpH can be achieved at low K+ concentration (Mitchell and Moyle 1968; Nicholls 1974; Nicholls 2005). It is noteworthy that ΔpH in low K+ medium is about 0.6–0.8 pH unit, but at high K+ medium it is only 0.3 pH unit. In our experiments valinomycin caused matrix alkalization and concomitant ΔpH elevation. This observation can be explained by the fact that the valinomycin-induced entry of K+ into the mitochondrial matrix usually triggers H+ extrusion and Pi/OH− exchange (Garlid and Paucek 2003). The H+ extrusion generally mediates a compensatory decrease in Δψm and an elevation of respiration both in the succinate- or α-GP-supported mitochondria. Valinomycin-caused depolarisation led to inhibition of RET-supported H2O2 production.
Effects of pHextra on H2O2 production
In agreement with the observations of Selivanov (Selivanov et al. 2008), in non-phosphorylating mitochondria, the acidification of the mitochondrial matrix is followed by an elevation in ΔpH and a decrease in the succinate- and α-GP-driven H2O2 production. There is an inverse proportionality between ΔpH and H2O2 formation, which weakens the notion of Lambert and Brand that ΔpH would exhibit a stronger effect on RET than Δψm (Lambert and Brand 2004). Our measurements of pHin with BCECF have shown that ΔpH is greater at lower pH and varies with pHextra. Banh and Treberg observed an analogous pattern in glutamate and malate-respiring, non-phosphorylating, rat skeletal muscle mitochondria, where the H2O2 generation was enhanced upon alkalization (Banh and Treberg 2013).
What mechanisms are behind the effects of Δψm and ΔpH on mitochondrial H2O2 production?
To understand the effects of Δψm and ΔpH on the RET-evoked H2O2 generation, we need to be aware of the production of superoxide (O2.-) by the CI. CI predominantly generates O2.- (Ohnishi et al. 2005; Grivennikova and Vinogradov 2006). Two mechanistic models exist for the explanation of mtROS production by the CI: (1) the one-site model states that the O2.- production site, during both FET and RET, is ultimately the reduced flavin (Galkin and Brandt 2005; Pryde and Hirst 2011), whereas (2) the two-site model suggests that during FET, the flavin of CI is responsible for O2.- formation, while, under RET, the SQ.- species, synthetized at the ubiquinone-binding Q-site (Q-binding site) of CI, are liable for the elevated O2.- release (Brand 2010; Treberg et al. 2011). Both theories agree that the greatest drop in redox potential in the CI occurs between the N2 subunit and the ubiquinone (Q), whose interaction initiates conformational changes that are coupled to the proton translocation (Treberg et al. 2011).
Δψm: There are speculations that the above mentioned conformational changes of the CI might also depend on Δψm (Brandt 2006; Dlaskova et al. 2008). When Δψm is adequately high, it decelerates the proton pumping activity of the CI, which may favour SQ.- formation and hence O2.- generation.
ΔpH and pHextra: Our results do not support the hypothesis that ΔpH would influence the RET-initiated ROS production to a higher degree than Δψm. The theory that tries to explain the influence of absolute pH on the H2O2 formation assigns a potential role to SQ.- formation at the Q-site of the CI (Ohnishi et al. 2005; Treberg et al. 2011). At the Q-site, Q is reduced by a single electron to SQ.-. SQ.- can react further in two possible ways (Selivanov et al. 2008): (1) with a single electron plus two H+ to form ubiquinol (QH2) (SQ.- + e− + 2 H+ ↔ QH2), or (2) with O2 to form the highly reactive O2.- (SQ− + O2 ↔ Q + O2.-). At acidic pH, the first reaction is shifted towards QH2 formation according to the Le Chatelier’s principle (Selivanov et al. 2008).
Potential significance of our results: Mild uncoupling
In succinate-respiring mammalian mitochondria, mild uncoupling lowers Δψm and consequently also the rate of ROS generation (Skulachev 1996; Korshunov et al. 1997; Miwa and Brand 2003). Mild uncoupling is a special condition where oxidative phosphorylation occurs at a relatively higher conductance of the inner mitochondrial membrane, this results in lowered pmf and a minor stimulation of respiration (Skulachev 1996; Brand et al. 2004). Our results support the notion that a minor decrease in Δψm leads to a diminution of the succinate-evoked, RET-initiated H2O2 release. Uncoupling proteins (UCP; like UCP1–3) and the adenine nucleotide transporter are also involved in mild uncoupling processes (Andreyev et al. 1988; Jezek 2002). Interestingly, O2.- can activate UCPs in the matrix with the contribution of fatty acids resulting in mild uncoupling (Echtay et al. 2002) and consequently a slower ROS production. Although it is likely that in vivo, under physiological conditions, ATP synthesis caused depolarisation of Δψm is sufficient to decrease ROS generation (Votyakova and Reynolds 2001; Starkov and Fiskum 2003), effects on mtROS of mild uncoupling and of Δψm are possibly relevant to patological states.
In fact, it has been hypothesized that initiation of mild uncoupling might be beneficial in oxidative stress-related diseases characterized by high Δψm such as in ischemia-reperfusion injury (Kadenbach et al. 2011). This hypothesis has been corroborated by a report showing that under ischemia, succinate can accumulate in mouse heart owing to the reversal of SDH (Chouchani et al. 2014). In reperfusion, SDH returns to oxidize the accumulated succinate and this has been claimed to result in an enhanced RET-mediated mtROS formation (Chouchani et al. 2014).
In summary, data from our laboratory provided evidence that the succinate- or α-GP-evoked, RET-initiated H2O2 production is more dependent on Δψm than on ΔpH. Our findings have helped elucidating mechanisms underpinning mtROS production and support consideration of the therapeutic applications of mild uncoupling, which can be initiated by e.g. mitochondria-targeted antioxidants.
Acknowledgements
The authors thank Vera Ádám-Vizi for her useful comments, and Katalin Takács and Andrea Várnagy for their excellent technical assistance.
Abbreviations
- α-GP
alpha-glycerophosphate
- α-GPDH
alpha-glycerophosphate dehydrogenase
- CI
complex I (NADH:ubiquinone oxidoreductase)
- CII
complex II (succinate dehydrogenase; succinate:coenzyme Q reductase)
- CIII
complex III (coenzyme Q:cytochrome c oxidoreductase)
- ΔpH
transmembrane pH gradient
- Δψm
mitochondrial membrane potential
- mtROS
mitochondrial reactive oxygen species
- O2.-
superoxide
- pHextra
extramitochondrial pH
- pHin
intramitochondrial pH
- pmf
proton motive force
- Q
ubiquinone
- QH2
ubiquinol
- RET
reverse electron transport
- ROS
reactive oxygen species
- SDH
succinate dehydrogenase, Complex II, CII
- SQ.-
semiquinone
- TPP+
tetraphenylphosphonium cation
- TPP+Cl−
tetraphenylphosphonium chloride
- UCP
uncoupling protein
Funding
This work was supported by the Hungarian Brain Research Program (KTIA_13_NAP-A-III/6 and 2017–1.2.1-NKP-2017-00002), OTKA (K 112230), and the Hungarian Academy of Sciences (MTA TKI 02001), all to Vera Adam-Vizi.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Highlights
Reverse electron transport (RET)-evoked ROS production is dependent predominantly on Δψm.
RET-evoked ROS formation is dependent more on absolute value of pH than on ΔpH.
Higher extramitochondrial pH is followed by enhanced RET-evoked ROS production
References
- Akerman KE, Wikstrom MK. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Andreyev A, Bondareva TO, Dedukhova VI, Mokhova EN, Skulachev VP, Volkov NI. Carboxyatractylate inhibits the uncoupling effect of free fatty acids. FEBS Lett. 1988;226(2):265–269. doi: 10.1016/0014-5793(88)81436-4. [DOI] [PubMed] [Google Scholar]
- Banh S, Treberg JR. The pH sensitivity of H2O2 metabolism in skeletal muscle mitochondria. FEBS Lett. 2013;587(12):1799–1804. doi: 10.1016/j.febslet.2013.04.035. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6(5):661–666. doi: 10.1016/S0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79(4):1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of proteein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45(7–8):466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37(6):755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Brandt U. Energy converting NADH:quinone oxidoreductase (complex I) Annu Rev Biochem. 2006;75:69–92. doi: 10.1146/annurev.biochem.75.103004.142539. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlaskova A, Hlavata L, Jezek J, Jezek P. Mitochondrial complex I superoxide production is attenuated by uncoupling. Int J Biochem Cell Biol. 2008;40(10):2098–2109. doi: 10.1016/j.biocel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415(6867):96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Galkin A, Brandt U. Superoxide radical formation by pure complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica. J Biol Chem. 2005;280(34):30129–30135. doi: 10.1074/jbc.M504709200. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P. Mitochondrial potassium transport: the K(+) cycle. Biochim Biophys Acta. 2003;1606(1–3):23–41. doi: 10.1016/S0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial complex I. Biochim Biophys Acta. 2006;1757(5–6):553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Henderson PJ, McGivan JD, Chappell JB. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek P. Possible physiological roles of mitochondrial uncoupling proteins-UCP. Int J Biochem Cell Biol. 2002;34(10):1190–1206. doi: 10.1016/S1357-2725(02)00061-4. [DOI] [PubMed] [Google Scholar]
- Jung DW, Davis MH, Brierley GP. Estimation of matrix pH in isolated heart mitochondria using a fluorescent probe. Anal Biochem. 1989;178(2):348–354. doi: 10.1016/0003-2697(89)90651-9. [DOI] [PubMed] [Google Scholar]
- Kadenbach B, Ramzan R, Moosdorf R, Vogt S. The role of mitochondrial membrane potential in ischemic heart failure. Mitochondrion. 2011;11(5):700–706. doi: 10.1016/j.mito.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Komary Z, Tretter L, Adam-Vizi V. H2O2 generation is decreased by calcium in isolated brain mitochondria. Biochim Biophys Acta. 2008;1777(7–8):800–807. doi: 10.1016/j.bbabio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15–18. doi: 10.1016/S0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch Biochem Biophys. 1998;350(1):118–126. doi: 10.1006/abbi.1997.0489. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382(Pt 2):511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeti E, Fonyo A. Competitive inhibition of valinomycin-induced K+-transport by Mg2+-ions in liver mitochondria. FEBS Lett. 1977;79(1):33–36. doi: 10.1016/0014-5793(77)80344-X. [DOI] [PubMed] [Google Scholar]
- Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci Rep. 1997;17(3):259–272. doi: 10.1023/A:1027328510931. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80(5):780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Mela L, Seitz S. Isolation of mitochondria with emphasis on heart mitochondria from small amounts of tissue. Methods Enzymol. 1979;55:39–46. doi: 10.1016/0076-6879(79)55006-X. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Moyle J. Proton translocation coupled to ATP hydrolysis in rat liver mitochondria. Eur J Biochem. 1968;4(4):530–539. doi: 10.1111/j.1432-1033.1968.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans. 2003;31(Pt 6):1300–1301. doi: 10.1042/bst0311300. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Commentary on: 'old and new data, new issues: the mitochondrial Deltapsi' by H. Tedeschi. Biochim Biophys Acta. 2005;1710(2–3):63–65. doi: 10.1016/j.bbabio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J Bioenerg Biomembr. 2005;37(1):1–15. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- Orr AL, Quinlan CL, Perevoshchikova IV, Brand MD. A refined analysis of superoxide production by mitochondrial sn-glycerol 3-phosphate dehydrogenase. J Biol Chem. 2012;287(51):42921–42935. doi: 10.1074/jbc.M112.397828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde KR, Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem. 2011;286(20):18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe DF, Hulbert AJ, Brand MD. Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen-consuming tissues of the rat. Biochim Biophys Acta. 1994;1188(3):405–416. doi: 10.1016/0005-2728(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab. 1987;7(6):752–758. doi: 10.1038/jcbfm.1987.130. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984;81(2):127–138. doi: 10.1007/BF01868977. [DOI] [PubMed] [Google Scholar]
- Rottenberg H, Lee CP. Energy dependent hydrogen ion accumulation in submitochondrial particles. Biochemistry. 1975;14(12):2675–2680. doi: 10.1021/bi00683a601. [DOI] [PubMed] [Google Scholar]
- Selivanov VA, Zeak JA, Roca J, Cascante M, Trucco M, Votyakova TV. The role of external and matrix pH in mitochondrial reactive oxygen species generation. J Biol Chem. 2008;283(43):29292–29300. doi: 10.1074/jbc.M801019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos H, Torocsik B, Tretter L, Adam-Vizi V. Impaired regulation of pH homeostasis by oxidative stress in rat brain capillary endothelial cells. Cell Mol Neurobiol. 2005;25(1):141–151. doi: 10.1007/s10571-004-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29(2):169–202. doi: 10.1017/S0033583500005795. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86(5):1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- Treberg JR, Quinlan CL, Brand MD. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I) J Biol Chem. 2011;286(31):27103–27110. doi: 10.1074/jbc.M111.252502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Moderate dependence of ROS formation on DeltaPsim in isolated brain mitochondria supported by NADH-linked substrates. Neurochem Res. 2007;32(4–5):569–575. doi: 10.1007/s11064-006-9130-y. [DOI] [PubMed] [Google Scholar]
- Tretter L, Mayer-Takacs D, Adam-Vizi V. The effect of bovine serum albumin on the membrane potential and reactive oxygen species generation in succinate-supported isolated brain mitochondria. Neurochem Int. 2007;50(1):139–147. doi: 10.1016/j.neuint.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Tretter L, Takacs K, Hegedus V, Adam-Vizi V. Characteristics of alpha-glycerophosphate-evoked H2O2 generation in brain mitochondria. J Neurochem. 2007;100(3):650–663. doi: 10.1111/j.1471-4159.2006.04223.x. [DOI] [PubMed] [Google Scholar]
- Tretter L, Takacs K, Kover K, Adam-Vizi V. Stimulation of H(2)O(2) generation by calcium in brain mitochondria respiring on alpha-glycerophosphate. J Neurosci Res. 2007;85(15):3471–3479. doi: 10.1002/jnr.21405. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79(2):266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- Zoccarato F, Cavallini L, Alexandre A. Respiration-dependent removal of exogenous H2O2 in brain mitochondria: inhibition by Ca2+ J Biol Chem. 2004;279(6):4166–4174. doi: 10.1074/jbc.M308143200. [DOI] [PubMed] [Google Scholar]
- Zoccarato F, Miotto C, Cavallini L, Alexandre A. The control of mitochondrial succinate-dependent H2O2 production. J Bioenerg Biomembr. 2011;43(4):359–366. doi: 10.1007/s10863-011-9363-6. [DOI] [PubMed] [Google Scholar]