Abstract
Purpose
To examine the role of sex in abnormal white matter microstructure after soccer heading as identified by using the diffusion-tensor imaging (DTI) metric fractional anisotropy (FA).
Materials and Methods
In this prospective cross-sectional study, 98 individuals who were enrolled in a larger prospective study of amateur soccer players (from 2013 to 2016) were matched 1:1 for age and history of soccer heading in the prior 12 months. Among the subjects, 49 men (mean age, 25.7 years; range, 18–50 years) and 49 women (mean age, 25.8 years; range, 18–50 years) with median total soccer headings per year of 487 and 469, respectively, underwent 3.0-T DTI. Images were registered to the Johns Hopkins University template. A voxelwise linear regression was fitted for FA with terms for the number of headings during the previous 12 months and its interaction with sex after controlling for the following potential confounders: age, years of education, number of lifetime concussions, and handedness. In the resulting statistical maps, P < .01 indicated a statistically significant difference, with a threshold cluster size larger than 100 mm3.
Results
Among men, three regions were identified in which greater heading exposure was associated with lower FA; eight such regions were identified among women (>100 contiguous voxels, P < .01). In seven of the eight regions identified in women, the association between heading and FA was stronger in women than in men. There was no significant difference of heading with FA between the sexes for any region in which heading was associated with FA among men (P > .01, <100 contiguous voxels).
Conclusion
With similar exposure to heading, women exhibit more widespread evidence of microstructural white matter alteration than do men, suggesting preliminary support for a biologic divergence of brain response to repetitive trauma.
© RSNA, 2018
Introduction
Soccer is a sport with known risk for concussion, which is commonly attributed to player-to-player collision and falls (1). It is also the only sport in which players purposefully use the head to deflect the ball in play, a move called “heading.” While heading-related impacts have been considered subconcussive, heading is associated with central nervous system symptoms in the short term (within 2 weeks of heading) (2), independent of collisions or recognized concussion. Moreover, cumulative heading over a 1-year period is associated with cognitive dysfunction (3,4) as well as microstructural alteration of white matter on images from diffusion-tensor imaging (DTI), similar to that reported in traumatic axonal injury (3). Long-term consequences of repeated exposure to heading is an area of concern because repetitive head injury in athletes has been associated with cognitive decline and behavioral changes attributed to chronic traumatic encephalopathy (5). Understanding the modifying factors that reduce susceptibility to brain injury and detecting subclinical pathologic changes before overt clinical manifestations of chronic traumatic encephalopathy are essential prerequisites to developing preventive strategies.
Female athletes are at greater risk than male athletes for poor outcomes after acute traumatic brain injury, including concussion (6–9). Although concussion is more common in men owing to their greater participation in high-risk activities, women are more likely to have persistent sequelae (8–11). Despite this, less research has addressed the effects of traumatic brain injury in women. Moreover, the finding of worse clinical outcomes in women after concussion has been controversial because women may be more likely to report symptoms than are men, perhaps due to cultural or sociologic pressures that lead men to underreport (12). In baseline assessments obtained before a season of play, female athletes tend to report more concussion-related symptoms than do male athletes (13,14). However, women do not experience a delayed return to normal activity or miss more days of work after concussion compared with men, despite reporting more postconcussive symptoms (8). With so much subjectivity involved in the reporting of symptoms, there is a need for more objective approaches for detecting and characterizing sex-based differences in the manifestations of brain injury, including subclinical changes that precede overt clinical dysfunction. With a growing population of women participating in sports such as soccer (15,16), sex-based differences in vulnerability to brain injury merit further study.
Neuroimaging offers an objective means to characterize subclinical variation in brain structure and function. Although DTI has been shown to robustly depict traumatic axonal injury abnormalities in concussion (17–19) and in subconcussive injury (3,20), to our knowledge only one study, which was conducted in 69 patients with mild traumatic brain injury, has reported on the effect of sex at DTI (21). We tested the hypothesis that DTI, an objective means to measure microstructure, would reveal sex-based differences in the association of repetitive head trauma by comparing the microstructural alteration of white matter in the brain. The purpose of our study was to characterize the effect of biologic sex on the relationship between repetitive heading and cerebral white matter microstructure in amateur soccer players.
Materials and Methods
Participants
This prospective study (participant enrollment from 2013 to 2016) was reviewed and approved by the local institutional review board and compliant with the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all participants.
As part of an ongoing longitudinal study of adult amateur soccer players, 78 women and 212 men were recruited from amateur and collegiate teams from 2013 to 2016 (2,4,22). Subjects were considered to be eligible for inclusion in the source sample if they had played soccer at the amateur level for more than 5 years, were aged 18–55 years, and had been actively playing soccer for at least 6 months per year. Exclusion criteria were presence of neurologic disorder; presence of mental disorder such as schizophrenia, bipolar disorder, or psychosis; illicit drug use within the past 30 days; positive urine test for substance abuse; and contraindications to MRI.
During a single enrollment visit, participants completed online questionnaires to ascertain demographic characteristics; handedness (23); exposure to soccer heading; history of concussion (4); medical, neurologic, and psychiatric history; and medication use. Selection of participants is detailed in Figure 1.
Figure 1:
Flowchart shows selection of amateur soccer players included in analysis. With exclusion of participants who did not meet image quality standards, the paired participant was also removed, resulting in removal of six pairs (n = 12) from the analysis.
To achieve similar groups for comparison, women and men included in this analysis were paired 1:1 on the basis of age and the total number of heading events during the previous 12 months. Specifically, each woman was paired with a man who was within 2 years of her age and the closest match with respect to reported 12-month heading exposure. Because of the wide range of heading events among our study participants, an absolute cutoff for the difference in heading was not used to determine pairing. Ultimately, 55 men and 55 women were matched according to age and heading exposure. Because of deficient image quality in one or both members of a matched pair, six pairs were subsequently excluded, leaving a final cohort of 49 men (mean age, 25.7 years; range, 18–50 years) and 49 women (mean age, 25.8 years; range, 18–50 years) with median total soccer heading events per year of 487 and 469, respectively. The similarity of the male and female players regarding heading exposure was confirmed by using the Mann-Whitney test as well as the median differences and interquartile ranges.
Heading Exposure
The frequency of heading during the previous 12 months was estimated with use of a previously validated structured questionnaire (3,4). Briefly, participants were asked about soccer activity, including practice and competitive games, in both indoor and outdoor settings. Participants were asked how often they headed the ball on average during each type of session (practice vs competition) and in each setting (indoor vs outdoor), how many times per week they participated in each, and how many months per year they played soccer. For each individual, the average number of heading events in each setting and session type was multiplied by the corresponding number of sessions per week, converted to months, and then multiplied by the number of months of play in a year, yielding four separate subtotals (indoor and outdoor settings, practice and competitive games). The sum of these subtotals was used as the 12-month total heading exposure.
Image Acquisition
Whole-brain MRI was performed at the same study visit described earlier by using a 3.0-T MR unit (Achieva TX; Philips Medical Systems, Best, the Netherlands) and a 32-channel head coil (Philips Medical Systems). T1-weighted magnetization-prepared rapid acquisition three-dimensional gradient-echo imaging (9.9/4.6/900 [repetition time msec/echo time msec/inversion time msec]; flip angle, 8°; isotropic 1-mm resolution; field of view, 240 × 188 × 220; number of signals acquired, one), T2-weighted fluid-attenuated inversion-recovery imaging (11 000/120 [effective]/2800; section thickness, 2 mm; matrix, 352 × 206; field of view, 240 × 240; number of signals acquired, one), and susceptibility-weighted imaging (16/23 [repetition time msec/echo time msec]; flip angle, 15°; section thickness, 1 mm; matrix, 268 × 210; field of view, 240 × 190; number of signals acquired, one) were performed. In addition, DTI was performed with two-dimensional single-shot echo-planar imaging (repetition time, 10 seconds; echo time, 65 msec; flip angle, 90°; section thickness, 2 mm; matrix, 128 × 116; field of view, 256 × 232; 32 diffusion directions; b value, 800 sec/mm2). An auxiliary three-dimensional B0 field map was obtained by using a dual-echo, gradient-echo technique (20/2.4; difference in echo times, 2.3 msec; isotropic resolution, 4 mm3; flip angle, 20°) to correct echo-planar imaging distortions on DTI images and small distortions in T1-weighted images. Trained raters (T.G.R. and N.L., with 2 years of experience) viewed all images, and images that were degraded by artifact were excluded from the analyses.
A board-certified neuroradiologist with 20 years of experience (M.L.L.) reviewed all images to detect structural abnormalities, including evidence of prior trauma. Images for each participant were reviewed at the time the respective MR image was obtained. To preserve participant anonymity, the reviewing neuroradiologist was blinded to all participant details because images were completely anonymized. As a result, the reviewing radiologist was also blinded to sex and heading exposure. No abnormal findings were noted.
Data Analysis
Demographic and exposure characteristics.—Data analysis was performed by T.G.R. (a graduate student with 2 years of experience) under the supervision of R.F. (an MR physicist with 15 years of experience) and M.L.L. Demographic and exposure characteristics were compared according to sex by using the Fisher exact, Mann-Whitney, and Student t tests. Analyses were performed by using software (SPSS Statistics for Mac OS, version 24.0; IBM, Armonk, NY).
Image processing.—Image processing was performed by using a high-performance computing system running a Community Enterprise Operating System, or CentOS, Linux distribution. The FSL software package (FSL v2.0.18, https://fsl.fmrib.ox.ac.uk/) (24) was used to perform correction for head motion and eddy current effects and to fit diffusion parameter images (fractional anisotropy [FA], radial diffusivity, mean diffusivity, and axial diffusivity) to a tensor model at each voxel. Diffusion parameter images were coregistered to the Johns Hopkins University brain template by using a multistep procedure that incorporates field map–based echo-planar imaging distortion correction as well as linear, within-participant, and nonlinear, across-participant, transformations, as described previously (25,26). Analysis was restricted to voxels within the white matter as defined by the Johns Hopkins University atlas (27).
Image quality and the results of each processing step were critically assessed by trained research personnel, under the supervision of M.L.L., with use of a standardized approach. Brain extraction or registration errors were corrected manually where possible. In the event of irreparable errors, pairs were excluded from analysis. Six matched participant pairs were excluded from the analysis because of imaging artifact or registration error.
Statistical Analysis
Imaging analysis was performed by T.G.R. in consultation with R.F., M.K., and M.L.L. Whole-brain voxelwise analysis of white matter FA was performed by using the Automatic Registration Toolbox (28,29) VANCOVA module to fit a linear regression for FA, with terms for the number of soccer headings and its interaction with sex after controlling for the following potential confounders: age, years of education, number of lifetime concussions, and handedness (laterality quotient). The interaction term was used to test for the influence of sex on the relationship between the number of headings and FA. For a more detailed description of the statistical model used, see Appendix E1 (online).
The model was applied separately in the same manner for the DTI parameters radial diffusivity, mean diffusivity, and axial diffusivity. To mitigate the possibility of a type I error, we only considered clusters comprising more than 100 contiguous voxels, within which each voxel met a cutoff of P < .01 (3,26,30). The volume of each cluster was computed and its anatomic location verified by M.L.L.
To assess the possibility that our results may have been driven by a small number of participants with the largest number of heading events, we conducted a series of sensitivity analyses in which the same statistical models were refit after sequentially eliminating the five pairs of participants with the most heading exposure. This resulted in sample sizes of 88–96 participants. In the group of 98 participants, the number of headers reported ranged from 0 to 6604. For the group with 88 subjects, the greatest number of heading events over the previous 12 months was 2611.
Results
Participant Characteristics and Heading Exposure
Enrolled men and women did not differ significantly by age (P = .97) or 12-month heading exposure (P = .80), the two criteria used for matching (Table 1). The median number of heading events per year was 487 (interquartile range, 221–1103) for men and 469 (interquartile range, 261–1008) for women. The median difference in the 12-month heading frequency between the matched pairs (men-women) was 10 headers (interquartile range, −48 to 61). We found no significant difference between men and women with respect to the age when soccer play started (P = .62), number of years of soccer play (P = .83), or number of years of play at a similar frequency (P = .33) (Table 1). Participants also did not differ significantly with regard to years of education or handedness (P = .23 and P = .20, respectively), but women reported more prior concussions compared with men (P = .03). The number of lifetime concussions and the total number of headings in the past 12 months did not show a correlation (Pearson r = −0.069, P = .50). No visible abnormalities were identified during neuroradiologic review of images for any of the participants.
Table 1:
Baseline Participant Characteristics according to Sex

Note.—Comparisons of age, years of education, total number of soccer headings in the past year, number of lifetime concussions, and handedness were made by using the Mann-Whitney test. Comparisons of age at which participants began playing soccer, number of years playing soccer, and number of years played at a similar frequency were made with the Student t test.
*Data are means, with interquartile range in parentheses.
Heading Exposure and FA
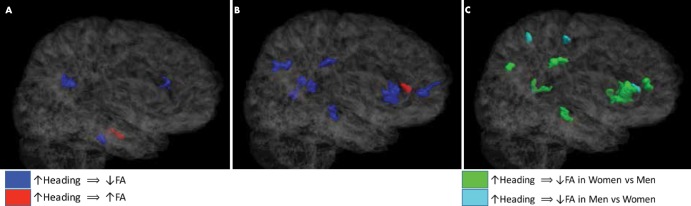
We independently assessed men and women to determine if there was an association between white matter FA and soccer heading. Among men, three regions were identified in which a greater number of heading events was significantly associated with lower FA (P < .01 across >100 contiguous voxels), including the genu and splenium of the corpus callosum and the pons (Fig 2, A). In men, we identified only one location, the left temporal white matter, in which a greater exposure to heading was associated with significantly higher FA (P < .01 across >100 contiguous voxels) (Fig 2, A). In women, we identified eight regions in which greater heading exposure showed a significant association with lower FA (P < .01 across >100 contiguous voxels), including the genu of the corpus callosum; left occipital, right parietal, and right orbitofrontal white matter; left superior longitudinal fasciculus; right cingulum; and right cerebral peduncle (Fig 2, B). Greater heading exposure among women showed a significant association with higher FA (P < .01 across >100 contiguous voxels) at a single location in the left frontal white matter (Fig 2, B). Overall, an association between greater heading exposure and FA was detected across 2121 mm3 of white matter in women, compared with 408 mm3 in men. The size, location, and significance (peak t value) for each abnormal region are presented in Table 2.
Figure 2:
A, B, Three-dimensional semitransparent images of the Johns Hopkins University template brain oriented facing the right hemisphere in, A, male, and, B, female soccer players demonstrate that lower fractional anisotropy (FA) is associated with heading more extensively in women than in men. Fewer regions of significant association of FA with heading are detected in men than in women. C, Image shows that significant differences in association of heading and FA between men and women are predominantly co-located with areas where women, but not men, show significant association of heading and FA.
Table 2:
Size, Location, and Peak t Values for Each Abnormal Region

Note.—Data are volumes (in cubic millimeters), with locations (X, Y, Z coordinates) in parentheses and peak t values in brackets. FA = fractional anisotropy, L = left, R = right.
We next identified locations where the relationship between heading and FA was different between men and women (Fig 2, C). In eight regions (green in Figs 2, C, E3 [online]), women showed a relationship between greater heading exposure and lower FA that was significantly different from that of men (P < .01 across >100 contiguous voxels); seven of these regions overlapped with the locations found when women alone were analyzed (Fig 2, B). Conversely, only four locations were identified in which men demonstrated a relationship between greater heading exposure and lower FA that was significantly different from that of women (P < .01 across >100 contiguous voxels) (light blue in Figs 2, C, E3 [online]). However, none of these four locations overlapped with the three regions in which greater heading exposure was significantly associated with lower FA when men were analyzed alone (Fig 2, A). This suggests that, in the three regions where men showed the strongest association between greater heading exposure and lower FA, women also showed similar trends (Fig E1 [online]), whereas in most of the regions where women showed the strongest associations between heading and lower FA, the results in men were weaker or in the opposite direction (Fig E2 [online]). For more information on the relationship of heading with FA at each of these locations, the average FA value at each location was plotted against heading for each individual (Figs E1–E3 [online]).
Results of sequential subgroup analyses examining the effect of participants who reported the highest heading exposure on overall results are presented in Figure E4 (online). At each sequential analysis, women maintained a greater number and volume of regions with a significant association of heading with lower FA (P < .01 across >100 contiguous voxels).
Heading Exposure and Other DTI Parameters
Heading was associated with changes in radial diffusivity, mean diffusivity, and axial diffusivity in both men and women. The total volume of regions where heading was associated with changes in DTI parameters for each analysis is shown in Figure 3. We detected moderate associations between radial diffusivity and mean diffusivity with heading, but neither differed greatly by sex. Greater heading exposure was associated with lower white matter axial diffusivity over a greater volume in men (1377 mm3) than in women (501 mm3). Figure 3, B–E, depicts each individual region identified in each group analysis; note that the points in these plots represent individual regions detected in the group analyses, not individual participants.
Figure 3:
Volume of regions where heading was associated with changes at diffusion-tensor imaging. AD = axial diffusivity, FA = fractional anisotropy, MD = mean diffusivity, RD = radial diffusivity, ΔMen-Women = difference between men and women. A, Graph shows total volume of all regions where imaging parameter (fractional anisotropy, radial diffusivity, axial diffusivity, mean diffusivity) demonstrates significant association with heading. Positive volumes correspond to positive association of parameter with heading, and negative volumes correspond to negative association of parameter with heading. B–E, Graphs show volume of individual regions where each parameter is associated with heading. Each point in plots represents volume of individual region (see Fig 1) found in group analysis, not individual participant. Values depicted in B–E were summed to generate graph in A.
Discussion
This study provides preliminary evidence regarding the influence of sex on the association of heading with differences in brain microstructure in soccer players, a finding that, to our knowledge, has not been previously established in the literature. We identified a sex difference in the effects of heading on white matter microstructure; similar levels of heading affected women more than men. The fivefold greater volume of affected white matter that we identified in women compared with men pointed to a higher burden of microstructural consequences in female headers. Previous reports of higher corpus callosum FA in women compared with men (31) served to strengthen our finding, because we identified lower corpus callosum FA associated with heading in women. Perhaps men and women head the ball differently, giving rise to different patterns of white matter changes. Under this hypothesis, we might have expected that men and women would each exhibit distinct areas of low FA. In fact, we found effects in female players that diverged from those in male players (co-located in sex-independent analysis and interaction) but did not identify any significant association of FA with heading in male players that was significantly different from that of female players. This suggests that women may be more sensitive than men to the effects of heading at the level of tissue microstructure. Our findings add to a growing body of evidence that men and women express distinct biologic responses to brain injury (7,21,32–34).
FA was the most prevalent indicator of microstructural alteration. Although we also observed changes in radial diffusivity, mean diffusivity, and axial diffusivity, FA accounted for the greatest volume of tissue associated with soccer heading. In addition, we observed a statistically significant sex difference in the relationship between heading and FA. Notably, at almost all locations where sex-based differences in the association between heading and FA were detected, a greater heading exposure was associated with lower FA, which has been widely reported as a hallmark of traumatic axonal injury (18,35). In addition, we did not find significant sex-based divergence where greater heading exposure was associated with higher FA. This finding extends previous evidence of diffusion abnormalities associated with concussion, which has almost exclusively focused on single, recognized injuries. The effect of ongoing repeated impacts to the head may be more complex.
One consideration in the interpretation of our results was whether the findings might have been driven by participants with the greatest heading exposure. To test this hypothesis, we performed identical analyses in a series of subgroups derived from our sample. For each sequential subgroup, we removed from the previous subgroup the participant pair (male-female) with the highest heading exposure. For each subgroup, more and larger regions in which greater heading exposure was associated with lower FA were seen in women compared with men. Moreover, for subgroups in which we excluded three or more pairs, men no longer exhibited any locations where a higher exposure to heading was associated with lower FA; women did. Furthermore, men in these groups showed an increasing association between greater heading exposure and higher FA as more high-exposure participants were excluded, but women did not exhibit this trend. These results indicate that the divergent response according to sex that we reported is a general characteristic of the players and strongly refutes the possibility that our finding was an artifact due to the inclusion of players with the highest heading exposure.
Higher FA has been reported less frequently in patients with concussion and has not been reported in association with exposure to soccer heading. Previous studies have attributed higher FA to cytotoxic edema and astrogliosis, predominantly in the setting of acute injury (8,36,37). FA also increases in response to motor training of healthy adults, which suggests that it may be a manifestation of neuroplasticity (38). In addition, higher FA was recently reported as a predictor of better long-term outcome after recognized concussion, suggesting it may be indicative of compensatory change (39). In our study, participants had been exposed to years of repetitive subconcussive heading and, in some cases, periodic impacts of greater severity (ie, recognized concussion). Thus, the low FA that we detected may be indicative of chronic traumatic axonal injury, whereas the areas of high FA could reflect more recent injury, a neuroplastic response to injury or athletic training, or perhaps be a manifestation of chronic neuroinflammation due to repetitive impacts to the head. Future studies should specifically measure activity and fitness levels to investigate these possibilities.
Notwithstanding the previously described investigation of the effect of the participants with highest heading exposure on our overall results, it is important to note that the participants excluded in the subgroup analyses were in fact not outliers with respect to heading exposure. Players enrolled in the larger study from which our sex-matched sample was derived exhibited a wide range of heading exposure over a 12-month period (mean = 1384 events, median = 611 events, range = 0–12 322 events). The players included in the current analysis thus fell well within the range of heading exhibited by the overall sample and were not composed of individuals with extreme exposure to heading.
The specific spatial pattern of heading-related changes found in this group likely reflected the heterogeneous nature of head impacts in soccer as well as the makeup and size of the specific sample enrolled. Because we analyzed participants by using a group comparison, however, we were more likely to uncover generalized features of brain injury across the group, rather than any individual’s specific injury profile. In this regard, some of the regions in which we identified a significant association between heading and FA, including the corpus callosum, the superior longitudinal fasciculus, and subcortical white matter, were consistent with previous studies of brain injury (3,20,40,41). Future application of individualized analysis of DTI may provide further insight into the individual injury burden.
The primary exposure variable relied on a structured questionnaire of the self-reported number of soccer headings in a 12-month period and thus represented an estimate. We did not have data on variation in heading acceleration and related biomechanics that could have mediated the sex differences we observed. Therefore, we cannot exclude the possibility that women in our sample more commonly experienced higher-magnitude impacts during heading compared with men. This possibility, however, seems implausible given the higher ball velocity in men’s soccer and the higher rate of collisions and other impacts in men compared with women, which speak to the generally more intense nature of soccer play among men (42). Several studies measured heading acceleration in men and women under controlled conditions and found greater acceleration in response to a similar force applied to women, which was attributed to anatomic differences between the sexes (43–45). Sex-based divergence of neck strength and anthropometrics (46) may have also played a role in our findings, but other factors such as genetics and hormonal influences may have contributed as well. Future studies examining each of these possibilities, and others, are necessary to uncover the mechanistic basis for the sex differences.
Although FA is the most widely reported parameter in studies of brain injury, it may not be the most sensitive or specific measure for detecting changes associated with subconcussive injury and may not best reflect sex differences. The most robust sex-specific finding in our study was related to FA, but other parameters, particularly axial diffusivity, exhibit an association with heading in a sexually divergent manner and warrant further investigation. Diffusivity measures hold promise for a more detailed understanding of underlying mechanisms associated with traumatic axonal injury, and multishell diffusion measures and animal studies can enhance the characterization of underlying pathologic mechanisms.
Although our study was quite large and evenly balanced between the sexes, its sensitivity was nonetheless constrained by the small sample size. Additional studies that include larger samples are required to characterize divergence of functional outcomes. We limited our analysis of DTI to white matter voxels by applying a mask based on the Johns Hopkins University white matter template. Nonetheless, some of the abnormal regions identified occurred in proximity to the gray matter–white matter junction. It is thus possible that registration errors could have led to comparison of gray matter voxels from some participants, potentially leading to spurious results. This limitation, which is generally not acknowledged in reports of quantitative image analysis, is an inherent feature of any template-based analysis but is likely to be minimized by averaging registration errors across participants (26). Notwithstanding this potential limitation, we performed a secondary analysis, excluding all regions proximate to the gray matter–white matter junction, and found no change in the pattern of sex-based divergence.
Although our cross-sectional finding represents a subclinical effect on brain tissue, such a finding remains paramount. As with chronic traumatic encephalopathy and environmental exposures in general, subclinical abnormalities develop before the onset of clinically overt functional effects. Identification of risk factors for cumulative injury, such as sex, before the onset of overt dysfunction represents the best opportunity to mitigate progression and maximize recovery, or at least ensure that injury remains subclinical.
The sex-based differences we identified underscore the need for studying the effects of soccer heading in both sexes to better characterize sex-specific profiles. Our study provides preliminary support that women are more sensitive to repetitive subconcussive head impacts at the level of brain tissue microstructure. Further investigation is warranted to confirm and further characterize sex differences in vulnerability to brain injury due to heading, with the hope of developing sex-specific guidelines for safer soccer play.
Summary
Our study provides preliminary support to the notion that women are more sensitive to repetitive subconcussive head impacts at the level of brain tissue microstructure.
Implications for Patient Care
■ Our findings provide preliminary evidence that, at similar levels of exposure to repeated head impacts, women experience excess microstructural change in white matter compared with men.
■ A focus on sex-based vulnerability to brain injury may inform care of injured athletes and enhance guidelines for safe play.
■ Sex-based differences in susceptibility to brain injury may provide an opportunity to better understand the biologic underpinnings of this difference and identify targets for intervention.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
The authors thank Chloe Ifrah, BA, and Kyle Friedman, MS, for their help with image processing, data management, and execution of statistical analyses.
Supported by the National Institutes of Health, National Institute of Neurologic Disorders and Stroke (R01 NS082432), and a grant from the Dana Foundation David Mahoney Neuroimaging Program.
T.G.R. and E.C. contributed equally to this work.
Disclosures of Conflicts of Interest: T.G.R. disclosed no relevant relationships. E.C. disclosed no relevant relationships. R.F. disclosed no relevant relationships. L.E.H. disclosed no relevant relationships. N.L. disclosed no relevant relationships. W.F.S. disclosed no relevant relationships. M.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Celgene. Other relationships: disclosed no relevant relationships. R.B.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Alder, Allergan, Amgen, Automatic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy’s Electocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta; receives royalties from Wiley and Informa; has stock/stock options in eNeura Therapeutics and Biohaven Holdings. Other relationships: disclosed no relevant relationships. M.L.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: received research support from Philips Medical Systems; receives travel support from GE Healthcare; receives royalties from Springer; receives payment for expert witness work; received speaker honoraria from academic institutions and venues.
Abbreviations:
- DTI
- diffusion-tensor imaging
- FA
- fractional anisotropy
References
- 1.Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr 2015;169(9):830–837. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Kim N, Ifrah CS, et al. Symptoms from repeated intentional and unintentional head impact in soccer players. Neurology 2017;88(9):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton ML, Kim N, Zimmerman ME, et al. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology 2013;268(3):850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitch CF, Zimmerman ME, Lubin N, et al. Recent and long-term soccer heading exposure is differentially associated with neuropsychological function in amateur players. J Int Neuropsychol Soc 2018;24(2):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 2011;30(1):179–188, xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R 2009;1(3):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT. Sex differences in outcome following sports-related concussion. J Neurosurg 2005;102(5):856–863. [DOI] [PubMed] [Google Scholar]
- 8.Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma 2010;27(3):527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berz K, Divine J, Foss KB, Heyl R, Ford KR, Myer GD. Sex-specific differences in the severity of symptoms and recovery rate following sports-related concussion in young athletes. Phys Sportsmed 2013;41(2):58–63. [DOI] [PubMed] [Google Scholar]
- 10.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004;36(43 Suppl):84–105. [DOI] [PubMed] [Google Scholar]
- 11.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004;36(43 Suppl):28–60. [DOI] [PubMed] [Google Scholar]
- 12.Laker SR. Epidemiology of concussion and mild traumatic brain injury. PM R 2011;3(10 Suppl 2):S354–S358. [DOI] [PubMed] [Google Scholar]
- 13.Shehata N, Wiley JP, Richea S, Benson BW, Duits L, Meeuwisse WH. Sport concussion assessment tool: baseline values for varsity collision sport athletes. Br J Sports Med 2009;43(10):730–734. [DOI] [PubMed] [Google Scholar]
- 14.Covassin T, Swanik CB, Sachs M, et al. Sex differences in baseline neuropsychological function and concussion symptoms of collegiate athletes. Br J Sports Med 2006;40(11):923–927; discussion 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senne JA. Examination of gender equity and female participation in sport. The Sport Journal. http://thesportjournal.org/article/examination-of-gender-equity-and-female-participation-in-sport/. Published February 26, 2016. Accessed January 17, 2017. [Google Scholar]
- 16.Kennedy CL. A new frontier for women’s sports (beyond title IX). Gend Issues 2010;27(1-2):78–90. [Google Scholar]
- 17.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 2010;25(4):241–255. [DOI] [PubMed] [Google Scholar]
- 18.Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 2013;34(11):2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6(2):137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahrami N, Sharma D, Rosenthal S, et al. Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology 2016;281(3):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fakhran S, Yaeger K, Collins M, Alhilali L. Sex differences in white matter abnormalities after mild traumatic brain injury: localization and correlation with outcome. Radiology 2014;272(3):815–823. [DOI] [PubMed] [Google Scholar]
- 22.Stewart WF, Kim N, Ifrah C, et al. Heading frequency is more strongly related to cognitive performance than unintentional head impacts in amateur soccer players. Front Neurol 2018;9(240):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2007;2(3):499–503. [DOI] [PubMed] [Google Scholar]
- 25.Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 2009;252(3):816–824. [DOI] [PubMed] [Google Scholar]
- 26.Suri AK, Fleysher R, Lipton ML. Subject based registration for individualized analysis of diffusion tensor MRI. PLoS One 2015;10(11):e0142288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oishi K, Faria A, Jiang H, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage 2009;46(2):486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H. A fully automatic multimodality image registration algorithm. J Comput Assist Tomogr 1995;19(4):615–623. [DOI] [PubMed] [Google Scholar]
- 29.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods 2005;142(1):67–76. [DOI] [PubMed] [Google Scholar]
- 30.Hoptman MJ, Nierenberg J, Bertisch HC, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res 2008;106(2-3):115–124. [DOI] [PubMed] [Google Scholar]
- 31.Kanaan RA, Allin M, Picchioni M, et al. Gender differences in white matter microstructure. PLoS One 2012;7(6):e38272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds BB, Patrie J, Henry EJ, et al. Effects of sex and event type on head impact in collegiate soccer. Orthop J Sports Med 2017;5(4):2325967117701708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farace E, Alves WM. Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg 2000;93(4):539–545. [DOI] [PubMed] [Google Scholar]
- 34.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma 2000;17(5):367–388. [DOI] [PubMed] [Google Scholar]
- 35.Ng TS, Lin AP, Koerte IK, et al. Neuroimaging in repetitive brain trauma. Alzheimers Res Ther 2014;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 2011;134(Pt 8):2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24(9):1447–1459. [DOI] [PubMed] [Google Scholar]
- 38.Fleet GH, Phaff HJ. Glucanases in Schizosaccharomyces. Isolation and properties of an exo-beta-glucanase from the cell extracts and culture fluid of Schizosaccharomyces japonicus var. versatilis. Biochim Biophys Acta 1975;410(2):318–332. [DOI] [PubMed] [Google Scholar]
- 39.Strauss SB, Kim N, Branch CA, et al. Bidirectional changes in anisotropy are associated with outcomes in mild traumatic brain injury. AJNR Am J Neuroradiol 2016;37(11):1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koerte IK, Ertl-Wagner B, Reiser M, Zafonte R, Shenton ME. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 2012;308(18):1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAllister TW, Ford JC, Flashman LA, et al. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 2014;82(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babbs CF. Biomechanics of heading a soccer ball: implications for player safety. Sci World J 2001;1:281–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caccese JB, Buckley TA, Tierney RT, et al. Head and neck size and neck strength predict linear and rotational acceleration during purposeful soccer heading. Sports Biomech 2017;1–15.. [DOI] [PubMed] [Google Scholar]
- 44.Dorminy M, Hoogeveen A, Tierney RT, Higgins M, McDevitt JK, Kretzschmar J. Effect of soccer heading ball speed on S100B, sideline concussion assessments and head impact kinematics. Brain Inj 2015;1–7. [DOI] [PubMed] [Google Scholar]
- 45.Tierney RT, Higgins M, Caswell SV, et al. Sex differences in head acceleration during heading while wearing soccer headgear. J Athl Train 2008;43(6):578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catenaccio E, Mu W, Kaplan A, et al. Characterization of neck strength in healthy young adults. PM R 2017;9(9):884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.