Abstract
The purpose of this study was to evaluate the accuracy of a mobile wireless digital automatic blood pressure monitor for clinical use and mobile health (mHealth). In this study, a manual sphygmomanometer and a digital blood pressure monitor were tested in 100 participants in a repetitive and sequential manner to measure blood pressure. The guidelines for measurement used the Korea Food & Drug Administration protocol, which reflects international standards, such as the American National Standard Institution/Association for the Advancement of Medical Instrumentation SP 10: 1992 and the British Hypertension Society protocol. Measurements were generally consistent across observers according to the measured mean ± SD, which ranged in 0.1 ± 2.6 mmHg for systolic blood pressure (SBP) and 0.5 ± 2.2 mmHg for diastolic blood pressure (DBP). For the device and the observer, the difference in average blood pressure (mean ± SD) was 2.3 ± 4.7 mmHg for SBP and 2.0 ± 4.2 mmHg for DBP. The SBP and DBP measured in this study showed accurate measurements that satisfied all criteria, including an average difference that did not exceed 5 mmHg and a standard deviation that did not exceed 8 mmHg. The mobile wireless digital blood pressure monitor has the potential for clinical use and managing one’s own health.
Keywords: Digital automatic blood pressure, Validation, Clinical use, Self-management
Introduction
Due to the recent increase in chronic diseases, such as cardiovascular diseases, diabetes, and hypertension, the management of blood pressure is emerging as an important factor. Blood pressure is the most fundamental clinical measurement to check a patient’s vital signs and represents a vital measure that patients with chronic diseases must measure and manage for themselves [1–4].
In general, blood pressure measurement methods include invasive and non-invasive methods, including the Korotkoff auscultatory method, which is a general and conventional method for measuring blood pressure. However, this method requires a person to measure blood pressure by hand and, therefore, it is difficult to measure blood pressure accurately and consistently due to the imprecision of the measurement method and the variability of blood pressure [5]. Additionally, it limits the number of patients whose blood pressure can be measured and the frequency of measurements.
Such limitations have led to the recent development and utilization of digital automatic blood pressure monitors [6]. Among them, blood pressure monitors using the non-invasive oscillometric technique [7] allows the patient to conveniently measure their blood pressure without the assistance of medical professionals. However, this device also requires a certain degree of consistency and accuracy in measuring blood pressure in order to produce reliable measurements for clinical use.
This study aimed to evaluate the accuracy of the mobile health (mHealth) device of the wireless digital automatic blood pressure monitor (SK Telecom Co., Ltd., South Korea) in order to determine its potential for clinical use and self-management in line with the Korea Food & Drug Administration (KFDA) protocol guidelines. These guidelines are a set of protocols reflecting international standards for testing methods to assess the accuracy of blood pressure monitors, such as American National Standard Institution (ANSI)/Association for the Advancement of Medical Instrumentation (AAMI) SP 10: 1992 [8] and the British Hypertension Society protocol [9].
Participants and methods
Device
The m-Healthcare device (SK Telecom Co., Ltd., South Korea) is a multi-functional medical measurement device capable of measuring the heart rate, oxygen saturation, activity, in addition to blood pressure, which largely consists of an automatic digital blood pressure monitor, the main device, and the management software (Table 1).
Table 1.
Device specification
| Type | Range | Specification | Device Design | |
|---|---|---|---|---|
| Automatic digital blood pressure monitor | Blood Pressure | Systolic: 40–270 mmHg | Communication: Bluetooth 4.0, NFC |
|
| Diastolic: 20–250 mmHg | Display: LED | |||
| Pulse rate: 30–300 BPM* | UI: 1 Button | 1. Body | ||
| Alarm: Speaker | 2. Cuff | |||
| Main device | Heart rate | 40–210 beat per minute | Communication: BT 4.0, NFC |

|
| Activity SpO2 | 4–10 km/h | Operating time: 5–24 h | 1. Body | |
| 60–100% | Charge time: 2 h | 2. Band | ||
| Display: LCD & 3-color LED | 3. SpO2 sensor | |||
| Alarm: Buzzer |
BPM beat per minute
This device measures blood pressure using the pressure oscillation caused by applying pressure on the cuff wrapped around the forearm. General sphygmomanometer is pressurized rapidly and measure while being depressurized, on the contrary, on this device, pressure is applied slowly so that it can detect even very small pulse amplitudes. As a result, the lowest blood pressure, the mean blood pressure, and the highest blood pressure values are measured and after the measurement, the cuff is quickly evacuated to minimize the tingling sensation on hands of patients. SpO2 is measured at the fingertip and the data is transmitted through a wire sensor which is connected to the main device by a PPG (Photo Plethysmography) method [10, 11].
The data collected through the device can be linked wirelessly through Bluetooth 4.0 transmission to the main device. The wireless communication range of the device is approximately 10 m like the general Bluetooth. However, if the distance between the patient with the device and the gateway exceeds 10 meters, the measured data cannot be directly transmitted, instead, it is stored in the device, and when the patient comes within the communication range, the stored data is transmitted to the server via the gateway.
The main device and the automatic digital blood pressure monitor is charged using a micro-USB charger within the set rating (5 V, 0.5A–2A, direct current).
The accuracy of the mHealth blood pressure monitor was compared to measurements taken using the UM-101 (A&D Co., Ltd., Japan) sphygmomanometer, which uses a manual measurement method.
Participants
The participants of this study consisted of subjects aged over 20 years who voluntarily agreed to the clinical trial. The subjects to be included in the study included 10% of all participants whose blood pressure was below 110 mmHg or above 160 mmHg for systolic blood pressure (SBP), and below 70 mmHg or above 100 mmHg for diastolic blood pressure (SBP). In terms of forearm circumference, 25–50% of all participants were selected with a forearm circumference of 20–30 cm, and 50–75% of all participants were selected with a forearm circumference of 30–40 cm. Criteria for exclusion included aortic stenosis or regurgitation that may cause abnormal waves, aortic dissection, and central or peripheral vascular malformation, such as aortic coarctation. A total of 100 participants were selected and agreed to the study, as they were all within the accepted range of blood pressure and forearm circumference, with none of them found to be applicable for the exclusion criteria or dropping out. Prior to blood pressure measurement, this study undertook a survey of demographic information, such as the gender, age, height, weight of the participants, as well as their medical history.
This study was approved by IRB of Seoul National University Bundang Hospital (IRB No: E-1607/354-001) and written informed consent was obtained from all participants.
Validation procedure
The investigators measured blood pressure by applying the test m-Healthcare device on one arm of the participant under a stable condition. In this study, two investigators who were trained about the proper use of blood pressure measurement devices were instructed to take measurements in a separate environment in order to prevent the collected data from being influenced by each other. The blood pressure of each participant was measured three times within 30 min. The measurement process was simultaneously monitored to check for any adverse reactions.
Following an adequate break of over 2 min, the two investigators each used the manual sphygmomanometer to consecutively measure blood pressure on the same arm where the test device was applied, and the measured values were recorded separately. Following another break of over 5 min, this process was repeated two more times in the same way. The blood pressure measured using the manual sphygmomanometer used the average of the values measured by the two investigators.
Since the test device measures the blood pressure while pressurizing and the manual sphygmomanometer measures while depressurizing, it is impossible to measure the blood pressure simultaneously. In this case, sequential measuring in the same arm is recommended according to the KFDA’s standards on electronic medical devices of automatic electronic blood pressure monitor. Therefore, in this study, according to the standard, blood pressure was measured by using the test device first and after resting for 2 min, the manual sphygmomanometer is applied in sequence to same patient.
Statistical analysis
In this study, the systolic and diastolic blood pressure data that were collected over a total of 300 readings (three measurements per participant) were analyzed. Then, the average and standard deviations of the differences in the observer measurement measured by the two investigators using the manual sphygmomanometer and the test mHealth device were calculated. In addition, the readings within 5 mmHg, 10 mmHg, and 15 mmHg as the three ranges of standard deviations are shown as percentages.
The results of the manual and device measurements are expressed using Bland–Altman plots.
Results
The total number of participants was 100 and a total of 14 (14%) participants were male and 86(86%) were female. Participants were aged from 22 to 73, 15 (15%) participants were aged between 20 and 29, 32 (32%) were 30–39, 27 (27%) were 40–49, 20 (20%) were between 50 and 59 and resting 6 (6%) participants were over the age of 60. The mean ± SD value of age was 41.9 ± 11.3. The mean ± SD of height and weight of all participants were 160.6 ± 6.4 cm and 57.9 ± 8.8 kg. And the mean ± SD of arm circumference was 27.7 ± 3.6 cm, ranging from 20 cm to 35 cm. The average systolic blood pressure (SBP) of all participants was 118.3 ± 19.8 mmHg and diastolic blood pressure (DBP) was 74.2 ± 13.3 mmHg (Table 2).
Table 2.
Participants’ characteristics
| Characteristics | Mean ± SD | Range |
|---|---|---|
| Age (years) | 41.9 ± 11.3 | 22–73 |
| Height (cm) | 160.6 ± 6.4 | 147–178 |
| Weight (kg) | 57.9 ± 8.8 | 43–83 |
| Arm circumference (cm) | 27.7 ± 3.6 | 20–35 |
| Systolic pressure (mmHg) | 118.3 ± 19.8 | 90–190 |
| Diastolic pressure (mmHg) | 74.2 ± 13.3 | 58–120 |
This study was conducted in 100 participants whose blood pressure was analyzed over 300 readings, which were generated by measuring each participant three sequential times using the test device and the manual sphygmomanometer.
The observer measurement between two investigators showed good agreement on the mean ± SD, ranged in 0.1 ± 2.6 mmHg for SBP and 0.5 ± 2.2 mmHg for DBP. In accordance with the recommendations of the British Hypertensive Society [9], the values measured simultaneously by the two investigators had to be identical within ± 10 mmHg for 95% and within ± 5 mmHg for 85%, which was satisfied by the measured values for both the SBP and DBP. For the test device and manual observer, the difference in the average blood pressure (mean ± SD) was 2.3 ± 4.7 for SBP, and 2.0 ± 4.2 for DBP. The SBP and DBP measured in this study satisfied the conditions of not exceeding 5 mmHg for the average difference and 8 mmHg for the standard deviation (Table 3).
Table 3.
Validation results of observer differences and device-observer differences
| Range (mmHg) | Mean ± SD (mmHg) | Percentage (%) of differences | |||
|---|---|---|---|---|---|
| ≤ 5 mmHg (%) | ≤ 10 mmHg (%) | ≤ 15 mmHg (%) | |||
| Observer differences | |||||
| Systolic pressure | 84–196 | 0.1 ± 2.6 | 96.0 | 99.7 | 100.0 |
| Diastolic pressure | 53–118 | 0.5 ± 2.2 | 97.0 | 100.0 | 100.0 |
| Device-observer differences | |||||
| Systolic pressure | 84–195 | 2.3 ± 4.7 | 77.7 | 95.0 | 99.4 |
| Diastolic pressure | 55–119 | 2.0 ± 4.2 | 80.7 | 97.0 | 100.0 |
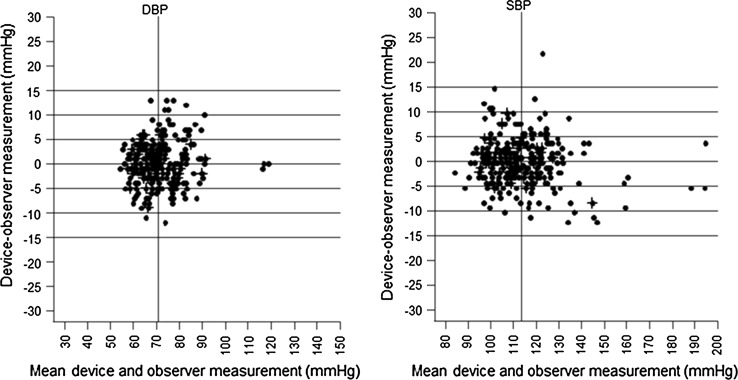
The average differences in the values measured using the test device and the observer are expressed as a scatter plot for SBP and DBP, which agrees with the analytical method presented by Bland–Altman. In the SBP, 99.4% of measured values of the test device and the observer showed a difference of less than 15 mmHg, 95.0% showed a difference of less than 10 mmHg and 77.7% of values showed a difference of less than 5 mmHg. And in the case of the DBP, 100% of values had a difference within 15 mmHg, and 97.0%, 80.7% of measured values showed a difference less than 10 mmHg, 5 mmHg, respectively (Fig. 1).
Fig. 1.
Bland-Altman plots of the diastolic blood pressure (left) and the systolic blood pressure (right) differences between the mHealth device and observers (y-axis) against the average of the test device and observer’ pressure values (x-axis)
Discussion
According to the outcome of this study, the m-Healthcare device satisfied every requirement outlined in the KFDA guidelines reflecting international protocols, and has been verified as a device capable of accurate blood pressure measurements.
The SBP and DBP measurement results, as evaluated through the device and manual observer measurements, showed accurate results. Therefore, the device can accurately be used as a blood pressure monitor in normal adults in both the home and the clinic.
The m-Healthcare device examined in this study is a non-invasive blood pressure monitor that can be used easily and conveniently by any user, and has the advantage of being able to link measured values wirelessly using Bluetooth communication technology. The linked value can be monitored and controlled centrally using a separate program, which allows measurement intervals and alarms to be set and managed for each program.
In the current healthcare market, wireless blood pressure monitors with similar functions have already been developed and commercialized. Among them, there are devices that can be easily measured with a single click and can connect to the smartphone through Bluetooth which make it effective and convenient to manage data. However, there is a limitation that they cannot guarantee the accuracy of the measured values [12, 13]. Therefore, most of the wireless blood pressure monitors currently on the market can only be used at home for health monitoring purposes. On the other hand, the newly developed device by reflecting the needs of the medical staff in this study have obtained the approval from the KFDA by verifying the accuracy of the measured value through the clinical experiment so that it can be used in clinical setting as well as at home. The m-Healthcare device received final approval from the KFDA in July 2017.
It is expected that while transferring patients (e.g., transfers to a different ward or lab) or post-operative patients who require periodic blood pressure monitoring, the m-Healthcare device can be used conveniently in the clinic and the home.
Conclusion
The m-Healthcare blood pressure monitor has been verified and found to satisfy all requirements presented by international standard protocols. To confirm its clinical feasibility and usability, clinical trials across disciplines may be needed in the future.
Acknowledgements
This work was funded by SK Telecom Co. Ltd and supported by the Technology Innovation Program funded By the Ministry of Trade, Industry and Energy (MOTIE) of Korea (10049785, Development of ‘medical equipment using (ionizing or non-ionizing) radiation’-dedicated R&D platform and medical device technology). The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for consultation on the statistical analyses.
Conflicts of interest
There are no conflicts of interest. This work was supported by SK Telecom Co. Ltd.
Ethical statement
This study was funded by SK Telecom Co. Ltd. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertensiona systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185–194. doi: 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Lu Y, Singh GM, Carnahan E, Stevens GA, Cowan MJ, et al. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2(8):634–647. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee G. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21(6):1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 ESH-ESC practice guidelines for the management of arterial hypertension: ESH-ESC task force on the management of arterial hypertension. J Hypertens. 2007;25(9):1751–1762. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 5.Celler BG, Le P, Basilakis J, Ambikairajah E. Improving the quality and accuracy of non-invasive blood pressure measurement by visual inspection and automated signal processing of the Korotkoff sounds. Physiol Meas. 2017;38(6):1006. doi: 10.1088/1361-6579/aa6b7e. [DOI] [PubMed] [Google Scholar]
- 6.Kakkad KM, Damor P, Parmar B, Patel S, Prajapati V, Dhivar N. Comparative study of blood pressure measurement by aneroid and digital manual sphygmomanometer. Natl J Community Med. 2016;7(8):700–702. [Google Scholar]
- 7.Meidert AS, Saugel B. Techniques for noninvasive monitoring of arterial blood pressure. Front Med. 2017;4:231. doi: 10.3389/fmed.2017.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association for the Advancement of Medical Instrumentation. American national standard for electronic or automated sphygmomanometers. Arlington, Virginia: 1992. Report No.
- 9.O’Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altman DG, Bland M, Atkins N. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11(suppl 2):S43–S62. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Hertzman AB. Observations on the finger volume pulse recorded photoelectrically. Am J Physiol. 1937;119:334–335. [Google Scholar]
- 11.Shelley KH. Photoplethysmography: beyond the calculation of arterial oxygen saturation and heart rate. Anesth Analg. 2007;105(6):S31–S36. doi: 10.1213/01.ane.0000269512.82836.c9. [DOI] [PubMed] [Google Scholar]
- 12.Medaval. The Standard for Medical Device Evaluation. Blood pressure monitors for home use. http://www.medaval.org/. Accessed 21 June 2018.
- 13.Parati G, Asmar R, Stergiou GS. Self blood pressure monitoring at home by wrist devices: a reliable approach? J Hypertens. 2002;20(4):573–578. doi: 10.1097/00004872-200204000-00005. [DOI] [PubMed] [Google Scholar]