Abstract
This study suggested a new EMG-signal-based evaluation method for knee rehabilitation that provides not only fragmentary information like muscle power but also in-depth information like muscle fatigue in the field of rehabilitation which it has not been applied to. In our experiment, nine healthy subjects performed straight leg raise exercises which are widely performed for knee rehabilitation. During the exercises, we recorded the joint angle of the leg and EMG signals from four prime movers of the leg: rectus femoris (RFM), vastus lateralis, vastus medialis, and biceps femoris (BFLH). We extracted two parameters to estimate muscle fatigue from the EMG signals, the zero-crossing rate (ZCR) and amplitude of muscle tension (AMT) that can quantitatively assess muscle fatigue from EMG signals. We found a decrease in the ZCR for the RFM and the BFLH in the muscle fatigue condition for most of the subjects. Also, we found increases in the AMT for the RFM and the BFLH. Based on the results, we quantitatively confirmed that in the state of muscle fatigue, the ZCR shows a decreasing trend whereas the AMT shows an increasing trend. Our results show that both the ZCR and AMT are useful parameters for characterizing the EMG signals in the muscle fatigue condition. In addition, our proposed methods are expected to be useful for developing a navigation system for knee rehabilitation exercises by evaluating the two parameters in two-dimensional parameter space.
Electronic supplementary material
The online version of this article (10.1007/s13534-018-0078-z) contains supplementary material, which is available to authorized users.
Keywords: Muscle fatigue, Knee rehabilitation, EMG signals
Introduction
Surgical interventions necessitated by knee pathologies are becoming more common in society today as the aged population increases. Following knee surgery, most patients would be required to perform rehabilitation exercises at home by themselves. However, because they have to perform the exercises without the therapist’s instructions and without feedback on their movements, the risk of secondary injury exists because of immoderate or insufficient exercise without a concrete goal [1, 2]. Thus, a monitoring device is required that will allow patients to monitor their movements or exercise status.
So far, some researchers have tried to monitor rehabilitation exercises using an accelerometer or a gyroscope [3–7]. For instance, Chen used an accelerometer to evaluate several knee rehabilitation exercises [3]. He evaluated movements using a decision tree and classified improper posture using only angle information. He also developed a mobile sensor system with an accelerometer and a gyroscope to measure the range of motion; this system is used by therapists for assessing the degree of recovery [4]. Masdar developed a knee joint angle measurement system for rehabilitation by fusing a flex sensor and gyroscope [6]. Milenkovic proposed an accelerometer-based portable rehabilitation system [7]. However, most of these studies focus on the analysis of motion features such as movement kinematics. The problem with movement kinematics is that it cannot quantify causal muscle activities (i.e., motor commands) because of the well-known redundancy of the musculoskeletal system. In general, a rehabilitation exercise is aimed at normalizing motor commands. In particular, evaluations based on only movement kinematics have the limitations of being unable to not only catch slight changes in the muscle activities but also evaluate the degree of rehabilitation for specific muscles. Accordingly, it is crucial to capture changes in the muscle activities as well as the resultant movement.
Electromyography (EMG) signals are useful to understand the muscle activities because they are correlated with the activities of alpha motor neurons, which represent the final motor commands from the central nervous system. These motor commands generate muscle contraction, which results in muscle tension. It has been established that a second-order low-pass filter is sufficient for estimating muscle tension from EMG signals [8–10]. In addition, some researchers have examined the relationship between EMG signals and muscle fatigue [11, 12]. The mean frequency or median frequency in raw EMG signals has been used to estimate muscle fatigue [13]. However, muscle fatigue parameters have not been used as evaluation parameters in the field of rehabilitation. In addition, muscle fatigue affects exercise performance [14, 15], and muscle fatigue around the knee affects the functions of daily life [16]. Considering that rehabilitation is aimed at enabling patients to resume normal activity, muscle fatigue parameters should be seriously considered as evaluation parameters for rehabilitation. In particular, repetitive and intensive training during rehabilitation causes muscle fatigue [17]. To avoid secondary injury and maximize the effectiveness while exercising moderately, muscle fatigue parameters should be used as evaluation parameters for rehabilitation.
Therefore, in this study, we suggested a new method for knee rehabilitation system in which muscle fatigue was monitored from four EMG signals. In particular, we monitored two feature parameters that characterized muscle fatigue based on the frequency and magnitude of the EMG signals. We then verified the effectiveness of these parameters in quantitatively evaluating muscle fatigue in the knee rehabilitation monitoring system.
Materials and methods
Experimental setup and movement task
Nine healthy subjects (male, age: 22–43 years, mean age: 26.88 years) participated in this study. In particular, because females are more resistant to muscle fatigue than males, we selected male subjects who are vulnerable to muscle fatigue in this study [18]. All the subjects provided written informed consent prior to participation. The experimental protocol was approved by the ethics committees of Handong Global University and was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki.
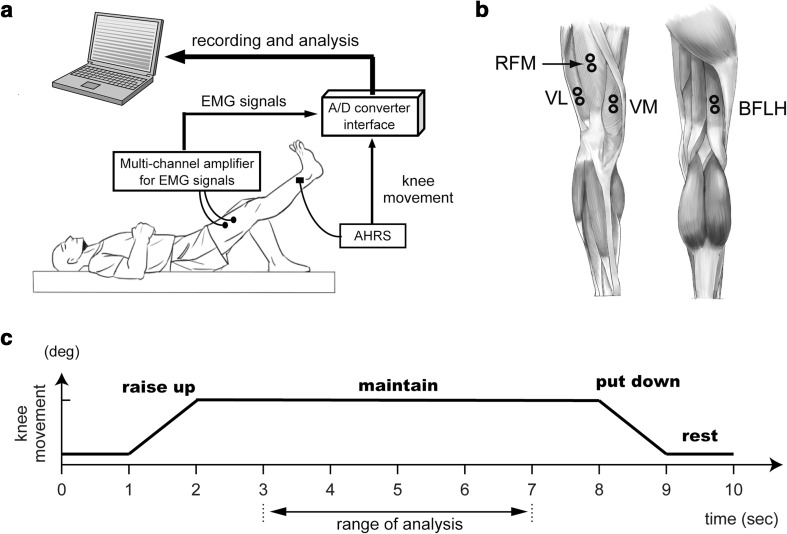
Figure 1a shows the outline of the recording system used for the analysis of knee rehabilitation exercises. The system consisted of four elements: an attitude heading reference system (AHRS), a notebook computer, a small universal serial bus (USB) analog-to-digital (A/D) converter interface, and a multichannel amplifier for surface electromyography (EMG) signals. In other words, a subject’s rehabilitation exercise information was measured as EMG signals using surface electrodes and the movements (i.e., joint angle) of the leg were measured using the AHRS. The data were recorded using the notebook computer through the USB A/D converter interface, NIDAQ USB-6009 (http://www.ni.com/en-us/support/model.usb-6009.html).
Fig. 1.
Experimental protocol. a Outline of the system for the analysis of knee rehabilitation exercises. b Muscles related to the knee joint and their recording positions with surface electrodes. The four prime knee movers whose activities were recorded: rectus femoris (RFM), vastus lateralis (VL), vastus medialis (VM), and biceps femoris (BFLH). c Procedure of the straight leg raise (SLR) as an experimental task. To initiate the exercise, the subject was first asked to lie on a mat. The subject then rested for 1 s. After 1 s, the subject was required to lift his right leg within 1 s (raise up). Then, the subject was asked to hold his right leg at that position for 6 s (maintain). After that, the subject was required to lower his right leg on to the mat (put down) and rest for the next trial (rest). In particular, to extract the characteristics of muscle fatigue more accurately, we limited the analysis of each parameter to the stable range (range of analysis) within the maintain range
We asked the subject to perform the straight leg raise (SLR) as a knee rehabilitation exercise. The SLR exercise can make subjects feel fatigued more easily than other knee rehabilitation exercises. The subject lifted his right leg. We did not consider the distinction of the dominant leg in this study, because a study by Lindström [19] reported that the dominant leg does not show significant differences. To initiate the exercise, the subject was first asked to lie on a mat, as illustrated in Fig. 1a. After 1 s, the subject was required to lift his right leg from the mat and hold that position for 6 s (Fig. 1c). Then, the subject was asked to lower the leg down and advance to the next trial. Each subject performed 15 trials per set. In addition, the SLR exercise was continued until the subject felt fatigued. As in Vøllestad’s study [20], we defined the criterion for muscle fatigue as decreased performance. In other words, when a subject could not raise his leg to the desired height in the SLR exercise, the result was judged as muscle fatigue with decreased performance. All trials were done at the same day.
Data acquisition and processing
During the exercise, we recorded the joint angle of the right leg and EMG signals from four prime movers of the leg—rectus femoris (RFM), vastus lateralis (VL), vastus medialis (VM), and biceps femoris (BFLH)—at 1 kHz. The EMG signals were measured using the Bagnoli EMG system (DELSYS, Inc., https://www.delsys.com/products/desktop-emg/bagnoli-desktop/). The locations of the surface electrodes for each muscle are shown in Fig. 1b. Based on the figure, the individual location was slightly adjusted to decrease the signal-to-noise ratio. In particular, the active electrodes in the system enable us to change the appropriate location for each muscle to show large-amplitude EMG signals in real time. In addition, since the distance between two active electrodes (Ag/Cl) was 1 cm, we only had to select one attachment site. Finally, we cleaned the skin with alcohol before sensor attachment. The EMG signals were digitally rectified and filtered using a second-order low-pass filter [21] (cut-off frequency: 2.2 Hz) that is sufficient to estimate muscle tensions from surface EMG signals. The tension of each muscle was normalized based on the muscular activity while the subject was generating the maximum voluntary contraction during isometric contraction for the preferred direction of each muscle. Then, we used the normalized EMG signals to analyze the magnitude of EMG signals in the state of muscle fatigue. In addition, we used the raw EMG signals to analyze the frequency of EMG signals in the state of muscle fatigue.
Data analysis and extracted parameters
From the EMG signals of the four prime movers of the leg, we captured the characteristics of the muscle activities in the state of muscle fatigue. In other words, to monitor the muscle fatigue in the knee rehabilitation system, we extracted two parameters that characterized the frequency and amplitude of EMG signals: the zero-crossing rate (ZCR) and amplitude of muscle tension (AMT). The ZCR was defined as the rate of zero crossing in the raw EMG signals. In other words, to calculate the ZCR for each muscle, we counted the number of points whose value changed from negative to positive in the raw EMG signals. The AMT was defined as the mean value of the normalized muscle tension for each muscle.
In particular, to extract the characteristics of muscle fatigue more accurately, we limited the analysis of each parameter to the stable range (4 s) of the leg’s holding period, as shown in Fig. 1c. In this study, we examined the change in each parameter between the first set of 15 trials (as the data for the non-fatigue condition) and the set of 15 trials prior to the set that was judged as reflecting fatigue (as the data for the fatigue condition) for verifying the effectiveness of these parameters and hence obtaining a quantitative evaluation of muscle fatigue in the knee rehabilitation monitoring system. We checked the significant change in the parameters between the two conditions of muscle fatigue using a paired-sample t test (ttest function in the statistics toolbox of MATLAB Ver. 7.14.0.739 (R2012a). The threshold of statistical significance was set at 5%. Also, we provide a supplementary Table 1 of abbreviations for more convenient referencing to the abbreviations.
Table 1.
Rate of change in the ZCR for each muscle after muscle fatigue
| Muscle | Case | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 (%) | S2 (%) | S3 (%) | S4 (%) | S5 (%) | S6 (%) | S7 (%) | S8 (%) | S9 (%) | |
| RFM | − 14.7 | − 15.8 | − 15.5 | − 7.9 | − 3.6 | − 2.8 | − 9.8 | 0.7 | − 6.6 |
| VL | − 3.0 | − 2.0 | − 10.0 | − 12.5 | − 2.7 | 4.5 | − 2.3 | 1.7 | − 4.4 |
| VM | 2.0 | − 13.9 | 2.4 | − 16.3 | − 8.6 | 14.1 | − 14.7 | 0.2 | − 18.3 |
| BFLH | − 2.4 | − 1.5 | − 30.5 | − 18.7 | − 24.6 | − 1.1 | − 20.3 | − 6.4 | − 8.2 |
RFM rectus femoris, VL vastus lateralis, VM vastus medialis, BFLH biceps femoris long head
Results
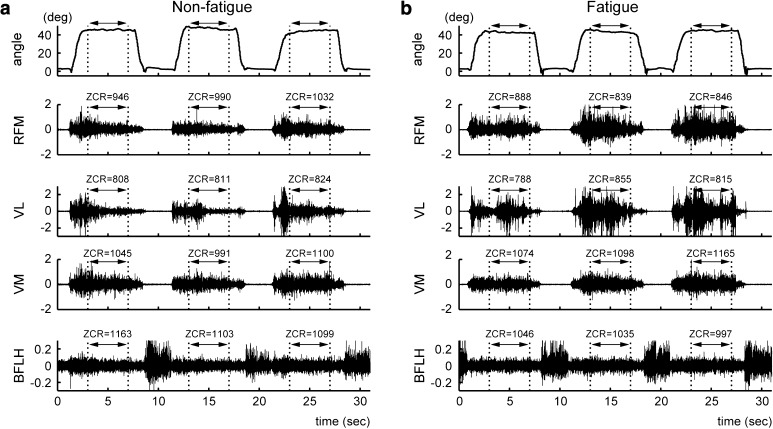
Figure 2 shows the leg movements and raw EMG signals obtained from the SLR exercise. Figure 2a and b present the data for the non-fatigue condition (i.e., three trials in the first set) and muscle fatigue condition (i.e., three trials in the last set), respectively. As shown in Fig. 2a, the RFM, VL, and VM were activated while the leg was being lifted, and their activities continued while the leg was being held. In contrast, the BFLH was activated when the subject lowered the leg. In particular, a comparison of the EMG signals between the non-fatigue and muscle fatigue conditions indicated that the amplitude of the EMG signal in the muscle fatigue condition tends to be bigger than that in the non-fatigue condition. Accordingly, we examined the characteristics of the EMG signals in the muscle fatigue condition based on the ZCR and AMT.
Fig. 2.
Movement trajectories and EMG signals for the SLR exercise. a An example of the non-fatigue condition. The top trace shows the angle of the knee joint. The bottom four traces show the EMG signals for the RFM, VL, VM, and BFLH. The ranges indicated by the arrows represent the range of analysis for muscle fatigue in the SLR exercise. b A corresponding example of the muscle fatigue condition. Note that the amplitude of the EMG signal in the muscle fatigue condition tends to be bigger than that in the non-fatigue condition. In addition, the ZCR values in the muscle fatigue condition decrease compared to those in the non-fatigue condition
Quantitative evaluation of muscle fatigue using ZCR
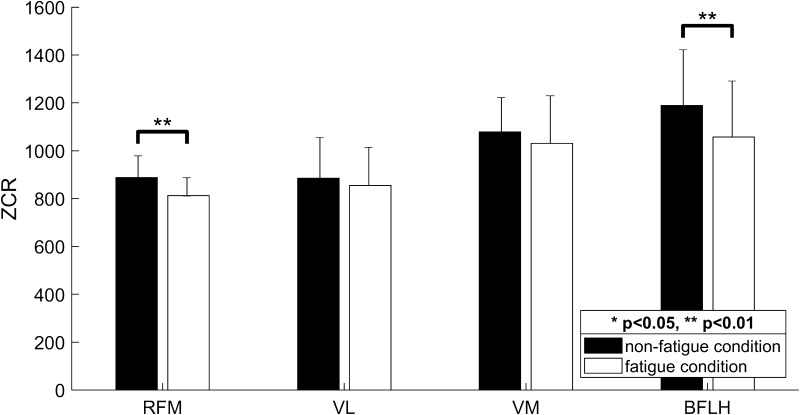
Figure 2 shows an example of the change in the ZCR between the data for the non-fatigue condition and fatigue condition. For the RFM, the ZCR values in the non-fatigue condition (Fig. 2a; ZCR = 946, 990, 1032) decreased compared to those in the muscle fatigue condition (Fig. 2b; ZCR = 888, 839, 846). As listed in Table 1, most of the subjects showed a decrease in the ZCR for the RFM. We also confirmed a significant difference between the two conditions (see RFM in Fig. 3; p = 0.0021). Similarly, for the BFLH, the ZCR values in the non-fatigue condition (Fig. 2a; ZCR = 1163, 1103, 1099) decreased compared to those in the muscle fatigue condition (Fig. 2b; ZCR = 1046, 1035, 997). As listed in Table 1, we confirmed a decrease in the ZCR for the BFLH in the muscle fatigue condition for most of the subjects. BFLH showed a significant difference between the two conditions (see BFLH in Fig. 3; p = 0.0046). On the other hand, for the VL, the ZCR decreased in the muscle fatigue condition for seven of the nine subjects, whereas for the VM, the ZCR decreased in the muscle fatigue condition for five of the nine subjects (Table 1). However, there are no significant differences in the ZCR in the muscle fatigue condition for the VL and VM (Fig. 3). Overall, these results suggest that the ZCR is a useful parameter for representing the characteristics of EMG signals in the muscle fatigue condition. In particular, the ZCR for the RFM and BFLH should be monitored further to analyze muscle fatigue when using the SLR as a knee rehabilitation exercise.
Fig. 3.
Comparison of the ZCR between non-fatigue and muscle fatigue conditions for the four prime knee movers during the SLR exercise. Black and white bars represent the ZCR values in the non-fatigue and muscle fatigue conditions, respectively. Note that the ZCR significantly decreases in the muscle fatigue condition, particularly for the RFM and BFLH (p < 0.01)
Quantitative evaluation of muscle fatigue using AMT
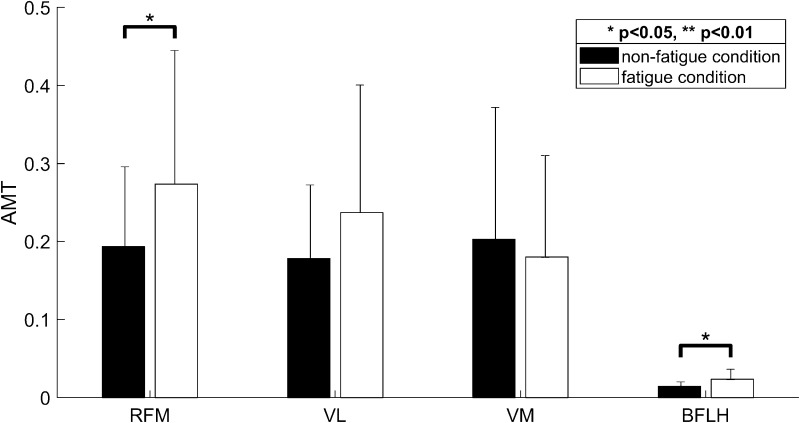
The EMG signals in the muscle fatigue condition showed an increase in the AMT (Fig. 2). In other words, for the RFM, the AMT values in the non-fatigue condition (Fig. 2a; AMT = 0.332, 0.262, 0.356) increased, compared to those in the muscle fatigue condition (Fig. 2b; AMT = 0.41, 0.612, 0.653). As listed in Table 2, eight of the nine subjects showed increases in the AMT for the RFM. RFM showed a significant difference between the two conditions (see RFM in Fig. 4; p = 0.0388). Similarly, the AMT for the BFLH showed increases for seven of the nine subjects. BFLH showed a significant difference between the two conditions (see BFLH in Fig. 4; p = 0.0391). For the VL, the AMT increased in the muscle fatigue condition for seven of the nine subjects. The VM showed an increase in the AMT in the muscle fatigue condition for five subjects (Table 2). However, there is no significant increase in the AMT for the VL and the VM in the muscle fatigue condition (see VL and VM in Fig. 4). These results suggest that the AMT is also a useful parameter for representing the characteristics of EMG signals in the muscle fatigue condition. In particular, for the AMT, the RFM and BFLH should be monitored further to analyze muscle fatigue when using the SLR as a knee rehabilitation exercise.
Table 2.
Rate of change in the AMT for each muscle after muscle fatigue
| Muscle | Case | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 (%) | S2 (%) | S3 (%) | S4 (%) | S5 (%) | S6 (%) | S7 (%) | S8 (%) | S9 (%) | |
| RFM | 30.0 | 42.5 | 47.4 | 16.3 | − 204.1 | 42.5 | 9.9 | 24.6 | 61.2 |
| VL | 21.8 | 1.3 | 49.2 | − 39.6 | − 117.8 | 1.3 | 34.7 | 38.4 | 46.6 |
| VM | − 17.9 | 38.7 | − 14.6 | − 63.7 | − 122.6 | 38.7 | 32.8 | 34.8 | 57.5 |
| BFLH | − 47.4 | 30.1 | 63.9 | 39.8 | 25.2 | 30.1 | 59.6 | 21.1 | − 15.9 |
RFM rectus femoris, VL vastus lateralis, VM vastus medialis, BFLH biceps femoris long head
Fig. 4.
Comparison of the AMT between non-fatigue and muscle fatigue conditions for the four prime knee movers during the SLR exercise. Black and white bars represent the AMT values in the non-fatigue and muscle fatigue conditions, respectively. Note that the AMT significantly increases in the muscle fatigue condition, particularly for the RFM and BFLH (p < 0.05)
Relationship between ZCR and AMT
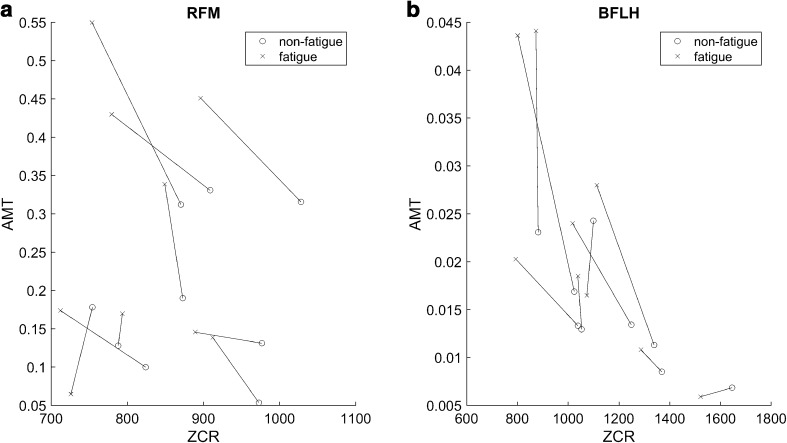
We examined the relationship between the ZCR and AMT in the non-fatigue and muscle fatigue conditions. In particular, Fig. 5 shows the analysis results for the RFM and BFLH, which showed marked changes in the two parameters in the muscle fatigue condition (Figs. 3, 4). The EMG signals in the non-fatigue condition had higher ZCR values and lower AMT values, whereas the EMG signals in the muscle fatigue condition had lower ZCR values and higher AMT values. In addition, our proposed methods are expected to be useful for developing a navigation system for knee rehabilitation exercises by evaluating the two parameters in two-dimensional parameter space.
Fig. 5.
Relationship between the ZCR and AMT in the non-fatigue and muscle fatigue conditions for whole subject. a Change in the two parameters for the RFM in the muscle fatigue condition. b Change in the two parameters for the BFLH in the muscle fatigue condition. In both (a) and (b), the circle markers indicate the non-fatigue condition, whereas the × markers represent the muscle fatigue condition
Discussion
Many researchers have studied knee rehabilitation monitoring. Previous studies focused on the data of an external coordinate (i.e., joint angle). However, these data do not directly reflect muscle conditions. Therefore, our study focused on the EMG signals of internal coordinates. EMG signals have been used in rehabilitation for providing EMG biofeedback [22–24]. Holtermann used EMG biofeedback to check muscle imbalance [25], and Middaugh used it for treating cervical and shoulder pain [26]. Sturma used biofeedback for the rehabilitation of amputees [27]. Overall, rehabilitation using EMG biofeedback can be used to not only identify a patient’s movement but also gather internal information such as muscle contraction parameters. However, EMG biofeedback has been used in rehabilitation mainly to obtain information about strength. Our study used EMG signals to obtain information about not only strength (i.e., muscle tension) but also muscle fatigue. With respect to the magnitude of the EMG signals obtained from muscles, we confirmed that muscle fatigue causes the AMT to increase (see RFM and BFLH in Fig. 4 and Table 2). This result is in agreement with those of previous studies, which show that large motor unit populations cause the EMG amplitude to increase rapidly [28, 29]. Our study quantitatively evaluated muscle fatigue in terms of the AMT. With respect to the frequency of the EMG signals, we found that muscle fatigue causes the ZCR to drop. In general, the mean frequency or median frequency has mainly been used to check muscle fatigue. However, our study used the ZCR to check muscle fatigue for convenience. Because we confirmed that the ZCR and median frequency are highly correlated to knee rehabilitation exercises in a previous experiment [30], low-frequency components of EMG signals, such as the ZCR, can also represent muscle fatigue [28]. We investigated the relationship between the magnitude and frequency of EMG signals using the AMT and ZCR. As shown in Fig. 5, muscle fatigue causes the AMT to increase and the ZCR to decrease. This implies that a high AMT value shows the activation of large motor units. Large motor units have low ZCR values. Because large motor units are activated, the frequency of small motor units decrease and low ZCR values are obtained. In the non-fatigue condition, smaller motor units are activated more frequently. In the fatigue condition, motor units corresponding to ‘×’ marker in Fig. 5 disappear. Instead, large motor units appear at ‘o’ marker in Fig. 5. This implies that motor units change to perform the same task. This result can be the basis for the creation of muscle activation patterns, for example, the rotation of motor units during fatigue [31]. In conclusion, our study is significant because we have suggested a more quantitative method to evaluate muscle fatigue using the ZCR and AMT.
In general, the mean or median frequency has mainly been used to assess muscle fatigue. However, with the assumption that smaller motor units are more activated in non-fatigue conditions, while larger motor units are activated in fatigue conditions, we adopted the magnitude of EMG signals (parameter AMT) to assess muscle fatigue in this study. As shown in Figs. 4, 5, we found that most subjects exhibited similar trends of AMT response from non-fatigue to fatigue (7 or 8 of 9 subjects). However, since the parameter AMT indicates the amplitude of the EMG signal (muscle tension), it is affected by the normalization method used for the EMG signal. In this study, maximum voluntary contraction (MVC) was performed for EMG normalization. However, because of intra-subject and inter-subject variability in MVC, the level of the AMT was different for each subject in Fig. 5. Therefore, to prevent individual differences in AMT values, we will use several methods to normalize EMG signals, such as the simple normalization method in the study by Shin [32] in future research. In addition, for the occasional subject who shows responses opposite to those of others in AMT changes, we will examine the relationship between non-fatigue and fatigue conditions for each muscle in terms of the change of main agonist under fatigue conditions in a future study.
Among the several knee rehabilitation exercises that exist, only the SLR was considered in this study. Therefore, exercises other than the SLR should be investigated in future. During the SLR, we found that the RFM and BFLH are important muscles for the evaluation of muscle fatigue. The VL shows a similar tendency to the RFM and BFLH, but the differences in the EMG signals are not as clear as those for the RFM and the BFLH; thus, the VL is considered to be inappropriate for the evaluation of the SLR. Because the VM and VL may be useful for evaluating other knee rehabilitation exercises, these muscles should continue to be investigated. In this study, evaluations were carried out only for the moment when the leg was held up. If other types of information are investigated and such information is collected in a database, knee rehabilitation patients can monitor their exercises through a smart device. Because the evaluation of muscle fatigue using the ZCR is more advantageous than that using the median frequency in terms of the computational load, the former is suitable for a rehabilitation monitoring device that is to be used at home. Through muscle fatigue evaluation, patients can avoid secondary injuries and perform exercises effectively. Through muscle power evaluation, patients can know their rehabilitation progress, and we expect this aspect to be a motivating factor for patients. A feasibility test should be performed for methodologies such as movement tasks, muscle measurements, and EMG parameters before patients are asked to perform these. This study confirmed that two parameters (AMT and ZCR) can be used to assess muscle fatigue during rehabilitation exercise.
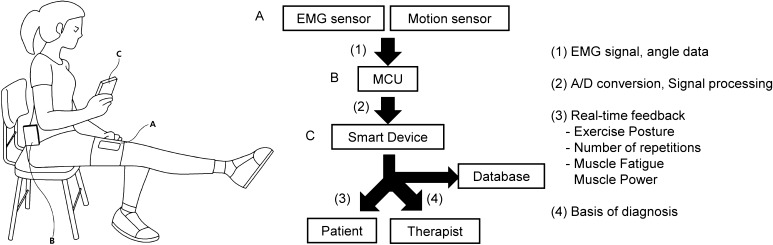
Figure 6 shows our suggested system. The system measures angle data and EMG data using a motion sensor and an EMG sensor, respectively. The obtained signals are processed through a microcontroller unit (MCU) and then transferred to a smart device. The system provides instructions to patients that help them to perform several exercises alone at home without the assistance of therapists. Proper feedback encourages patients to continue with their exercises [33]. The system decides whether the exercises performed by the patient is appropriate or not and provides proper information pertaining to parameters such as angle or velocity. In addition, the system uses EMG signals to instruct patients as to whether they are properly exercising the target muscle and to provide muscle fatigue information. Muscle fatigue can be an indicator to decide the proper amount of exercises. The rehabilitation process details and status of patients are collected in the database and evaluated. This information will help therapists to better diagnose knee pathologies.
Fig. 6.
Suggested knee rehabilitation system that monitors muscle fatigue. The system measures knee angle data using a motion sensor and EMG signals using an EMG sensor (A). The measured data are processed through an MCU (B) and then transferred to a smart device (C). The rehabilitation data of patients are collected in a database, and the data will help therapists to guide the rehabilitation process in an appropriate manner for each patient
Electronic supplementary material
Below is the link to the electronic supplementary material.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical statement
All the subjects provided written informed consent prior to participation. The experimental protocol was approved by the ethics committees of Handong Global University and was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki.
References
- 1.Holliday RC, Antoun M, Playford ED. A survey of goal-setting methods used in rehabilitation. Neurorehabilit Neural Repair. 2005;19(3):227–231. doi: 10.1177/1545968305279206. [DOI] [PubMed] [Google Scholar]
- 2.White G, Cordato D, O’Rourke F, Mendis R, Ghia D, Chan D. Validation of the Stroke Rehabilitation Motivation Scale: a pilot study. Asian J Gerontol Geriatr. 2012;7(2):80–87. [Google Scholar]
- 3.Chen K-H, Chen P-C, Liu K-C, Chan C-T. Wearable sensor-based rehabilitation exercise assessment for knee osteoarthritis. Sensors-Basel. 2015;15(2):4193–4211. doi: 10.3390/s150204193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen K-H, Tseng W-C, Liu K-C, Chan C-T. Using gyroscopes and accelerometers as a practical rehabilitation monitor system after total knee arthroplasty. 2015 International microwave workshop series on RF and wireless technologies for biomedical and healthcare applications (IMWS-BIO), 2015 IEEE MTT-S; 2015. p. 58–9.
- 5.Taylor PE, Almeida GJ, Kanade T, Hodgins JK. Classifying human motion quality for knee osteoarthritis using accelerometers. In: 2010 Annual international conference of the IEEE engineering in medicine and biology; 2010. p. 339–43. [DOI] [PubMed]
- 6.Masdar A, Ibrahim B, Hanafi D, Jamil MMA, Rahman K. Knee joint angle measurement system using gyroscope and flex-sensors for rehabilitation. In: Biomedical engineering international conference (BMEiCON), 2013 6th.; 2013. p. 1–4.
- 7.Milenkovic M, Jovanov E, Chapman J, Raskovic D, Price J. An accelerometer-based physical rehabilitation system. In: 2002 Proceedings of the thirty-fourth southeastern symposium on system theory; 2002. p. 57–60.
- 8.Herzog W, Sokolosky J, Zhang Y, Guimarães A. EMG-force relation in dynamically contracting cat plantaris muscle. J Electromyogr Kinesiol. 1998;8(3):147–155. doi: 10.1016/S1050-6411(97)00015-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu MM, Herzog W, Savelberg HH. Dynamic muscle force predictions from EMG: an artificial neural network approach. J Electromyogr Kinesiol. 1999;9(6):391–400. doi: 10.1016/S1050-6411(99)00014-0. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Martel F, Rancourt D, Clancy EA, Brown DR. Fingertip force estimation from forearm muscle electrical activity. 2014 IEEE international conference on acoustics, speech and signal processing (ICASSP); 2014. p. 2069–73.
- 11.Weir JP, Wagner LL, Housh TJ. Linearity and reliability of the IEMG v torque relationship for the forearm flexors and leg extensors. Am J Phys Med Rehabil. 1992;71(5):283–287. doi: 10.1097/00002060-199210000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lykholt LE, Ganeswarathas S, Thota AK, Harreby KR, Jung R. Information on ankle angle from intramuscular EMG Signals during development of muscle fatigue in an open-loop functional electrical stimulation system in rats. In: Winnie J, Ole KA, Metin A, editors. Replace, repair, restore, relieve-bridging clinical and engineering solutions in neurorehabilitation. Berlin: Springer; 2014. pp. 529–536. [Google Scholar]
- 13.Cifrek M, Medved V, Tonković S, Ostojić S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin Biomech (Bristol, Avon) 2009;24(4):327–340. doi: 10.1016/j.clinbiomech.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88(1):287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 15.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586(1):161–173. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harput G, Kilinc HE, Ozer H, Baltaci G, Mattacola CG. Quadriceps and hamstring strength recovery during early neuromuscular rehabilitation after ACL hamstring-tendon autograft reconstruction. J Sport Rehabil. 2015;24(4):398–404. doi: 10.1123/jsr.2014-0224. [DOI] [PubMed] [Google Scholar]
- 17.Hawley JA, Myburgh K, Noakes TD, Dennis S. Training techniques to improve fatigue resistance and enhance endurance performance. J Sports Sci. 1997;15(3):325–333. doi: 10.1080/026404197367335. [DOI] [PubMed] [Google Scholar]
- 18.Hicks Audrey L, Kent-Braun Jane, Ditor David S. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;9(3):109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Lindström B, Karlsson S, Gerdle B. Knee extensor performance of dominant and non-dominant limb throughout repeated isokinetic contractions, with special reference to peak torque and mean frequency of the EMG. Clin Physiol Funct Imaging. 1995;15(3):275–286. doi: 10.1111/j.1475-097X.1995.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 20.Vøllestad NK. Measurement of human muscle fatigue. J Neurosci Methods. 1997;74(2):219–227. doi: 10.1016/S0165-0270(97)02251-6. [DOI] [PubMed] [Google Scholar]
- 21.Koike Y, Kawato M. Estimation of arm posture in 3D-space from surface EMG signals using a neural network model. IEICE Trans Inf Syst. 1994;77(4):368–375. [Google Scholar]
- 22.Christanell F, Hoser C, Huber R, Fink C, Luomajoki H. The influence of electromyographic biofeedback therapy on knee extension following anterior cruciate ligament reconstruction: a randomized controlled trial. BMC Sports Sci Med Rehabil. 2012;4(1):1. doi: 10.1186/1758-2555-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draper V. Electromyographic biofeedback and recovery of quadriceps femoris muscle function following anterior cruciate ligament reconstruction. Phys Ther. 1990;70(1):11–17. doi: 10.1093/ptj/70.1.11. [DOI] [PubMed] [Google Scholar]
- 24.Giggins OM, Persson UM, Caulfield B. Biofeedback in rehabilitation. J Neuroeng Rehabil. 2013;10(1):1. doi: 10.1186/1743-0003-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtermann A, Mork P, Andersen L, Olsen HB, Søgaard K. The use of EMG biofeedback for learning of selective activation of intra-muscular parts within the serratus anterior muscle: a novel approach for rehabilitation of scapular muscle imbalance. J Electromyogr Kinesiol. 2010;20(2):359–365. doi: 10.1016/j.jelekin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Middaugh S, Thomas KJ, Smith AR, McFall TL, Klingmueller J. EMG biofeedback and exercise for treatment of cervical and shoulder pain in individuals with a spinal cord injury: a pilot study. Top Spinal Cord Inj Rehabil. 2013;19(4):311. doi: 10.1310/sci1904-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturma A, Göbel P, Herceg M, Gee N, Roche A, Fialka-Moser V, et al. Advanced rehabilitation for amputees after selective nerve transfers: EMG-guided training and testing. In: Winnie J, Ole KA, Metin A, et al., editors. Replace, repair, restore, relieve-bridging clinical and engineering solutions in neurorehabilitation. Berlin: Springer; 2014. pp. 169–177. [Google Scholar]
- 28.Holtermann A, Grönlund C, Karlsson JS, Roeleveld K. Motor unit synchronization during fatigue: described with a novel sEMG method based on large motor unit samples. J Electromyogr Kinesiol. 2009;19(2):232–241. doi: 10.1016/j.jelekin.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Hunter SK, Enoka RM. Changes in muscle activation can prolong the endurance time of a submaximal isometric contraction in humans. J Appl Physiol. 2003;94(1):108–118. doi: 10.1152/japplphysiol.00635.2002. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Koo K, Lee Y, Lee J, Kim J. Spatiotemporal analysis of EMG signals for muscle rehabilitation monitoring system. In: 2013 IEEE 2nd global conference on consumer electronics (GCCE); 2013. p. 1–2.
- 31.Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72(5):1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- 32.Shin D, Kim J, Koike Y. A myokinetic arm model for estimating joint torque and stiffness from EMG signals during maintained posture. J Neurophysiol. 2009;101:387–401. doi: 10.1152/jn.00584.2007. [DOI] [PubMed] [Google Scholar]
- 33.Flores E, Tobon G, Cavallaro E, Cavallaro FI, Perry JC, Keller T. Improving patient motivation in game development for motor deficit rehabilitation. In: Proceedings of the 2008 international conference on advances in computer entertainment technology; 2008. p. 381–4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.