Abstract
This is the first study demonstrating the efficacy of menstrual blood-derived stem cell (MenSC) transplantation via decellularized human amniotic membrane (DAM), for the promotion of skin excisional wound repair. The DAM was seeded with MenSCs at the density of 3 × 104 cells/cm2 and implanted onto a rat’s 1.50 × 1.50 cm2 full-thickness excisional wound defect. The results of wound closure and histopathological examinations demonstrated that the MenSC-seeded DAM could significantly improve the wound healing compared with DAM-treatment. All in all, our data indicated that the MenSCs can be a potential source for cell-based therapies to regenerate skin injuries.
Keywords: Amniotic membrane, Wound healing, Menstrual blood-derived stem cells
Introduction
The coverage of large skin defects remains a major challenge when the defect area exceeds a critical size [1, 2]. A varieties of skin grafting methods have been exploited successfully. Despite showing clinical benefits, skin grafts have many drawbacks such as limited harvestable tissue, donor site morbidity, and risk of disease transmission in case of allogenic skin grafts [3]. These drawbacks have driven efforts to search for alternative structures to serve as skin substitutes. Skin tissue engineering is a therapeutic approach in regenerative medicine that offers new solutions for patients suffering from skin tissue loss [4]. This technology is based on synergistic combination of scaffolds, cells, and growth factors to induce de novo skin formation [5]. Among various scaffold materials we used Decellularized human Amniotic Membrane (DAM) because it can facilitate the fibroblasts recruitment and vessel invasion for subsequent skin tissue remodeling [6, 7]. It consists of several extracellular matrix (ECM) components and growth factors such as laminin, fibronectin, different types of collagen, elastin, hyaluronic acid, nerve growth factor (NGF), keratinocyte growth factor (KGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and transforming growth factor-β (TGF-β) [8–11]. These components help the DAM to mimic the stem cell niche and be an ideal carrier for stem cell transplantation [12, 13]. Stem cells possess several therapeutic potentials to regenerate the damaged skin, largely through their trophic and paracrine activities [14]. Among candidate cell populations, menstrual blood-derived stem cells (MenSCs) have gained new attention for clinical applications [15, 16]. They are highly proliferative and easily accessible stem cells without any surgical intervention [17, 18]. MenSCs not only have a high differentiation potential but also can be expanded in clinically sufficient numbers and a relatively short time without compromising their healing potential or developing genetic abnormalities [19–21]. Based on these well-established principals, we were encouraged to investigate the healing potential of MenSC-seeded DAM (MenSCs-DAM) on full-thickness skin defect of rats. We investigated the level of wound healing using the assessment methods of wound closure analysis and histopathological examinations.
Materials and methods
Preparation of MenSCs-DAM
MenSCs were isolated and characterized as described previously [22]. Briefly, the menstrual blood was harvested from four healthy donors aged 25–35 years during the first 2 days of menstruation at Avicenna infertility clinic (Tehran, Iran). The MenSCs were isolated by Ficoll–Hypaque (GE-Healthcare, Uppsala, Sweden) density gradient centrifugation and after rinsing with phosphate-buffered saline (PBS; Cat. No. P4417, Sigma-Aldrich, St. Louis, USA), were cultivated in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F12; Cat. No. 32500-035, Gibco, Grand Island, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Cat. No. 10270, Gibco, Grand Island, USA), 100 unit/mL of penicillin (Cat. No. P3032, Sigma-Aldrich, St. Louis, USA), 100 μg/mL of streptomycin (Cat. No. S9137, Sigma-Aldrich, St. Louis, USA) and 0.25 μg/mL of Amphotericin B (Cat. No. A2942, Sigma-Aldrich, St. Louis, USA). After 2 days of incubation in a humidified incubator at 37 °C with 5% CO2, the non-adherent cells were discarded and the adherent cells (~ 70% confluence) were passaged. The DAM was purchased from Sabz Biomedical Company (Tehran, Iran) and seeded with third-passages MenSCs at the density of 3 × 104 cells/cm2 and remained in the same culture medium for 48 h.
Wound healing model
Animal experiments were approved by the ethics committee of Shahroud University of Medical Sciences and were performed in accordance with the university’s guidelines. A full-thickness excisional skin wound model was used to investigate the effects of prepared membranes on wound healing. Twelve healthy adult male Wistar rats (3 months old, weighing 250–270 g) were purchased from Pasteur Institute (Tehran, Iran). The animals were anesthetized by intraperitoneal injection of 70 mg Ketamine and 6 mg Xylazine (both from Alfasan Co., Woerden, Netherlands) per kg of body weight. The back of each rat was shaved and after disinfection with povidone-iodine (Partkimia pharmaceutical Co., Gorgan, Iran), was excised (1.50 × 1.50 cm2) using a scalpel blade. The animals were divided into two groups (6 rats per group) and the wounds were treated with DAM and MenSCs-DAM.
Wound healing analysis
For wound closure study, overall appearance of the wound area was photographed by a digital camera (Canon Inc., Tokyo, Japan) at 3, 7 and 14 days after surgical procedure and the level of wound closure was measured using an image analyzing software (Digimizer, Ostend, Belgium) with the following equation [23].
After 14 days of surgery, the animals were euthanized under anesthesia and the wounded skin tissue was excised for histopathological examinations. The skin specimens were fixed in 10% buffered formalin and after processing and embedding in paraffin, were cut into 5 µm sections and stained with hematoxylin–eosin (H&E) stain kit (ScyTek Laboratories Inc., Logan, USA) and Masson’s trichrome (MT) stain kit (Cat. No. HT15, Sigma-Aldrich, St. Louis, USA). The prepared tissue slides were imaged under a light microscope (Carl Zeiss, Thornwood, USA) with a digital camera (Olympus, Tokyo, Japan). Epithelialization was evaluated semi-quantitatively on a 5-point scale: 0 (without new epithelialization), 1 (25%), 2 (50%), 3 (75%), and 4 (100%). Samples were also semi-qualitatively analyzed with regard to angiogenesis according to the number of new vessels within the scar tissue using a 5-point scale as follows: 0 (none), 1 (few), 2 (moderate), 3 (many) or 4 (considerable). For these parameters, results were validated by comparative analysis of one independent observer blinded to the treatment groups.
Statistical analysis
The results were statistically analyzed by Minitab 17 software using Student’s t test and the data were expressed as the mean ± standard deviation (SD). In all evaluations, P < 0.05 was considered as the statistically significant.
Results
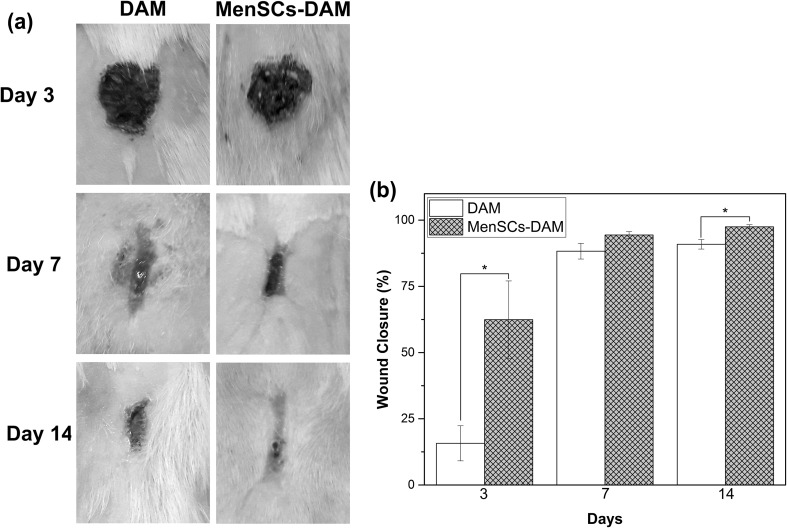
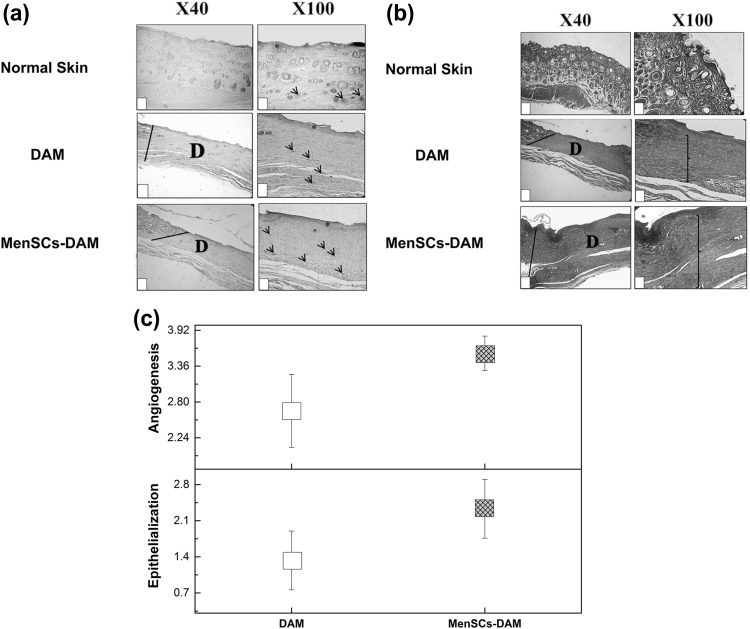
Figure 1a illustrates the macroscopic appearances of the skin wounds treated with DAM and MenSCs-DAM. At the end of 3th post-wounding day, most wounds in both groups were covered by scab but at the end of 7th day, some of the DAM-treated wounds were observed to be still fresh. The MenSCs-DAM-treated wounds had the best cosmetic appearance after 14 days. In order to quantify the wound size reduction, the wound closure was measured from the corresponding images (Fig. 1b). The DAM-treated group had the wound closure of 15.74 ± 6.63%, 88.29 ± 2.96% and 90.91 ± 1.80% at 3, 7 and 14 days post-wounding, respectively. These values for the MenSCs-DAM treated group were 62.43 ± 14.62%, 94.40 ± 1.29% and 97.49 ± 0.78% after 3, 7 and 14 days, respectively which were significantly (P < 0.05) higher than those of the DAM-treated group after 3 and 14 days. The H&E histopathological examination of both treated groups (Fig. 2a) revealed a well-recovered epidermal layer with higher thickness in MenSCs-DAM group compared with the DAM group, indicating its higher repairing activity. However, the epidermal papillae could not be observed in any of the two groups. To further investigate the effect of treatments on the wound healing, the samples were stained with MT staining. The MT stained sections (Fig. 2b) exhibited loosely packed collagen fibers with irregular arrangement at the edge of both treated groups. The MenSCs-DAM group had higher collagen fiber synthesis, maturation and arrangement. The results obtained from the histomorphometric analysis (Fig. 2c) revealed that the MenSCs-DAM group had higher angiogenesis and epithelization scores compared with the DAM group, although the results were not statistically significant.
Fig. 1.
a Macroscopic appearances of the skin wounds treated with DAM and MenSCs-DAM 3, 7, and 14 days post-wounding. b Histogram comparing the wound closure of MenSCs-DAM and DAM treated wounds at the end of 3rd, 7th, and 14th post-wounding days. Values represent the mean ± SD, *P < 0.05 obtained by Student’s t-test
Fig. 2.
a Histopathological examination of the hematoxylin–eosin stained wounds at the end of 14th post-wounding day compared with the normal skin. b Histopathological examination of the Masson’s trichrome stained wounds at the end of 14th post-wounding day compared with the normal skin. c Histogram comparing the level of epithelialization and angiogenesis at the end of 14th post-wounding day. Dotted lines indicate the border between epidermis and dermis; D: defect, star: epithelialization, arrow: angiogenesis, and bracket: thickness of the collagen
Discussion
Mesenchymal stem cells (MSCs) possess self-renewal and multi-lineage differentiation potential, and their ability to promote skin wound healing has been well documented in previous studies [24–26]. Acting via their strong paracrine secretions, MSCs accelerate the wound healing, promote angiogenesis, modulate inflammatory responses and encourage restoration of skin tissue following damages [27–29]. MenSCs are a group of MSCs which show a high rate of cell proliferation and differentiation potential [22]. Additionally, MenSCs express a relatively lower rate of major histocompatibility complex class II and co-stimulatory molecules on their surface which reduce the risk of immune rejection [16, 30]. Direct stem cell injection into the wound bed or systemic administration have been investigated in some studies as potential routes of stem cell transplantation into the wounded animal [24, 31, 32]. However, stem cell survival and retention in the harsh environment of fresh wound and entrapment in the lung or other organs in case of systemic injection, can compromise the efficacy of stem cell-based therapies in wound healing [33–35]. To avoid these possibilities, we exploited DAM as a platform to implant stem cells into the damaged skin. Taking together, our data showed that the best results for regeneration was obtained in MenSCs-DAM group. This may be due to the following reasons. First, MenSCs exert their therapeutic effects through the constant secretion of paracrine factors and matrix proteins that can aid the wound healing. Miranda et al. reported that there was a significant amount of angiogenic factors which have therapeutic benefits in wound healing including vascular endothelial growth factor (VEGF) and bFGF in the conditioned media of MenSCs [17, 36]. Wang et al. reported that bFGF can positively regulate the migration ability of fibroblasts via the activation of wnt/β cantenin signaling pathway [37]. This can increase the recruitment of fibroblasts from surrounding tissues and subsequently enhance the wound closure [38]. Second, as we previously reported, MenSCs can differentiate into epidermal/keratinocyte lineage when they were co-cultured with foreskin-derived keratinocytes. Dermal cells developed from MenSCs may replace the lost cells in the wounded skin tissue [39]. Finally, the immunomodulatory properties of MenSCs may support their favorable effect on the wound healing response [40, 41]. Although, their ability to modulate the inflammatory responses in the wounds yet to be studied.
Conclusion
Taken together, the evidences presented in this study indicate that the transplantation of MenSCs via DAM can promote the wound healing. However, long term side effects of MenSCs transplantation should be addressed in future studies. Our data demonstrate that the MenSCs can be a potential candidate for cell therapy to enhance the wound healing.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Animal experiments were approved by the ethical committee of Shahroud University of Medical Sciences and were carried out in accordance with the university’s guidelines.
Contributor Information
Saeed Farzamfar, Phone: +98 9224350431, Email: farzamfar.saeed@razi.tums.ac.ir.
Majid Salehi, Phone: +989107646451, Phone: +989302655158, Email: msalehi.te1392@gmail.com.
Arian Ehterami, Phone: +989132886274, Email: arian.ehterami@srbiau.ac.ir.
Mahdi Naseri-Nosar, Phone: +989331002713, Email: naseri.iranica@aut.ac.ir.
Ahmad Vaez, Phone: +989302655158, Email: avaez@razi.tums.ac.ir.
Amir Hassan Zarnani, Phone: +989302655158, Email: zarnani@ari.ir.
Hamed Sahrapeyma, Phone: +989113005822, Email: hamed.sahrapeyma@srbiau.ac.ir.
Mohammad-Reza Shokri, Phone: +989302655158, Email: shokri.mr@tak.iums.ac.ir.
Mehdi Aleahmad, Phone: +989302655158, Email: m_aleahmad@razi.tums.ac.ir.
References
- 1.Chandika P, Ko S-C, Jung W-K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int J Biol Macromol. 2015;77:24–35. doi: 10.1016/j.ijbiomac.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 2.Sundaramurthi D, Krishnan UM, Sethuraman S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym Rev. 2014;54(2):348–376. doi: 10.1080/15583724.2014.881374. [DOI] [Google Scholar]
- 3.Alrubaiy L, Al-Rubaiy KK. Skin substitutes: a brief review of types and clinical applications. Oman Med J. 2009;24(1):4. doi: 10.5001/omj.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansbridge J. Skin tissue engineering. J Biomater Sci Polym Ed. 2008;19(8):955–968. doi: 10.1163/156856208784909417. [DOI] [PubMed] [Google Scholar]
- 5.Priya SG, Jungvid H, Kumar A. Skin tissue engineering for tissue repair and regeneration. Tiss Eng Part B Rev. 2008;14(1):105–118. doi: 10.1089/teb.2007.0318. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, et al. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 2009;336(1):59. doi: 10.1007/s00441-009-0766-1. [DOI] [PubMed] [Google Scholar]
- 7.Ilic D, et al. Human amniotic membrane grafts in therapy of chronic non-healing wounds. Br Med Bull. 2016;117(1):59–67. doi: 10.1093/bmb/ldv053. [DOI] [PubMed] [Google Scholar]
- 8.Ishino Y, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45(3):800–806. doi: 10.1167/iovs.03-0016. [DOI] [PubMed] [Google Scholar]
- 9.Niknejad H, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater. 2008;15:88–99. doi: 10.22203/eCM.v015a07. [DOI] [PubMed] [Google Scholar]
- 10.Dua HS, et al. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Cooper LJ, et al. An investigation into the composition of amniotic membrane used for ocular surface reconstruction. Cornea. 2005;24(6):722–729. doi: 10.1097/01.ico.0000154237.49112.29. [DOI] [PubMed] [Google Scholar]
- 12.Motamed S, et al. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am J Surg. 2017;214(4):762–769. doi: 10.1016/j.amjsurg.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48(6):631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Duscher D, et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016;62(2):216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues Maria Carolina Oliveira, Lippert Trenton, Nguyen Hung, Kaelber Sussannah, Sanberg Paul R., Borlongan Cesar V. Advances in Experimental Medicine and Biology. Cham: Springer International Publishing; 2016. Menstrual Blood-Derived Stem Cells: In Vitro and In Vivo Characterization of Functional Effects; pp. 111–121. [DOI] [PubMed] [Google Scholar]
- 16.Faramarzi H, et al. The potential of menstrual blood-derived stem cells in differentiation to epidermal lineage: a preliminary report. World J Plast Surg. 2016;5(1):26. [PMC free article] [PubMed] [Google Scholar]
- 17.Alcayaga-Miranda F, et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6(1):32. doi: 10.1186/s13287-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, et al. Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl Med. 2017;6(1):272–284. doi: 10.5966/sctm.2015-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, et al. Plasticity of human menstrual blood stem cells derived from the endometrium. J Zhejiang Univ-Sci B. 2011;12(5):372–380. doi: 10.1631/jzus.B1100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vu Ngoc Bich, Trinh Van Ngoc-Le, Phi Lan Thi, Phan Ngoc Kim, Van Pham Phuc. Regenerative Medicine. London: Springer London; 2014. Human Menstrual Blood-Derived Stem Cell Transplantation for Acute Hind Limb Ischemia Treatment in Mouse Models; pp. 205–215. [Google Scholar]
- 21.Verdi J, et al. Endometrial stem cells in regenerative medicine. J Biol Eng. 2014;8(1):20. doi: 10.1186/1754-1611-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farzamfar S, et al. Sciatic nerve regeneration by transplantation of menstrual blood-derived stem cells. Mol Biol Rep. 2017;44(5):407–412. doi: 10.1007/s11033-017-4124-1. [DOI] [PubMed] [Google Scholar]
- 23.Naseri-Nosar M, et al. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: in vitro and in vivo evaluation. Mater Sci Eng C. 2017;81:366–372. doi: 10.1016/j.msec.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki M, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa H, et al. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153(1):29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- 28.Maxson S, et al. Concise review: role of mesenchymal stem cells in wound repair. Stem cells translational medicine. 2012;1(2):142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter M, et al. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316(7):1271–1281. doi: 10.1016/j.yexcr.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Xiang B, et al. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int J Mol Sci. 2017;18(4):689. doi: 10.3390/ijms18040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badiavas EV, et al. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196(2):245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 32.Kim W-S, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Li L, et al. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016;2016:14. doi: 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun JS, et al. Enhancing stem cell survival in vivo for tissue repair. Biotechnol Adv. 2013;31(5):736–743. doi: 10.1016/j.biotechadv.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends in molecular medicine. 2010;16(5):203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3(10):647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, et al. Feedback activation of basic fibroblast growth factor signaling via the Wnt/β-catenin pathway in skin fibroblasts. Front Pharmacol. 2017;8:32. doi: 10.3389/fphar.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreier T, Degen E, Baschong W. Fibroblast migration and proliferation during in vitro wound healing. Res Exp Med. 1993;193(1):195–205. doi: 10.1007/BF02576227. [DOI] [PubMed] [Google Scholar]
- 39.Akhavan-Tavakoli M, et al. In vitro differentiation of menstrual blood stem cells into keratinocytes: a potential approach for management of wound healing. Biologicals. 2017;48:66–73. doi: 10.1016/j.biologicals.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Therapy. 2016;7(1):37. doi: 10.1186/s13287-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikoo S, et al. Effect of menstrual blood-derived stromal stem cells on proliferative capacity of peripheral blood mononuclear cells in allogeneic mixed lymphocyte reaction. J Obstet Gynaecol Res. 2012;38(5):804–809. doi: 10.1111/j.1447-0756.2011.01800.x. [DOI] [PubMed] [Google Scholar]