Abstract
Purpose
For over 30 years, the place of consolidation high-dose chemotherapy in Ewing sarcoma (ES) has been controversial. A randomized study was conducted to determine whether consolidation high-dose chemotherapy improved survival in patients with localized ES at high risk for relapse.
Methods
Randomization between busulfan and melphalan (BuMel) or standard chemotherapy (vincristine, dactinomycin, and ifosfamide [VAI], seven courses) was offered to patients if they were younger than 50 years of age with poor histologic response (≥ 10% viable cells) after receiving vincristine, ifosfamide, doxorubicin, and etoposide (six courses); or had a tumor volume at diagnosis ≥ 200 mL if unresected, or initially resected, or resected after radiotherapy. A 15% improvement in 3-year event-free survival (EFS) was sought (hazard ratio [HR], 0.60).
Results
Between 2000 and 2015, 240 patients classified as high risk (median age, 17.1 years) were randomly assigned to VAI (n = 118) or BuMel (n = 122). Seventy-eight percent entered the trial because of poor histologic response after chemotherapy alone. Median follow-up was 7.8 years. In an intent-to-treat analysis, the risk of event was significantly decreased by BuMel compared with VAI: HR, 0.64 (95% CI, 0.43 to 0.95; P = .026); 3- and 8-year EFS were, respectively, 69.0% (95% CI, 60.2% to 76.6%) versus 56.7% (95% CI, 47.6% to 65.4%) and 60.7% (95% CI, 51.1% to 69.6%) versus 47.1% (95% CI, 37.7% to 56.8%). Overall survival (OS) also favored BuMel: HR, 0.63 (95% CI, 0.41 to 0.95; P = .028); 3- and 8-year OS were, respectively, 78.0% (95% CI, 69.6% to 84.5%) versus 72.2% (95% CI, 63.3% to 79.6%) and 64.5% (95% CI, 54.4% to 73.5%) versus 55.6% (95% CI, 45.8% to 65.1%). Results were consistent in the sensitivity analysis. Two patients died as a result of BuMel-related toxicity, one after standard chemotherapy. Significantly more BuMel patients experienced severe acute toxicities from this course of chemotherapy compared with multiple VAI courses.
Conclusion
BuMel improved EFS and OS when given after vincristine, ifosfamide, doxorubicin, and etoposide induction in localized ES with predefined high-risk factors. For this group of patients, BuMel may be an important addition to the standard of care.
INTRODUCTION
Treatment advances in Ewing sarcoma (ES) have largely resulted from a multidisciplinary approach to clinical trials conducted by national and international cooperative groups. Over the years, these trials have answered key chemotherapy questions and better defined risk groups, allowing tailored treatment strategies. Prolonged disease-free survival is achieved in the majority of patients with localized ES after treatment with multiagent chemotherapy and primary tumor control.1-4 Larger initial tumor volume is a well-defined adverse prognostic factor,5-8 the predictive power of which is over-ridden by the extent of histologically determined chemotherapy-induced necrosis assessed in resected primary tumors.4,8-11 These observations can be used to define subgroups of patients with localized ES in whom treatment interventions to improve survival may be examined.
The rationale for high-dose therapy (HDT) in ES was developed to exploit an alkylating agent dose-response relationship. Several single-arm studies have indicated high-dose chemotherapy efficacy using historical controls in newly diagnosed metastatic or recurrent ES, using various drugs or combinations with or without total-body irradiation.12-18 Two national nonrandomized studies evaluated the role of busulfan-melphalan (BuMel) in patients with poor histologic response and suggested its potential benefit compared with historical controls treated with conventional chemotherapy.19,20 Because these encouraging results were based on uncontrolled comparisons, a randomized comparison of high-dose chemotherapy against standard chemotherapy was therefore incorporated in two multinational controlled trials, Euro-E.W.I.N.G.99 and EWING-2008. In the selected high-risk population, termed R2Loc, the primary objective was to evaluate whether HDT using BuMel improved event-free survival (EFS) compared with standard chemotherapy (vincristine, dactinomycin, ifosfamide [VAI]).
METHODS
Study Design
The R2Loc trial was an international, randomized, superiority trial comparing consolidation treatment with BuMel or seven VAI courses in a two-parallel-group design. The R2Loc randomized trial was a component of the Euro-E.W.I.N.G.99 study (ClinicalTrials.gov identifier: NCT00020566) recruiting all patients with ES at diagnosis, enrolled by four cooperative groups: European Organization for Research and Treatment of Cancer (EORTC), Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH), French Society of Pediatric Oncology and French Sarcoma Group, and the UK Children’s Cancer and Leukaemia Group.1,21,22 From May 2010, patients from GPOH were recruited in the same R2Loc randomized trial conducted through the EWING-2008 study (ClinicalTrials.gov identifier: NCT00987636).
Patients
Eligibility requirements were age younger than 50 years; enrollment at diagnosis either in Euro-E.W.I.N.G.99 or EWING-2008 studies for newly diagnosed biopsy-proven ES; localized disease classified as high risk because of poor histologic response to induction chemotherapy (residual viable cells ≥ 10%) for patients undergoing surgery after induction chemotherapy alone or because of large tumor volume at diagnosis (≥ 200 mL) in unresected or initially resected tumors or resected tumors with preoperative irradiation, but patients with a small unresected tumor were also eligible in case of poor clinical response to induction chemotherapy (< 50% radiologic reduction in soft tissue disease component); and no medical contraindication to treatment. After amendment, because of busulfan-related radiosensitivity,23 patients expected to receive radiotherapy > 30 Gy to the spinal cord (Amendment July 2004) or > 45 Gy to large intestinal volume (Amendment November 2008) were no longer eligible. Written informed consent was obtained from all patients and/or their parents/guardians before enrollment.
Treatments
Induction chemotherapy consisted of six chemotherapy courses combining vincristine, ifosfamide, doxorubicin, and etoposide (VIDE).21,24 After one VAI course, allocated consolidation treatment was either seven VAI courses (VAI arm) or one course of high-dose BuMel chemotherapy with autologous stem-cell transplant (BuMel arm). Treatment schedule and chemotherapy details are in the Data Supplement.
Local therapy was tailored to patient and tumor characteristics, and included surgery with complete surgical removal wherever feasible, radiotherapy, or a combination of both (Data Supplement). Stem-cell harvest was undertaken according to local practice after VIDE course 2.
Randomization
Random assignment was performed after six VIDE courses plus one VAI consolidation course, after surgery and assessment of histologic response when applicable. Randomization was balanced and stratified according to cooperative group, sex, age (younger than 25 years), and local treatment (resection after chemotherapy alone with or without postoperative radiotherapy v initial surgery v resection after chemotherapy and radiotherapy v radiotherapy only). Centralized randomization software was used in all data centers, ensuring the concealment of the next patient allocation. The GPOH data center used permuted blocks of four. In the other data centers, randomization was also balanced by the treating center using dynamic allocation of treatment (minimization with a random factor set at 0.8).
End Points and Assessments
The primary end point was EFS, defined as the time from randomization to the date of the first failure assessed by the investigator (progression, relapse, second malignancy, or death, whatever the cause). Follow-up was planned every 3 months during the first 3 years, every 6 months during years 4 and 5, then yearly, regardless of treatment compliance. Overall survival (OS) since randomization was a secondary efficacy end point, considering all deaths, whatever the cause.
Central imaging review of tumor volume and response, and pathologic review were not undertaken. Compliance with treatment and toxicity were monitored. All chemotherapy doses were recorded, as well as the reasons for dose reduction or delay. Acute toxicity related to chemotherapy was assessed after each course, using a list of 22 selected items from the National Cancer Institute Common Toxicity Criteria version 2.0 and Bearman’s criteria for sinusoidal obstruction syndrome.25 Other adverse reactions were specified. A modified list was used to evaluate toxicity after radiotherapy, using Radiation Therapy Oncology Group classification for 17 toxicities.26 For each toxicity type, the maximum grade observed over the whole maintenance treatment was computed, including radiotherapy to the primary site. Grade 4 hematologic toxicities and grade 3 or higher of all nonhematologic toxicities were considered severe.
Statistical Analysis
The study was designed to ensure 80% power for a 40% reduction in the risk of an event in the BuMel arm compared with the VAI arm (expected 3-year EFS, 70% v 55%; hazard ratio [HR], 0.60) with a two-sided log-rank test α of .05. The initial target sample size was 328 patients (124 events).27 With support from the independent data monitoring committee, recruitment was stopped before reaching this target because of low accrual. This is the final analysis, on the basis of data as of July 2016.
Preplanned efficacy stopping rules were defined using the α spending function approach with O'Brien-Fleming boundaries.28,29 These analyses were only disclosed to the independent monitoring committee. Survival rates (EFS and OS) were estimated using the Kaplan-Meier method with Rothman’s 95% CIs. Median follow-up was estimated using the reverse Kaplan-Meier method. The HR of event (EFS) and the HR of death (OS) were estimated in Cox models. The point estimate of the HR of event, its CI, and the P value were corrected for the four previous interim analyses using the inverse normal method.31 The primary efficacy analysis was performed according to the patients’ randomly assigned treatments (ie, by intention-to-treat population). Post hoc sensitivity analyses were performed, (1) adjusting for age (in four categories: < 12, 12 to 18, 18 to 25, > 25 years) and (2) excluding patients with a major treatment modification (as-treated; Data Supplement). The heterogeneity of treatment effect (BuMel v VAI) on EFS according to stratification variables and tumor volume, tumor site, and histologic response (post hoc exploratory analysis) were evaluated in multivariable models, including interaction terms, and illustrated in a forest plot. Because the EFS is a composite end point, a competing risk approach was also used to estimate the effect of treatment on the risk of metastases using subdistribution HRs considered competing events: local progression/relapse without concomitant metastases, secondary malignancy, and death without prior metastases (post hoc analysis).32,33
Safety analyses were performed on the safety set, excluding patients who did not receive the assigned treatment (as-treated population). For each toxicity category, the relative risk of having experienced a severe toxicity in BuMel versus VAI was estimated.
Estimates are provided with 95% CIs. All tests are two sided. The analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
Study Oversight
Study protocols were approved by an independent ethics committee and the appropriate institutional review boards. The studies were conducted in accordance with the ethical principles of the Declaration of Helsinki and with Good Clinical Practice guidelines. The trial was designed jointly by the senior academic authors from the participating cooperative groups. The protocols are available online. Data were analyzed by the biostatisticians at Institut Gustave Roussy and reviewed by the statisticians’ board. The first draft of this article was written by M.C.L.D., O.O., and J.W. All authors contributed to subsequent drafts and made the decision to submit the manuscript for publication. None of the funders had a role in study design, collection, analysis, or interpretation of data.
RESULTS
Patients
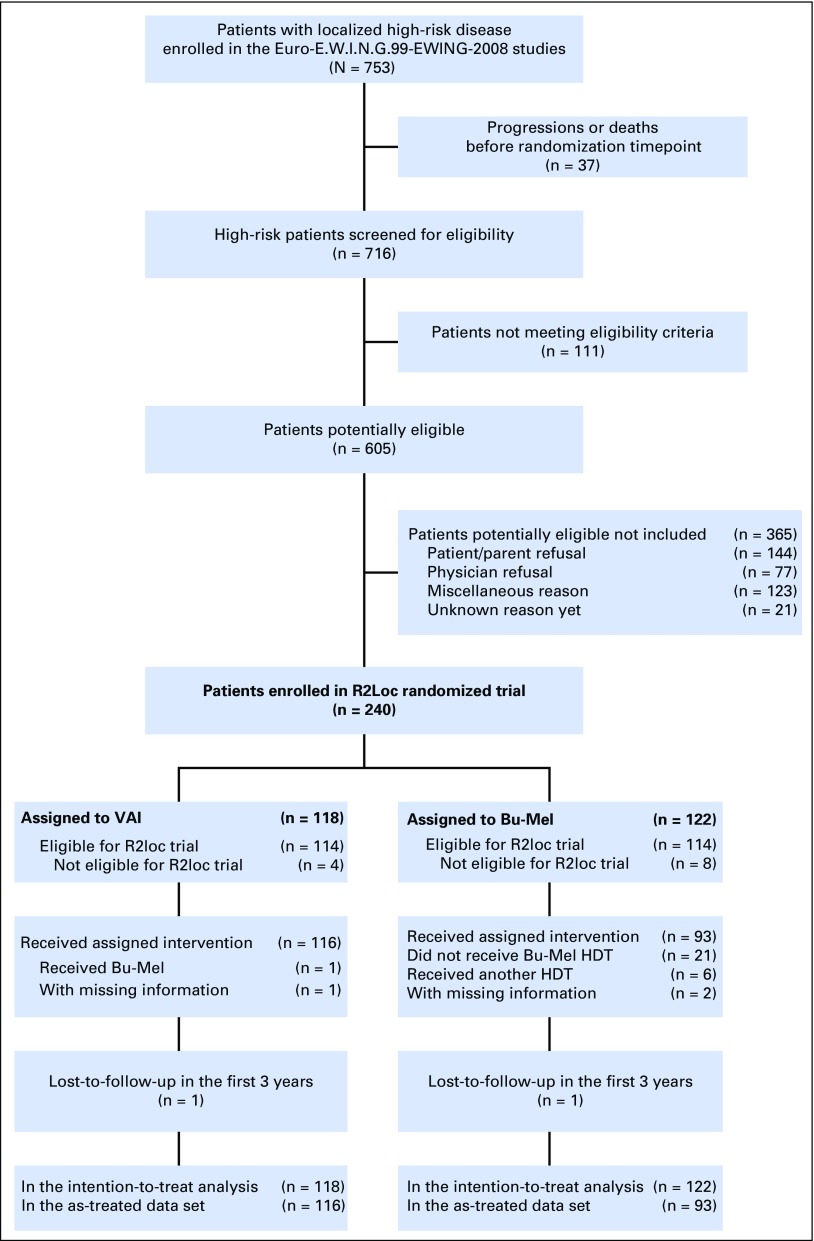
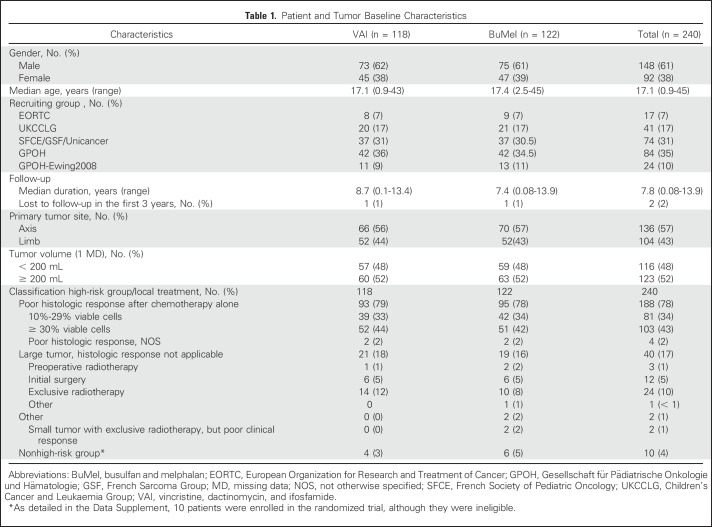
Between February 2000 and December 2015, 716 patients with a localized tumor fulfilling the predefined high-risk criteria, enrolled in 131 centers from 13 countries, were assessed for eligibility. Among them, 111 did not meet eligibility criteria (details in the legend of Fig 1). Of 605 eligible patients, 221 were not enrolled because of patient/parent (n = 144) or physician (n = 77) refusal, 123 for other reasons, and 21 for whom the reason was unknown; 240 patients were included in the randomized trial: 118 assigned to VAI and 122 assigned to BuMel (Fig 1). The median age was 17.1 years (range, 11 months to 44.7 years). The baseline characteristics were well balanced between the two groups (Table 1), although over the course of the study, some changes were notable, with a greater proportion with less favorable histologic response represented (Data Supplement). Overall, 188 of the 240 patients (78%) entered the trial because of poor histologic response after chemotherapy alone. Median follow-up was 7.8 years and was not significantly different between treatment groups (Data Supplement). Among the 240 enrolled patients, four patients in the VAI arm and eight patients in the BuMel arm were not eligible (Data Supplement).
Fig 1.
Trial profile. A total of 111 patients with disease classified as localized high-risk disease assessed for eligibility did not meet eligibility criteria: insufficient diagnosis criteria or diagnosis rejected (n = 14); persisting toxicity related to previous treatment and/or contraindication to planned treatment (n = 78), including contraindication to busulfan and melphalan (BuMel) because of planned radiotherapy to an axial site (n = 36); early radiotherapy (n = 13); psychological problems (n = 6); 123 patients meeting eligibility criteria were not enrolled because of other reasons. HDT, high dose therapy; R2Loc, selected high-risk population; VAI, vincristine, dactinomycin, and ifosfamide.
Table 1.
Patient and Tumor Baseline Characteristics
One patient allocated to VAI received BuMel on his request. In the BuMel arm, 21 patients did not receive any HDT because of patient refusal (n = 11), medical reason (n = 7), physician decision (n = 2), or failure to collect peripheral stem cells (n = 1); six patients received HDT other than BuMel because of physician decision in five patients and BuMel contraindication in one patient (Data Supplement). These 28 patients were excluded in the as-treated analysis.
When grouped by selected factors, the proportions of patients randomly assigned to those not randomly assigned varied (data not shown). There were differences by study year, study group, age, tumor volume, histologic response, and treatment with radiotherapy.
Efficacy
A total of 105 events were reported (VAI arm, 60; BuMel arm, 45): 13 local progressions or local relapses, 80 distant metastases (including lung metastases in 51 patients), five secondary malignancies, and seven deaths as first events (Data Supplement), including three treatment-related deaths.
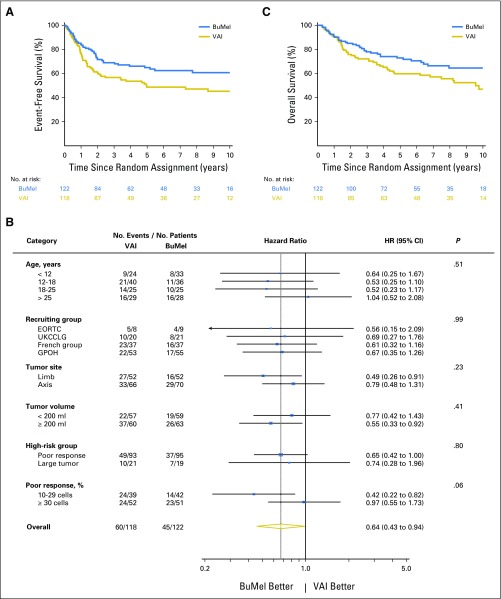
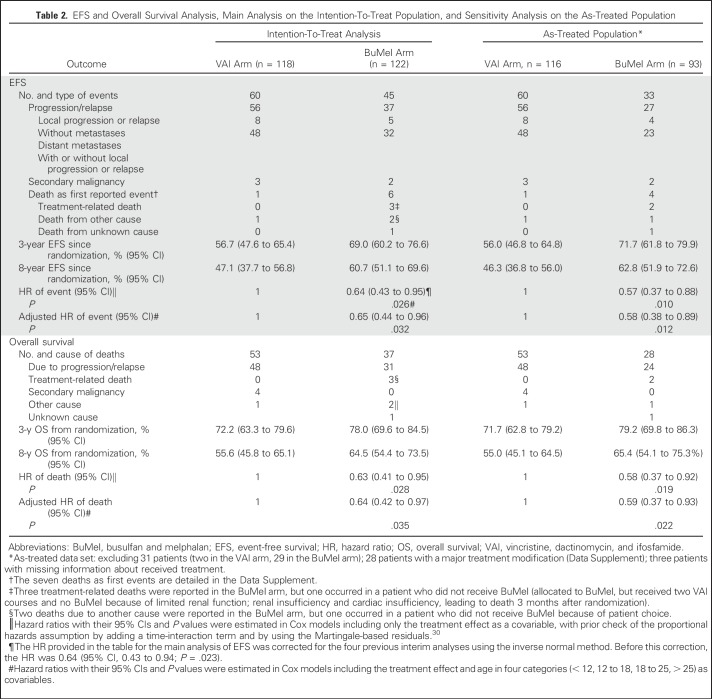
EFS for all 240 randomly assigned patients was 62.9% (95% CI, 56.6% to 68.9%) at 3 years and 54.0% (95% CI, 47.2% to 60.7%) at 8 years. The treatment effect of BuMel was estimated as an HR of 0.64 (95% CI, 0.43 to 0.95; P = .026), corrected for the four previous interim analyses. Three-year EFS rates for VAI and BuMel were 56.7% (95% CI, 47.6% to 65.4%) and 69.0% (95% CI, 60.2% to 76.6%), respectively, with this improvement being sustained at 8 years: 47.1% (95% CI, 37.7% to 56.8%) versus 60.7% (95% CI, 51.1% to 69.6%; Fig 2A; Table 2), respectively. The treatment effect estimate seems greater in the sensitivity analysis, when patients with protocol violations were excluded (Table 2). As illustrated in Figure 2B, no significant heterogeneity of the treatment effect was observed according to cooperative group, tumor site, tumor volume, or eligibility criteria for high-risk classification. However, patients with an intermediate poor response may benefit more than those with a very poor response (10% to 29% v ≥ 30% viable cells; interaction test, P = .06), as well as older patients (≤ 25 v > 25 years of age; P = .12). Improvement in EFS was mostly related to a reduction in the risk of metastases (subdistribution-HR, 0.58; 95% CI, 0.37 to 0.90; P = .02; Data Supplement).
Fig 2.
(A and B) Event-free survival (EFS) and (C) overall survival. (A) Kaplan-Meier estimates of EFS by treatment group, on the intention-to-treat (ITT) population. At the time of this analysis (cutoff date, Jan 1, 2016), 105 events were reported: 60 in the vincristine, dactinomycin, and ifosfamide (VAI) group and 45 in the busulfan and melphalan (BuMel) group. (B) Forest plot of EFS according to subgroups. The hazard ratio of events by subgroup were estimated in a Cox proportional hazards model, on the ITT population including all patients, except for the (1) assessment of treatment effect according to tumor volume: we excluded one patient with missing data; (2) assessment of treatment effect by the high-risk group definition (poor response v large tumor): we excluded 10 patients who were not classified as high risk (listed in the Data Supplement) and two patients who entered the trial for a small tumor with exclusive radiotherapy, but poor clinical response (too small category); and (3) assessment of treatment effect according to the grading of histologic response in patients with poor response: we focused the analysis on the 188 patients who entered the trial because of a poor histologic response and excluded four patients with poor response not otherwise specified. French group: French Society of Pediatric Oncology/French Sarcoma Group/Unicancer. Concerning the interaction between treatment effect and age, the P value was .12 when age was considered in two categories using the cutoff of 25 years as defined at the design stage to stratify the randomization. (C) Kaplan-Meier estimates for overall survival by treatment group on the ITT population. EORTC, European Organization for Research and Treatment of Cancer; GPOH, Gesellschaft für Pädiatrische Onkologie und Hämatologie; UKCCLG, UK Children’s Cancer and Leukaemia Group
Table 2.
EFS and Overall Survival Analysis, Main Analysis on the Intention-To-Treat Population, and Sensitivity Analysis on the As-Treated Population
Benefit from BuMel was also observed in OS. Ninety deaths were reported (VAI, 53; BuMel, 37), leading to an HR of 0.63 (95% CI, 0.41 to 0.95; P = .028). Three-year OS was 72.2% (95% CI, 63.3% to 79.6%) and 78.0% (95% CI, 69.6% to 84.5%) for VAI and BuMel, respectively (Fig 2C), and 75.1% (95% CI, 69.2% to 80.2%) overall. Eight-year OS was 55.6% (95% CI, 45.8% to 65.1%) versus 64.5% (95% CI, 54.4% to 73.5%).
Safety
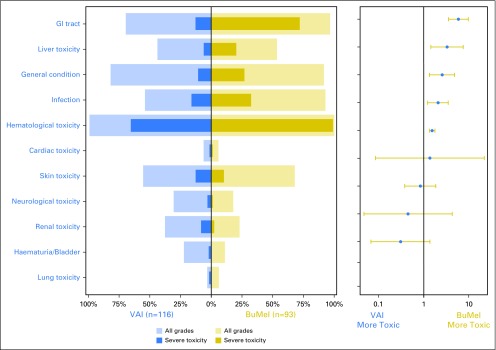
Significantly more BuMel patients experienced severe acute toxicities (Fig 3), but toxicity arose from a single high-dose course versus multiple VAI courses (Data Supplement). The effect of BuMel on the risk of severe acute toxicity did not differ between patients older than 25 years and younger patients (Data Supplement). Three treatment-related deaths were reported in the BuMel arm, although one was in a patient who did not receive BuMel because of renal dysfunction. One patient with a cervical spine primary site died as a result of myelopathy related to radiotherapy administered after BuMel, and one patient with a chest wall primary site died as a result of acute respiratory distress syndrome associated with pancytopenia 5 months after receiving BuMel.
Fig 3.
Adverse events. The panel on the left is a butterfly plot showing the proportion of patients experiencing an adverse event, whatever the grade (gold for busulfan and melphalan [BuMel] and blue for the vincristine, dactinomycin, and ifosfamide [VAI] arm), and a severe adverse event (dark gold for BuMel and dark blue for VAI arm) according to the randomization group. The panel on the right displays the relative risk of a severe adverse event in patients with BuMel relative to patients with VAI, with 95% CIs for a 2 × 2 table. The acute toxicity related to chemotherapy was assessed after each course, using a list of 22 selected items from the National Cancer Institute Common Toxicity Criteria version 2.0. A modified list of items was used to evaluate toxicity after radiotherapy, using Radiation Therapy Oncology Group classification for eight types of specific toxicities. A free text area was available to document other adverse reactions. The toxicity items were then pooled by category: bladder toxicity, cardiac toxicity, GI toxicity, general deterioration, hematologic toxicity, infection, liver toxicity, lung toxicity, neurologic toxicity (including mood alteration), renal toxicity, and skin toxicity. The respiratory tract toxicity (larynx, pharynx, salivary gland) reported after radiotherapy was pooled within the category GI toxicity because of small numbers and because they were usually associated. Details are provided in the Data Supplement. For each adverse event type, the analysis is based on the maximum grade observed over the whole maintenance treatment duration. A grade 4 hematologic toxicity and a grade ≥ 3 nonhematologic toxicity were classified as severe toxicities. The categories of adverse event are ordered by decreasing value of the relative risk of severe toxicity. This analysis was performed on the safety set (116 VAI patients and 93 BuMel patients), excluding patients who did not receive the treatment allocated by randomization, similar to the as-treated population. The number of chemotherapy courses followed by toxicity over the whole maintenance treatment duration is detailed in the Data Supplement.
DISCUSSION
In the subgroup of patients with localized ES specifically defined for this study, treatment with BuMel conferred a significant, sustained, and clinically meaningful improvement (European Society for Medical Oncology–Magnitude of Clinical Benefit Scale grade A) in EFS and OS.34 This outcome was despite the final analysis being conducted after recruitment of only 240 of a planned 328 patients and was sustained with prolonged follow-up. This finding is particularly significant, given the observation that cure rates in this important cancer of children and young people have improved modestly at best over recent decades and that the expanding knowledge of the biology of ES has, to date, not resulted in the introduction of new agents into standard care.35
Several factors deserve more detailed consideration. First, this was a complex but pragmatic trial performed by an experienced multinational cooperative group that encompassed a large proportion of major pediatric and sarcoma-treating centers in Europe. Despite this, recruitment fell short of the recruitment rates proposed at study initiation. The study was closed before the planned event/patient target was reached because of some investigators’ concerns that rates of participation in the study were too low to be sustainable. Of patients within the study registration cohort, one third of those fulfilling the definition of localized high-risk disease were ultimately randomly assigned, with evidence of low acceptability of the study question to both clinicians and patients. We acknowledge that this limits the external validity of our findings. However the description of a screened population and the estimate of randomization rate are missing from other studies, and sets this intervention into a clinically meaningful context.
The use of histologic response as the main selection criterion identifies a population of patients with some degree of initial drug resistance. In R2Loc, this is supplemented by other high-risk patients, accounting for 20% of randomly assigned patients, for whom histologic response was not available, those with tumors > 200 mL who had undergone primary surgery or had early radiotherapy or exclusive radiotherapy. This subset of patients may include patients with unidentifiable good response to induction chemotherapy. Overall, the BuMel effect seemed consistent across main strata (cooperative group, tumor site, tumor volume, or eligibility criteria for high-risk classification). Within the poor histologic response spectrum, it is possible that BuMel may have less benefit in those with poor response, although, given that this was an observation from a post hoc exploratory analysis, it is insufficient evidence to be used alone to exclude patients from treatment with BuMel if all other criteria for possible benefit are fulfilled. It is also possible that there may be less benefit for older patients, although this observation may be by chance, and the test for interaction was not significant.
BuMel was chosen as HDT based on international transplant registry data16,18 and previous work by the French Society of Pediatric Oncology.20 Concerns about interaction with radiotherapy, including fatal toxicity, led to a protocol amendment after which patients requiring large-volume or high-dose radiotherapy to critical organs were excluded from randomization.23 There is little evidence to define thresholds for concurrent use of radiotherapy and busulfan. Others have reported its use after whole-lung irradiation without significant toxicity.36 There are no data to support equivalent efficacy with treosulfan, which, although closely related to busulfan, is less radiosensitizing.
We chose to compare BuMel against seven cycles of maintenance chemotherapy only. Given that the effect of HDT in this series is seen in a reduction in the incidence of subsequent metastases, which were often pulmonary, we cannot exclude that whole-lung irradiation would have similar benefits.37,38
Extrapolation of benefit to other settings in ES is to be cautioned against. A similar effect of BuMel on survival in patients presenting with pulmonary metastases was not observed when bilateral pulmonary irradiation was added to conventional chemotherapy.37 Uncontrolled studies of HDT in patients with recurrent ES describe some patients with far superior survival than would be expected from conventionally dosed chemotherapy alone, but no randomized studies have been conducted.39,40 It is possible that different induction regimens may select different groups of patients for which the benefits of BuMel may also differ. Whether results similar to those reported here are achievable with dose-compressed vincristine, doxorubicin, cyclophosphamide/ifosfamide and etoposide is an open question.2
As expected, more patients experienced severe toxicity in the BuMel arm than in the VAI arm, mostly hematologic, digestive, and hepatic. However, these types of toxicity were transient and occurred only once after BuMel compared with repetitive hematologic toxicity with VAI courses. Two patients died as a result of BuMel toxicity, including one as a result of interaction with radiotherapy. Recommendations for the use of radiotherapy with BuMel as adopted during this study should limit this risk. Long-term toxicity from this and similar cohorts has yet to be reported.
These results are a further illustration of the value of international collaboration in rare cancers such as ES. Although the study was challenging to undertake, patients and their clinicians can now benefit from randomized evidence of the value of this treatment. Although the benefit was shown for a relatively small subgroup, the reliable demonstration of EFS and OS improvement indicates that BuMel may be considered as a standard of care for patients with localized ES fulfilling the definition of high-risk disease used in this trial and no contraindication to BuMel.
ACKNOWLEDGMENT
We are indebted to all the patients and parents who agreed to participate in this study; Carolyn Douglas, Sue Ablett, Paul Donachie, Children’s Cancer and Leukaemia Group Data Centre, Leicester, United Kingdom; Davina Scott, Jennifer Anderton, Nicola Fenwick, Keith Wheatley, Veronica Moroz, Cancer Research UK Clinical Trials Unit, University of Birmingham, United Kingdom; Susanne Ahrens, Martina Blankschän, Gabriele Braun-Munzinger, Susanne Jabar, Andreas Ranft, Gesellschaft für Pädiatrische Onkologie und Hämatologie data center, Münster, Germany; Eva Sorz, Austrian-Gesellschaft für Pädiatrische Onkologie und Hämatologie data center, Vienna, Austria; Anthony Mangin, Jean-François Leforestier, Yulia Belikova, Noel Ny Tovo, Zakia Idir, Fatima Bizeul, Julien Marandet, Muriel Wartelle, Nathalie Cozic, Institut Gustave Roussy, Villejuif; Marta Jimenez, Céline Mahier Aït Oukhatar, Naïma Bonnet, Jessy Delaye, Jean Genève, Unicancer, Paris, France; Christine Olungu, Anne Kirkpatrick, Saskia Litiere, Sandrine Marreaud, European Organization for Research and Treatment of Cancer data center, Brussels, Belgium, for data management and study coordination assistance; Robert Souhami, Paul Meyers, and Abdel Babiker for participating in the Independent Data Monitoring Committee; and all investigators who participated in the trial.
Appendix
All investigators who participated in the trial:
From Austria: Ch. Urban, University Children's Hospital, Graz; B. Meister and F.M. Fink, University Children's Hospital, Innsbruck; R. Kerbl, Leoben Clinical Centre, Children's Hospital Leoben; O. Stollinger, Children’s Hospital Barmherzige Schwestern, Linz; K. Schmitt and G. Ebetsberger, Linz Clinical Centre, Children's Hospital, Linz; N. Jones, University Hospital, Salzburg, R. Ladenstein and H. Gadner, St Anna Children's Hospital, Wien; B. Ausserer, Children`s Hospital, Dornbirn;
From Belgium: P. Maes, AZM Children’s Cancer Centre, Antwerpen; B. Brichard, University Children's Hospital, Brussels; F. Mazzeo, University Hospital, Clinical Oncology, Brussels; T. Gil, Hôpitaux universitaires Bordet-Erasme, Brussels; C. Dhooge, Universitair Ziekenhuis, Gent; J.B. Vermorken, Universitair Ziekenhuis, Antwerpen; A. Klein, Hôpital Universitaire des Enfants Reine Fabiola, Brussels;
From Czech Republic: J. Kruseova, University Hospital Motol, Prague;
From Denmark: A. Krarup-Hansen, Herlev Hospital-University, Copenhagen; O. Nielsen, Aarhus University Hospital, Aarhus;
From Ireland: M. Capra, Our Lady's Children's Hospital, Dublin;
From Finland: J. Kanerva, Pediatric Oncology, Helsinki University Children’s Hospital, Helsinki, Finland;
From France: C. Devoldere, University Hospital, Amiens; I. Pellier, University Hospital, Angers; O. Collard, Institut de Cancerologie de l’Ouest, Saint Priest en Jarez; P. Soulie, Centre Paul Papin, Angers; E. Plouvier, University Hospital, Besançon; C. Vérité, University Hospital, Bordeaux; B. Bui N'guyen, Institut Bergonié, Bordeaux; G. Dabouis, University Hospital, Nantes; F. Aubier, Hôpital d’enfants Margency, Margency; O. Minckes, University Hospital, Caen; C. Delcambre, Centre François Baclesse, Caen; J.O. Bay, Centre Jean Perrin, Clermont-Ferrand; J. Kanold, University Hospital, Clermont-Ferrand; E. Colomb, University Hospital, Dijon; N. Isambert, Centre Jean François Leclerc, Dijon; D. Plantaz, University Hospital, Grenoble; A.S Defachelles, Centre Oscar Lambret, Lille; C. Piguet, University Hospital, Limoges; P. Marec-Bérard, Centre Léon Bérard, Lyon; J.Y. Blay, Centre Léon-Bérard, Lyon; J.C. Gentet, University Hospital, Marseille; F. Duffaud, University Hospital, Marseille; F. Bertucci, Institut Paoli Calmettes, Marseille; J.P Lotz, Hôpital Tenon, Paris; L. Saumet, University Hospital, Montpellier; C. Schmitt, University Hospital, Nancy; M. Rios, Centre Alexis Vautrin, D. Cupissol, Institut du Cancer de Montpellier, Val d’Aurelle, Montpellier; Nancy; N. Corradini, University Hospital, Nantes; F. Rolland, Centre René Gauducheau, Nantes; A. Deville, University Hospital, Nice; A. Thyss, Centre Antoine Lacassagne, Nice; J. Michon, Institut Curie, Paris; M.D. Tabone, Trousseau University Hospital, Paris; V. Laurence, Institut Curie, Paris; F. Millot, University Hospital, Poitiers; S. Gorde GrosJean, University Hospital, Reims; S. Taque, University Hospital, Rennes; J.P. Vannier, University Hospital, Rouen; C. Guillemet, Centre Henri Becquerel, Rouen; L. Chauvenet, Hotel Dieu, Paris; E. Brain, Centre René Huguenin, St Cloud; C. Berger, University Hospital, St Etienne; P. Lutz, University Hospital, Strasbourg; M.P. Castex, University Hospital, Toulouse; H. Roche, Institut Claudius Regaud, Toulouse; O. Lejars, University Hospital, Tours; C. Linassier, University Hospital, Tours; O. Oberlin, Institut Gustave Roussy, Villejuif; N. Gaspar, Institut Gustave Roussy, Villejuif; A. Le Cesne, Institut Gustave Roussy, Villejuif;
From Germany (EORTC): S. Bauer, P. Ebeling, and M. Flasshove, Essen University Hospital, Essen; J.T. Hartmann, Eberhard Karls University, Tuebingen; P. Reichardt, Charité Universitaetsmedizin, Campus Berlin- Bush, Berlin
From Germany (GPOH): R. Mertens, U. Kontny, University Children's Hospital, Aachen; R. Osieka, University Hospital, Medical Oncology, Aachen; A. Gnekow, Augsburg Clinical Centre, Children’s Hospital, Augsburg; G. Schlimok, Medical Oncology, Augsburg Clinical Centre, Augsburg; G. Henze, P. Hundsdörfer, Charité Virchow Clinical Centre, Children's Hospital, Berlin; P. Thuss-Patience, A. Kunitz, Charité Virchow Clinical Centre, Berlin; L. Wickmann, L. Schweigerer, Berlin-Buch Children’s Hospital, Berlin; P. Reichardt, Medical Oncology, Berlin-Buch Clinical Centre, Berlin; K. Possinger, Medical Oncology, Charité Central Campus, Berlin; J. Potenberg, Ev. Waldkrankenhaus, Berlin; P. Reichardt, Charité Universitaetsmedizin, Campus Berlin-Buch, Berlin; N. Jorch, Ev. Children’s Hospital Bielefeld; U. Krümpelmann, Medical Oncology Ev. Hospital, Bielefeld; H. Weh, Medical Oncology, Franziskus Hospital, Bielefeld; J. Baier, Medical Oncology, St Josef’s Hospital, Bochum; W. Schmiegel, Medical Oncology, Ruhr-University Hospital, Bochum; U. Bode, D. Dilloo, University Children's Hospital, Bonn; I.G.M. Schmidt-Wolf, V. Janzen, Medical Oncology I, University Hospital Bonn; J. Nolting, University Hospital, Medical Oncology III, Bonn; W. Eberl, Children’s Hospital, Braunschweig; B. Wörmann, Medical Oncology, City Hospital, Braunschweig; W. Hoffmann, Radiology and Radiotherapy, City Hospital, Braunschweig; Th. Wolff, J. Kullmer, Ev. Diakonie Hospital, Bremen; A. Pekrun, Bremen Mitte Children’s Hospital, Bremen; A. Hofmann, Children’s Hospital, Chemnitz; G. Geißler, Internal Medicine III, Chemnitz; E. Hohlfeld; Carl-Thiem-Hospital, Cottbus; W. Andler, T. Wiesel, Children's Hospital, Datteln; D. Schneider, B. Bernbeck, Children's Hospital, Dortmund; I. Lauterbach, M. Suttorp, University Children’s Hospital, Dresden; S. Richter, G. Ehninger, University Hospital Dresden; P. Zickler, Children’s Hospital, Duisburg; U. Göbel, A. Borkhardt, University Children's Hospital, Düsseldorf; J.-N. Machatschek, Medical Oncology, University Hospital, Düsseldorf; A. Lemmer, A. Sauerbrey, Children's Hospital, Erfurt; W. Holter, M. Metzler, University Children's Hospital, Erlangen; N. Meidenbauer, University Hospital, Erlangen; S. Seeber, S. Bauer, P. Ebeling, and M. Flasshove, University Hospital, Essen; A. Eggert, G. Fleischhack, University Children's Hospital, Essen; T. Klingebiel, University Children's Hospital Frankfurt, M. Ahrens University Hospital Frankfurt; C. Niemeyer, J. Rößler, University Children's Hospital, Freiburg; J. Heinz, Medical Oncology, University Hospital, Freiburg; A. Reiter, W. Wössmann University Children's Hospital, Giessen; M. Rummel, W. Blau, Medical Oncology, University Hospital, Giessen; V. Runde, Goch Clinical Centre, Goch; M. Lakomek, C. Kramm University Children's Hospital, Göttingen; L. Trümper, D. Hertramph, Medical Oncology, University Hospital, Göttingen; J. Beck, H. Lode University Children's Hospital, Greifswald; G. Dölken, Medical Oncology, University Hospital, Greifswald; H.-W. Lindemann, Hagen Clinical Centre, Hagen; G. Günther, D. Körholz, T. Bernig, University Children's Hospital, Halle; H.-J. Schmoll, Medical Oncology, University Hospital, Halle; R. Schneppenheim, W.A. Hassenpflug, UKE University Children's Hospital, Hamburg; D.K. Hossfeld, C. Bokemeyer, Medical Oncology, UKE University Hospital, Hamburg; W. Alberti, Radiology and Radiotherapy, UKE University Hospital, Hamburg; H. Keles, Medical Oncology, Asklepios Hospital Altona, Hamburg; N. Schmitz, Haematology, Asklepios Hospital St Georg, Hamburg; N. Brüllke, Medical Oncology, Asklepios Hospital Barmbek, Hamburg; H. Schmidt, Internal Medicine, Regional Hospital, Hameln; C. Dürk, Medical Oncology, St Marien Hospital, Hamm; K. Welte, C. Klein, B. Maecker-Kohlhoff, Pediatric Oncology, Hannover Medical School, Hannover; Ch. Reuter, Medical Oncology, Hannover Medical School, Hannover; H. Kirchner, Medical Oncology III, Siloah Hospital, Hannover; A. Kulozik, W. Behnisch, University Children's Hospital, Heidelberg; G. Egerer, Medical Oncology, University Hospital, Heidelberg; Ewerbeck, Orthopedic, University Hospital, Heidelberg; M. Thomas, Medical Oncology, Thorax Hospital, Heidelberg; J. Cyran, Clinical Centre, Heilbronn; Kachel, Pediatrics, SLK Hospital, Heilbronn; U. Martens, Pediatric, Haematology, SLK Hospital Heilbronn; Ch. Tautz, General Hospital, Herdecke; D. Strumberg, St Marien Hospital, Herne; W. Freier, Oncology Practice, Hildesheim; U. Kaiser, Medical Oncology II, St Bernward, Hildesheim; N. Graf, University Children's Hospital, Homburg; M. Pfreundschuh, Medical Oncology, University Hospital, Homburg; F. Zintel, B. Gruhn, University Children's Hospital, Jena; E. Eigendorff, University Hospital, Jena; H. Link, Medical Oncology, Westpfalz Hospital, Kaiserslautern; R. Germann, A. Leipold, Children's Hospital, Karlsruhe; Th. Fischer, M. Bentz, Medical Oncology, Karlsruhe Clinical Centre, Karlsruhe; J. Mezges, Medical Oncology, St Vincentius Hospital, Karlsruhe; H. Wehinger, M. Nathrath, Children's Hospital, Kassel; M. Wolf, Medical Oncology, Kassel Hospital, Kassel; H. Meye, Radiotherapy, Kassel Hospital, Kassel; Prümmer, Internal Medicine III, Kempten Ober-Allgäu Hospital, Kempten; M. Schrappe, A. Claviez, University Children's Hospital, Kiel; M. Lamprecht, Medical Oncology II, University Hospital, Kiel; H. Nolte, Kemperhof Clinical Centre, Koblenz; M. Rister, M. Jakob, Kemperhof Koblenz, Koblenz; F. Berthold, T. Simon, University Children's Hospital, Cologne; V. Diehl, J. Wolf, Medical Oncology, University Hospital, Cologne; W. Sternschulte, A. Prokop, Children's Hospital, Cologne; E. Stoelben, Pulmonary Hospital, Cologne; S. Voelpel, T. Imschweiler, Children’s Hospital, Krefeld; T. Frieling, Medical Oncology, Krefeld Clinical Centre, Krefeld; J. Moessner, D. Niederwiese, Medical Oncology, University Hospital, Leipzig; U. Bierbach, H. Christiansen, University Children’s Hospital, Leipzig; J. Bennek, Pediatric Surgery, University Hospital, Leipzig; L. Mantovani, Medical Oncology, St Georg Hospital, Leipzig; D. Selle, St Annastift Children's Hospital, Ludwigshafen; P. Bucsky, T. Langer, University Children’s Hospital, Lübeck; H. Bartels, Medical Oncology, Clinical Centre Lübeck South, Lübeck; Th. Wagner, Medical Oncology I, University Hospital, Lübeck; G. Heil, Medical Oncology, Reginal Hospital, Lüdenscheid; M. Mohren, Th. Fischer, E. Schalk, Medical Oncology, University Hospital, Magdeburg; U. Kluba, P. Vorwerk, University Children's Hospital, Magdeburg; P. Gutjahr, M. Dittrich, J. Faber, University Children's Hospital, Mainz; H.-J. Beck, Medical Oncology III, University Hospital, Mainz; S. Reiter, B. Kasper, Medical Oncology III, University Hospital, Mannheim; M. Dürken, University Children’s Hospital, Mannheim; A. Neubauer, J. Beyer, Medical Oncology, University Hospital, Marburg; B. Schütz, H. Christiansen, University Children's Hospital, Marburg; B. Erdlenbruch, Pediatrics Johannes Wesling Hospital, Minden; M. Griesshammer, Medical Oncology, Johannes Wesling Hospital, Minden; Reis, Medical Oncology, St,. Franziskus Hospital, Mönchengladbach; S. Burdach, A. Wawer, TU Technical University (TU) Clinical Centre, Children's Hospital, München; I. Schmid, Ludwig-Maximilians-University (LMU) Children's Hospital, Munich; C. Meyer zum Büschenfelde, C. Peschel, S. Lorenzen, Medical Oncology, TU Clinical Centre, Munich; B. Emmerich, M. Schlemmer, F. Oduncu, R. Issels, Medical Oncology, LMU Clinical Centre, Munich; H. Jürgens, University Children's Hospital, Münster; W. Berdel, A. Kerkhoff, Medical Oncology, University Hospital, Münster; H. Held, Medical Oncology, Clinical Centre, Neumünster; G. Hofmann-Wackersreuther, W. Scheurlen, M. Augustin, Medical Oncology, Clinical Centre, Nürnberg; H. Müller, Children’s Hospital, Oldenburg; C.-H. Köhne, D. Krämer, Medical Oncology, Oldenburg Hospital, Oldenburg; A. Rickers, Pediatrics, Marienhospital, Osnabrück; T. Wolff, Medical Oncology, St Josef Hospital, Paderborn; U. Loss, Internal Medicine, Knappschaftshorpital, Recklinghausen; O. Peters, St Hedwig Children's Hospital, Regensburg; R. Andreesen, S. Krause, M. Grube, Medical Oncology, University Hospital, Regensburg; Kreuser, Medical Oncology, Barmherzige Brüder Hospital, Regensburg; S. Corbacioglu, University Children’s Hospital, Regensburg; C.-F. Classen, University Children’s Hospital, Rostock; M. Freund, Medical Oncology, University Hospital Rostock; J. Potratz, F. Heits, Medical Oncology, Rotenburg Clinical Centre, Rotenburg; R. Geib-König, Winterberg Clinical Centre, Children's Hospital, Saarbrücken; J. Weis, Radiology and Radiotherapy, Saarbrcken Hospital, Saarbrücken; M. Kasbohm, Helios Clinical Centre, Children’s Hospital, Schwerin; D. Hähling, Medical Oncology, Helios Hospital, Schwerin; D. Bürger, R. Burghard, Deutsches Rotes Kreuz Children's Hospital, Siegen; R. Dickerhoff, H. Reinhard, Asklepios Clinical Centre, Children's Hospital, St Augustin; S. Bielack, K. Apel, S. Simon-Klingenstein, Stuttgart Cancer Center, Pediatrics 5 (Oncology, Hematology, Immunology), Klinikum Stuttgart – Olgahospital, Stuttgart; H.G. Mergenthaler, G. Illerhaus, Stuttgart Cancer Center, Medical Oncology, Klinikum Stuttgart - Katharinenhospital, Stuttgart; C. Denzlinger, Internal Medicine III, Marienhospital, Stuttgart; I. Feddersen, S. Weis, Rauh Mutterhaus der Borromäerinnen, Children's Hospital, Trier; M.R. Clemens, Hospital “Barmherzige Brüder”, Trier; Waladkhani, Medical Oncology I, Mutterhaus der Borromäerinnen, Trier; H. Kirchen, Medical Oncology, Barmherzige Brüder Hospital, Trier; R. Handgretinger, M. Ebinger, University Children's Hospital, Tübingen; Brossart, J.T. Hartmann, H.-G. Kopp, Eberhard Karls Universitaet, Tuebingen; ; K.-M. Debatin, University Children's Hospital, Ulm; Döhner, R. Mayer-Steinacker, University Cancer Centre, Ulm; M. Müller, A. Schoengen, Internal Medicine I, Bundeswehrkrankenhaus, Ulm; St Brettner, Medical Oncology, Regional Hospital, Waldbröhl; N. Frickhofen, Internal Medicine III, Dr.-Horst-Schmidt-Hospital, Wiebaden; Dohrn, Pediatrics, HELIOS Hospital, Wuppertal; M. Sandmann, Medical Oncology, St Antonius Hospital, Wuppertal; P. Schlegel, University Children's Hospital, Würzburg;
From Hong Kong: V. Lee and K-W Chik, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong;
From Hungary: P. Hauser, Semmelweis University Children’s Hospital, Budapest, Hungary;
From the Netherlands: H. van den Berg, AMC Emma Children's Hospital/Academic Medical Centre, Amsterdam; S. Rodenhuis, The Netherlands Cancer Institute-Antoni Van Leeuwenhoekziekenhuis, Amsterdam; A.J. Gelderblom, J. Anninga, Leiden University Medical Centre, Cancer Centre, Leiden; P. Hoogerbrugge and JPM. Bökkerink, UMC St Radboud, Nijmegen; R. Pieters, Sophia Children’s Hospital - Erasmus Medical Centre, Rotterdam;
From New Zealand: R, Corbett, Christchurch Hospital, Christchurch
From Sweden: G. Österlundh, Queen Silvia’s Children’s Hospital, Sahlgrenska Hospital, Gothenburg; M. Behrendtz and B.-M. Holmqvist, Department of pediatrics, Linköping University Hospital, Linköping; L. Hjorth, Children’s Hospital, Skåne University Hospital, Lund; C. Petersen and Å. Jakobson, Astrid Lindgren’s Children’s Hospital, Karolinska Hospital, Stockholm; U. Hjalmars, Department of pediatrics, Umeå University Hospital, Umeå; G. Ljungman, Academic Children’s Hospital, Academic Hospital, Uppsala;
From Switzerland: R. Angst, Kantonsspital Aarau; T. Kühne and M. Paulussen, University Children's Hospital, Basel; S. Leyvraz, Lausanne Cancer Centre, Lausanne; J. Rischewski, Luzerner Kantonsspital, Luzern; A. Feldges and J. Greiner, Ostschweizer Kinderspital, St Gallen, U. Hess, Clinical Centre, Medical Oncology, S. Gallen; G.U. Exner, Orthopedic Hospital Balgrist, Zürich; F. Niggli, University Children’s Hospital, Zürich; A. Knuth, University Hospital, Medical Oncology, Zürich;
From United Kingdom: D. King and H. Bishop, Royal Aberdeen Children's Hospital, Aberdeen; A. McCarthy, Royal Belfast Hospital for Sick Children, Belfast; P. Henry, Belfast City Hospital, Belfast; B. Morland, Birmingham Children's Hospital, Birmingham; H. Rees, University Hospitals Bristol NHS Foundation Trust, Bristol; J. Nicholson, Addenbrooke’s Hospital, Cambridge; H. Traunecker, Children's Hospital for Wales, Cardiff; H. Wallace, Royal Hospital for Sick Children, Edinburgh; M. Ronghe and E. Simpson, Royal Hospital for Sick Children, Glasgow; F. Cowie and J. White, NHS Greater Glasgow and Clyde-Western Infirmary, Glasgow; S. Picton and I. Lewis, Leeds General Infirmary, Leeds; M. Leahy, D Stark and PJ. Selby, St James University Hospital, Leeds; D. Heney and E. Ross, Leicester Royal Infirmary, Leicester; B. Pizer and H. McDowell, Royal Liverpool Children's Hospital NHS Trust, Alder Hey, Liverpool; A. Michalski, Great Ormond Street Hospital for Children, London; J. Whelan, University College Hospital NHS Trust, London; J. Chisholm and K. Pritchard-Jones, Royal Marsden NHS Trust, London; I. Judson, Royal Marsden Hospital, London; B. Brennan, Royal Manchester Children's Hospital and The Christie NHS Foundation Trust, Manchester; J. Hale and Q. Campbell-Hewson, Royal Victoria Infirmary, Newcastle upon Tyne; M. Verrill, Newcastle Upon Tyne, Newcastle; D. Walker, Queens Medical Centre, Nottingham University Hospitals NHS Trust, Nottingham; M. Sokal, Nottingham University Hospital NHS Trust, Nottingham; K. Wheeler, Oxford Radcliffe Hospitals, Oxford; V. Lee and M. Gerrard, Sheffield Children's Hospital, Sheffield; P. Woll, P. Lorigan and M. Robinson, Weston Park Hospital, Sheffield; J. Kohler and R. Ramanujachar, Southampton General Hospital, Southampton.
Footnotes
Euro-E.W.I.N.G.99 is an academic clinical trial funded through multiple national and international government agencies and cancer charities: FP7-EURO EWING Consortium; Germany: Deutsche Krebshilfe (Grants No. 70-2551-Jue3 and 108128), and Bundesministerium für Bildung und Forschung (Grants No. BMBF 01GM0869 and BMBF/Era-Net 01KT1310); France: Association Enfants et Santé, Société Française de Lutte Contre les Cancers et les Leucémies de l’Enfant et de l’Adolescent, Unicancer; United Kingdom: Cancer Research UK (Grant No. CRUK/02/014), National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Presented at the ASCO Annual Meeting, Chicago, IL, June 3 to 7, 2016; and the Connective Tissue Oncology Society Annual Meeting, Lisbon, Portugal, November 9 to 12, 2006.
Clinical trial information: NCT00987636
See accompanying Editorial on page 3072
AUTHOR CONTRIBUTIONS
Conception and design: Jeremy Whelan, Marie-Cecile Le Deley, Douglas S. Hawkins, Stefan Burdach, Ruth Ladenstein, Jean Michon, Michael Paulussen, Andreas Ranft, Hendrik van den Berg, Ian Lewis, Alan Craft, Heribert Juergens
Administrative support: Marta Jimenez
Provision of study materials or patients: Uta Dirksen, Gwénaël Le Teuff, Stefan Bielack, Jean-Yves Blay, Angelika Eggert, Hans Gelderblom, Jean-Claude Gentet, Wolfgang Hartmann, Wolf-Achim Hassenpflug, Lars Hjorth, Marta Jimenez, Thomas Klingebiel, Valerie Laurence, Perrine Marec-Berard, Jean Michon, Michael Paulussen, Andreas Ranft, Hendrik van den Berg, Ian Lewis, Alan Craft
Data analysis and interpretation: Jeremy Whelan, Marie-Cecile Le Deley, Uta Dirksen, Gwénaël Le Teuff, Bernadette Brennan, Nathalie Gaspar, Douglas S. Hawkins, Susanne Amler, Sebastian Bauer, Stefan Bielack, Jean-Yves Blay, Stefan Burdach, Marie-Pierre Castex, Dagmar Dilloo, Hans Gelderblom, Jean-Claude Gentet, Wolfgang Hartmann, Wolf-Achim Hassenpflug, Lars Hjorth, Thomas Klingebiel, Udo Kontny, Jarmila Kruseova, Ruth Ladenstein, Valerie Laurence, Cyril Lervat, Perrine Marec-Berard, Sandrine Marreaud, Bruce Morland, Michael Paulussen, Andreas Ranft, Peter Reichardt, Hendrik van den Berg, Keith Wheatley, Ian Judson, Ian Lewis, Alan Craft, Heribert Juergens, Odile Oberlin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
High-Dose Chemotherapy and Blood Autologous Stem-Cell Rescue Compared With Standard Chemotherapy in Localized High-Risk Ewing Sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jeremy Whelan
No relationship to disclose
Marie-Cecile Le Deley
No relationship to disclose
Uta Dirksen
Consulting or Advisory Role: Eli Lilly (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Gwénaël Le Teuff
No relationship to disclose
Bernadette Brennan
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Nathalie Gaspar
Travel, Accommodations, Expenses: EISAI
Douglas S. Hawkins
Travel, Accommodations, Expenses: Loxo, Bayer, Bristol-Myers Squibb, Celgene
Susanne Amler
No relationship to disclose
Sebastian Bauer
Honoraria: Novartis, Pfizer, Bayer, Pharmamar, GlaxoSmithKline
Consulting or Advisory Role: Blueprint Medicines, Bayer, Eli Lilly, Deciphera, Nanobiotix
Research Funding: Blueprint Medicines, Novartis, Incyte (Inst)
Travel, Accommodations, Expenses: Pharmamar
Stefan Bielack
Consulting or Advisory Role: Clinigen Group, Bayer, Eli Lilly, Pfizer, Novartis, Isofol Medical, Sensorion
Research Funding: Janssen-Cilag (Inst), Amgen (Inst), EISAI (Inst), Loxo
Jean-Yves Blay
No relationship to disclose
Stefan Burdach
Stock or Other Ownership: PDLI
Marie-Pierre Castex
No relationship to disclose
Dagmar Dilloo
Stock or Other Ownership: Pfizer, Amgen (I), Johnson & Johnson, Merck (I)
Angelika Eggert
No relationship to disclose
Hans Gelderblom
No relationship to disclose
Jean-Claude Gentet
No relationship to disclose
Wolfgang Hartmann
Travel, Accommodations, Expenses: Menarini
Wolf-Achim Hassenpflug
Consulting or Advisory Role: Shire, CSL Behring, SOBI
Travel, Accommodations, Expenses: Shire, Novo Nordisk
Lars Hjorth
Stock or Other Ownership: Bioinvent
Marta Jimenez
No relationship to disclose
Thomas Klingebiel
No relationship to disclose
Udo Kontny
Consulting or Advisory Role: Eisai
Jarmila Kruseova
No relationship to disclose
Ruth Ladenstein
Honoraria: Apeiron Biologics, Boehringer Ingelheim, EUSA Pharma
Consulting or Advisory Role: Apeiron Biologics, Boehringer Ingelheim, EUSA Pharma (Inst)
Research Funding; Apeiron Biologics (Inst), EUSA Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Apeiron Biologics (Inst)
Expert Testimony: Apeiron Biologics, EUSA Pharma
Travel, Accommodations, Expenses: Apeiron Biologics, EUSA Pharma
Valerie Laurence
No relationship to disclose
Cyril Lervat
Travel, Accommodations, Expenses: Chugai Pharma
Perrine Marec-Berard
No relationship to disclose
Sandrine Marreaud
No relationship to disclose
Jean Michon
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Bruce Morland
Consulting or Advisory Role: Bayer. Clinigen Group
Michael Paulussen
No relationship to disclose
Andreas Ranft
No relationship to disclose
Peter Reichardt
Honoraria: Novartis, Pfizer, Bayer, PharmaMar, Eli Lilly, GSK, Amgen
Consulting or Advisory Role: GSK, Novartis, Clinigen Group, Eli Lilly, Merck, Bayer, Roche, BMS
Research Funding: Novartis (Inst)
Hendrik van den Berg
No relationship to disclose
Keith Wheatley
Research Funding: Novartis (Inst)
Ian Judson
Honoraria: Eli Lilly
Consulting or Advisory Role: Eli Lilly
Research Funding: AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Rewards to inventors relating to patent for abiraterone acetate
Travel, Accommodations, Expenses: Eli Lilly
Ian Lewis
No relationship to disclose
Alan Craft
No relationship to disclose
Heribert Juergens
No relationship to disclose
Odile Oberlin
No relationship to disclose
REFERENCES
- 1.Le Deley M-C, Paulussen M, Lewis I, et al. : Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J Clin Oncol 32:2440-2448, 2014 [DOI] [PubMed] [Google Scholar]
- 2. doi: 10.1200/JCO.2011.41.5703. Womer RB, West DC, Krailo MD, et al: Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J Clin Oncol 30:4148-4154, 2012 [Erratum: J Clin Oncol 33:814, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulussen M, Craft AW, Lewis I, et al. : Results of the EICESS-92 Study: Two randomized trials of Ewing’s sarcoma treatment--Cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol 26:4385-4393, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bacci G, Forni C, Longhi A, et al: Long-term outcome for patients with non-metastatic Ewing’s sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur J Cancer40:73-83, 2004. [DOI] [PubMed]
- 5.Cotterill SJ, Ahrens S, Paulussen M, et al. : Prognostic factors in Ewing’s tumor of bone: Analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol 18:3108-3114, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Göbel V, Jürgens H, Etspüler G, et al. : Prognostic significance of tumor volume in localized Ewing’s sarcoma of bone in children and adolescents. J Cancer Res Clin Oncol 113:187-191, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Marina N, Granowetter L, Grier HE, et al: Age, tumor characteristics, and treatment regimen as event predictors in Ewing: A Children’s Oncology Group report. Sarcoma 2015:927123, 2015. [DOI] [PMC free article] [PubMed]
- 8.Oberlin O, Deley MC, Bui BN, et al. : Prognostic factors in localized Ewing’s tumours and peripheral neuroectodermal tumours: The third study of the French Society of Paediatric Oncology (EW88 study). Br J Cancer 85:1646-1654, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulussen M, Ahrens S, Dunst J, et al. : Localized Ewing tumor of bone: Final results of the cooperative Ewing’s Sarcoma Study CESS 86. J Clin Oncol 19:1818-1829, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Bacci G, Ferrari S, Bertoni F, et al. : Prognostic factors in nonmetastatic Ewing’s sarcoma of bone treated with adjuvant chemotherapy: Analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol 18:4-11, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Le Deley MC, Ahrens S, Paulussen M, et al. : Histological response is the main prognostic factor of survival in localised Ewing tumor treated with chemotherapy alone before surgery. International Society of Pediatric Oncology (SIOP). Brisbane. Pediatr Blood Cancer 85:1646-1654, 2001 [Google Scholar]
- 12.Cornbleet MA, Corringham RE, Prentice HG, et al. : Treatment of Ewing’s sarcoma with high-dose melphalan and autologous bone marrow transplantation. Cancer Treat Rep 65:241-244, 1981 [PubMed] [Google Scholar]
- 13.Hartmann O, Benhamou E, Beaujean F, et al. : High-dose busulfan and cyclophosphamide with autologous bone marrow transplantation support in advanced malignancies in children: A phase II study. J Clin Oncol 4:1804-1810, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Hartmann O, Oberlin O, Beaujean F, et al: Role of high-dose chemotherapy followed by bone marrow autograft in the treatment of metastatic Ewing’s sarcoma in children [in French]. Bull Cancer 77:181-187, 1990. [PubMed] [Google Scholar]
- 15.Burdach S, Jürgens H, Peters C, et al. : Myeloablative radiochemotherapy and hematopoietic stem-cell rescue in poor-prognosis Ewing’s sarcoma. J Clin Oncol 11:1482-1488, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Ladenstein R, Lasset C, Pinkerton R, et al: Impact of megatherapy in children with high-risk Ewing’s tumours in complete remission: A report from the EBMT Solid Tumour Registry. Bone Marrow Transplant 15:697-705, 1995 [Erratum: Bone Marrow Transplant 15:697-705, 1995] [PubMed] [Google Scholar]
- 17.Atra A, Whelan JS, Calvagna V, et al. : High-dose busulphan/melphalan with autologous stem cell rescue in Ewing’s sarcoma. Bone Marrow Transplant 20:843-846, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Ladenstein R, Hartmann O, Pinkerton R, et al: A multivariate and matched pair analysis on high-risk Ewing tumor (ET) patients treated by megatherapy (MGT) and stem cell reinfusion (SCR) in Europe. Proc Am Soc Clin Oncol 18:555, 1999 (abstr) [Google Scholar]
- 19.Ferrari S, Sundby Hall K, Luksch R, et al. : Nonmetastatic Ewing family tumors: High-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann Oncol 22:1221-1227, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Gaspar N, Rey A, Bérard PM, et al: Risk adapted chemotherapy for localised Ewing’s sarcoma of bone: The French EW93 study. Eur J Cancer 48:1376-1385, 2012. [DOI] [PubMed]
- 21.Juergens C, Weston C, Lewis I, et al. : Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer 47:22-29, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Ladenstein R, Pötschger U, Le Deley MC, et al. : Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J Clin Oncol 28:3284-3291, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Toxicity of high-dose chemotherapy (busulfan-melphalan) followed by radiation therapy (RT) in Ewing’s axial tumours: Results of the French study. in International Society of Paediatric Oncology (SIOP). Pediatr Blood Cancer 49:555, 2007 (abstr) [Google Scholar]
- 24.Strauss SJ, McTiernan A, Driver D, et al. : Single center experience of a new intensive induction therapy for Ewing’s family of tumors: Feasibility, toxicity, and stem cell mobilization properties. J Clin Oncol 21:2974-2981, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Bearman SI, Anderson GL, Mori M, et al. : Venoocclusive disease of the liver: Development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol 11:1729-1736, 1993 [DOI] [PubMed] [Google Scholar]
- 26. RTOG Foundation: RTOG/EORTC Late Radiation Morbidity Scoring Schema. https://www.rtog.org/ResearchAssociates/AdverseEventReporting/RTOGEORTCLateRadiationMorbidityScoringSchema.aspx.
- 27.Freedman LS: Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1:121-129, 1982 [DOI] [PubMed] [Google Scholar]
- 28.Lan KG, DeMets DL: Discrete sequential boundaries for clinical trials. Biometrika 70:659-663, 1983 [Google Scholar]
- 29.O’Brien PC, Fleming TR: A multiple testing procedure for clinical trials. Biometrics 35:549-556, 1979 [PubMed] [Google Scholar]
- 30. McTiernan AM, Cassoni AM, Driver D, et al: Improving outcomes after relapse in Ewing’s sarcoma: Analysis of 114 patients from a single institution. Sarcoma 2006:83548, 2006. [DOI] [PMC free article] [PubMed]
- 31.Wassmer G: Planning and analyzing adaptive group sequential survival trials. Biom J 48:714-729, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Kalbfleisch J, Prentice R: The Statistical Analysis of Failure Time Data (ed 2). New York, NY, Wiley, 2002. [Google Scholar]
- 33.Fine JP, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 34.Cherny NI, Sullivan R, Dafni U, et al. : A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 26:1547-1573, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Gaspar N, Hawkins DS, Dirksen U, et al. : Ewing sarcoma: Current management and future approaches through collaboration. J Clin Oncol 33:3036-3046, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Luksch R, Tienghi A, Hall KS, et al. : Primary metastatic Ewing’s family tumors: Results of the Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV Study including myeloablative chemotherapy and total-lung irradiation. Ann Oncol 23:2970-2976, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Whelan JS, Burcombe RJ, Janinis J, et al. : A systematic review of the role of pulmonary irradiation in the management of primary bone tumours. Ann Oncol 13:23-30, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Razek A, Perez CA, Tefft M, et al. : Intergroup Ewing’s Sarcoma Study: Local control related to radiation dose, volume, and site of primary lesion in Ewing’s sarcoma. Cancer 46:516-521, 1980 [DOI] [PubMed] [Google Scholar]
- 39.Barker LM, Pendergrass TW, Sanders JE, et al. : Survival after recurrence of Ewing’s sarcoma family of tumors. J Clin Oncol 23:4354-4362, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Rasper M, Jabar S, Ranft A, et al. : The value of high-dose chemotherapy in patients with first relapsed Ewing sarcoma. Pediatr Blood Cancer 61:1382-1386, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Lin DY, Wei L-J, Ying Z: Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 80:557-572, 1993 [Google Scholar]