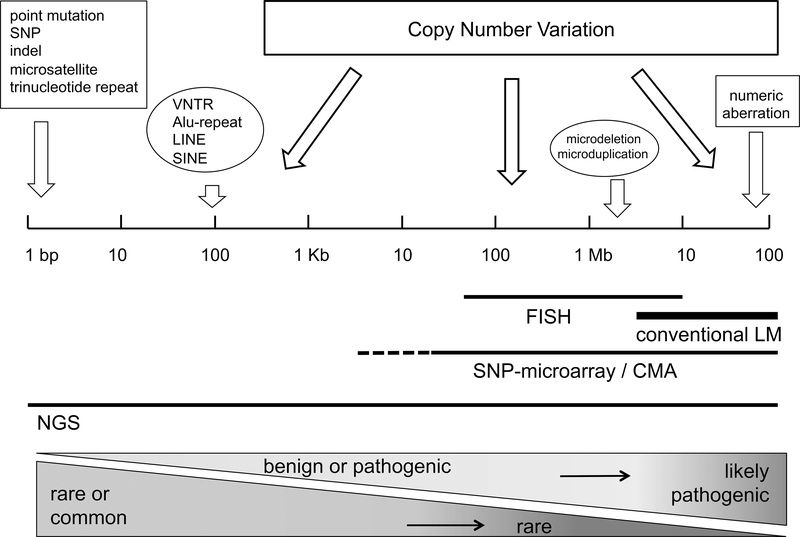
Copy number variation (CNV) describes both genomic deletions, defined as “loss” of genetic material, and genomic duplications, defined as a “gain” of an additional copy of an existing DNA sequence. As illustrated in Figure 1, CNV can range in size from 50 base pairs (bp) up to several megabases (Mb) or even entire chromosomes, in contrast to a single nucleotide polymorphism (SNP) altering only a single nucleotide base. CNV occurs ubiquitously throughout the genome and constitutes an important part of the genetic diversity in the human population with increasingly recognized clinical impact.1 Presently, the identification of CNV is limited by several factors including DNA-quality, the data generation platform, and computational analysis. As a consequence, the study of CNV in the clinical setting lags far behind the analysis of SNPs.
Figure 1. Types of genetic variation, ranked according to size (length of DNA in base pairs), detection methods, clinical impact and population frequency.
SNP = single nucleotide polymorphism; indel = short insertion / deletion; VNTR = variable number of tandem repeats; LINE/SINE = long / short interspersed repetitive elements; FISH = fluorescent-labeled in-situ hybridization; LM = light-microscopy. NGS = next generation sequencing.
CNV-detection and interpretation
Different data generation platforms (SNP-microarrays, chromosome microarrays (also known as comparative genomic hybridization or CGH) and next generation sequencing) allow genome-wide detection of CNV.1 The current review will focus on SNP-microarray-based CNV analysis, since large collections of stroke patients have previously been genotyped using SNP-microarrays.2 From an efficiency standpoint, these samples can be directly re-utilized for CNV-analysis without extra material costs. However, the use of SNP arrays for CNV detection has some limitations:
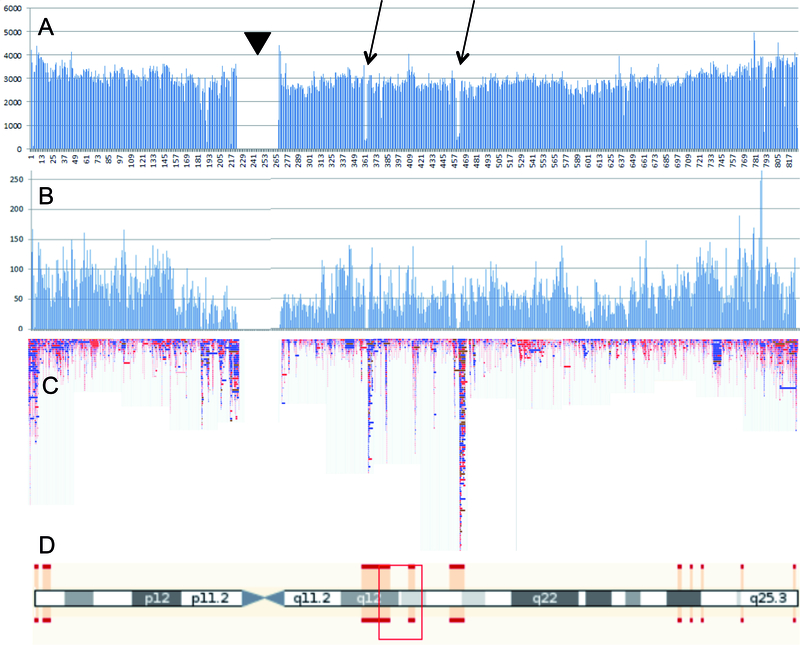
The resolution of SNP-microarray analyses (i.e. the minimal size of the variants that can be reliably detected) is inversely related to SNP-density of the platform. Hence, low SNP-density arrays are less useful. Moreover, as shown in Figure 2, the distribution of annotated SNPs across the genome (Figure 2A) and on a typical microarray (Figure 2B) is highly non-random. The SNP density is particularly low in genomic regions with segmental duplications, as well as around the centromere (Figure 2D). These regions are prone to CNV, as indicated in Figure 2C. As a consequence, the resolution of SNP-microarray studies varies across the genome and is particularly low in regions with high frequency of CNV.
Noise in microarray datasets is an important source of false-positive CNV-findings. Some noise components can be adjusted for by pair-wise comparison of samples or by more sophisticated identification of independent noise components. 3,4
Figure 2. CNV detection using SNP-microarrays.
Fig. 2A. All annotated short variants from dbSNP (https://www.ncbi.nlm.nih.gov/SNP/), distributed over 100 Kb bins along chromosome 17; Fig. 2B: all SNPs from chromosome 17 of the Illumina Omni 5 exome platform; Fig 2C. All CNVs of human chromosome 17 from the Database of Genomic Variants (DGV: http://dgv.tcag.ca/dgv/app/home); Fig. 2D. Idiogram of chromosome 17. Red bars delineate regions of uncertain mapping due to segmental duplications. Arrows point to regions with low SNP density in dbSNP. The low SNP-density is not outbalanced by SNP selection for the Illumina platform. Regions with low SNP density on the Illumina array appear to be very rich in CNV. Arrowhead indicates peri-centromeric region.
The clinical interpretation of a specific CNV-finding may be difficult or uncertain.5 In general, very large (> 500 kilobase) and rare (<1%) CNVs are more likely to be disease-associated than small and common ones, but size alone is not crucial. Large CNVs can be benign while small ones can be clinically important.5,6 Rather than the physical length, the total number of genes within the CNV, as well as the function of the affected genes (protein-coding or non-coding, coding for dosage-sensitive or dosage-insensitive proteins) are likely to determine the clinical importance.5,7 Currently, the clinical interpretation of many CNV-findings remains unclear.7,8 Moreover, in large CNVs covering many different genes, it may be difficult to pinpoint the specific disease-causing gene.
Anticipating the disclosure of incidental (“unsolicited”) pathogenic findings is an ongoing challenge for all genome-wide diagnostic methods, including CNV detection. For example, well-established pathogenic variants may be found in genes not related to the phenotype of interest. Genetic counseling of patients prior to CNV analysis, as usually offered to patients in a clinical context, could anticipate and assist with incidental findings as well as unclear findings (variants of unknown significance).9 However, patient-data in large epidemiologic studies are usually anonymized –with neither the scientists nor participants well prepared to deal with incidental findings.
CNV in stroke patients
As listed below and as demonstrated in Table 1, ischemic stroke has been associated with several different types of CNV-findings:
Common risk-variants: The lipoprotein (a) gene (LPA) contains a repeated domain of 114 amino-acids that occurs in a highly variable number of copies (1 to >40). Individuals with lower copy numbers (<22 repeats) have an approximately two-fold higher risk of ischemic stroke than those with larger isoforms.25 Multiplex ligation-dependent probe amplification (MLPA) is the gold-standard to analyze this CNV.
Rare disease-causing variants: A large (749,000 bp) duplication encompassing six protein-coding genes (COL4A1, COL4A2, RAB20, NAXD, CARS2, ING1) genes as well as several non-protein-coding genes was found in a young patient with recurrent lacunar infarcts due to small vessel disease and in eleven affected family members.16 The CNV was identified during next-generation sequencing analysis and was confirmed by array comparative genome hybridization.
Global genomic imbalance: An excess burden of large, gene-disrupting CNVs was found in stroke patients with unfavorable functional outcome after three months, compared to patients with favorable outcome.26 SNP-microarrays from previous GWAS were re-utilized to study CNV.
Variants of unknown significance (VUS): a large (> 3.1 Mb) duplication encompassing eight protein-coding genes (SCOC, CLGN, ELMOD2, TBC1D9, RNF150, ZNF330, IL15, INPP4B) was detected in a 19-year-old boy with ischemic stroke due to spontaneous carotid artery dissection.11 This finding should be conservatively considered as variant of unknown significance (VUS), since the variant was novel and sporadic (i.e. non-familial) and since none of the duplicated genes are known candidate genes for cervical artery dissection.
Table 1. CNV-findings associated with ischemic stroke.
CADASIL: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy; EDS: Ehlers Danlos syndrome; SAO: small arterial occlusive disease; LVD: large vessel disease; CeAD: cervical artery dissection; CCM: cerebral cavernous malformations; VNTR: variable number of tandem repeats.
| Phenotype | CNV | affected/disrupted genes | Ref |
|---|---|---|---|
| CNV-findings in stroke due to a Mendelian disorder | |||
| CADASIL | 100 bp deletion | NOTCH3 | [10] |
| vascular EDS | 2q32 deletion | COL3A1, COL5A2 | [11] |
| CNV associated with subtypes of ischemic stroke | |||
| CeAD | enrichment of various CNVs affecting arterial development | [12] | |
| CeAD | 16p13.1 duplication | MYH11 /ABCC6 locus | [12] |
| Moya-moya | 6pter duplications | [13,14] | |
| CCM | exonic CNVs | CCM1; CCM2; CCM3 | [15] |
| SAO | 13q34 duplication | COL4A1/COL4A2 locus | [16,17] |
| SAO | low (<4) copy number | DEFB4 | [18] |
| LVD | low (<4) copy number | DEFB4 | [18] |
| CNV associated with complex developmental retardation phenotypes and pediatric stroke | |||
| 1q24 /10q26 deletions | SERPINC1 | [19] | |
| CNV associated with stroke risk factors | |||
| Atrial fibrillation | intronic duplication | KCNIP1 | [20] |
| Obesity | CNV burden | [21] | |
| Obesity | 16p11.2/22q11.2 deletion | [22] | |
| Obesity | low copy number | AMY1 | [23] |
| Hyperlipidemia | VNTR | LDLR, LPA | [24] |
Stroke patients with chromosome aberrations
Chromosome aberrations that are large enough to be analyzed by microscopy are rare in patients with non-syndromic cardiovascular diseases or stroke.27,28 An abnormal chromosome 13 was detected by light microscopic analysis of lymphocyte metaphase chromosomes in a young patient with recurrent stroke. Parallel SNP-microarray analysis revealed a complex rearrangement with multiple duplications.29 Some studies associated stroke with numerical aberrations including trisomy 21,30 Klinefelter syndrome31 and Turner syndrome.32 Somatic loss of the Y-chromosome is a common acquired aberration in male blood cells associated with age and smoking.33 An association between loss of the Y-chromosome and cardiovascular outcomes is suspected,34 but rigorous analysis of mosaicism in cardiovascular patients is lacking.
CNV and ischemic stroke
Common CNV in several candidate genes was tested for association with ischemic stroke. A lower copy number of the DEFB4 gene was associated with ischemic stroke in a single study.18 A meta-analysis of four CNV studies did not replicate prior CNV associations of GSTM1 and GSTT1 with stroke.35 Positive associations with ischemic stroke were reported with common CNVs in LPA and LDLR.36
As early as 2008, the impact of CNV was explored in 263 ischemic stroke patients and 275 control subjects by analyzing microarray data from the first GWAS in ischemic stroke.37 A total of 408,000 SNPs were genotyped in each study subject, resulting in a resolution of CNV-detection of about 50,000 bp. In the stroke cohort, 231 CNVs were identified, widely distributed throughout the genome, with sizes up to 2.1 Mb. All reported variants were low-frequency findings. None of the observed variants were unequivocally linked to ischemic stroke. In the stroke cohort, ischemic strokes were classified according to TOAST criteria,38 but subtype analysis was not performed as the numbers were small and power was deemed insufficient.
CNV and cervical artery dissection (CeAD)
Dissection of the carotid or vertebral artery is a major cause of ischemic stroke in patients younger than 50 years. Dissection can occur spontaneously in young adults without known vascular risk factors, suggesting an underlying structural defect of the arterial wall, which was subsequently confirmed by electron-microscopic investigation of arterial biopsies.39 Genetic analysis revealed rare point-mutations in different candidate genes associated with inherited connective tissue diseases, but this was only in a minority of the patients.40,41
In an early CNV study, 70 CeAD patients were phenotyped by an electron-microscopic analysis of a skin biopsy in order to detect connective tissue alterations.11 One patient with carotid artery dissection and a history of aortic disease had a large deletion covering the entirety of the COL3A1 and COL5A2 genes.11 Another patient carried a large recurrent duplication of chromosome 16p13 including the MYH11 and ABCC6 genes, a rare finding in the normal population that predisposes to aortic aneurysm and dissection.42,43 Four further patients with CNV of the MYH11/ABCC6 locus were identified in a subsequent exploration of 833 CeAD patients.12 This latter CNV-study of CeAD did not detect association with variation in a particular locus, but found association with variation in a predefined set of genes involved in cardiovascular system development.
CNV and hemorrhagic stroke
CNV was studied in 23 Korean patients with ruptured intracranial aneurysms by comparative genomic hybridization (CGH) with 4,030 BAC (bacterial artificial chromosome)-clones covering the entire human genome at a resolution of 1 Mbp. Each patient was analyzed separately and compared with a pooled reference DNA sample from 10 gender-matched healthy subjects, but no definitive risk CNVs were detected.44 The CGH system used in this study was designed for the detection of very large (>1Mb) variants, which are usually rare. Further, for the study of rare variants, the sample size was notably small.
In another study, high-density (300K Illumina) SNP-microarrays from a GWAS of 191 Japanese patients with aneurysmal subarachnoid hemorrhage and 282 controls were used for CNV exploration.45 CNV-findings were carefully validated by visual examination of the genoplot images and overlapping analysis with the Database of Genomic Variants (DGV - http://dgv.tcag.ca/dgv/app/home ). Moreover, selected findings were validated by quantitative PCR. Most CNV findings were distributed evenly across the chromosomes, but common variants in two regions (chr4:153210505–153212191 and chr10:6265006–6267388) were significantly associated with the risk of SAH. These findings are pending replication in independent studies. No subsequent genome-wide CNV-studies of intracranial aneurysm have been published. Systematic explorations of the impact of CNV on intracerebral hemorrhage have yet to be published.
CNV and stroke pharmacokinetics
CNV occurs in many genes associated with drug absorption, distribution, metabolism and excretion, but until recently the influence of CNV on drug response was not well recognized.46,47 Currently, the impact of CNV on drug response is increasingly perceived as a potential driver of drug efficacy. This may lead in the near future to more precise pharmacological targeting, including stroke-specific medications.
Outlook
Worldwide, stroke researchers have collected large numbers well-characterized stroke patients and genotyped them on high-density SNP-microarrays for genome-wide association studies of common SNPs.2 These microarrays could be re-analyzed to detect CNV without the need for extra material costs. Unfortunately, several obstacles may prevent scientists from initiating such large-scale CNV studies using these data, including: 1) the high proportion of false positive CNV-calls when using current CNV-detection algorithms; 2) the huge work-load of pair-wise comparing each sample with a referent sample; 3) the uncertain clinical interpretation of many CNV-findings, and; 4) the lack of a universally accepted reference set of CNV-findings (size, frequency) across ethnically-diverse human populations. Moreover, a significant fraction of the large CNV-findings seen in SNP-microarray based CNV studies is expected to be rare (i.e. population frequency <0.01), which may have power implications regarding associations with specific phenotypes.48,49
In closing, the concept of CNV describes a large and highly heterogeneous set of genomic variants, including rare vs. common, benign vs. pathogenic, and inherited vs. de-novo. While current genetic epidemiological methods for the analysis of common variants with small relative risks are well-suited for large patient populations, optimal analytical methodologies for rare disease-causing mutations such as CNV remain uncertain. Rather than mere risk factors, pathogenic CNVs may also be considered as “inborn errors”. Thus, the study of rare pathogenic CNVs may lead stroke genetics back to a more individual or patient-centered clinical focus.
Acknowledgments
Sources of funding
Dr. Cole’s efforts on this project were supported in part by NIH grants U01 NS069208, R01 NS100178, and R01 NS105150; the U.S. Department of Veterans Affairs, and the American Heart Association Cardiovascular Genome-Phenome Study (Grant# 15GPSPG23770000), and an American Heart Association Discovery Grant supported by Bayer Group (Grant# 17IBDG33700328).
Abbreviation of gene names
- ABCC6
ATP Binding Cassette Subfamily C Member 6
- AMY1
Amylase, Alpha 1A (Salivary)
- CARS2
Cysteinyl-TRNA Synthetase 2, Mitochondrial
- CLGN
Calmegin
- COL3A1
Collagen Type III Alpha 1 Chain
- COL4A1
Collagen Type IV Alpha 1 Chain
- COL4A2
Collagen Type IV Alpha 2 Chain
- COL5A2
Collagen Type V Alpha 2 Chain
- DEFB4
Defensin Beta 4A
- ELMOD2
ELMO Domain Containing 2
- GSTM1
Glutathione S-Transferase Mu 1
- GSTT1
Glutathione S-Transferase Theta 1
- IL15
Interleukin 15
- ING1
Inhibitor Of Growth Family Member 1
- INPP4B
Inositol Polyphosphate-4-Phosphatase Type II B
- KCNIP1
Potassium Voltage-Gated Channel Interacting Protein 1
- LDLR
Low Density Lipoprotein Receptor
- LPA
Lipoprotein(A)
- MYH11
Myosin Heavy Chain 11
- NAXD
NAD(P)HX Dehydratase
- NOTCH3
Notch (Drosophila) Homolog 3
- RAB20
RAB20, Member RAS Oncogene Family
- RNF150
Ring Finger Protein 150
- SCOC
Short Coiled-Coil Protein
- SERPINC1
Serpin Family C Member 1
- TBC1D9
TBC1 Domain Family Member 9
- ZNF330
Zinc Finger Protein 330
Footnotes
Conflict of Interest Disclosures
None.
Social media handles for Stroke posts:
University of Heidelberg Medical School:
Facebook: www.klinikum.uni-heidelberg.de/facebook
Twitter: https://twitter.com/uniklinik_hd
University Hospital Basel:
Facebook: https://www.facebook.com/unispitalbasel
Twitter: https://twitter.com/unispitalbasel
Felix-Platter Hospital Basel:
Facebook: https://www.facebook.com/felixplatterspital/
Twitter: https://twitter.com/hashtag/felixplatterspital
University of Maryland Medical Center:
Facebook: @UMDMedCenter https://www.facebook.com/UMDMedCenter
Twitter: @UMMC https://twitter.com/UMMC
University of Maryland School of Medicine:
Facebook: @Maryland.Medicine https://www.facebook.com/Maryland.Medicine?sk=wall
Twitter: @UMmedschool https://twitter.com/ummedschool
References
- 1.Valsesia A, Macé A, Jacquemont S, Beckmann JS, Kutalik Z. The Growing Importance of CNVs: New Insights for Detection and Clinical Interpretation. Front Genet. 2013;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diskin SJ, Li M, Hou C, Yang S, Glessner J, Hakonarson H, et al. Adjustment of genomic waves in signal intensities from whole-genome SNP genotyping platforms. Nucleic Acids Res. 2008;36,e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsbach P, Chen B, Jiang Y, Engelter ST, Grond-Ginsbach C. Copy Number Studies in Noisy Samples. Microarrays (Basel). 2013;2:284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korbel JO, Kim PM, Chen X, Urban AE, Weissman S, Snyder M, et al. The current excitement about copy-number variation: how it relates to gene duplications and protein families. Curr Opin Struct Biol. 2008;18:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowakowska B: Clinical interpretation of copy number variants in the human genome: J appl Genetics. 2017;58:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice AM, McLysaght A. Dosage sensitivity is a major determinant of human copy number variant pathogenicity. Nat Commun. 2017;8:14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itsara A, Cooper GM, Baker C, Girirajan S, Li J, Absher D, et al. Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet. 2009;84:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke W, Antommaria AH, Bennett R, Botkin J, Clayton EW, Henderson GE, et al. Recommendations for returning genomic incidental findings? We need to talk! Genet Med. 2013;15:854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiming F, Yuliang W, Youjie L, Xinsheng L, Shuyang X, Zhaoxia L. A novel Notch3 deletion mutation in a Chinese patient with cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). J Clin Neurosci. 2013;20:322–323. [DOI] [PubMed] [Google Scholar]
- 11.Grond-Ginsbach C, Chen B, Pjontek R, Wiest T, Jiang Y, Burwinkel B, et al. Copy number variation in patients with cervical artery dissection. Eur J Hum Genet. 2012;20:1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grond-Ginsbach C, Chen B, Krawczak M, Pjontek R, Ginsbach P, Jiang Y,et al. Genetic Imbalance in Patients with Cervical Artery Dissection. Curr Genomics. 2017;18:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg RE, Egan M, Rodgers S, Harter D, Burnside RD, Milla S, et al. Complex chromosome rearrangement of 6p25.3->p23 and 12q24.32->qter in a child with moyamoya. Pediatrics. 2013;.131: e1996–2001. [DOI] [PubMed] [Google Scholar]
- 14.Toldo I, Po’ C, Morao V, Talenti G, Causin F, D’Avella D, et al. Moyamoya syndrome and 6p chromosome rearrangements: Expanding evidences of a new association. Eur J Paediatr Neurol. 2016;20:766–771. [DOI] [PubMed] [Google Scholar]
- 15.Felbor U, Gaetzner S, Verlaan DJ, Vijzelaar R, Rouleau GA, Siegel AM. Large germline deletions and duplication in isolated cerebral cavernous malformation patients. Neurogenetics. 2007;8:149–153. [DOI] [PubMed] [Google Scholar]
- 16.Saskin A, Sillon G, Palfreeman N, Buhas D. Col4A1/2 CNVs and cerebral small vessel disease. Neurology. 2018;90:1026–1028. [DOI] [PubMed] [Google Scholar]
- 17.Renard D, Miné M, Pipiras E, Labauge P, Delahaye A, Benzacken B, et al. Cerebral small-vessel disease associated with COL4A1 and COL4A2 gene duplications. Neurology. 2014;83:1029–1031. [DOI] [PubMed] [Google Scholar]
- 18.Tiszlavicz Z, Somogyvári F, Szolnoki Z, Sztriha LK, Németh B, Vécsei L, et al. Genetic polymorphisms of human β-defensins in patients with ischemic stroke. Acta Neurol Scand. 2012;126:109–115. [DOI] [PubMed] [Google Scholar]
- 19.Kibe T, Mori Y, Okanishi T, Shimojima K, Yokochi K, Yamamoto T. Two concurrent chromosomal aberrations involving interstitial deletion in 1q24.2q25.2 and inverted duplication and deletion in 10q26 in a patient with stroke associated with antithrombin deficiency and a patent foramen ovale. Am J Med Genet A. 2011;155A:215–220. [DOI] [PubMed] [Google Scholar]
- 20.Tsai CT, Hsieh CS, Chang SN, Chuang EY, Ueng KC, Tsai CF, et al. Genome-wide screening identifies a KCNIP1 copy number variant as a genetic predictor for atrial fibrillation. Nat Commun. 2016;7:10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macé A, Tuke MA, Deelen P, Kristiansson K, Mattsson H, Nõukas M, et al. CNV-association meta-analysis in 191,161 European adults reveals new loci associated with anthropometric traits. Nat Commun. 2017;8:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voll SL, Boot E, Butcher NJ, Cooper S, Heung T, Chow EW, et al. Obesity in adults with 22q11.2 deletion syndrome. Genet Med. 2017;19:204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falchi M, El-Sayed Moustafa JS, Takousis P, Pesce F, Bonnefond A, Andersson-Assarsson JC, et al. Low copy number of the salivary amylase gene predisposes to obesity. Nat Genet. 2014;46:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacocca MA, Hegele RA. Role of DNA copy number variation in dyslipidemias. Curr Opin Lipidol. 2018;29:125–132. [DOI] [PubMed] [Google Scholar]
- 25.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55:2160–2167. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer D, Chen B, Bevan S, Jern C, Jimenez-Conde J, Lee JM, et al. Genetic imbalance is associated with poorer outcome after ischemic stroke. Neurol Genet. 2017; 3(1 Suppl 1): S12–S18.28428978 [Google Scholar]
- 27.Nik-Zainal S, Cotter PE, Willatt LR, Abbott K, O’Brien EW. Ring chromosome 12 with inverted microduplication of 12p13.3 involving the Von Willebrand Factor gene associated with cryptogenic stroke in a young adult male. Eur J Med Genet. 2011;54:97–101. [DOI] [PubMed] [Google Scholar]
- 28.Luukkonen TM, Mehrjouy MM, Pöyhönen M, Anttonen AK, Lahermo P, Ellonen P, et al. Breakpoint mapping and haplotype analysis of translocation t(1;12)(q43;q21.1) in two apparently independent families with vascular phenotypes. Mol Genet Genomic Med. 2018;6:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnside RD, Harris A, Speyer D, Burgin WS, Rose DZ, Sanchez-Valle A. Constitutional Chromoanagenesis of Distal 13q in a Young Adult with Recurrent Strokes. Cytogenet Genome Res. 2016;150:46–51. [DOI] [PubMed] [Google Scholar]
- 30.Sobey CG, Judkins CP, Sundararajan V, Phan TG, Drummond GR, Srikanth VK. Risk of Major Cardiovascular Events in People with Down Syndrome. PLoS One. 2015;10:e0137093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calogero AE, Giagulli VA, Mongioì LM, et al. Klinefelter syndrome: cardiovascular abnormalities and metabolic disorders. J Endocrinol Invest. 2017;40:705–712. [DOI] [PubMed] [Google Scholar]
- 32.Irioka T1, Mizusawa H. Ischemic stroke in a young adult with Turner syndrome. Neurol Sci. 2011;32:317–319. [DOI] [PubMed] [Google Scholar]
- 33.Forsberg LA. Loss of chromosome Y (LOY) in blood cells is associated with increased risk for disease and mortality in aging men. Hum Genet. 2017;136:657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuster JJ, Walsh K. Somatic Mutations and Clonal Hematopoiesis: Unexpected Potential New Drivers of Age-Related Cardiovascular Disease. Circ Res. 2018;122:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nørskov MS, Frikke-Schmidt R, Loft S, Sillesen H, Grande P, Nordestgaard BG,et al. Copy number variation in glutathione S-transferases M1 and T1 and ischemic vascular disease: four studies and meta-analyses. Circ Cardiovasc Genet. 2011;4:418–428. [DOI] [PubMed] [Google Scholar]
- 36.Iacocca MA, Hegele RA. Role of DNA copy number variation in dyslipidemias. Curr Opin Lipidol. 2018;29:125–132. [DOI] [PubMed] [Google Scholar]
- 37.Matarin M, Simon-Sanchez J, Fung HC, Scholz S, Gibbs JR, Hernandez DG, et al. Structural genomic variation in ischemic stroke. Neurogenetics. 2008;9:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 39.Brandt T, Morcher M, Hausser I. Association of cervical artery dissection with connective tissue abnormalities in skin and arteries. Front Neurol Neurosci. 2005;20:16–29. [DOI] [PubMed] [Google Scholar]
- 40.Grond-Ginsbach C, Brandt T, Kloss M, Aksay SS, Lyrer P, Traenka C, et al. Next generation sequencing analysis of patients with familial cervical artery dissection. Eur Stroke J. 2017;2:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pezzini A, Drera B, Del Zotto E, Ritelli M, Carletti M, Tomelleri G, et al. Mutations in TGFBR2 gene cause spontaneous cervical artery dissection. J Neurol Neurosurg Psychiatry. 2011;82:1372–1374. [DOI] [PubMed] [Google Scholar]
- 42.Kuang SQ, Guo DC, Prakash SK, McDonald MLN, Johnson RJ, Wang M, et al. Recurrent chromosome 16p13.1 duplications are a risk factor for aortic dissections. PLoS Genetics. 2011:7, e1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erhart P, Brandt T, Straub BK, Hausser I, Hentze S, Böckler D, et al. Familial aortic disease and a large duplication in chromosome 16p13.1. Mol Genet Genomic Med. 2018;6:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi JS, Kim SR, Jeon YW, Lee KH, Rha HK. Identification of DNA copy number aberrations by array comparative genomic hybridization in patients with ruptured intracranial aneurysms. J Clin Neurosci. 2009;16:295–301. [DOI] [PubMed] [Google Scholar]
- 45.Bae JS1, Cheong HS, Park BL, Kim LH, Park TJ, Kim JY, et al. Genome-wide association analysis of copy number variations in subarachnoid aneurysmal hemorrhage. J Hum Genet. 2010;55:726–730. [DOI] [PubMed] [Google Scholar]
- 46.Santos M, Niemi M, Hiratsuka M, Kumondai M, Ingelman-Sundberg M, Lauschke VM, et al. Novel copy-number variations in pharmacogenes contribute to interindividual differences in drug pharmacokinetics. Genet Med. 2018;20:622–629. [DOI] [PubMed] [Google Scholar]
- 47.Willyard C Copy number variations’ effect on drug response still overlooked. Nat Med. 2015;21:206. [DOI] [PubMed] [Google Scholar]
- 48.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45 (D1): D353–D361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M, Lin S. Detecting associations of rare variants with common diseases: collapsing or haplotyping? Brief Bioinform. 2015;16:759–768.. [DOI] [PMC free article] [PubMed] [Google Scholar]