Abstract
Neuroblastoma, an embryonal cancer of neural crest origin, shows metastases frequently at diagnosis. Delloye-Bourgeois and colleagues demonstrate that neuroblastoma cell lines and patient-derived xenografts engraft and adopt a metastatic program in chick embryos. They identify Sema3C as a molecular switch that regulates metastatic dissemination.
Preview
Chick embryos have been at the forefront of developmental biology research since Aristotle’s first dissection in 350 BC. While many animal embryos cannot be visualized readily during development, the chick embryo forms in a contained, self-sufficient environment allowing easy access to perform experiments and visualize their consequences. The chick embryo can be efficiently manipulated genetically via viral infection and in ovo electroporation, enabling powerful gain-of-function or loss-of-function experiments (Stern, 2005). The application of advanced imaging, including confocal light sheet imaging, also make the chick embryo an attractive model in neurodevelopment.
From the early 1900s, chick embryos were used to study the neural crest, a developmental structure at the origin of the peripheral nervous system and non-neural cell types including melanocytes, smooth muscle, craniofacial bones, cartilage and connective tissue (Bronner and Simões-Costa, 2016). The neural crest is a dynamic population of pluripotent cells which, upon undergoing epithelial to mesenchymal transition, delaminate from the dorsal neuroepithelium of the neural tube to migrate long distances and ultimately reach widespread destinations in the embryo. Such migration requires interaction among neural crest cells as well as with their environment, directing collective migration (cells moving in concert) in response to signals delivered by guidance molecules (Bronner and Simões-Costa, 2016). These characteristics of neural crest cell migration lend themselves to the biology of cancer cells (Figure 1), as metastases are similarly characterized by an epithelial-to-mesenchymal transition, cell migration, invasion, and acquisition of stem cell-like properties (Shibue and Weinberg, 2017).
Figure 1.
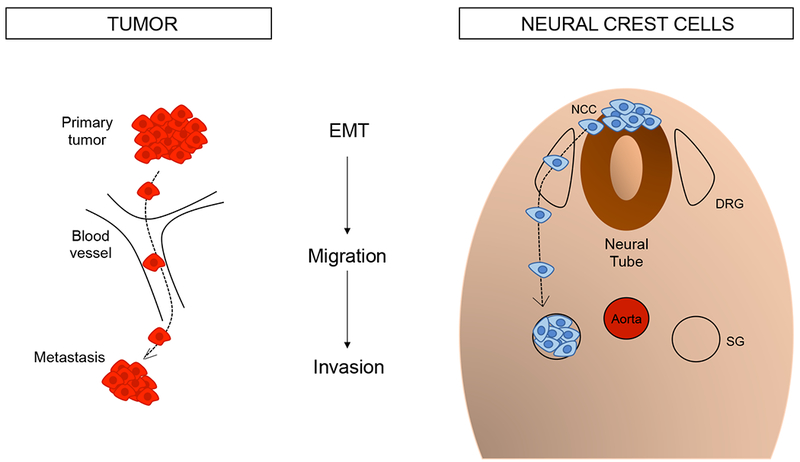
Parallels between cancer metastasis and neural crest migration. Both cell populations undergo epithelial to mesenchymal transition (EMT), migration, and ultimately a form of invasion, with cells settling in distant tissues. NCC: neural crest cells, DRG: dorsal root ganglia, SG: sympathetic ganglia.
Melanoma originates from neural crest-derived melanocytes. As the natural immunodeficiency of chick embryos enables engraftment of human tumor cells in the developing embryo, Kulesa and colleagues were previously able to analyze the behavior of human metastatic melanoma cells in the environment of migrating neural crest, by transplanting these cells into the neural tubes of developing chick embryos. They observed migration of melanoma cells along the typical cranial neural crest migratory pathway as well as colonization of peripheral sites. These observations suggested that melanoma cell lines retained their ability to respond to the neural crest-microenvironment in the developing embryo. However, human cells formed tumors only when implanted into non-neural crest environments such as the retina (Kulesa et al., 2006).
Neuroblastoma, an embryonal childhood malignancy derived from the neural crest, is the most common extracranial malignancy of childhood. Half of patients present with metastatic disease, often to regional lymph nodes, bone marrow, bone, liver, and skin (Matthay et al., 2016). As in most cancers, metastatic spread correlates with high-risk, stage 4 disease and poor survival. In this issue of Cancer Cell, Delloye-Bourgeois and colleagues present a novel model to capture the embryonic microenvironment and subsequent tumor development in chick embryos engrafted with human stage 4 neuroblastoma lines and human patient-derived tumors (PDXs). They grafted fluorescently-labeled neuroblastoma cells into the dorsal neural tube of chick embryos and conducted immunofluorescent and time-lapse imaging, and confocal light sheet microscopy. Neuroblastoma cells followed neural crest migration to reach sympathetic ganglia where they subsequently formed dense, proliferating masses that often spread multifocally (metastasized) to distant sites by 7 days post-engraftment. The metastatic spread proceeded through the dorsal aorta and peripheral nerves, a novel finding demonstrated in multiple human cell lines.
Delloye-Bourgeois et al. then conducted RNA-seq to characterize gene expression changes occurring between engraftment and primary (premetastatic) tumors. Forty-six neural crest genes were differentially expressed. Twenty-four were related to cell movement, and three of these were Semaphorins. The Semaphorin gene family regulates axon guidance and cardiac neural crest cell migration (Schwarz et al., 2009). They also contribute to cell migration and angiogenesis, functions that also can use to either suppress or drive tumors, depending on cell type (Tamagnone, 2012).
Levels of Semaphorin3C (Sema3C) decreased by half in neuroblastoma tumor masses compared to naïve pre-engraftment cells. Sema3C siRNA knockdown during neural crest migration hindered collective migration and targeting of neuroblastoma cells to sympathetic structures, and led to increased dissemination (failure to reach sympathoadrenal sites). Importantly, the authors validated that the effect of inducible Sema3C shRNA knockdown in already established primary tumors (from human cell lines) led to increased metastatic spread in the embryos. This effect was rescued by expressing an exogenous Sema3C construct. Additionally, the authors examined potential paracrine actions of Sema3C by mixing high-Sema3C and low-Sema3C cells. Inclusion of 25% high-Sema3C cells was sufficient to block the dissemination of the neuroblastoma cells. These results clearly demonstrate the role of Sema3C in the cohesion of neuroblastoma cells and their collective migration, suggesting Sema3C as a metastasis suppressing gene.
The canonical receptors for secreted Semaphorin ligands are Neuropilins, which must complex to transmembrane Plexins to transduce downstream signals (Neufeld et al., 2012). Delloye-Bourgeois and colleagues showed that – like Sema3C – the expression of Neuropilin 1 (NRP1) and PlexinA4 (PLXNA4) were both decreased significantly in tumor masses relative to pre-engraftment cells. Knockdown of NRP1/2 or PLXNA4 also drastically impaired cell aggregation and increased metastasis from the tumor core in vivo, mimicking the effect of Sema3C knockdown. These results correlated with retrospective data from neuroblastoma patients showing that low levels of Sema3C, NRP1, and PLXN4 were associated with poorer event free survival. This work highlights the importance of Semaphorin/Neuropilin/Plexin signaling in neuroblastoma, with functional knockdown experiments suggesting a role in metastatic spread. Additional studies are needed to validate the functional importance of this candidate metastatic signaling pathway in neuroblastoma tumors in mammals.
Finally, Delloye-Bourgeois and colleagues show that neuroblastoma patient-derived xenografts (PDXs) could be grafted into their chick embryo model and, like the cell lines, targeted sympathoadrenal derivatives to form tumors in the embryo. Impressively, distant metastases occurred only using cells derived from patients with metastatic neuroblastoma. Some metastatic neuroblastoma cells were also observed in the aorta, again confirming this as a preferential route for dissemination of neuroblastoma in the chick.
This work by Delloye-Bourgeois and colleagues introduces a novel xenograft model in which neuroblastoma cell lines and PDXs engrafted in the neural crest environment of chick embryos, and followed migration patterns of endogenous avian neural crest cells. This study opens the possibility for studying a variety of cell behaviors, and testing potential therapeutics for relevance and efficacy in human disease. Additionally, although this study focused on the role of Sema3C in neuroblastoma cell cohesion, deeper analysis of the RNA-seq data generated here could identify other transcriptional changes contributing to metastatic neuroblastoma in children. While RNA-seq analysis was performed in this work between pre-engraftment cells and the primary tumor, it was lacking between the primary tumor and metastatic cells, which could provide further insights on the mechanisms of metastatic spread. However, the difficulty of isolating metastatic cells makes this experiment challenging.
The mechanisms driving metastasis in neuroblastoma are challenging to investigate, and poorly recapitulated to date in existing murine models. Delloye-Bourgeois et al. highlight the considerable power of the chick embryo model for neuroblastoma. Zhu and colleagues have also shown that they can model metastasis in zebrafish, where overexpressing the transcriptional regulators LMO1 and MYCN led to metastatic spread (Zhu et al., 2017). These non-mammalian vertebrate models are already improving our understanding of metastatic neuroblastoma, and have potential to identify candidate targets for future clinical trials.
References
- Bronner ME, and Simões-Costa M (2016). The Neural Crest Migrating into the Twenty-First Century. Curr. Top. Dev. Biol 116, 115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor REB, and Hendrix MJC (2006). Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl. Acad. Sci. U.S.a 103, 3752–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, and Weiss WA (2016). Neuroblastoma. Nat Rev Dis Primers 2, 16078. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Sabag AD, Rabinovicz N, and Kessler O (2012). Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med 2, a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Maden CH, Vieira JM, and Ruhrberg C (2009). Neuropilin 1 signaling guides neural crest cells to coordinate pathway choice with cell specification. Proc. Natl. Acad. Sci. U.S.a 106, 6164–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, and Weinberg RA (2017). EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD (2005). The chick; a great model system becomes even greater. Dev. Cell 8, 9–17. [DOI] [PubMed] [Google Scholar]
- Tamagnone L (2012). Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer Cell 22, 145–152. [DOI] [PubMed] [Google Scholar]
- Zhu S, Zhang X, Weichert-Leahey N, Dong Z, Zhang C, Lopez G, Tao T, He S, Wood AC, Oldridge D, et al. (2017). LMO1 Synergizes with MYCN to Promote Neuroblastoma Initiation and Metastasis. Cancer Cell 32, 310–323. e315. [DOI] [PMC free article] [PubMed] [Google Scholar]


