Abstract
Background and Purpose:
Endovascular thrombectomy (EVT) is effective for acute ischemic stroke with large vessel occlusion (LVO) and NIHSS ≥6. However, EVT benefit for mild deficits LVOs (NIHSS<6) is uncertain. We evaluated EVT efficacy and safety in mild strokes with LVO.
Methods:
A retrospective cohort of patients with anterior circulation LVO and NIHSS<6 presenting within 24hours from last-seen-normal were pooled. Patients were divided into 2 groups: EVT or medical management. 90day mRS=0–1 was the primary outcome; mRS=0–2 was the secondary. Symptomatic intracerebral hemorrhage (sICH) was the safety outcome. Clinical outcomes were compared through a multivariable logistic regression after adjusting for age, presentation NIHSS, time-last-seen-normal-to-presentation, center, IV-alteplase, ASPECTS, and thrombus location. We then performed propensity score matching as a sensitivity analysis. Results were also stratified by thrombus location.
Results:
214 patients (EVT-124, medical management-90) were included from 8 US and Spain centers between January/2012 and March/2017. The groups were similar in age, ASPECTS, IV-alteplase rate and time-last-seen-normal-to-presentation. There was no difference in mRS=0–1 between EVT and medical management (55.7% versus 54.4%, respectively, aOR=1.3, 95%CI=0.64–2.64, p=0.47). Similar results were seen for mRS=0–2 (63.3% EVT versus 67.8% medical management, aOR=0.9, 95%CI=0.43–1.88, p=0.77). In a propensity matching analysis, there was no treatment effect in 62 matched pairs (53.5%EVT, 48.4% medical management; OR=1.17, 95%CI=0.54–2.52, p=0.69). There was no statistically significant difference when stratified by any thrombus location; M1 approached significance (p=0.07). sICH rates were higher with thrombectomy (5.8% EVT versus 0% medical management, p=0.02).
Conclusions:
Our retrospective multicenter cohort study showed no improvement in excellent and independent functional outcomes in mild strokes (NIHSS<6) receiving thrombectomy irrespective of thrombus location, with increased sICH rates, consistent with the guidelines recommending the treatment for NIHSS≥6. There was a signal towards benefit with EVT only in M1 occlusions; however this needs to be further evaluated in future RCTs.
Keywords: Ischemic stroke, Large vessel occlusion, Mild stroke, Endovascular thrombectomy
Introduction
Endovascular thrombectomy (EVT) improved reperfusion rates and clinical outcomes over best medical management, including IV-alteplase, in multiple randomized control trials (RCTs) for selected stroke patients with large vessel occlusion (LVO).1–9 Based on these trials, current guidelines endorse EVT as the standard of care for patients with LVO in the anterior circulation (ICA and MCA/M1) with a National Institutes of Health Stroke Scale (NIHSS) score of 6 or more.10 This NIHSS cutoff was established because the previous RCTs’ eligibility criteria mostly excluded patients with NIHSS <6. One exception was MR CLEAN, which enrolled patients with NIHSS as low as 2.1 Consequently, only a restricted group of patients with mild strokes and LVO were included in those RCTs as illustrated in the HERMES pooled meta-analysis which had limited number of patients with NIHSS <10 and even fewer had NIHSS <6.11 Thus, there is a lack of evidence of EVT effectiveness and safety in this important subpopulation.
Many LVO patients presenting with NIHSS <6, however, can end up with significant disability. In the absence of data, physicians face uncertainty how to manage these patients, since relatively milder deficits may not seem to justify the risks of EVT. To help fill this gap in information, we explored the effectiveness and safety of EVT in LVO patients with mild strokes (NIHSS <6) in a large, multicenter dataset that reflects worldwide daily practice.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design, Settings and Participants
We retrospectively pooled a cohort of acute ischemic stroke (AIS) patients from 8 USA and Spain centers with anterior circulation LVO and NIHSS <6 presenting within 24 hours from last-seen-normal between January, 2012 and March, 2017. LVO was defined as an occlusion of the anterior circulation (ICA, MCA: M1, M2, M3, M4, and, ACA) identified on the coronal CT angiogram, anterior-posterior projection of the magnetic resonance angiogram, or anterior-posterior conventional angiogram as read and adjudicated locally by the participating center. The study was approved by the institutional review boards of the participating centers (University of Texas, Houston, Texas; Valley Baptist Hospital, Harlingen, Texas; Kaiser Permanente, Los Angeles, California; University of Tennessee, Memphis; Kansas University, Kansas City; Baylor College of Medicine, Dallas, Texas; Rush University, Chicago, Illinois; and University Hospital Vall d’Hebron, Barcelona, Spain), which determined that informed consent was not required for this retrospective analysis.
Study Treatments, Exposures and Intervention
Patients were divided into EVT and medical management groups based on their locally determined treatment. Endovascular treatment included mechanical thrombectomy by means of retrievable stents, with or without adjunctive aspiration techniques. Primary aspiration or intra-arterial thrombolytics were given in some cases. Medical management was based on current AHA guidelines, including IV-alteplase in patients presenting within the first 4.5 hours from last-seen-normal and meeting other guideline criteria. In patients not eligible for IV-alteplase, an antiplatelet agent was administered on day 1, unless there was an indication for early anticoagulation.
Demographics, Variables, and Measurements
Information on baseline demographics, vascular risk factors, last-seen-normal, admission blood glucose level, NIHSS score (range, 0–42, with higher scores indicating severe stroke), IV-alteplase administration and time, if applicable, and EVT center arrival time were obtained from the prospectively collected stroke registries of the different centers. Other clinical endpoints obtained included symptomatic intracerebral hemorrhage (sICH) (defined as a parenchymal hematoma grade 2 associated with worsening neurologic status thought to be related to the hematoma), neurologic deterioration (defined as a ≥4-point increase in the NIHSS score), and functional outcome at 90 days as measured by modified Rankin Scale (range, 0–6, with lower scores indicating better outcomes). Type of mechanical thrombectomy device, intra-arterial thrombolytic, conscious sedation (CS) or general anesthesia (GA), groin puncture time, time to recanalization (mTICI ≥ 2b if achieved or end of procedure), and duration of the procedure were also reported from the respective centers’ databases.
Imaging Analysis
Early ischemic changes were measured by the Alberta Stroke Program Early Computed Tomographic Score (ASPECTS)12 on non–contrast-enhanced CT head scans. For recanalization, the modified Thrombolysis in Ischemic Stroke score (mTICI (range, 0 [no perfusion] to 3 [full perfusion with filling of all distal branches])13 was used; successful reperfusion (partial and complete) was defined as a modified score ≥ 2b at the end of the procedure. The CT and angiographic images were adjudicated locally at the treatment center.
Study Outcomes
90 day excellent outcome (mRS 0–1) was chosen as the primary outcome as it is a more appropriate goal for mild strokes than mRS 0–2. Independent outcome (mRS 0–2) was a secondary outcome. Rates of hemorrhage and symptomatic hemorrhage (SITS-MOST criteria14) were safety outcomes. Other outcomes included rates of asymptomatic hemorrhage (parenchymal hematoma (PH) type 1 and 2, and reperfusion in the EVT group measured by the modified Thrombolysis in Ischemic Strokes core (mTICI).13
Statistical Analysis
Demographics, baseline characteristics, and clinical outcomes in EVT patients were compared to those who had medical management by two sample t-test or Wilcoxon rank sum test as appropriate for continuous variables and Chi-square test or Fisher’s exact test as appropriate for categorical variables. Multivariable logistic regression was conducted to evaluate the differences in outcome between the two groups after adjustment for pre-specified variables including age, presentation NIHSS, time last-seen-normal to EVT center arrival, center, IV-alteplase, ASPECTS, and thrombus location. We also explored whether there was any interaction between treatment differences and treatment time windows (0–6 hours and >6–24 hours) from last-seen-normal to treatment time. Unadjusted odds ratio (OR) and adjusted OR (aOR) along with their 95% confidence intervals (CI) were reported.
We assessed the interaction between thrombectomy treatment effect and thrombus location. Further exploratory analysis was done to evaluate thrombus-location-specific differences in outcomes between the two treatment groups (EVT versus medical management) and thrombus location (M1, ICA, M2 and M3/M4/ACA) as well as dichotomized thrombus location (proximal: M1+ICA versus distal: M2+M3+M4+ACA).
Furthermore, we matched EVT and medical management patients using propensity scores. A multivariable logistic regression model was built to calculate propensity score for each patient. The model included the following pre-specified variables: age, NIHSS, IV-alteplase, transfer status, wake-up stroke status, onset to arrival time, and thrombus location. We applied digit-based greedy approach15 to match patients. Initially, we matched EVT patients to medical management patients within all thrombus locations. In addition, we matched EVT and medical management patients within each dichotomized thrombus locations (proximal and distal). We assessed the distribution of patient characteristics between two groups in the matched samples through two sample t-test or Wilcoxon rank sum test as appropriate for continuous variables and Chi-square test or Fisher’s exact test for categorical variables. The odds ratio along with its 95% confidence interval based on conditional logistic regression were reported for the matched samples. All analyses were performed using SAS 9.4 (Cary, NC) and a two-sided p-value less than 0.05 was considered as statistically significant.
Results
Baseline Characteristics
214 patients were included; 124 received EVT and 90 medical management. The two groups had similar baseline age, ASPECTS, % IV-alteplase and median time (mins, IQR range) to EVT center as illustrated in Table 1. Medical management patients had milder strokes (median NIHSS: 3 versus 4, p=0.005) than EVT. Most patients had MCA occlusion (M1 or M2) (EVT 54.8%-M1 and 26.6%-M2) as compared to medical management (23.3%-M1 and 48.9%-M2), while 16.9% of EVT and 13.3% of medical management patients had ICA occlusions (Table 1).
Table 1.
Baseline characteristics of EVT and medical management patients
| Variable | EVT (N=124) | Medical Management (N=90) | P value |
|---|---|---|---|
| Age, years (mean ±SD) | 65.8±15.5 | 65.4±16.2 | 0.85* |
| Males, % | 58.1 | 56.7 | 0.84† |
|
NIHSS at presentation Median (Q1, Q3) |
4.0 (2.0, 5.0) | 3.0 (2.0, 4.0) | 0.005‡ |
| NIHSS breakdown, % | |||
| 0 | 7.3 | 0.0 | |
| 1 | 4.0 | 18.9 | |
| 2 | 16.1 | 16.7 | |
| 3 | 10.5 | 24.4 | |
| 4 | 25.8 | 22.2 | |
| 5 | 36.3 | 17.8 | |
|
Blood glucose level, mg/dL Median (Q1, Q3) |
118.0 (101.0, 158.0) | 115.0 (101.0, 150.0) | 0.92‡ |
| Hypertension, % | 70.2 | 80.0 | 0.10† |
| Type II Diabetes, % | 29.0 | 30.0 | 0.88† |
| Wake up strokes, % | 17.7 | 13.3 | 0.40† |
| Transfers, % | 20.5 | 45.6 | <.001† |
| IV-alteplase given, % | 31.4 | 32.2 | 0.90† |
| Left sided occlusion, % | 45.5 | 52.2 | 0.76§ |
| Thrombus location, % | <.001† | ||
| Proximal (M1+ ICA) M1 ICA |
71.8 54.8 16.9 |
36.7 23.3 13.3 |
|
| Distal (M2+M3+M4+ACA) M2 M3 / M4/ ACA |
28.2 26.6 1.6 |
63.3 48.9 14.4 |
|
| ASPECTS (mean ±SD) | 9.7±0.9 | 9.4±1.1 | 0.11* |
|
Onset to EVT arrival time, minutes Median (Q1, Q3) |
183.5 (83.0, 312.0) | 226.0 (94.0, 401.0) | 0.15‡ |
| mTICI Pre-Procedure, % | |||
| 0 | 83.1 | − | − |
| 1 | 9.2 | − | |
| 2a | 7.7 | − | |
|
Last-seen-normal to reperfusion time, minutes Median (Q1, Q3) |
350.0 (268.0, 574.0) | − | − |
denotes p-values obtained by two sample t-test
denotes p-values obtained by Chi-square test
denotes p-values obtained by Wilcoxon rank sum test
denotes p-values obtained by Fisher’s exact test
NIHSS: National Institute of Health Stroke Scale, EVT: Endovascular Thrombectomy, ICA: Internal Carotid Artery, M1: Middle Cerebral Artery/M1 segment occlusion, M2: Middle Cerebral Artery/M2 segment occlusion, M3: Middle Cerebral Artery/M3 segment occlusion, M4: Middle Cerebral Artery/M4 segment occlusion, mTICI: the modified Thrombolysis in Ischemic Stroke score, ASPECTS: The Alberta stroke program early CT score, IV-alteplase: Intra Venous Tissue Plasminogen Activator
Clinical Outcomes Comparison
Tables 2 summarizes the ORs and aORs through multivariable regression analyses in the whole cohort and after stratification by thrombus location for excellent outcomes. There was no difference in excellent (mRS 0–1) outcome between the groups (55.7% EVT versus 54.4% medical management, Figure 1A), aOR 1.30 (95% CI 0.64–2.64, p=0.47, Table 2). Similar results were seen for independent outcome (63.3% EVT versus 67.8% medical management), aOR 0.90 (95%CI=0.43–1.88, p=0.77)] (Table 1). There was no interaction between outcome differences with EVT versus medical management and time from last-seen-normal to treatment time; early (0–6 hrs) versus late (>6–24 hrs) time windows (p=0.98).
Table 2.
Excellent outcome rates (mRS 0–1), unadjusted and adjusted ORs, between EVT and medical management only stratified by thrombus location
| Excellent outcome (mRS 0–1) % | Excellent outcome (mRS 0–1) | |||||
|---|---|---|---|---|---|---|
| Thrombus Location | EVT | Medical Management | OR (95% CI) | P value | aOR (95%CI) | P value |
| All (n=214) | 55.7 | 54.4 | 1.05 (0.57, 1.93) | 0.87 | 1.30 (0.64, 2.64) | 0.47 |
| M1 (n=89) | 52.8 | 23.8 | 3.58 (1.07, 11.97) | 0.04 | 3.31 (0.92, 11.94) | 0.07 |
| ICA (n=33) | 53.8 | 41.7 | 1.63 (0.33, 8.05) | 0.54 | 1.95 (0.35, 10.77) | 0.44 |
| Proximal*(n=122) | 53.1 | 30.3 | 2.60 (1.02, 6.64) | 0.05 | 2.68 (0.98, 7.32) | 0.05 |
| M2 (n=77) | 64.3 | 72.7 | 0.68 (0.24, 1.88) | 0.45 | 0.68 (0.23, 2.01) | 0.48 |
| M3/M4/ACA (n=15) | 0 | 53.8 | − | − | − | − |
| Distal† (n=92) | 60 | 68.4 | 0.69 (0.27, 1.75) | 0.43 | 0.70 (0.26, 1.87) | 0.47 |
Proximal occlusions included M1+ICA
Distal occlusions included M2+M3/M4/ACA
Figure 1A.
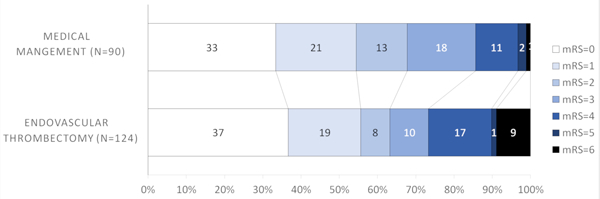
Modified Rankin Scale scores (mRS) at 90 days compared between endovascular thrombectomy and medical management for all thrombus locations
Clinical Outcome Comparison by Thrombus Location
Patients with mild strokes due to proximal occlusions (M1 and ICA) were more likely to receive thrombectomy (54.8% and 16.9% EVT versus 23.3% and 13.3% medical management, respectively, p<0.001) while those with more distal occlusions (M2, M3/M4) were more likely to receive medical management only (26.6% and 1.6% EVT versus 48.9% and 14.4% medical management).
There was no statistically significant interaction between thrombectomy treatment effect and thrombus location (p=0.15). Since patients with more proximal occlusions are more likely to have larger areas at risk despite of mild deficits, we further explored if there was a potential treatment effect difference by the different thrombus location strata. The rates of excellent outcomes were higher with EVT in patients with M1 occlusions (52.8% EVT versus 23.8% medical management, Figure 1B) and ICA (53.8% EVT versus 41.7% medical management, Figure 1C). For M1 occlusions, the aOR for excellent outcome was 3.31 (95%CI=0.92–11.94, p=0.07) (Table 2). The difference approached but not did reach statistical significance for M1 occlusions, suggesting that patients with M1 occlusions might potentially benefit from thrombectomy. There was no statistical significance for ICA occlusions (aOR=1.95, 95% CI=0.35, 10.77, p=0.44) (Table 2).
Figure 1B.
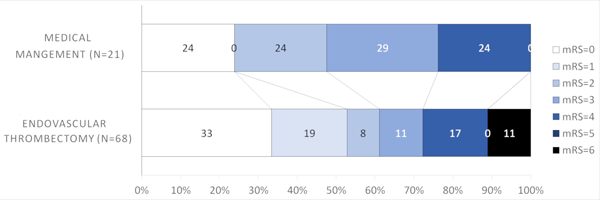
Modified Rankin Scale scores (mRS) at 90 days compared between endovascular thrombectomy and medical management for M1 thrombus location
Figure 1C.
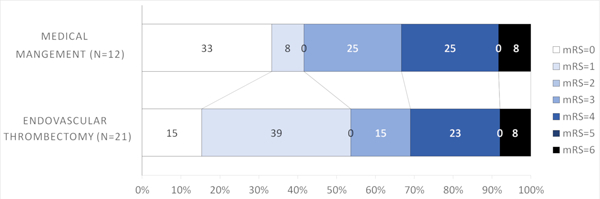
Modified Rankin Scale scores (mRS) at 90 days compared between endovascular thrombectomy and medical management for ICA thrombus location
Conversely, it appeared that patients with more distal occlusions did not benefit with thrombectomy; M2 (64.3% EVT versus 72.7% medical management, Figure 1D) and M3/M4/ACA (0% EVT versus 53.8% medical management, Figure 1). Similar results were observed for the secondary outcome (mRS=0–2) (Table 1). When dichotomizing thrombus location into proximal (M1+ICA) and distal (M2+M3+M4+ACA), patients with proximal occlusions had more excellent outcomes with EVT that approached but did not reach statistical significance (53.1% EVT versus 30.3% medical management, aOR =2.68, 95%CI=0.98–7.32, p=0.05) (Figure 2A, Table 2). There was no difference in excellent outcome rates in patients with distal occlusions (60% EVT versus 68.4% medical management, Figure 2B).
Figure 1D.
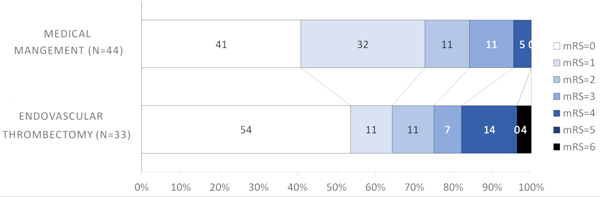
Modified Rankin Scale scores (mRS) at 90 days compared between endovascular thrombectomy and medical management for M2 thrombus location
Figure 2A.
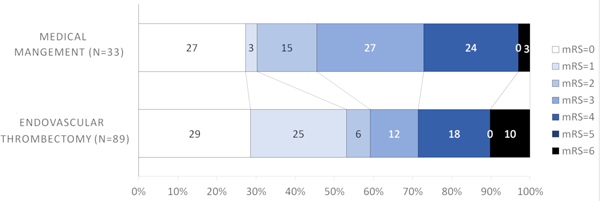
Modified Rankin Scale scores (mRS) at 90 days compared between endovascular thrombectomy and medical management for proximal (M1+ICA) thrombus location
Figure 2B.
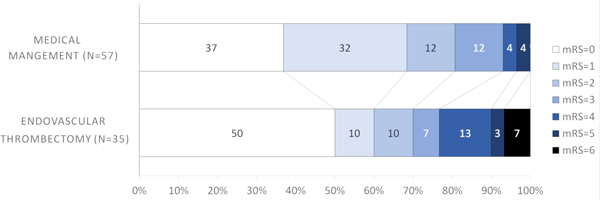
Modified Rankin Scale scores (mRS) at 90 days compared between endovascular thrombectomy and medical management for distal (M2+M3+M4+ACA) thrombus location
Clinical Outcome Comparison with Propensity Score Matching
We matched 62 pairs of EVT and medical management patients. The distributions of patient characteristics between two groups in the matched sample were similar (Table 2). There was no treatment effect for excellent outcome in the matched sample (53.5% EVT, 48.4% medical management; OR=1.17, 95%CI=0.54–2.52, p=0.69). Similar results were seen when matching within proximal and distal thrombus locations, respectively (Tables 3 and 4 show baseline characteristics of the matched pairs in the two treatment groups). 32 pairs were matched within proximal thrombus location and 29 pairs were matched within distal with no difference in excellent outcomes with EVT for proximal thrombi (47.1% EVT, 31.3% medical management; OR=1.67; 95%CI=0.40–6.97, p=0.48) or distal thrombi (56% EVT, 69% medical management; OR=0.78; 95%CI=0.29–2.09, p=0.62).
Table 3.
Clinical and radiographical outcomes
| Variable | EVT | Medical Management | Total | P value |
|---|---|---|---|---|
| Asymptomatic hemorrhage, % | 7.9 | 10.0 | 8.8 | 0.60† |
| Symptomatic hemorrhage, % | 5.8 | 0.0 | 3.3 | 0.02§ |
| PH1, % | 5.3 | 10.0 | 7.4 | 0.20† |
| PH2, % | 94.7 | 90.0 | 92.6 | 0.20† |
| 90 day mRS, % | ||||
| 0 | 36.7 | 33.3 | 34.9 | |
| 1 | 19.0 | 21.1 | 20.1 | |
| 2 | 7.6 | 13.3 | 10.7 | |
| 3 | 10.1 | 17.8 | 14.2 | |
| 4 | 16.5 | 11.1 | 13.6 | |
| 5 | 1.3 | 2.2 | 1.8 | |
| 6 | 8.9 | 1.1 | 4.7 | |
| Excellent outcome at 90 days, % | 55.7 | 54.4 | 55.0 | 0.87† |
| Independent outcome at 90 days, % | 63.3 | 67.8 | 65.7 | 0.54† |
| Post procedure mTICI score, % | ||||
| 0 | 12.3 | 12.3 | ||
| 1 | 0.9 | 0.9 | ||
| 2a | 8.8 | 8.8 | ||
| 2b | 21.9 | 21.9 | ||
| 3 | 56.1 | 56.1 |
Denotes p-values obtained by Chi-square test
denotes p-values obtained by Fisher’s exact test
mRS: Modified Rankin Scale, EVT: Endovascular Thrombectomy, mTICI: the modified Thrombolysis in Ischemic Stroke sxscore
Table 4.
Characteristics of EVT patients with sICH and without sICH
| Variable | sICH | Non-sICH | P value |
|---|---|---|---|
| Age, Mean ± SD | 68.0±14.7 | 65.6±15.6 | 0.69* |
| ASPECTS, Mean ± SD | 9.5±0.8 | 9.7±0.9 | 0.67* |
| IV-alteplase given, % | 14.3 | 32.4 | 0.43§ |
| Last-seen-normal to reperfusion time, Median (Q1, Q3) | 497.5 (453.5, 802.5) | 340.0 (261.0, 574.0) | 0.11‡ |
| NIHSS at presentation, Median (Q1, Q3) | 3.0 (0.0, 4.0) | 4.0 (2.0, 5.0) | 0.17‡ |
| Hypertension, % | 57.1 | 70.2 | 0.44§ |
| Thrombus Location, % | 0.49§ | ||
| M1 MCA | 57.1 | 54.4 | |
| M2 MCA | 42.9 | 25.4 | |
| M3/M4 ACA | 0.0 | 1.8 | |
| Proximal or Distal ICA | 0.0 | 18.4 | |
| Number of Passes, % | 0.26§ | ||
| Single | 28.6 | 52.6 | |
| Multiple | 71.4 | 47.4 |
denotes p-values obtained by two sample t-test
denotes p-values obtained by Wilcoxon rank sum test
denotes p-values obtained by Fisher’s exact test
Safety Outcomes
There was no difference in the incidence of PH1 and PH2 (5.3% EVT versus 10% medical management, p=0.20) (Table 3). However, 5.8% of patients in the EVT group had sICH, as compared to none in the medical management group (p=0.02). Further analysis of patients who had sICH did not show any difference in IV-alteplase rate, ASPECTS, age, reperfusion times, NIHSS, history of HTN, or thrombus location as compared to those who did not have sICH (Table 4). We observed increased sICH with multiple passes during EVT (as compared to a single pass), but this difference did not reach significance (p=0.26).
There was a statistically significant association between sICH and mortality (p=0.01) and patients undergoing EVT had higher mortality (8.9% EVT versus 1.1 % medical management, p=0.03).
Discussion
Patients with milder strokes usually have independent outcome, but those with LVOs may face a different fate. In our cohort of patients with baseline NIHSS <6, one third (32.2%) of those receiving medical management had 90 day mRS >2. The benefit of EVT for LVO in milder stroke patients remains uncertain. A patient-level pooled meta-analysis of 5 RCTs showed no clear benefit of EVT in 177 patients across the entire NIHSS range 0 to 10 (aOR 1.67 95%CI 0.80–3.50, p=0.45)11 but results in the cohort of patients with NIHSS <6 was not reported. These results are affected by the small number of patients pooled, since most of these RCTs’ inclusion criteria started at NIHSS threshold of 6 2−5 and MR CLEAN at 2.1 This illustrates the need for data specifically in this subgroup of patients with mild strokes.
Prior studies have attempted to address outcomes with EVT in milder stroke patients but were limited by single center reporting,16 small sample size16–18 or lack of a concurrent medical management group.19 In a single center retrospective study, Haussen et al. reported a shift towards better discharge NIHSS in EVT patients (−2.5 versus 0, p<0.01) but there was no statistical difference in 90 day mRS 0–2 (100% EVT versus 77% medical management, p=0.15) or mRS 0–1 (70% versus 57% respectively p=0.19).16 An analysis of the GESTOR and STOPstroke databases with 26 matched pairs showed increased rates of independence with EVT at 90 days (93% versus 69%, p=0.04).18 In a 3 center single arm study of 138 patients with NIHSS ≤7, Dargazanli et al. reported excellent outcomes with EVT in 65% and independent outcomes in 78%,19 but there was no superiority of EVT when compared to medical management when the authors further evaluated data from the Endovascular Treatment in Ischemic Stroke (ETIS) cohort.20 Similarly, an analysis of 78 patients with mild LVO stroke (NIHSS ≤5) from the multicenter SONIIA registry17 showed no benefit of EVT over medical treatment (58.8% EVT versus 68.2% medical management, p=0.39) with an excess of sICH in patients receiving EVT (11.8%). IV thrombolysis was the single independent predictor of independent outcome in that cohort. With 3 studies reporting no adjunctive benefit with EVT in this population and one reporting increased rates of independent outcomes, these inconclusive results emphasize the need for additional higher quality data in this population.
Our results showed there was no overall EVT treatment benefit in patients with NIHSS <6. In a secondary analysis comparing the two treatments stratified by thrombus location, patients with more proximal occlusions (ICA and M1) were more likely to receive EVT compared to those with more distal occlusions. Since patients with more proximal occlusions would, theoretically, have a larger area at risk, it is plausible that there would be an interaction between thrombectomy treatment effect and thrombus location. Our data did not support treatment effect modification by the overall thrombus location. We, however, conducted further exploratory analyses to look at treatment effect difference after stratifying by thrombus location. Although not statistically significant, and limited by small numbers, our analyses suggested that patients with more distal occlusions have better outcome with medical management. Furthermore, our data showed that there might be a potential treatment effect for patients with M1 occlusions as the difference approached statistical significance. These analyses need to be verified in other cohorts, hopefully with randomized data. If thrombectomy is to be studied further in LVO patients with NIHSS <6, our data suggest that only patients with proximal occlusions should be the target population. Furthermore, we performed propensity matching as a sensitivity and confirmatory analysis and the results remained the same with no statistical significance in outcome differences between EVT and medical management groups.
In regards to safety outcomes, while the rate of disability was the same in both groups, EVT was associated with higher rates of sICH and mortality compared to medical management. These findings support the concerns around the safety of the intervention in this population and advocate for a careful approach when entertaining mild strokes with LVO for thrombectomy.
Our findings are limited by the retrospective design and lack of a randomized comparison group. While a randomized trial would provide the highest level of evidence, it may have significant feasibility challenges. RCT in this population would be difficult to power since there was no treatment effect when including all thrombus locations, difficult to enroll given the limited number of patients would meet the eligibility criteria if the trial was restricted to only proximal occlusions, and difficult to execute given the difficulty differentiating patients with one or two points differences in NIHSS (<6 versus ≥6). The lack of randomized data in this population is probably due to the fact that this is a challenging population to conduct an RCT on. Having said that, there are two RCTs that are currently being planned. These trials will bring conclusive answers and complement our data. In the meanwhile, however, our data serve as a good level of foundational evidence. The other limitation of our study is that although the EVT and medical management groups were mostly similar in baseline characteristics, the patients were from 8 centers with different EVT criteria where selection for thrombectomy was not randomized and was based on institutional protocols and treating physicians’ decisions. Selection bias might have occurred since those patients who were viewed as more likely to benefit from EVT were the ones treated with the intervention. Furthermore, there were differences in baseline characteristics and given the nature of the retrospective study, treatment decision was not randomized or prospectively protocolized. We attempted to account for these treatment patterns by utilizing multivariable regression models for adjustment and performing propensity matching analyses but this might not have completely accounted for selection biases, as patients who were felt to benefit from thrombectomy by the treating physicians were undoubtedly selected for the treatment. While we adjusted for variables known to affect clinical outcomes, the lack of randomization prevented us from accounting for unmeasured covariates. We only have data on CT changes (ASPECTS) as a baseline imaging modality and we considered that in our analyses. Other advanced imaging modalities, which further evaluate ‘at risk’ tissue, such as CT Perfusion, magnetic resonance perfusion, and collateral status might have been taken into consideration to guide treatment decisions.
The strength of our data is that it represents a large cohort derived from real world daily practice with a concurrent medical management group.
Summary/Conclusions
Our retrospective multicenter cohort study showed no increase in excellent and independent functional outcomes in patients receiving EVT, with increased rates of sICH and mortality rates. These findings are consistent with the current guidelines that recommend the treatment only in patients with NIHSS ≥6.
Supplementary Material
Acknowledgments
Funding
This study had no funding. Sean I Savitz was supported by National Institutes of Health funding
Footnotes
Conflict of Interest and Disclosures
Amrou Sarraj is the Principal Investigator of the SELECT and SELECT 2 trials with unrestricted grant from Stryker Neurovascular; consultant, speaker bureau, and advisory board member for Stryker Neurovascular; as well as site PI for DEFUSE 3 trial which was funded by the National Institutes of Health. Michael Abraham is consultant for Stryker Neurovascular and Penumbra Inc. Michael Chen is consultant for Medtronic and Penumbra, as well as an advisory board member for Stryker Neurovascular and Genentech.
Contributor Information
Amrou Sarraj, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Ameer Hassan, Department of Interventional Neurology, University of Texas - Rio Grande Valley, Harlingen, TX.
Sean I Savitz, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
James C Grotta, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Chunyan Cai, Department of Internal Medicine, University of Texas Health Science Center at Houston, Houston, TX.
Kaushik N Parsha, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Christine M Farrell, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Bita Imam, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Clark W Sitton, Department of Diagnostic & Interventional Imaging, University of Texas Health Science Center at Houston, Houston, TX.
Sujan T Reddy, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Haris Kamal, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Nitin Goyal, Department of Neurology & Neurosurgery, University of Tennessee Health Sciences Center, Memphis, TN.
Lucas Elijovich, Department of Neurology & Neurosurgery, University of Tennessee Health Sciences Center, Memphis, TN.
Katelin Reishus, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Rashi Krishnan, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN.
Navdeep Sangha, Department of Neurology, Kaiser Permanente Los Angeles, CA..
Abel Wu, Department of Neurology, Kaiser Permanente Los Angeles, CA..
Renata Costa, Department of Neurology, Rush University Medical Center, Chicago, IL.
Ruqayyah Malik, Department of Neurology, Rush University Medical Center, Chicago, IL.
Osman Mir, Department of Neurology, Baylor University Medical Center, Texas A&M University, Dallas, TX.
Rashedul Hasan, Department of Neurology, Baylor University Medical Center, Texas A&M University, Dallas, TX.
Lindsay M Snodgrass, Department of Neuroscience, Baylor University Medical Center, Dallas, TX.
Manuel Requena, Department of Neurology, Vall d’Hebron University Hospital, Barcelona, Spain..
Dion Graybeal, Department of Neurology, Baylor University Medical Center, Texas A&M University, Dallas, TX.
Michael Chen, Department of Neurology, Rush University Medical Center, Chicago, IL.
Michael Abraham, Department of Neurology and Radiology, Kansas University Hospital, Kansas, MO.
Louise D McCullough, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX.
Marc Ribo, Department of Neurology, Vall d’Hebron University Hospital, Barcelona, Spain..
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N Engl J Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med. 2015; 372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N Engl J Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 6.Muir KW, Ford GA, Messow CM, Ford I, Murray A, Clifton A, et al. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 10.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 12.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. [DOI] [PubMed] [Google Scholar]
- 13.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITSMOST): An observational study. Lancet. 2007;369:275–282. [DOI] [PubMed] [Google Scholar]
- 15.Parsons L Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. SAS Users Group International. 2004; 26:214–226. [Google Scholar]
- 16.Haussen DC, Bouslama M, Grossberg JA, Anderson A, Belagage S, Frankel M, et al. Too good to intervene? Thrombectomy for large vessel occlusion strokes with minimal symptoms: an intention-to-treat analysis. J NeuroInterventional Surg. 2017;9:917–921. [DOI] [PubMed] [Google Scholar]
- 17.Urra X, San Román L, Gil F, Millán M, Cánovas D, Roquer J, et al. Medical and Endovascular Treatment of Patients with Large Vessel Occlusion Presenting with Mild Symptoms: An Observational Multicenter Study. Cerebrovasc Dis. 2014;38:418–424. [DOI] [PubMed] [Google Scholar]
- 18.Haussen DC, Lima FO, Bouslama M, Grossberg JA, Silva GS, Lev MH, et al. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: an analysis from STOPStroke and GESTOR cohorts. J Neurointerv Surg. 2018;10:325–329. [DOI] [PubMed] [Google Scholar]
- 19.Dargazanli C, Consoli A, Gory B, Blanc R, Labreuche J, Preda C, et al. Is Reperfusion Useful in Ischaemic Stroke Patients Presenting with a Low National Institutes of Health Stroke Scale and a Proximal Large Vessel Occlusion of the Anterior Circulation? Cerebrovasc Dis. 2017;43:305–312. [DOI] [PubMed] [Google Scholar]
- 20.Dargazanli C, Arquizan C, Gory B, Consoli A, Labreuche J, Redjem H, et al. Mechanical Thrombectomy for Minor and Mild Stroke Patients Harboring Large Vessel Occlusion in the Anterior Circulation: A Multicenter Cohort Study. Stroke. 2017;48:3274–3281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


