Abstract
Disruption of the gut microbiota, termed gut dysbiosis, has been described in animal models of hypertension and hypertensive patients. We have shown that gut dysbiosis plays a causal role in the development of hypertension in a rat model of obstructive sleep apnea (OSA). Functional analysis of the dysbiotic microbiota in OSA demonstrates a loss of short chain fatty acid (SCFA) producing bacteria. However, measurements of SCFA concentrations and testing of their role in blood pressure regulation is lacking. We hypothesized that reduced SCFAs in the gut are responsible for OSA-induced hypertension. OSA significantly increased SBP at 7 and 14 days (p<0.05), an effect that was abolished by the probiotic Clostridium butyricum or the prebiotic Hylon VII. 16S rRNA analysis identified a number of SCFA producing bacteria that were significantly increased by C.butyricum and Hylon treatment. Acetate concentration in the cecum was decreased by 48% following OSA (p<0.05), an effect that was prevented by C.butyricum and Hylon. C.butyricum and Hylon reduced OSA-induced dysbiosis, epithelial goblet cell loss, mucus barrier thinning, and activation of brain microglia (p<0.05 for each). To examine the role of acetate in OSA-induced hypertension, we chronically infused acetate into the cecum during 2 weeks of sham or OSA. Restoring cecal acetate concentration prevented OSA-induced gut inflammation and hypertension (p<0.05). These studies identify acetate as a key player in OSA-induced hypertension. We demonstrate that various methods to increase cecal acetate concentrations are protective from the adverse effects of OSA on the microbiota, gut, brain, and blood pressure.
Keywords: Obstructive sleep apnea, hypertension, short chain fatty acid, dysbiosis, acetate
Introduction
In the last decade it has become increasingly apparent that disruption of the native gut microbiota is strongly linked to a number of disease states including metabolic disorders (obesity and diabetes), neurological diseases (stroke outcome and Alzheimer’s), and cardiovascular diseases (atherosclerosis and hypertension).1–14 Evidence for the role of the gut microbiota in development of hypertension has been obtained in four different animal models of hypertension and to a lesser extent for hypertension in humans.1–4, 6, 15, 16 While there is strong evidence for a role of the gut microbiota in hypertension, the mechanism how the microbiota induce hypertension is not well understood. Although circumstantial, several studies suggest that decreases in bacterial-derived short chain fatty acids (SCFAs) may have a prominent role in the onset of hypertension.2–4, 17,18
The majority of the SCFAs (primarily acetic, propionic, and butyric acids) in the host are produced via fermentation of dietary undigestible fibers and starches by bacteria residing in the lower gastrointestinal tract.19 These SCFAs can affect the host through activation of G protein coupled receptors or inhibition of histone deacetylases.19–21 Working through either or both of these pathways, SCFAs stabilize the gut epithelial barrier, modulate cytokine secretion, alter T-lymphocyte populations, increase the protective mucus layer, and modulate antibody secretion.19, 22 In addition to having an effect on the gut, SCFAs can influence tissues and organs beyond the gut if they gain access to the circulation.18, 21, 23–25
We recently reported that a high fat diet (HFD) synergizes with apnea in a rat model of obstructive sleep apnea (OSA) to elicit hypertension.2 When the cecal and colonic contents of hypertensive rats were gavaged into rats, which were otherwise not destined for hypertension, hypertension developed over the course of two weeks.2 This key experiment proved that something in the gut contents (i.e., microbiota) was necessary to elicit hypertension. We found that this OSA-induced hypertension was accompanied by decreases in bacteria that produced SCFAs.2 Similarly, decreased SCFA producing bacterial abundance has also been described in spontaneously hypertensive rats.1, 4 While these observations are suggestive, we do not know if SCFAs are actually decreased in our model of OSA-induced hypertension.
Given that a bacterially-derived SCFA could have a role in the development of hypertension, we tested the hypothesis that decreases in SCFAs are responsible for hypertension in a rat model of OSA. We report that hypertension in our model is accompanied by a 48% decrease in acetate in the cecum without any significant change in butyrate or propionate. Additionally, we prevented OSA-induced hypertension using highly translatable pre- and probiotic treatments that maintained normal acetate concentrations in OSA rats. To demonstrate a definitive role for decreased acetate in OSA-induced hypertension, we abolished hypertension in our model by restoring acetate levels by directly infusing acetate into the cecum though a chronically inserted cannula. To examine a potential mechanistic link between dysbiosis and hypertension we examined the effects of OSA, pre-, and probiotics on neuroinflammation, a key component of OSA-induced hypertension.26–28 We show that OSA increased neuroinflammation, which was prevented by pre- and probiotic treatments.
Materials and Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Experimental Animals:
Animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, published by the National Institutes of Health (NIH) and were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine, Houston, TX. Eight weeks old male Long Evans rats, purchased from Charles Rivers Laboratories, were allowed to acclimate for 2 weeks with a 12 hr light (6AM-6PM): 12 hr dark (6PM-6AM) cycle before being randomized to HFD, HFD+Hylon, or HFD+C.butyricum.
Statistics:
Line and bar plot data is expressed as mean ± SEM. One-way analysis of variance was performed followed by Tukey’s Tukey’s multiple comparison test (Figs. 2C-E, Figs. S2A-B). Two-way analysis of variance was performed followed by Holm-Sidak post-hoc analysis when main effects were found to be significant (Figs. 4A, 4C, 4E, 5A-E, 6B, 6C, S1B, S3A, S3B, S3D, S3E, S4A-F). When analyzing blood pressure over several timepoints, a two-way repeated measures ANOVA was used followed by a Holm-Sidak test for individual comparisons when appropriate (Fig. 1 and 5F). Differences were considered statistically significant if p≤0.05.
Figure 2.
C.butyricum and Hylon alter the makeup of the gut microbiota. Weighted Unifrac principal coordinate analysis shows separation of HFD+Hylon treated microbiota from HFD and HFD+C.butyricum (A). LEFSe analysis identified several taxa that characterize HFD vs HFD+C.butyricum vs HFD+Hylon. Major SCFA produced by genera are shown to the right of each bar (B; A=acetate, B=butyrate, P=propionate). Relative abundance of taxa identified by LEFSe analysis (LDA Score >4) to characterize HFD (C, RC4–4, Akkermansia, and Coprococcus), C.butyricum (D, Clostridiales and Parabacteroides), and Hylon (E, Bifidobacterium, Ruminococcus, Blautia, and Colinsella). Data are shown as the mean ± S.E.M. n=6 (HFD and HFD+Hylon) or 7 (HFD+C.butyricum) for all analyses, *p<0.05 vs HFD alone, **p<0.01 vs HFD alone, ****p<0.0001 vs HFD alone.
Figure 4.
Adverse effects of OSA on the cecum wall are reduced by C.butyricum and Hylon. A and B, Goblet cells (arrowhead) per crypt were reduced in the cecum following OSA in HFD, but not HFD+C.butyricum or HFD+Hylon rats. C and D, Separation of luminal bacteria (red, EUB338 FISH probe) and epithelium (blue, DAPI) was reduced in the cecum following OSA in HFD, but not HFD+C.butyricum or HFD+Hylon rats. E, Epithelial barrier integrity was impaired in HFD and HFD+C.butyricum OSA rats, as compared to shams. Data are shown as the mean ± S.E.M. n=6 for A, n=5 (HFD Sham and OSA), 6 (HFD+Hylon Sham and HFD+C.butyricum OSA) or 7 (HFD+C.butyricum Sham and HFD+Hylon OSA) in E, n=3 for C. *p<0.05 vs sham of same treatment, **p<0.01 vs sham of same treatment.
Figure 5.
OSA reduces cecal acetate concentration, which is prevented by C.butyricum, Hylon, and acetate infusion. OSA reduced cecal acetate concentrations in HFD alone, but not HFD+C.butyricum or HFD+Hylon treated rats (A). Portal plasma concentration of acetate (B) was not significantly different between sham and OSA in any treatment group. Hylon, independent of OSA, increased cecal and plasma acetate concentrations (A and B). PBS (vehicle) treated rats had decreased cecal acetate concentrations following OSA, which was prevented by cecal acetate infusion (C). OSA significantly increased cecal IL-1α and IL-6 mRNA, which was prevented with cecal acetate infusion (D and E). Cecal acetate infusion prevented OSA-induced increase in SBP at 7 and 14 days of OSA (F). Data are shown as the mean ± S.E.M. n=5 (HFD+C.butyricum OSA) or 6 (all other groups) for A and B, n=6 for all groups in C-F, *p<0.05 vs sham of same treatment, #p<0.05 vs HFD.
Figure 6.
OSA increases activated microglia in brain, which is prevented by C.butyricum and Hylon treatment.A, Gating strategy used to identify activated microglia in brain by flow cytometry.B, Activated microglia were increased in brain following 2 weeks of OSA in HFD treated rats. No difference in activated microglia was observed following OSA in HFD+C.butyricum or HFD+Hylon treated rats.C, The percentage of T regulatory cells was not different between sham and OSA in any of the treatment groups. Two-way ANOVA showed a main effect of treatment, with Hylon treatment increasing T regulatory cells in brain. Data are shown as the mean ± S.E.M. n=4 (HFD OSA), or 5 (HFD Sham, HFD+C.butyricum Sham and OSA), or 6 (HFD+Hylon Sham and OSA) for all analyses. *p<0.05 vs sham, #p<0.05 vs. HFD sham.
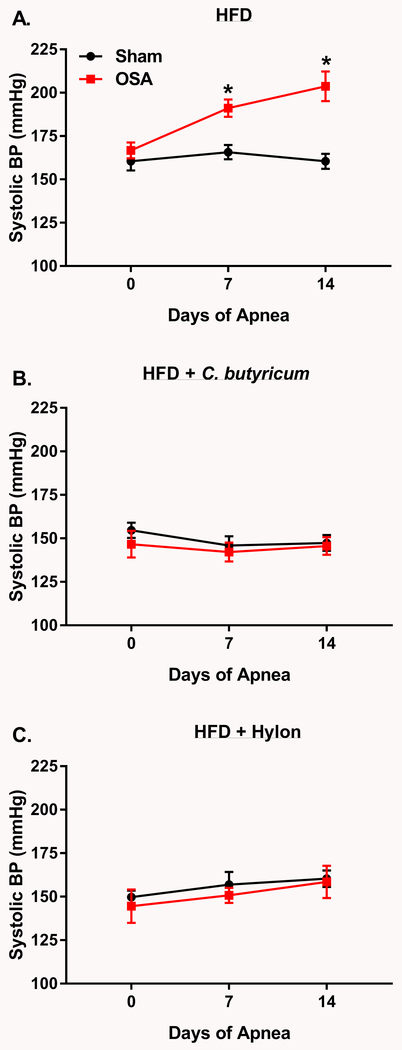
Figure 1.
C.butyricum and Hylon prevent OSA-induced hypertension.A, On HFD OSA rats exhibited significantly higher SBP, as compared to sham rats, after 7 and 14 days of OSA (n=7 per group). OSA-induced hypertension was prevented by C.butyricum (B, n=7 (OSA) and 13 (Sham)) or Hylon treatment (C, n=6). Data are shown as the mean ± S.E.M., *p<0.05 for sham vs. OSA.
Figure 3.
Effects of OSA on the microbiota of HFD, HFD+C.butyricum, and HFD+Hylon treated rats. Weighted Unifrac principal coordinate analysis shows separation of Hylon treated microbiota, but no effect of OSA (A). Relative abundance of major phyla in HFD, HFD+C.butyricum, and HFD+Hylon sham and OSA rats (B). OSA altered the relative abundance of multiple bacterial taxa in HFD alone and HFD+C.butyricum, but not HFD+Hylon (C). LEFSe analysis was used to identify and calculate a linear discriminate analysis (LDA) score for taxa that characterize sham vs. OSA. In panel C, positive LDA scores indicate the enrichment of taxa in sham relative to OSA (green), and negative LDA scores indicate the enrichment of taxa in OSA relative to sham (red). Data are shown as the mean. n=6 (HFD and HFD+Hylon) or 7 (HFD+C.butyricum) for all analyses, *p<0.05 vs. HFD group.
Results
C.butyricum and Hylon prevented OSA-induced hypertension
In HFD fed rats, SBP significantly increased by 24 and 44mmHg after 1 and 2 weeks of OSA, respectively, as compared to sham rats. (Fig. 1A). Given that multiple animal models of hypertension exhibit a decrease in SCFA producing bacteria, we treated sham and OSA rats with the probiotic C.butyricum or the prebiotic Hylon VII. C.butyricum is capable of producing both acetate and butyrate, and is found in commercially available probiotic supplements.29 Hylon VII is a resistant corn starch that serves as a substrate for bacterial fermentation and SCFA generation, and has been shown to increase fecal acetate and butyrate concentrations.30 OSA-induced hypertension was prevented by treatment with either C.butyricum or Hylon VII (Figs. 1B and C). Two-way repeated measures ANOVA of the 6 groups presented in Figure 1 showed no significant effect of treatment (i.e., probiotic, prebiotic) on sham SBP. This indicates that probiotic and prebiotic were capable of preventing increased SBP due to OSA, but did not affect SBP in normotensive sham rats (Fig. 1).
C.butyricum and Hylon altered the gut microbiota composition
Compared to normotensive controls the microbiota has been shown to be altered in multiple models of hypertension.1, 2, 4, 6 Microbiota transplants between normotensive and hypertensive models have demonstrated that the microbiota plays a causal role in the development of hypertension.1, 2 Therefore, we initially examined the effects of C.butyricum and Hylon on the microbiota in the absence of OSA (i.e., shams alone). Community richness and diversity were not different between HFD alone and HFD+C.butyricum. Compared to HFD alone, HFD+Hylon significantly reduced community richness and diversity (Fig. S2A-B). Similarly, HFD+Hylon, but not HFD+C.butyricum, showed clear separation from HFD alone in both unweighted and weighted unifrac principal coordinate analysis (Fig. S2C and Fig. 2A). LEfSe analysis was performed in order to identify key taxa that characterized HFD alone vs. HFD+C.butyricum vs. HFD+Hylon. Figure 2B shows that 9, 12, and 4 taxa were found to be characteristic of HFD alone (blue bars), HFD+C.butyricum (red bars), and HFD+Hylon (orange bars), respectively. The relative abundance of taxa found to be the most characteristic (LDA score of >4.0; Fig. 2B) of each treatment group are presented in figures 2C thru E. Of particular interest, HFD alone was characterized by an increased abundance of RC4-4 and Akkermansia, as compared to HFD+C.butyricum or HFD+Hylon (Fig. 2C). Increased RC4-4 abundance has been associated with obesity.31 Studies suggest Akkermansia is beneficial in the setting of obesity and diabetes.32 However, increased Akkermansia abundance has been observed in patients and animal models of multiple sclerosis.33, 34 Additionally, the relative abundance of several SCFA producing bacteria was increased with C.butyricum and Hylon. For example, Clostridiales trended (p=0.08) to be increased in HFD+C.butyricum, while Bifidobacterium, Ruminococcus, Blautia, and Colinsella were each significantly increased in HFD+Hylon, as compared to HFD alone (Figs. 2D and E). In total, of the 13 genera found to be characteristic of HFD+C.butyricum and HFD+Hylon, 6 have been identified as SCFA producers in the gut (Fig. 2B).
The effects of OSA on the gut microbiota of HFD alone, HFD+C.butyricum, and HFD+Hylon are presented in supplemental figure S3 and figure 3. Measures of alpha and beta diversity did not show significant differences due to OSA in any of the treatment groups (Figs. S3A-C and Fig. 3A). Richness and diversity were significantly reduced in both sham and OSA of HFD+Hylon versus HFD alone and HFD+C.butyricum (Figs. S3A-B). Similarly, HFD+Hylon sham and OSA samples clustered separate from HFD alone and HFD+C.butyricum samples in unweighted and weighted Unifrac PCoA analysis (Fig. S3C and Fig 3A). Relative abundance of the major phyla is presented in Figure 3B. OSA did not result in any significant changes in the major phyla in any of the treatment groups. HFD+Hylon treated rats showed a significant increase in Actinobacteria abundance, as compared to HFD alone and HFD+C.butyricum (Fig. 3B and Figure S3D). The relative abundance of the phylum Verrucomicrobia was significantly greater in HFD rats than in HFD+Hylon, and trended to be greater than HFD+C.butyricum (Fig. 3B and Figure S3E). Figure 3C shows the bacterial taxa that were altered by OSA on the HFD alone, HFD+C.butyricum, and HFD+Hylon according to LEfSe analysis. On HFD alone, 5 taxa were shown to be enriched in sham vs. OSA (green bars) and 3 taxa enriched in OSA vs. sham (red bars). C.butyricum and Hylon diminished the effects of OSA on the microbiota, both in the number of taxa altered and the magnitude of the alteration. OSA had no significant effect on any taxa in HFD+Hylon treated rats.
OSA-induced damage to the gut wall was reduced by C.butyricum and Hylon
We next sought to examine the effects of OSA on the gut wall, with and without C.butyricum and Hylon. Figure 4 demonstrates that OSA caused a significant decrease in the number of mucus producing goblet cells in the cecum. C.butyricum and Hylon prevented the loss of goblet cells in cecum following OSA (Figs. 4A-B). Compared to HFD alone, HFD+Hylon significantly increased cecum goblet cells independent of OSA (Figs. 4A-B). Since OSA decreased the number of mucus producing goblet cells, we next examined the proximity of bacteria to the epithelial layer in cecum. Using the EUB 338 FISH probe to unbiasedly label bacteria, we measured the mucus barrier thickness as the distance separating luminal bacteria from the underlying epithelium. In the HFD alone group OSA decreased the separation of luminal bacteria from the epithelium by 30% (Fig. 4C and D). Epithelium-bacteria separation was not altered by OSA in C.butyricum or Hylon treated rats. We tested intestinal barrier function by gavaging rats with 4 kDa FITC-dextran on day 10 of sham or OSA. We found a significant increase in plasma FITC of HFD OSA and HFD+C.butyricum OSA rats as compared to shams of the respective groups, indicating gut barrier impairment in these two groups (Fig. 4E)
OSA reduced cecal acetate, which was prevented by C.butyricum and Hylon
We next tested whether the OSA-induced changes to the microbiota led to changes in microbial metabolites that may contribute to the adverse effects of OSA on the gut and blood pressure. Microbiota analysis revealed a number of short chain fatty acid producers that were altered by OSA, C.butyricum, and Hylon (Figs. 2–3). For this reason, we measured SCFA concentrations in cecal content and portal plasma. OSA led to a significant decrease in cecal acetate concentration in HFD rats (Fig. 5A). C.butyricum and Hylon prevented the OSA-induced decrease in cecal acetate concentration (Fig. 5A). Additionally, as compared to HFD alone, HFD+Hylon led to a significant increase in cecal acetate and butyrate concentration, independent of OSA (Fig. 5A and Fig. S4B). OSA did not alter cecal concentration of propionate or butyrate (Fig. S4A and B). OSA did not alter acetate, propionate, or butyrate concentrations in portal plasma (Fig. 5B and Figs. S4C and D). However, similar to observations in cecum, HFD+Hylon increased portal plasma butyrate concentrations independent of OSA (Fig. 5B).
Cecal acetate treatment prevented adverse effects of OSA on gut and blood pressure
Following the observation that OSA is associated with a decrease in cecal acetate concentration, we attempted to deliver acetate to the cecum and colon through a chronic indwelling catheter. By using a chronic infusion throughout the 2 weeks of sham or OSA we were able to elevate acetate concentrations continuously, as opposed to the acute transient increases in acetate levels that would follow gavage or drinking water administration. In addition, catheter infusion ensured delivery to the site of interest, in this case the cecum and colon. Cecal infusion of 20µmol/(kg-min) sodium acetate or PBS vehicle began 2 days prior to sham or OSA and continued throughout the study. Following 2 weeks of sham or OSA, OSA rats treated with PBS had a significant reduction in cecal acetate concentration (Fig. 5C). Rats treated with cecal acetate showed an approximate 2-fold increase in cecal acetate relative to sham rats receiving PBS, and there was no significant difference between sham and OSA rats treated with acetate (Fig. 5C). There was no significant effect of OSA on portal plasma acetate concentrations in PBS or acetate treated rats. However, acetate infusion did significantly increase portal plasma acetate levels by approximately 2-fold (Fig. S4E). There was no significant effect of OSA or cecal acetate treatment on systemic plasma acetate concentrations (Fig. S4F).
We next assessed the effects of OSA and acetate on inflammatory markers in the cecum wall. In PBS treated rats, OSA led to an increase in IL-1α and IL-6 mRNA levels (Fig. 5D and E). Acetate infusion prevented the OSA-induced increases in IL-1α and IL-6 mRNA levels, which were not significantly different from sham rats treated with PBS (Fig. 5D and E).
SBP was measured during the 2 weeks of sham or OSA in PBS and acetate treated rats. Similar to our previous findings (Fig. 1A), OSA led to significant increases in SBP at 1 and 2 weeks in PBS treated rats, as compared to sham rats treated with PBS (Fig. 5F). SBP of sham rats treated with acetate was not different from sham rats treated with PBS. However, cecal acetate infusion prevented any significant changes in SBP due to OSA (Fig. 5F).
Neuroinflammation associated with OSA was prevented by C.butyricum and Hylon
Neuroinflammation plays a key role in the etiology of hypertension in patients and multiple animal models. Additionally, shifts in the gut microbiota, such as those described with OSA (Fig. 3), have been shown to influence brain homeostasis and neuroinflammation through the microbiota-gut-brain axis. Therefore, in an effort to examine potential mechanistic links between OSA-induced gut dysbiosis and hypertension we assessed the effects of OSA, C.butyricum, and Hylon on neuroinflammation. Figure 6A illustrates the gating strategy used to measure activated microglia (CD11b+CD45lowMHCIIhigh) in brain by flow cytometry. We found that OSA increased activated microglia in brain by approximately 3-fold (Fig. 6B). As further evidence that the gut influences brain, we found that C.butyricum and Hylon (both administered orally) prevented OSA-induced increases in activated microglia in brain. We did not observe any significant effect of OSA on T-regulatory cells in the brain of any of the treatment groups (Fig. 6C). However, there was a significant main effect of treatment, with those rats receiving Hylon having increased T-regulatory cells as compared to HFD alone (Fig. 6C).
Discussion
In a rat model of OSA, we have previously demonstrated that OSA alters the normal makeup of the gut microbiota.2 Through microbiota transplant studies we have shown that OSA-induced dysbiosis contributes to the development of hypertension in this model.2 We and others have gone on to show that this connection between gut dysbiosis and blood pressure is not unique to OSA, and in fact exists in multiple models of hypertension.1, 4–6, 15 Furthermore, dysbiosis associated with models of hypertension are characterized by decreased abundance of SCFA-producing bacteria.1–5 We hypothesized that decreases in SCFAs are responsible for hypertension in a rat model of OSA. We report that (1) OSA results in decreased cecal acetate concentration, epithelial dysfunction, increased neuroinflammation, and hypertension. (2) In OSA rats, the probiotic C.butyricum and prebiotic Hylon increase cecal acetate concentration, reduce dysbiosis and epithelial damage, and prevent neuroinflammation and hypertension. (3) Cecal infusion of acetate prevents OSA-induced epithelial inflammation and hypertension. These findings demonstrate a key role for impaired acetate production in the development of OSA-induced hypertension, and suggest that treatments targeted to increase microbial acetate production may prove efficacious in the treatment of hypertension.
Fiber intake has been shown to be inversely associated with the risk of cardiovascular disease and hypertension.35–37 These dietary fibers can be fermented by bacteria residing in the gut to generate SCFAs. Studies from our lab and others have demonstrated that the relative abundance of many SCFA producing bacteria is reduced in animal models of hypertension.1, 2, 4, 5, 15 In addition, the relative abundance of several SCFA producing bacteria, including Coprococcus, Blautia, and Roseburia were shown to be lower in pre-hypertensive and hypertensive patients relative to healthy controls.16 In the current study, we show that C.butyricum and Hylon treatment significantly increase the relative abundance of numerous SCFA producing genera including, Parabacteroides, Roseburia, Clostridium, Bifidobacterium, Ruminococcus, and Blautia. Additionally, Hylon and C.butyricum treatment during the 2 weeks of sham or apnea reduce the effects of OSA on altering the microbiota. LEfSe analysis, to identify taxa altered by OSA, showed 7 taxa that were significantly different between sham and OSA with HFD alone. However, only 2 taxa were different between sham and OSA in HFD + C.butyricum, and no significant differences were observed between sham and OSA in HFD + Hylon treated rats. We conclude that HFD + C.butyricum and HFD + Hylon treated rats had a more stable microbiota when subjected to OSA than HFD alone. Thus, these treatments prevented the decrease in SCFA producing bacteria to potentially maintain levels of cecal acetate.
SCFAs serve as the main energy source for epithelial cells and have many beneficial effects including improving barrier function and reducing mucosal inflammation.25 We found that OSA significantly reduces acetate, but not propionate or butyrate, concentrations in the cecum. C.butyricum and Hylon prevented the decrease in acetate concentration due to OSA. Studies by Pluznick et al have demonstrated that activation of the G-protein-coupled receptors olfactory receptor 78 (Olfr78) and Gpr41, which bind acetate and propionate, have pressor and anti-pressor effects respectively.24 These effects have largely been attributed to effects on the vasculature and kidney. Since we did not observe any changes in SCFA concentrations in plasma following OSA, we examined the possibility that decreased acetate concentrations in the cecum were having localized effects on the epithelium. The number of mucus producing goblet cells and mucus barrier thickness is reduced following OSA, and this is prevented with Hylon and C.butyricum treatment. Similarly, goblet cells in the colon are reduced in SHR and AngII rat models of hypertension.5 SHR and AngII rats also exhibit significant increases in gut permeability.5 We found OSA rats to have modest, albeit significant, increases in gut permeability. However, the effects of OSA on permeability are not improved by C.butyricum and a trend for increased permeability still exists with Hylon treated OSA rats. These findings suggest that impaired barrier function alone may not be sufficient to induce hypertension. Since C.butyricum and Hylon shifted the microbial makeup, it is possible that barrier disruption and the presence of select microbial members are both required to lead to hypertension.
Hylon and C.butyricum treatment increased cecal acetate concentrations, but also altered the microbiota. Additionally, sucrose, which can increase gut permeability, was eliminated from the HFD+ Hylon diet to maintain a similar caloric density.38 These secondary effects of our treatments made it difficult to precisely understand the mechanism by which benefits on gut epithelium and SBP occur. To precisely address the role of acetate we chronically infused acetate into the cecum to elevate acetate concentrations and prevent the decrease in acetate due to OSA. Cecal acetate infusion prevened OSA-induced epithelial inflammation and hypertension. In line with our findings, previous studies have shown that acetate in the drinking water reduced BP in DOCA treated mice.15 While further studies are needed to fully understand the protective effects of acetate, our findings support the idea that the primary site of action is in the gut, since portal and systemic acetate concentrations are not decreased with OSA. Acetate in the gut could signal via SCFA receptors on enteric neurons to the brain resulting in blood pressure changes.
We have previously proposed the hypothesis that OSA-induced hypertension involves neuroinflammation that originates from the gut.3 In support of this hypothesis we find that OSA increases activated microglia in brain, and this is prevented by C.butyricum and Hylon treatment. Further studies are required to understand the mechanistic links between gut dysbiosis and neuroinflammation, but there is mounting evidence that communication between these two sites exist. This could involve (1) breakdown of the gut barrier allowing bacteria or immunogenic molecules access to the systemic circulation and potentially the brain, (2) activation of immune cells in the gut that migrate to brain, (3) microbial metabolites in the systemic circulation acting on the brain, (4) signaling through the enteric and central nervous system (as described above with acetate), or (5) yet unidentified mechanism of gut-to-brain signaling.
Perspectives
Growing evidence demonstrates that gut dysbiosis can play a causal role in the development of hypertension. This is supported by data from several animal models as well as humans. Using a model of OSA-induced hypertension, we demonstrate gut dysbiosis that is associated with gut wall pathology and neuroinflammation. Understanding of the mechanistic links between gut dysbiosis and hypertension are lacking. We provide evidence of decreased acetate production by the dysbiotic OSA microbiota. Finally, we demonstrate that various methods to maintain cecal acetate concentrations prevent the adverse effects of OSA on the gut, brain, and blood pressure. These studies identify acetate as a key player in OSA-induced hypertension. Treatment strategies to maintain a healthy gut microbiota (i.e., diet, pre- or probiotics) may prove effective in the prevention and treatment of hypertension.
Supplementary Material
Novelty and Significance
What is new?
OSA-induced hypertension is associated with gut dysbiosis, gut wall pathology, decreased cecal acetate concentration, and neuroinflammation.
We demonstrate the ability of a probiotic, prebiotic, and microbial metabolite to prevent the adverse effects of OSA on the microbiota, gut, brain, and blood pressure.
What is relevant?
Understanding interactions between the microbiota, gut, and brain is a critical area of investigation to fully understand the pathophysiology of hypertension.
Summary
OSA-induced hypertension involves disruption to the microbiota, gut, and brain. Treatment strategies to normalize the microbiota prevented the adverse effects of OSA on the gut, brain, and blood pressure. Therapeutic interventions to manipulate the gut microbiota may offer a promising approach in the prevention and treatment of hypertension.
Acknowledgments
Sources of Funding
This project was funded by grants from the American Heart Association 16SDG29970000, Public Health Service DK56338, NHLBI R01HL134838 (DJD), and by NINDS R01NS080531 (RMB).
Footnotes
Conflict(s) of Interest / Disclosure(s)
none
References
- 1.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM Jr. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durgan DJ. Obstructive sleep apnea-induced hypertension: Role of the gut microbiota. Curr Hypertens Rep. 2017;19 Available from: 10.1007/s11906-017-0732-3. [DOI] [PubMed]
- 4.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the dahl rat. Physiol Genomics. 2015;47:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83. [DOI] [PubMed] [Google Scholar]
- 8.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36:7428–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB, INDIA-FBP Group. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. [DOI] [PubMed] [Google Scholar]
- 14.Brandscheid C, Schuck F, Reinhardt S, Schafer KH, Pietrzik CU, Grimm M, Hartmann T, Schwiertz A, Endres K. Altered gut microbiome composition and tryptic activity of the 5xFAD alzheimer’s mouse model. J Alzheimers Dis. 2017;56:775–788. [DOI] [PubMed] [Google Scholar]
- 15.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14–016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pluznick J A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G-protein coupled receptor 41. Physiol Genomics. 2016;48:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 20.Peti-Peterdi J, Kishore BK, Pluznick JL. Regulation of vascular and renal function by metabolite receptors. Annu Rev Physiol. 2016;78:391–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pluznick JL. Extra sensory perception: The role of gpr receptors in the kidney. Curr Opin Nephrol Hypertens. 2014;23:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol. 2014;10:416–424. [DOI] [PubMed] [Google Scholar]
- 23.Rajkumar P, Aisenberg WH, Acres OW, Protzko RJ, Pluznick JL. Identification and characterization of novel renal sensory receptors. PLoS One. 2014;9:e111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapin E, Peyron C, Roche F, Gay N, Carcenac C, Savasta M, Levy P, Dematteis M. Chronic intermittent hypoxia induces chronic low-grade neuroinflammation in the dorsal hippocampus of mice. Sleep. 2015;38:1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U. Brain inflammation and hypertension: The chicken or the egg? J Neuroinflammation. 2015;12:85-015-0306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boone DR, Castenholz RW, Garrity GM. Bergey’s Manual of Systematic Bacteriology. New York: Springer; 2001. [Google Scholar]
- 30.Le Leu RK, Hu Y, Brown IL, Young GP. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr Metab (Lond). 2009;6 Available from: 10.1186/1743-7075-6-11. [DOI] [PMC free article] [PubMed]
- 31.Zietak M, Kovatcheva-Datchary P, Markiewicz LH, Stahlman M, Kozak LP, Backhed F. Altered microbiota contributes to reduced diet-induced obesity upon cold exposure. Cell Metab. 2016;23:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, Kumpfel T, Hohlfeld R, Krishnamoorthy G, Wekerle H. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114:10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topcuolu BD, Holden J, Kivisakk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7 Available from: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: A meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–481. [DOI] [PubMed] [Google Scholar]
- 36.Streppel MT, Arends LR, van ‘t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: A meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165:150–156. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, Darfeuille-Michaud A, Barnich N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116–124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.