Summary
During mammalian ovary formation, the production of ovarian follicles is accompanied by an enormous loss of germ cells. It is not known how this loss is regulated. We have investigated the role of the Trk tyrosine kinase receptors, primarily TrkB, in this process. The ovaries of TrkB−/− and TrkC−/− mice with a mixed (129Sv × C57BL/6) genetic background were examined shortly after birth. Around 50% of TrkB−/− mice had grossly abnormal ovaries that contained greatly reduced numbers of follicles. No defects were found in the ovaries of TrkC−/− mice. Congenic TrkB−/− mice were generated on 129Sv and C57BL/6 backgrounds: whereas the former had a mixed ovarian phenotype similar to that of the original colony of mice, the ovaries of all offspring of the C57BL/6 congenic line contained reduced numbers of follicles. RT-PCR showed that mRNA encoding TrkB and its two ligands, neurotrophin 4 (NT4) and brain-derived neurotrophic factor (BDNF), were present throughout the period of follicle formation in the mouse. In situ hybridisation showed that TrkB was expressed primarily in the germ cells before and after follicle formation. Mouse neonatal and fetal ovaries and human fetal ovaries were cultured in the presence of K252a, a potent inhibitor of all Trk receptors. In mice, K252a inhibited the survival of germ cells in newly formed (primordial) follicles. This effect was rescued by the addition of basic fibroblast growth factor (bFGF) to the culture medium. Combined addition of both BDNF and NT4 blocking antibodies lowered germ-cell survival, indicating that these TrkB ligands are required in this process. The results indicate that signalling through TrkB is an important component of the mechanism that regulates the early survival of female germ cells.
Keywords: Trk, Oogonia, Oocyte, Survival, Human, Mouse, Neurotrophin
Introduction
Female embryonic mice produce tens of thousands of germ cells as the ovary forms. Shortly before birth, germ cells (termed oogonia at this stage) stop mitosis, initiate meiosis (which is halted at the diplotene stage) and associate closely with somatic pregranulosa cells to form primordial follicles. There is now a finite supply of female germ cells (now termed oocytes), which cannot be replenished if lost. Concurrent with these processes, there is a massive wave of cell death that results in the death of 80-90% of oocytes in mice and humans (Brambell, 1927; Baker, 1963; Hirshfield, 1991). The same general pattern of oocyte loss occurs in all mammals. In mice, this wave of oocyte death is most pronounced around the time of birth, when follicle formation is at its peak; in humans, it occurs at around five months of gestation. This process is vital to the reproductive potential of all female mammals because their reproductive lifespan is determined by the supply of primordial follicles, but its regulation is not understood.
Primordial follicles consist of an oocyte surrounded by flattened granulosa cells, and are considered to be at a ‘resting’ stage of development. Follicles can remain at this stage throughout the reproductive lifespan of a female. The first sign of further development of the primordial follicle is the rounding up of granulosa cells. When follicles contain primarily rounded granulosa cells, they are considered to have entered the growth phase and are termed ‘primary follicles’. This process first occurs shortly after birth in the mouse.
The neurotrophins are a small family of closely related peptide factors. Nerve growth factor (NGF) was the first to be discovered; BDNF, NT3, NT4 and NT6 have since been identified (Snider, 1994). The neurotrophins act on both high and low affinity cell-surface receptors. Many of the effects of the neurotrophins on cell survival and neuronal growth are mediated by high affinity glycoprotein tyrosine receptor kinases, or Trk receptors. Trk receptors consist of an extracellular domain, which contains the neurotrophin-binding site, a short transmembrane segment, and an intracellular domain that encodes a tyrosine kinase. The neurotrophins bind selectively to the high affinity Trk receptors, which form homodimers and autophosphorylate to trigger the intracellular cascade (Segal and Greenberg, 1996). There are three members of the Trk receptor family: TrkA, the receptor for NGF; TrkB, the receptor for BDNF and NT4; and TrkC, the receptor for NT3. The functions of truncated forms of the TrkB and TrkC receptors, which lack the intracellular tyrosine kinase domains (Klein et al., 1990; Dechant, 2001), are unclear. In addition to the Trk receptors, all neurotrophins bind with relatively equal, low affinity to a membrane receptor known as p75, a member of the tumour necrosis receptor superfamily. The p75 receptor lacks tyrosine kinase activity, but it does appear to have signalling capabilities. It might modulate cellular responses to the neurotrophins by enhancing the sensitivity of the Trk receptors (Hantzopoulos et al., 1994), whereas in the absence of Trk receptors it can induce cell death (Friedman, 2000).
The neurotrophins are implicated in a variety of developmental processes at numerous neural sites. Their best-known roles are in the regulation of cell survival. Thus, neurons that contain one or more of the Trk receptors might require the presence of sufficient concentrations of the appropriate neurotrophin(s) for their continued survival. They might also be involved in the regulation of neuronal differentiation, growth and migration (Ghosh and Greenberg, 1995; Segal and Greenberg, 1996).
All three Trk receptors are expressed around the time of follicle formation in rats and humans (Dissen et al., 1995; Anderson et al., 2002). In rats, expression of TrkB mRNA increases sharply and TrkA mRNA decreases abruptly during the period of follicle formation whereas TrkC remains constant throughout. Expression of NT4 mRNA increases concomitantly with that of its ligand TrkB. In humans, the expression pattern of NT4 mRNA changes as follicles start to form, with expression, which is predominantly in oogonia before follicle formation, switching predominantly to the somatic pregranulosa cells around the time of follicle formation (Anderson et al., 2002). Thus, the location of NT4 mRNA production moves from the germ cell to the somatic cell just as germ cells undergo the massive wave of apoptosis. Together, this indicates the possible involvement of TrkB signalling in regulating germ-cell survival as follicles form.
Here, we report evidence that TrkB plays an important role in the survival of germ cells in mouse and human ovaries around the time of follicle formation. We have examined the ovaries of transgenic mice with a mutation in the catalytic domain of the TrkB and TrkC receptors (Klein et al., 1993; Klein et al., 1994), and show the results of culturing fetal and neonatal mouse ovaries and fetal human ovaries in the presence of (1) K252a, a potent inhibitor of the Trk receptors, and (2) blocking antibodies against NT4 and BDNF.
Materials and methods
Animals
Mice were housed in an environmentally controlled room on a 14-hour light, 10-hour dark photoperiod. Animals were provided with food and water ad libitum, and kept in accordance with UK legal requirements. Transgenic mice had a mutation in the catalytic domain of either the TrkB (Klein et al., 1993) or TrkC (Klein et al., 1994) receptor. Heterozygous pairs were bred to provide TrkB+/+ and TrkB−/− offspring, and TrkC+/+ and TrkC−/− offspring. Offspring were earmarked for identification and DNA prepared from the material from the ear punches for subsequent genotyping.
Genotyping transgenic mice
For TrkB+/− x TrkB+/− offspring, sense primer 5′-TCGCGTAAAGACGGAACATGATCC and antisense primer 5′-AGACCATGATGAGTGGGTCGCC were used to amplify a 900 bp TrkB+/+ band, and sense primer 5′-CCAGCCTCTGAGCCCAGAAAGC and antisense primer 5′-GCTGAAGGACGCAGCGACAAT were used to amplify a TrkB−/− band of ~450 bp. PCR reactions for the TrkB+/+ band and the TrkB−/− band were performed separately. For TrkC+/− × TrkC+/− offspring, we used the sense primer 5′-CTGAAGTCACTGGCTAGAGTCTGGG together with antisense primers for TrkC+/+ (5′-GTCCCATCTTGCTTACCCTGAGG) and TrkC−/− (5′-CCAGCCTCTGAGCCCAGAAAGC), which amplified 400 bp and 500 bp bands, respectively (Schimmang et al., 1995). PCR reactions for the TrkC+/+ band and the TrkC−/− band were performed simultaneously.
Human fetal ovaries
Human fetal ovaries were obtained following medical termination of pregnancy. Women gave written consent according to national guidelines (Polkinghorne, 1989) and the study was approved by the Lothian Paediatrics/Reproductive Medicine Research Ethics SubCommittee. Termination of pregnancy was induced by treatment with mifepristone (200 mg orally) followed 48-hours later by prostaglandin E1 analogue (Gemeprost, Beacon Pharmaceuticals, Tunbridge Wells, UK) 1 mg 3-hourly per vaginam. None of the terminations were for reasons of fetal abnormality, and all fetuses appeared morphologically normal. Gestational age was determined by ultrasound examination prior to termination and confirmed by subsequent direct measurement of foot length.
Mouse ovary cultures
Neonatal C57BL/6 × CBA/Ca mice were killed by decapitation. Fetal ovaries were obtained from pregnant females killed by cervical dislocation 16.5 days after mating. Ovaries were removed aseptically and placed in watch glasses containing Liebovitz L-15 dissecting medium (Gibco-BRI, Renfrew, UK) supplemented with 0.3% (w/v) bovine serum albumin (BSA) (Fraction V, Sigma, Poole, UK). Tissue surrounding the ovaries was removed using sterile needles. Freshly dissected newborn ovaries were bisected using a sterile scalpel blade. In the first cultures with K252a alone (Calbiochem, Nottingham, UK) ovaries were subsequently halved again using fine-gauged needles. In the cultures with K252a and bFGF (R&D Systems, Abington, UK) and in those with the anti-NT4 and anti-BDNF blocking antibodies (Sigma), ovary halves were used because the quarter ovaries used in the earlier experiments were more difficult to handle and process for histological analysis. Whole ovaries were used with embryonic day 16.5 (E16.5) mice, as it was not possible to cut the E16.5 ovaries cleanly because of fragility of the tissue. Tissue was either fixed in Bouin’s for analysis (uncultured control) or cultured. Culture pieces were placed on a polycarbonate membrane on the base of a 96-well plate (Iwaki, Japan). Wells contained 100 μl of pregassed medium overlaid with 100 μl of silicone fluid (Gibco-BRL). Ovarian pieces were cultured in α-MEM (Gibco-BRI) supplemented with ascorbic acid (28 μM) and 0.3% (w/v) BSA, with additions as detailed below. The tissue was cultured in a humidified incubator (5% CO2, 37°C). Half of the used medium (50 μl) was exchanged for fresh medium every other day for the duration of the culture period. Upon fixation, ovarian pieces were washed in PBS containing polyvinyl pyrrolidone (3 mg ml−1) to remove any medium and fixed for 1.5-2 hrs in Bouin’s solution.
Human ovary cultures
Ovaries were dissected free of adherent tissues using sterile technique, bisected longitudinally and cut into ~0.5 mm-thick slices. Samples of fresh tissue were fixed for histological analysis. The remaining tissue fragments were cultured on 0.4 μm pore Millicell CM filters (Millipore, Bedford, MA, USA) in a 24-well plate (Transwell, Costar, High Wycombe, UK). Medium (0.4 ml) was added to each well to just cover the tissue fragments. Any remaining wells were partially filled with medium to maintain humidity in the culture vessel. The medium comprised α MEM containing 3 mg ml−1 BSA, 100 IU ml−1 penicillin, 100 μg ml−1 streptomycin sulphate, 0.125 μg ml−1 amphotericin, 5 μg ml−1 insulin, 5 μg ml−1 transferrin, 5 μg ml−1 sodium selenite, 2 mM glutamine and 2 mM pyruvate (all chemicals supplied by Sigma). Ovaries were cultured in the presence or absence of K252a. Because K252a was reconstituted in dimethylsulfoxide (DMSO), the equivalent amount of DMSO was added to control wells. The cultures were maintained at 37°C in a humidified incubator under 5% CO2 in air for 48 hours and fixed for histological analysis at the end of the culture period.
Histological assessment of mouse ovaries
After fixation in Bouin’s, ovaries or ovarian pieces were embedded in wax and 5 μm sections cut. Every third (cultured ovarian piece) or fifth (in vivo ovaries) section was analysed. Individual images were captured using the Leica Q5001W digital imaging microscope (Leica Microsystems, Milton Keynes, UK) using a 40×objective. Healthy oocytes containing a visible germinal vesicle were counted. In addition, in the experiment in which NT4 and BDNF activity was inhibited, a count was made of dead and dying oocytes. In some culture experiments, the maximum and minimum diameters of each oocyte were measured. All analyses were carried out blind.
Histological assessment of human ovaries
Sections of tissue were analysed to determine the density of germ cells in the ovary. Analysis was carried out blind using the Area Fraction Probe in the Stereologer software programme (Systems Planning and Analysis Inc, Alexandria, VA, USA) as previously described (Sharpe et al., 2002). A 121-point graticule was used to count the number of germ cells within a frame: only cells whose nuclei lay beneath the intersections on the grid were counted. Between 18 and 42 frames were used on each ovary piece, as determined by the programme. Tissue sections were at least 20 μm apart to ensure that no cell was counted twice. Data are presented as number of germ cells per frame.
RT-PCR
Ovaries were dissected from E16.5, postnatal day 0 (P0) and P4 C57BL/6 × CBA/Ca F1 mice, frozen in liquid nitrogen and mRNA subsequently extracted using a Quickprep micro mRNA purification kit (Pharmacia, St. Albans, UK). Brain tissue was collected from mice at P0. cDNA was prepared from mRNA using random primers (Promega, Southampton, UK). Separate PCR reactions were then carried out for cyclophilin, TrkB, NT4 and BDNF. Two separate TrkB reactions were carried out. The first set of primers were to the tyrosine kinase domain of the gene and, thus, recognised only full length transcripts of TrkB, the second were to the ligand-binding domain and recognised both full-length and truncated TrkB receptors. The following primers were used:
cyclophilin, 5′-CCAGGGTGGTGACTTTACAC-3′ (forward), 5′-CGGAAATGGTGATCTTCTTG-3′ (reverse);
TrkB (full length), 5′-ATGGCAGAGGGTAACCC-3′ (forward), 5′-CTCTCTGGAGGCATCCAT-3′ (reverse) (Singh et al., 1997);
TrkB (full length and truncated), 5′-CTCCGTGTGATTGGTAACATG-3′ (forward), 5′-AGTCCAGACACTCAGGATTTGGAC-3′ (reverse) (Anderson et al., 2002);
NT4, 5′-CCCTGCGTCAGTACTTCTTCGAGAC-3′ (forward), 5′-CTGGACGTCAGGCACGGCCTGTTC-3′ (reverse) (Botchkarev et al., 1999); and
BDNF, 5′-GTGAGAAGCTTGATGACCATCC-3′ (forward), 5′-AACAGAATTCCACTATCTTCCC-3′ (reverse) (Botchkarev et al., 1999).
In situ hybridisation
Ovaries from E16.5 and P4 C57BL/6×CBA/Ca F1 mice were fixed for 30 minutes in freshly-made 4% paraformaldehyde/PBS and embedded in wax. Sections (6 μm) were cut and mounted on TESPA-coated slides. Slides were then dewaxed, treated in proteinase K (20 μg ml−1 for 2 minutes at 37°C) and hybridised with digoxigenin-labelled riboprobes. The probe, which was cloned into pBluescript (Stratagene, La Jolla, CA, USA), has been described previously (Klein et al., 1990) and recognised both truncated and full length TrkB. For antisense probes, plasmids were digested with EcoRI (Roche, Lewes, UK) and transcribed in vitro with T7 (Roche). For sense probes, plasmids were digested with XhoI (Roche) and transcribed in vitro with T3 (Roche). Probes were labelled with digoxygenin using a DIG RNA-Labeling Mix (Roche) and then cleaned with 70% ethanol. The probe was revealed with an anti-digoxigenin alkaline phosphotase antibody (Roche) (100 μl made up to 50 ml with ddH2O and left overnight at 4°C). Colour detection was carried out the following day in nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indlyl phosphate, toluidine salt (Roche), with levamisole (Vector, Peterborough, UK). Slides were counterstained with nuclear fast red (Vector).
Immunocytochemistry
Ovaries were fixed in Bouin’s fixative for 1 hour then transferred to a 70% ethanol/eosin solution and embedded in wax. Sections (5 μm) were cut and mounted on electrostatically charged slides (BDH Laboratory Supplies), dried overnight in a 60°C oven and dewaxed. Endogenous peroxidases were quenched with a 3% hydrogen peroxidase solution in methanol for 30 minutes at room temperature. Immunocytochemistry was performed as described (Anderson et al., 2002). Briefly, slides were blocked with 20% normal donkey serum (NDS; Diagnostics Scotland, Carluke, UK) in TBS containing 5% BSA and 8 drops avidin solution per ml (Avidin/Biotin Blocking Kit, Vector) for 30 minutes at room temperature. Slides were blocked using biotin from the same kit in the same way as avidin. Chicken IgY primary antibody specific to full-length TrkB was diluted 1/10 in TBS/BSA/NDS, applied to the slides and incubated overnight at 4°C (Anti-TrkB In pAb, Promega, UK). Biotinylated donkey anti-chicken IGY secondary antibody was diluted 1/500 in TBS/BSA/NDS, applied to the slides and incubated at room temperature for 30 minutes, with avidin biotin horseradish peroxidase linked complex (DAKO) applied according to the manufacturers instructions. Bound antibody was visualised using 3,3′-diaminobenzidine tetrahydrochloride (DAKO). Sections were counterstained with haematoxylin.
Statistics
Data from mouse in vivo ovary counts were analysed with Mann-Whitney U tests. Total counts of mouse and human cultured ovaries were analysed with probability values (P) of differences in oocyte numbers determined by analysis of variance: where appropriate, paired comparisons were made using Student’s t-test. Where the data did not have a normal distribution, a Kruskal-Wallis test was used. The Kolmagorov Smirnov test was used to compare differences in proportions of oocytes with varying diameters in the cultured mouse ovaries.
Results
Transgenic mice on mixed genetic background
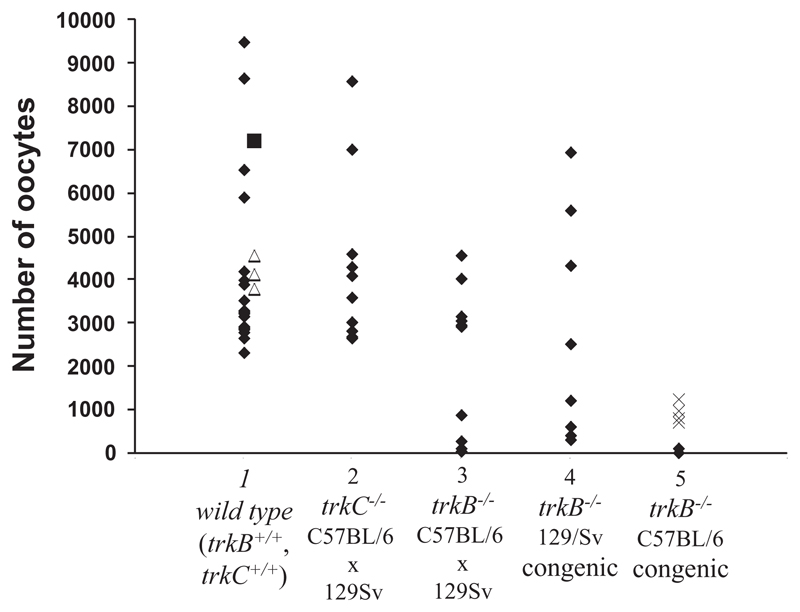
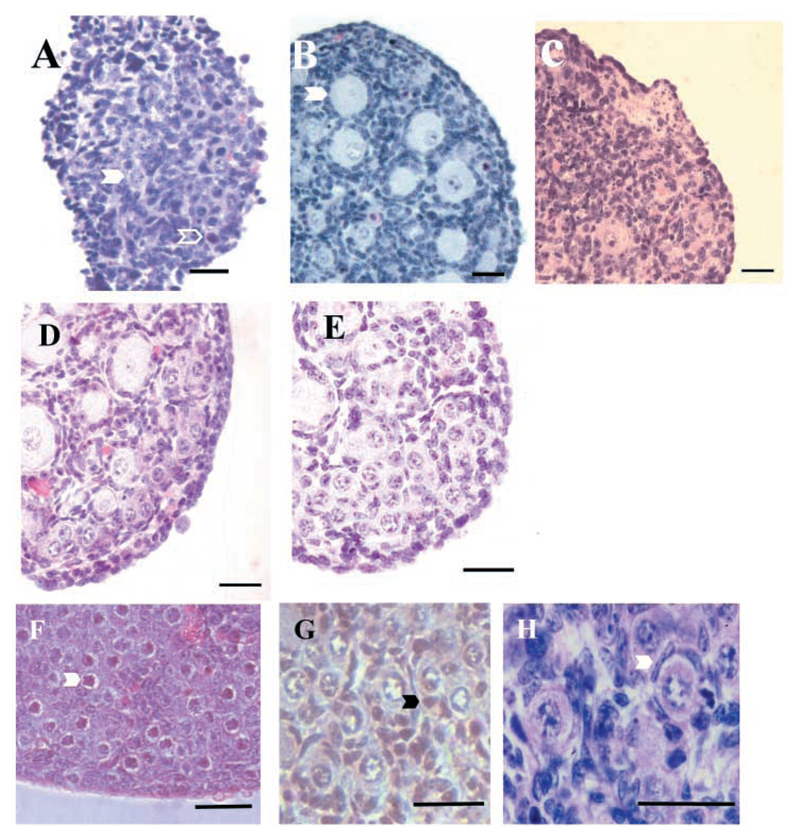
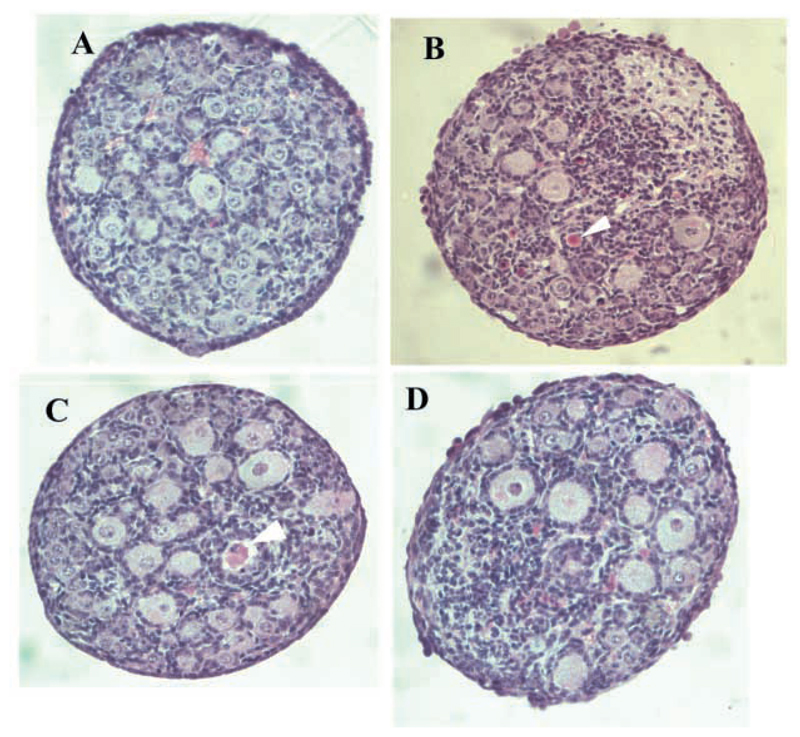
Ovaries were examined and the total number of oocytes estimated in 23 TrkB+/+,TrkC+/+ mice (P4-6), 11 TrkB−/− mice (P4-5) and 10 TrkC−/− mice (P6), all on a mixed C57BL/6 × 129/Sv genetic background. At this age, normal ovaries are full of follicles at the primordial and primary stage (Fig. 1A). In ~50% of TrkB−/− animals, the ovaries were grossly abnormal (Fig. 1B) and contained substantially fewer oocytes (<1000) than those of the wild-type animals (>2000) (Fig. 2). Sometimes, the follicles contained only a few granulosa cells or dark oocytes with abnormal looking nuclei. In all cases, the ovaries contained a large number of dark, apoptotic-looking granulosa cells and the ovary sections had a fuzzy appearance (Fig. 1B,D). This phenotype was not seen in the ~100 ovaries from wild-type mice that we have examined to date, including the 23 TrkB+/+,TrkC+/+ ovaries analysed in this experiment (Fig. 1A, Fig. 2). The ovaries of the remaining 50% of the TrkB−/− mice obtained in this experiment appeared normal (Fig. 1C, Fig. 2) and contained the expected number of follicles. The ovaries of all TrkC−/− mice looked normal and contained oocyte numbers comparable with those of the TrkB+/+,TrkC+/+ mice (Fig. 2).
Fig. 1.
Sections of ovaries from wild-type and mutant mice at P4-5 stained with haematoxylin and eosin. Ovaries from 50% of TrkB−/− mice from a mixed genetic background and all mice from the congenic C57BL/6 background have grossly abnormal ovaries that contain greatly reduced numbers of follicles. (A) TrkB+/+,TrkC+/+ ovary, containing many follicles. (B) TrkB−/− ovary on C57BL/6 × 129Sv background with only few follicles remaining (arrowheads). (C) Unaffected TrkB−/− ovary on C57BL/6 × 129Sv background, which contains as many follicles as (A). (D) Ovary from fourth generation C57BL/6 congenic TrkB−/− mouse, which contains large areas of cell death and only a few follicles (arrowhead). Scale bars: 20 μm.
Fig. 2.
Ovaries of TrkC−/− mice contain normal numbers of follicles but those of TrkB−/− mice contain few follicles. This phenomenon is found in ~50% of TrkB−/− mice on either the mixed genetic background or the 129/Sv congenic line, but all ovaries in the C57BL/6 congenic line are affected. Scattergram of number of oocytes in ovaries of TrkB+/+,TrkC+/+ mice (column 1); TrkC−/− mice (column 2); TrkB−/− mice from the original mixed genetic background colony (column 3); TrkB−/− mice from the 129/Sv congenic colony (column 4); and TrkB−/− mice from the C57BL/6 colony (column 5). In column 1, TrkB+/+, TrkC+/+ mouse on C57BL/6 background is represented with a filled square and those on 129/Sv background are represented with open triangles, other mice are of the mixed genetic background. In column 5, filled diamonds represent mice from the seventh generation of the C57BL/6 congenic line of TrkB−/− mice and crosses are mice from the fourth generation.
Congenic strains of TrkB−/− mice
The mixed genetic background of the mice in the original colony was a likely explanation of the variable effect of the TrkB−/− mutation on ovarian development described above. To test this, congenic strains of TrkB−/− mice were generated, based on either C57BL/6 or 129/Sv inbred strains. Breeding pairs were set up at the fourth (C57BL/6 and 129Sv) and seventh (C57BL/6) generation to generate TrkB−/− offspring for analysis. Examination of all the offspring collected from 10 pairs of fourth generation matings showed that a total of five out of 225 C57BL/6 pups were TrkB−/− (four of these were female), in contrast with 19 out of 207 129/Sv pups (nine of which were female). This indicates that C57BL/6 TrkB−/− mice are more severely affected in general (although the 5 C57BL/6 TrkB−/− mice that survived to P4 did not appear different from the TrkB−/− pups on a mixed or 129/Sv background). Fig. 2 shows the number of oocytes in ovaries of the C57BL/6 and 129/Sv congenic strain TrkB−/− females at P4. Of the six TrkB–/– female mice obtained in the C57BL/6 line, all had severely affected ovaries with large apoptotic-looking areas (Fig. 1D) and few follicles (Fig. 2). By contrast, the nine female 129/Sv TrkB–/– mice were similar to the original mixed-background mice, with some ovaries containing normal numbers of oocytes and others with areas of cell death and depleted stores of oocytes (Fig. 2). The Mann-Whitney test showed that there were significantly fewer oocytes in the ovaries of C57BL/6 TrkB−/− mice than in wild-type ovaries (P<0.0001).
Expression of TrkB, NT4 and BDNF mRNA and protein
Ovaries were obtained from female mice at E16.5, P0 and P4. RT-PCR showed that TrkB (both full length and truncated), NT4 and BDNF were expressed in mice at E16.5 (prior to the start of follicle formation), at P0 (in the middle of follicle formation) and at P4 (when follicle formation is complete) (Fig. 3). Full-length TrkB was present at very low levels at all times, compared to expression in a similar amount of brain tissue (as determined by equivalent expression of cyclophilin) (Fig. 3, lane 2), but when more ovary tissue was used in the reaction, the presence of the full length form in the ovary was seen clearly (Fig. 3 lane 3). By contrast, the PCR reaction that detected both full-length and truncated forms of TrkB showed expression at high levels in brain and in ovaries at all ages (Fig. 3, lane 4). Thus, truncated TrkB was expressed in the ovary at a much higher level than full-length TrkB.
Fig. 3.
TrkB (full length and truncated), NT4 and BDNF are expressed in mouse ovaries at E16, P0 and P4. Full-length TrkB is expressed at very low levels in all ovary samples. Each RT-PCR reaction was carried out on P0 brain and on ovary at E16.5, P0 and P4. RT-PCR was carried out for: (1) cyclophilin; (2) full-length TrkB using equivalent amounts of mRNA (in terms of cyclophlin expression); (3) full-length TrkB receptor with eight times more E16 ovary mRNA than brain mRNA and five times more P0 and P4 ovary mRNA than brain mRNA (in terms of cyclophlin expression); (4) TrkB receptor, both truncated and full length, using equivalent amounts of mRNA (in terms of cyclophlin expression); (5) NT4, using equivalent amounts of mRNA (in terms of cyclophlin expression); and (6) BDNF using equivalent amounts of mRNA (in terms of cyclophlin expression).
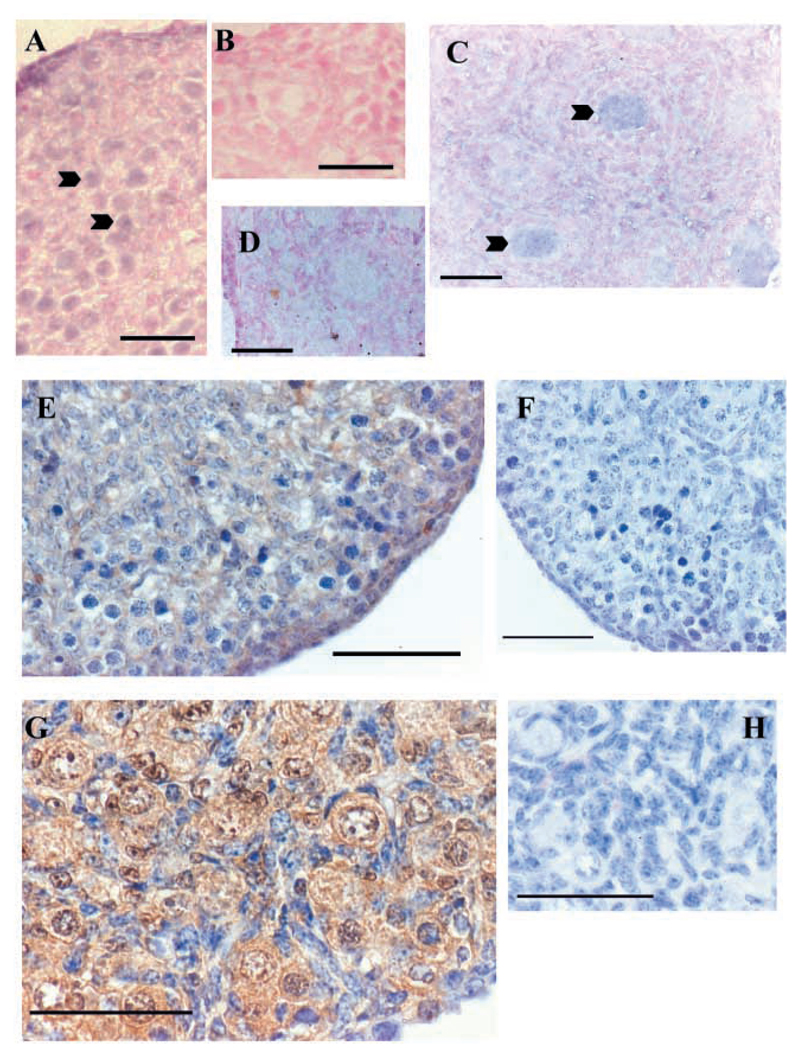
In situ hybridisation using a probe that recognised both full-length and truncated forms of TrkB showed that expression was primarily confined to the germ cells, in oogonia at E16.5 and oocytes at P4 (Fig. 4A-D). This probe was checked by in situ hybridisation using E19 and P0 brain, which showed the same expression pattern as described previously (Ringstedt et al., 1993). Immunocytochemistry was then carried out using an antibody specific for full-length TrkB. This showed a similar pattern of ovarian expression, but TrkB protein was much more abundant in P0 oocytes than in E16 oogonia (Fig. 4E-H).
Fig. 4.
TrkB is expressed in germ cells of ovaries both before and after follicle formation in the mouse. In situ hybridisation uses a probe that recognises truncated and full-length forms of TrkB, whereas immunocytochemistry is specific for full length TrkB. (A-D) In situ hybridisation. (A) Using antisense probe (staining is primarily in oogonia, arrowheads) and (B) sense probe on E16.5 ovaries. (C) Antisense probe (staining is primarily in oocytes, arrowheads) and (D) sense probe on P4 ovaries (D). (E-H) Immunocytochemistry. (E) On E16 ovary with primary antibody present and (F) negative control for E16 ovary with no primary antibody. There is little full-length TrkB present at E16, but some expression can be seen in oogonia. (G) P0 ovary with primary antibody present and (H) P0 ovary with no primary antibody (full-length TrkB is strongly expressed in oocytes). Scale bars: 20 μm in A-D; 50 μm in E-H.
Effect of K252a on newborn mouse ovaries
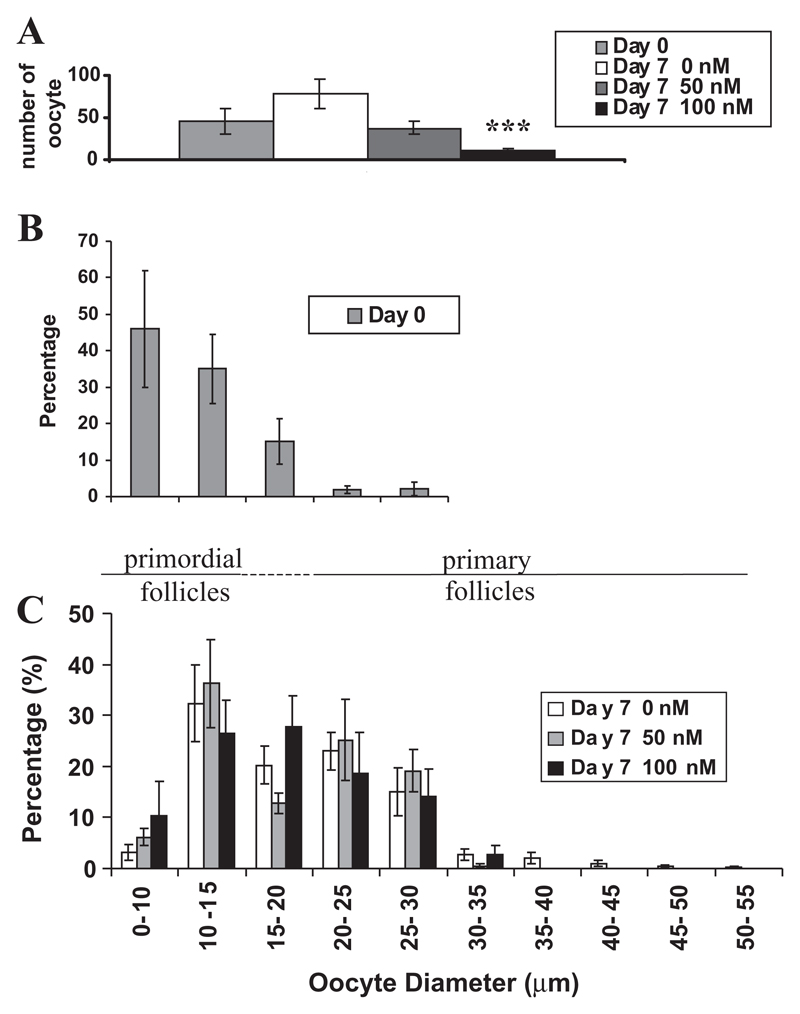
Ovarian quarters from P0 mice were either fixed immediately or cultured with medium containing K252a (0, 50 and 100 nM). At P0, ovaries contained primordial follicles. The development of follicles from the primordial to the primary, growing stage was supported in culture. This is shown in Fig. 5A,B and was confirmed by measurements of oocyte diameter (Fig. 6B,C). Ovaries from P0 mice cultured for 7 days in the presence of K252a exhibited areas of extensive cell death with few follicles present at the end of the culture period (Fig. 5C). There was a dose-dependent decrease in the total number of follicles in K252a-treated ovaries, compared to untreated cultured ovaries. The decrease in follicle number in ovaries cultured in the presence of 50 nM K252a was not statistically significant (P=0.08), but there was a highly significant decrease in the presence of 100 nM K252a (P<0.005) (Fig. 6A). Fig. 6C shows the proportion of oocytes at different diameters in control and K252a-treated cultured ovaries. Although >85% of oocytes were lost when ovaries were cultured in the presence of 100 nM K252a (Fig. 6A), the surviving oocytes had a similar distribution in diameter to those in ovaries cultured in control medium (Kolmogorov Smirnov test showed no significant difference in distribution). Thus, oocytes that survived in the presence of K252a grew to similar diameters to those in control cultures. K252a, therefore, inhibits the survival of follicles, but does not affect the growth of follicles that do survive.
Fig. 5.
Photomicrographs of sections of ovarian pieces stained with haematoxylin and eosin. (A) Uncultured ovary at P0 containing oogonia (open arrowhead) and primordial follicles (closed arrowhead). (B) P0 ovary cultured for 7 days, which contains many growing, primary follicles with larger oocytes (arrowhead). (C) P0 ovary cultured for 7 days in the presence of 100 nM K252a (which inhibits oocyte survival). The ovary contains some oocytes and large areas of cell death. (D) P0 ovary cultured for 7 days in the presence of 40 ng ml−1 bFGF contains many healthy follicles. (E) P0 ovary cultured for 7 days in the presence of 100 nM K252a and 40 ng ml−1 bFGF. The ovary has been rescued from the effect of K252a and contains many healthy follicles. (F) Uncultured ovary from E16.5 mouse containing oogonia (arrowhead) but no follicles. (G) E16.5 ovary cultured in control medium for 4 days contains many follicles, all of which are at the primary, growing stage with rounded granulosa cells (arrowhead). (H) Ovary at P0, an equivalent age for the cultured ovary shown in G, with follicles at the primordial, resting stage with flattened granulosa cells (arrowhead). Scale bars: 20 μm.
Fig. 6.
K252a reduces oocyte survival in newborn mouse ovaries in culture, but does not affect the distribution of oocyte diameters in surviving follicles. (A) The total number of follicles in uncultured day 0 ovaries and ovaries cultured in 0 nM, 50 nM and 100 nM K252a. Asterisks indicate significant difference compared with control culture (D7 0 nM), P<0.005. (B) The proportion of follicles containing oocytes of various diameters in uncultured day 0 ovaries. (C) The proportion of follicles containing oocytes of various diameters in ovaries cultured in 0 nM, 50 nM and 100 nM K252a. Line between B and C shows the follicle stage that corresponds to different diameters of oocytes.
Rescue of cultured ovaries with bFGF
Previous studies have shown that, at the doses used here, K252a blocks Trk receptors but not other tyrosine kinase receptors, including those for bFGF (Tapley et al., 1992). This was confirmed in our study by culturing P0 ovaries in media containing: (1) 100 nM K252a; (2) 40 ng ml−1 bFGF; and (3) 100 nM K252a and 40 ng ml−1 bFGF. bFGF acts via a non-Trk tyrosine kinase receptor (Wert and Palfrey, 2000) and has been shown to stimulate primordial follicle development (Nilsson et al., 2001). Ovaries cultured in the presence of bFGF with or without the addition of K252a looked healthy and contained many follicles (Fig. 5D,E). Fig. 7 shows the total number of oocytes in the different treatment groups. The increase in oocyte numbers in ovaries cultured in bFGF alone was not significant. Ovaries cultured in 100 nM K252a showed a large reduction in oocyte survival, with significantly fewer oocytes present at the end of the culture period (P<0.05). bFGF rescued follicles from the effect of K252a: there was no difference between the number of oocytes in untreated ovaries and in ovaries cultured in the presence of K252a and bFGF. K252a did not, therefore, block the function of all tyrosine kinase receptors nonspecifically.
Fig. 7.
bFGF rescues cultured newborn mouse ovaries from the effect of K252a. Histogram of the total number of follicles in ovaries cultured in control conditions and in 100 nM K252a, 50 ng ml−1 bFGF, and K252a plus bFGF. Asterisk indicates significant difference compared with untreated control culture, P<0.05.
Inhibition of BDNF and NT4 activity
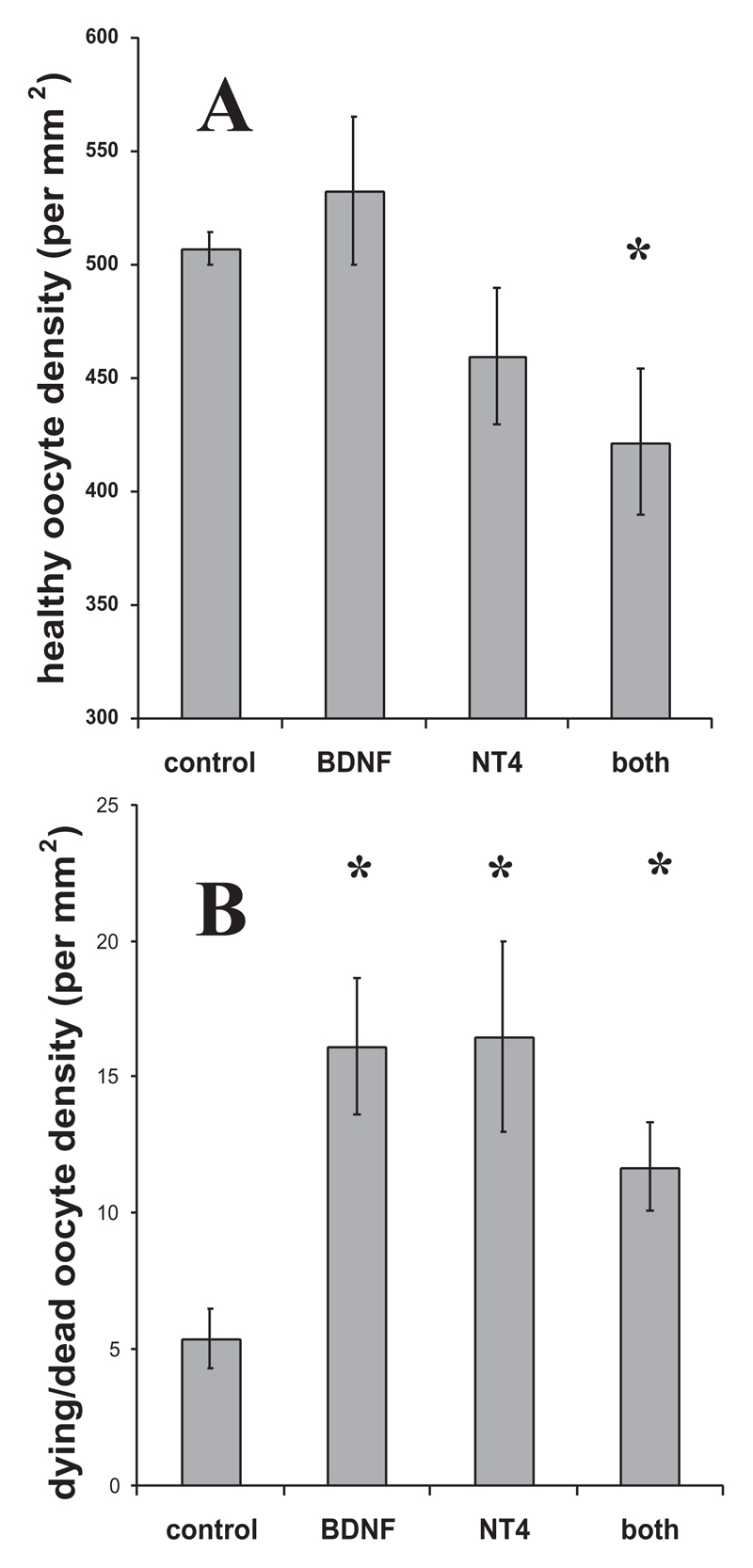
P0 ovaries were cultured for 7 days in control medium or in medium containing (1) 10 μg ml−1 anti-BDNF antibody, (2) 10 μg ml−1 anti-NT4 antibody, and (3) 10 μg ml−1 each of anti-BDNF and of anti-NT4 antibody. The addition of either anti-BDNF or anti-NT4 antibodies alone had no significant effect on oocyte survival, but when added in combination, oocyte survival was lowered significantly (Fig. 8A; P<0.05). Examination of the cultured ovaries showed that all treated ovaries, but not control ovaries, had large areas around the edge that contained no healthy oocytes (Fig. 9). The density of dying and dead oocytes was significantly higher in all three treatment groups than in control ovaries (Fig. 8B; P<0.05 in all cases).
Fig. 8.
Blocking the effects of BDNF and NT4 in culture decreases germ-cell survival and increases germ-cell death. (A,B) The density of healthy oocytes (A), and dying and dead oocytes (B) in ovaries cultured in control medium or in medium containing 10 μg ml−1 anti-BDNF, 10 μg ml−1 anti-NT4, and 10 μg ml−1 each of anti-BDNF and of anti-NT4. Asterisk indicates significant difference compared to untreated control culture, P<0.05.
Fig. 9.
Photomicrographs of sections of ovarian pieces stained with haematoxylin and eosin. (A-D) Ovaries were cultured for 7 days in control medium (A), medium containing 10 μg ml−1 anti-BDNF (B), medium containing 10 μg ml−1 anti-NT4 (C) and medium containing 10 μg ml−1 each of anti-BDNF and of anti-NT4 (D). Areas around the edge of ovaries cultured in the presence of antibodies had few if any germ cells. These ovaries also often contained dying/dead oocytes (white arrowheads).
Culture of E16.5 ovaries
At the start of the culture period, E16.5 ovaries contained oogonia but no primordial follicles (Fig. 5F). After culture for 4 days follicles formed, with many oocyte-enclosed follicles present by the end of the culture period (Fig. 5G). Examination of sections showed that cultured E16.5 ovaries appeared not to form follicles in the normal manner. This was confirmed by examination of an ovary from a P0 animal in vivo and of an E16.5 ovary cultured in control medium for 4 days. At P0, ovaries contain both oogonia and primordial follicles (and occasional primary follicles) (Fig. 5H). E16.5 ovaries that were cultured for 4 days (the in vitro equivalent of P0 ovaries) contained oogonia and follicles by the end of the culture period. However, in marked contrast to the situation in vivo, these follicles were virtually all at the primary stage, with rounded granulosa cells (Fig. 5G). Fig. 10a shows the results of a detailed comparison of in vivo and cultured ovaries. Both ovaries contained similar percentages of oogonia and oocytes. Oocytes were then further classified into those contained in primordial and primary follicles. This further classification was only possible where granulosa cells were clearly visible around the oocytes and clearly part of that follicle. This was not possible in ~65% of both in vivo and in vitro ovaries and these oocytes were excluded from further analysis. The oocytes from the in vivo ovary were predominantly at the primordial stage but those in the cultured ovary were virtually all at the primary stage. E16.5 ovaries were also examined after 1, 2 and 3 days in culture in control medium (results not shown). Examination of sections showed that in virtually no instances were primordial follicles found. It appears, therefore, that when ovaries containing only oogonia are cultured, follicles form directly at the primary (growing) stage, and completely bypass the primordial (resting) stage of development that occurs in vivo. This culture system was then used to examine the survival of primary follicles in culture.
Fig. 10.
Very few primordial follicles form in E16.5 fetal ovaries in culture and K252a has no effect on oocyte survival in this culture system. (Ai) The proportion of oogonia and oocytes in E16.5 ovaries cultured for four days and in P0 ovary without culture. (Aii) Further classification of oocytes into those contained in primordial and primary follicles, in E16.5 ovaries cultured for four days and in P0 ovary without culture. (B) The diameters of oocytes in E16.5 ovaries cultured in the presence or absence of 100 nM K252a.
Effect of K252a on E16.5 ovaries
E16.5 ovaries were cultured in medium containing 0 nM or 100 nM K252a for four days. There was no difference in oocyte number between ovaries cultured in the presence of K252a and those cultured in the absence of K252a (Fig. 10B). The way in which follicles formed in E16.5 cultured ovaries, bypassing the primordial follicle stage, might explain the lack of effect of K252a. K252a appears to affect follicles specifically at the primordial stage, which is represented in P0 cultured ovaries but not in E16.5 cultured ovaries.
Culture of fetal human ovaries in the presence of a Trk receptor blocker
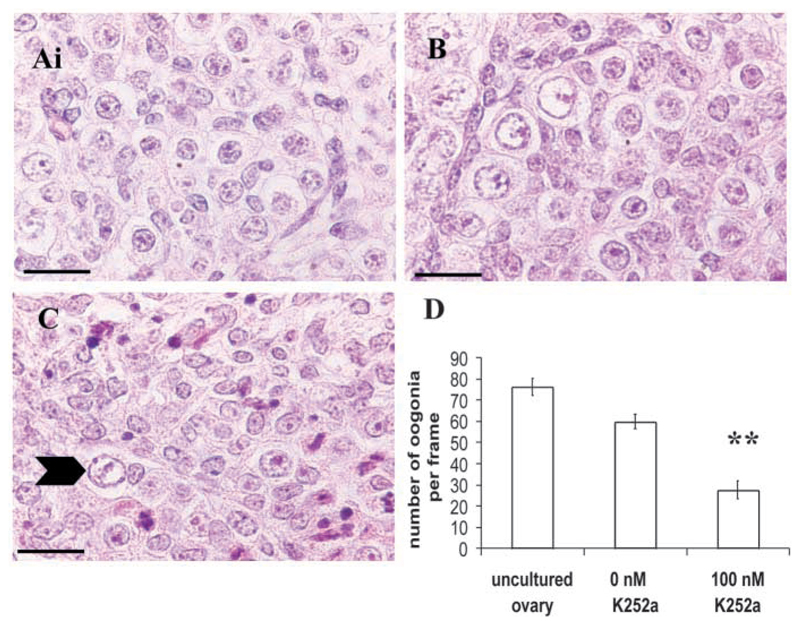
Ovaries from five fetuses, ranging from 13 to 16 weeks of gestation, were cultured in 0 nM or 100 nM K252a. At these ages, ovaries contained oogonia only (Fig. 11A). Even in control medium, follicles did not form but oogonia survived (Fig. 11B). In ovaries cultured in 0 nM K252a, a mean of 78% of oogonia survived after 48 hours in culture, whereas only 36% survived when ovaries were cultured in the presence of 100 nM K252a (P=0.01; Fig. 11C,D).
Fig. 11.
Effect of K252a on human ovaries in culture. (A-C) Photomicrographs of human fetal ovaries at 13-weeks gestation. (A) Uncultured ovary containing oogonia. (B) Ovary cultured in the absence of K252a with many oogonia surviving. (C) Ovary cultured in the presence of 100 nM K252a with few oogonia surviving (arrowhead). Scale bars: 20 μm. (D) Density of germ cells in uncultured ovaries, and ovaries cultured in the presence of 0 nM and 100 nM K252a. The number of oogonia was quantified by random stage microscopy. Data are the number of oogonia (mean±s.e.m.) per 121-point grid. A total of 18-42 grids per treatment were counted in each of five experiments. Asterisks indicate significant difference compared with control culture (0 nM K252a), P<0.01.
Discussion
The factors that determine whether oocytes survive or die as follicles form are unknown. We have investigated the possible role of the Trk receptors in this process by examining TrkB−/− and TrkC−/− mice, and interfering with the action of Trk receptors and their ligands in cultures of mouse and human ovaries.
In the original mixed-genetic background colony of TrkB−/− mice (C57BL/6 × 129/Sv), ~50% of ovaries contained reduced populations of oocytes, whereas the remainder appeared normal. Examination of congenic strains of TrkB−/− mice based on either 129/Sv or C57BL/6 lines of mice showed that, as with the original colony, ~50% of the ovaries of the 129/Sv congenic TrkB−/− mice had a normal complement of follicles, but that ovaries of all TrkB−/− C57BL/6 congenic mice had greatly reduced numbers of follicles. By contrast, the ovaries of all TrkC−/− mice were normal. These findings indicate that TrkB is an important factor in oocyte survival. The fact that, on certain backgrounds, its loss does not always have a significant effect indicates that other factors are also likely to be involved in oocyte survival. The efficacy of these factors might vary with background, being low in C57BL/6 and high in 129Sv mice. Similar background effects have been found in the development of transgenic α3 Connexin mice (Gong et al., 1999).
TrkB−/− offspring have retarded development in general and die within the first 10 days of birth. The paucity of oocytes in TrkB−/− mice was not caused by this general retardation because 50% of mice on the original mixed background and of the 129/Sv congenic mice had normal ovaries but retarded general development. In addition, TrkC−/− mice exhibited similarly retarded development but their ovaries were unaffected.
Ovaries were cultured either prior to (human), or during and shortly after (mouse) follicle formation, in the presence or absence of K252a. K252a is an indole carbazole and a potent, specific inhibitor of the intracellular protein-kinase domain of the Trk receptors (Tapley et al., 1992). K252a dramatically inhibited oocyte survival in newborn mouse ovaries in culture, inducing a loss of 85% of germ cells. In other systems, K252a is reported to block the activity of Trk receptors but not other tyrosine kinase receptors at the doses used here (Tapley et al., 1992). Evidence for this in the ovary was obtained by adding bFGF, which acts via a non-Trk tyrosine kinase receptor, to cultures containing K252a. bFGF rescued ovaries from the effects of K252a, indicating that K252a did not block bFGF receptors. These findings also demonstrate that ovarian cells are responsive to bFGF. It is conceivable that bFGF is another survival factor for oocytes acting in concert with neurotrophins.
RT-PCR showed that mRNA encoding NT4 and BDNF are both present throughout the period of follicle formation in the mouse. We have already shown this is the case in humans, where production of NT4 mRNA moves from a germ-cell to a somatic-cell location as follicles form (Anderson et al., 2002). Although NT4 expression increases in rat follicles as they start to form (Dissen et al., 1995), the fertility of NT4−/− mice appears normal (Conover et al., 1995). It is not possible to examine the fertility of BDNF−/− mice because, like TrkB−/− mice, they die shortly after birth (Conover et al., 1995).
The combined inhibition of NT4 and BDNF activities in culture using blocking antibodies lowered oocyte survival, but blocking either ligand alone had no detectable effect. Dead and dying oocytes were rare in control ovaries, but significantly increased in all treated ovaries, irrespective of whether blocking antibodies were added singly or in combination. The lack of a significant effect of blocking either BDNF or NT4 alone on oocyte survival indicates that the ligands are able to compensate for each other to a large extent. The combined addition of anti-BDNF and anti-NT4 was less effective than the addition of K252a, probably because the antibodies were less able to penetrate the tissue. This would explain the variation in the extent of oocyte loss from region to region within the treated ovaries. A less likely explanation is that other, as yet unidentified, ligand(s) are involved.
Newborn ovary cultures supported development of follicles from the primordial to the primary stage. By contrast, culture of E16.5 mouse ovaries supported formation of follicles from oogonia such that follicles formed directly at the primary stage, bypassing the primordial stage of development. K252a had no effect in this system, indicating that Trk receptors do not play a role in the survival of primary follicles. We conclude, therefore, that K252a inhibits the survival of primordial follicles in newborn ovary cultures. Thus, Trk receptors appear to play a role in the survival of follicles at the primordial but not the primary stage of development. Although we found no indication that neurotrophins affect primary follicle survival, they do appear to influence follicle function at that stage: NGF−/− mice have fewer follicles leaving the primordial follicle pool and undergoing growth initiation (Dissen et al., 2001), whereas early postnatal rat ovaries cultured with NGF have increased numbers of follicle stimulating hormone receptors (Romero et al., 2002).
The neurotrophins and their receptors also play a role in later ovarian function. TrkA and NGF are involved in the regulation of ovulation (Dissen et al., 1996) and BDNF might be involved in oocyte maturation in antral follicles (Seifer et al., 2002). In addition, there is recent evidence that neurotrophins play a role in testis development. TrkA−/− and TrkC−/− male foetuses have reduced numbers of germ cells and impaired seminiferous tubule development compared to wild-type mice (Cupp et al., 2002). Similarly, in human fetal testes, Trk-receptor signalling is involved in the regulation of germ cells and peritubular cells (Robinson et al., 2003).
TrkB was present in mouse ovaries throughout the period of follicle formation, with mRNA (both truncated and full length) and protein (full length only) located primarily in the germ cells. Immunocytochemistry of full-length TrkB was strongest in the oocytes of P0 ovaries. It is likely that the effect of K252a on germ-cell survival shown here is caused by inhibition of TrkB function. It is less likely that it results from either inhibition of TrkC receptors, given the absence of any ovarian phenotype in TrkC−/− mice, or inhibition of TrkA receptors, because expression of these virtually disappears as follicles form in the rodent (Dissen et al., 1995). Our data do not exclude a role of TrkA and TrkC in oocyte survival, because these receptors might become more important in the absence of TrkB signalling. This could be investigated in double mutants.
Truncated TrkB mRNA appeared to be more abundant than full-length TrkB mRNA in ovaries from E16.5 to P4. This pattern appears to be common in non-neuronal tissues (Wetmore and Olson, 1995) but the reason is unclear. The truncated receptors lack a tyrosine-kinase domain, and their effects are not well understood. However, the effects of mutations and antagonists on follicle survival described here are caused by interference with the low abundance, full-length Trk receptors. TrkB−/− mice have a mutation in the tyrosine-kinase domain. Whereas full length TrkB is not expressed in these mice, examining the head of newborn mice showed that levels of truncated TrkB receptors remained unchanged (Klein et al., 1993). Similarly, K252a blocks the tyrosine-kinase function of the Trk receptors, but does not interfere with potential activity of Trk receptors outwith the tyrosine-kinase domain. The role of the truncated receptors in the ovary remains to be determined.
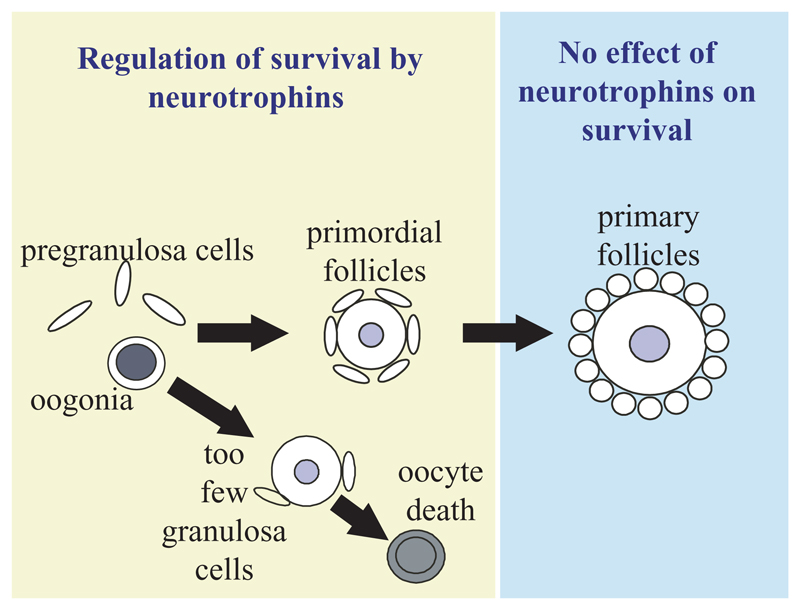
It is unclear why some follicles survive in TrkB–/– mice. In only two instances, both in C57BL/6 congenic mice, were ovaries with no follicles found. In all other cases, some follicles survived. Lundy et al. (Lundy et al., 1999) showed that primordial follicles contain a variable number of somatic cells, with the number of granulosa cells in sheep primordial follicles ranging from three to 52. It has been suggested that oocytes contained in primordial follicles require a particular number of associated granulosa cells for their continued survival (Sawyer et al., 2002). It is possible that an oocyte needs sufficient granulosa cells to sequester sufficient neurotrophins for its continued survival. Surviving oocytes in ovaries of TrkB−/− mice would, therefore, be the ones that have a sufficient number of granulosa cells attached (Fig. 12). In these instances, the increased numbers of granulosa cells might produce an increased amount of either neurotrophins (which might signal through other Trk receptors) or other factors that might compensate for a lack of neurotrophin signalling (possibly bFGF).
Fig. 12.
The possible effect of neurotrophins on folliculogenesis. Oogonia and pregranulosa cells associate into primordial follicles, with flattened granulosa cells, (a ‘resting’ stage of development). Oocytes with too few granulosa cells die at this point. Surviving oocytes are contained in follicles that have enough granulosa cells to provide the oocyte with sufficient neurotrophins. Follicles that leave the primordial stage and enter the primary stage exhibit rounding up of granulosa cells and undergo oocyte growth. Trk receptors have no effect on primary follicle survival.
Culture of human ovaries used foetuses with gestational ages ranging from 13 to 17 weeks. At these ages germ cells are proliferating, before primordial follicle formation. Primordial follicles first appear in the human ovary from approximately 19-weeks gestation. Lack of tissue and the slowness of human development with respect to that of rodents meant that we could not do experiments at the equivalent time to that of follicle formation in the mouse. The experiments on human ovaries examined the effect of K252a on prefollicular germ cells (oogonia) only. Human foetal ovaries cultured in the presence of K252a showed a decrease in oogonia, with K252a causing the loss of ~50% of oogonia over a 48-hour period. This indicates that Trk signalling might affect oogonial survival.
In conclusion, the data demonstrate that TrkB and its ligands are present in mouse ovaries as follicles form, as we have previously shown in human ovaries (Anderson et al., 2002). Our studies of mutant mice indicate that TrkB plays an important role in oocyte survival. Culture of mouse and human ovaries with a potent inhibitor of all Trk receptors, K252a, decreases germ-cell survival. This effect appears to be specific to oogonia and primordial follicles and does not occur in primary follicles. Blocking NT4 and BDNF also decreased germ-cell survival. Together, our results point to a role of the TrkB receptor and its ligands in the regulation of germ-cell survival at the oogonial and primordial follicle stage in mammalian ovaries. This pathway is therefore crucial to the determination of female reproductive lifespan.
Acknowledgments
The authors wish to thank Dr John West for helpful discussions and advice over the generation of congenic mice, Nasrin Taherkhani and Gillian Luther for help with the initial examination of the transgenic mice and Emily Otter for help with the in situ hybridisation. Thanks also to R. Klein for mutant mice and TrkB plasmid. The work was supported by the MRC and The Wellcome Trust. N.S. was a Royal Society University Research Fellow.
References
- Anderson RA, Robinson LL, Brooks J, Spears N. Neurotropins and their receptors are expressed in the human fetal ovary. J Clin Endocrinol Metab. 2002;87:890–897. doi: 10.1210/jcem.87.2.8221. [DOI] [PubMed] [Google Scholar]
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond (Biol) 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- Botchkaarev A, Botchkareva NV, Welker P, Metz M, Lewin GR, Subramaniam A, Bulfone-Paus S, Hagen E, Braun A, Lommatzsch M, Renz H, et al. A new role for neurotrophins: involvement of brain-derived neurotrophic factor and neurotrophin-4 in hair cycle control. FASEB J. 1999;13:395–410. doi: 10.1096/fasebj.13.2.395. [DOI] [PubMed] [Google Scholar]
- Brambell FWR. The Development and Morphology of the Gonads of the Mouse. – Part I. The Morphogenesis of the Indifferent Gonad and of the Ovary. Roy Soc Proc B. 1927;101 B:391–409. [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- Cupp AS, Tessarollo L, Skinner MK. Testis Developmental Phenotypes in Neurotropin Receptor trkA and trkC Null Mutations: Role in Formation of Seminiferous Cords and Germ Cell Survival. Biol Reprod. 2002;66:1838–1845. doi: 10.1095/biolreprod66.6.1838. [DOI] [PubMed] [Google Scholar]
- Dechant G. Molecular interactions between neurotrophin receptors. Cell Tissue Res. 2001;305:229–238. doi: 10.1007/s004410100378. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hirshfield AN, Malamed S, Ojeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinol. 1995;136:4681–4692. doi: 10.1210/endo.136.10.7664689. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Les Des CW, Lara HE, Ojeda SR. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinol. 1996;137:198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endrocrinol. 2001;142:2078–2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- Friedman EJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Gong X, Agopian K, Kumar NM, Gilula NB. Genetic factors influence cataract formation in α3 connexin knockout mice. Dev Genet. 1999;24:27–32. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<27::AID-DVG4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hantzopoulos PA, Suri C, Glass DJ, Goldfarb MP, Yancopoulos GD. The low affinity NGF receptor, p75, can collaborate with each of the Trks to potentiate functional responses to the neurotrophins. Neuron. 1994;13:187–201. doi: 10.1016/0896-6273(94)90469-3. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–99. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Lundy T, Smith P, O’Connell A, Hudson NL, McNatty KP. Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil. 1999;115:251–262. doi: 10.1530/jrf.0.1150251. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. doi: 10.1016/s0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- Polkinhorne J. Review of the guidance on the research and use of fetuses and fetal material. London: HMSO; 1989. [Google Scholar]
- Ringstedt T, Lagercrantz H, Persson H. Expression of members of the trk family in the developing postnatal rat brain. Brain Res Dev Brain Res. 1993;72:119–131. doi: 10.1016/0165-3806(93)90165-7. [DOI] [PubMed] [Google Scholar]
- Robinson LLL, Townsend J, Anderson RA. The human fetal testis is a site of expression of neurotrophins and their receptors: regulation of the germ cell and peritubular cell population. J Clin Endocrinol Metab. 2003;88:3943–3951. doi: 10.1210/jc.2003-030196. [DOI] [PubMed] [Google Scholar]
- Romero C, Paredes A, Dissen GA, Ojeda SR. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinol. 2002;143:1485–1494. doi: 10.1210/endo.143.4.8711. [DOI] [PubMed] [Google Scholar]
- Sawyer H, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Minichiello L, Vazquez E, San Jose I, Giraldez F, Klein R, Represa J. Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development. 1995;121:3381–3391. doi: 10.1242/dev.121.10.3381. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Ann Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab. 2002;87:655–659. doi: 10.1210/jcem.87.2.8213. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, Walker M. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. 2002;17:1692–1703. doi: 10.1093/humrep/17.7.1692. [DOI] [PubMed] [Google Scholar]
- Singh TD, Mizuno K, Kohno T, Nakamura S. BDNF and trkB mRNA expression in neurons of the neonatal mouse barrel field cortex: normal development and plasticity after cauterizing facial vibrissae. Neurochem Res. 1997;22:791–797. doi: 10.1023/a:1022075508176. [DOI] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- Wert MM, Palfrey HC. Divergence in the anti-apoptotic signalling pathways used by nerve growth factor and basic fibroblast growth factor (bFGF) in PC12 cells: rescue by bFGF involves protein kinase C delta. Biochem J. 2000;352(Pt 1):175–182. [PMC free article] [PubMed] [Google Scholar]
- Wetmore C, Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol. 1995;353:143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]