Abstract
For the investigation of retention and release of flavor components, various methods are available, which are mostly used on a case-to-case basis depending on the raw material. These effects that originate from kinetics and thermodynamics could be put in a much wider perspective if these fields were taken as a starting point of investigation in combination with rigorous data analysis. In this Review, we give an overview of experimental techniques and data analysis methods, and predictive methods using mass transfer techniques are also discussed in detail. We use this as a foundation to discuss the interactions between volatile flavors and the matrix of liquid foods/beverages. Lipids present in the form of an emulsion are the strongest volatile retainers due to the lipophilic nature of most of the volatile flavors. Proteins also have flavor retention properties, whereas carbohydrates hardly have a retention effect in beverages. Smaller components, such as sugars and salts, can change the water activity, thereby facilitating flavor release. Alternatively, salts can also indirectly affect binding sites of proteins leading to release (e.g., NaCl and Na2SO4) or retention (NaCSN and Cl3CCOONa) of flavors. Furthermore, the effects of temperature and pH are discussed. The Review concludes with a critical section on determination of parameters relevant to flavor release. We highlight the importance of accurate determination of low concentrations when using linearization methods and also show that there is an intrinsic preference for nonlinear regression methods that are much less sensitive to measurement error.
Keywords: aqueous food, experimental method, flavor release, modeling
1. Introduction
Aroma, taste, texture, and mouthfeel all contribute to the perception of flavors.1 When removing off-flavors or adding flavor to a product, separation of these components highly depends on their physicochemical interactions with other molecules, which are complex as discussed in various reviews.2,3,12−16,4−11
Flavor retention and release are mostly studied to design healthier food products (low-fat milk, alcohol-free beer, etc.) without compromising on traditional product acceptability, functional beverages (including drinkable meal replacers or sport supplements), and beverages with exotic features (exotic fruit tastes, cocktails, fusions, etc.). In skim milk, loss/lack of hydrophobic flavors challenges consumer’s acceptability compared to that of high-fat milk, whereas the potential health benefit of soy milk suffers from a beany off-flavor17 originating from lipoxygenase activity.18,19
Flavor release or retention is generally affected by the intrinsic chemical properties of the flavor (hydrophobicity, hydrophilicity (log P value), and volatility), the composition of the medium (lipid, protein, salt, sugar, etc.), and finally environmental conditions (temperature, pH). In other words, the interaction between flavor compounds and other food ingredients under given environmental conditions determines the intensity of flavor retention or release from a product. In this Review, we cover the thermodynamics and kinetics of flavor–matrix interactions in aqueous food systems starting with experimental and theoretical approaches; the actual human flavor perception is considered outside the scope of this Review.
In general, the driving force for flavor release from an aqueous phase is determined by the deviation from the thermodynamic equilibrium conditions between aqueous and gas phase. Such thermodynamic equilibrium obeys the following relationship:
| 1 |
where K is the partition coefficient or dimensionless Henry’s volatility coefficient, which is the reciprocal value of Henry’s solubility coefficient H, with CG and CL, the concentrations of the flavor in gas and liquid phase, respectively. The superscript cc indicates that concentrations are used. Although these coefficients are tabulated for binary aqueous systems (e.g., Sanders20), the available information is limited to simple systems, and even moving from binary to ternary systems makes the behavior quite complex.21 In the current Review, we will use the thermodynamic background to link the intrinsic chemical properties of flavors, matrix, and environmental conditions starting with measurement methods.
2. Methods to Determine Flavor Retention
Thermodynamics and transport phenomena can be investigated experimentally or mathematically to predict equilibrium and kinetics of flavor–matrix interactions. Understanding retention of flavors in a product requires measuring the variation of flavor present in at least one of the phases, liquid and/or gas, for which ample experimental methods are available for food matrices. For example, binding of volatiles to β-lactoglobulin has been investigated by O’Neill and Kinsella22 by equilibrium dialysis, Andriot et al.23 by headspace analysis, Relkin et al.24 by spectrofluorometric measurement, and Rogacheva et al.25 used a diffusion cell. In the food field, interpretation of data is complex, leading to the use of (over)simplified systems, whereas predictive methods have gained relevance due to experimental limitations or high costs.
First, we focus on experimental methods, after which data analysis is touched upon, followed by the mathematical models in use. In a dedicated section, specific liquid foods are discussed.
2.1. Experimental Approach
Flavor behavior can be assessed by sensory or instrumental analysis. Sensory analysis (performed by trained experts or ordinary assessors) gives an overall picture of the perceivable flavors, which implies that only a limited number of components play a role; the concentrations of a vast amount of volatile chemicals are simply below the limit of detection of the human sensory system. For example, in wine with more than 1000 identified compounds,26 only a few flavors contribute to sensory experiences. For the current review, we consider aspects related to sensory perception outsize our scope and focus on instrumental methods.
The current analytical methods are capable of tracking flavor behavior in great detail. Liquid chromatography,27 dynamic coupled liquid chromatography,28 and affinity chromatography29 have been used for different aqueous flavor systems often in combination with headspace analysis. One of the important parameters that may be obtained using chromatography is the octanol/water partition coefficient (log P) that can be used to parametrize hydrophilicity or lipophilicity of compounds.30,31
2.1.1. Static Headspace (SHS) Analysis
SHS is a standard procedure of collecting samples from a gas phase in equilibrium with a second phase (liquid or solid).19 Samples are collected by syringe, solid-phase microextraction,32 or single-drop microextraction16,33 and mostly analyzed by GC equipped with flame ionization detector (FID) or mass spectrometry (MS), thermal conductivity detector (TCD), and proton transfer reaction mass spectrometer (PTR-MS) that may be equipped with a switchable reagent (SRI-PTR-MS). For quantitative measurement of the concentration in gas phase, the total vaporization technique is particularly used for the preparation of calibration standards.34 The effective parameters in static headspace analysis are temperature, sample volume,35 and incubation time that can easily be controlled using an incubator and standard vials. In case of microextraction methods, the selection of adsorbent fiber or solvent has a significant effect on the quality of measurement.
2.1.2. Dynamic Headspace (DHS)
In this method, volatiles are continuously removed from the headspace by sweeping with an inert gas or taking multiple samples in time, leading to depletion of the matrix. The most important parameters are the sweep or purge gas volume and the extraction temperature.35
Multiple Headspace Extraction (MHE)
MHE was introduced by McAuliffe36 and uses multiple gas-phase withdrawal steps. This method was originally used to find the total concentration of a component in a matrix; because sampling times are not carried out ad infinitum, regression is used. The concentration in headspace and consequently peak area decrease exponentially
| 2 |
where C is volatile flavor concentration in time t, a is the proportionality parameter, and C0 is the initial concentration. Transforming this into peak area results in
| 3 |
where A1 is the peak area of the first measurement and i is the sample number. In linear form
| 4 |
Just like any linear regression, the equation heavily depends on A1, the first measurement taken, which can be prone to experimental error. Therefore, the quotient q = e–a has been introduced, leading to a new intercept A1*. The sum of all peak areas for a component defined as
| 5 |
Phase Ratio Variation (PRV)
PRV is an indirect method to determine the partition coefficient that is independent of liquid volume. The following constants are derived
| 6 |
| 7 |
where VV and VS are vial and sample volume, respectively, and AP is the peak area. By linear regression of α against β, the partition coefficient follows from the slope and intercept:
| 8 |
Because this method depends on the peak size differences resulting from changing the phase ratio, it is not suitable for components with high partition coefficients Kcc that give large peaks already at low concentrations of which the difference is hard to measure, leading to issues with linear regression. For more details on the method, we refer to the book of Kolb and Ettre.37
Exponential Dilution Technique (EDT)
EDT is a method where the liquid phase is exhausted by the continuous flow of an inert gas. The concentration in the liquid usually decreases exponentially (similarly as described for DHS analysis), and extraction kinetics can be compared between different liquid samples that contain flavor retainers or enhancers.
Multivolatile Method (MVM)
MVM is a sequential dynamic headspace method in combination with adsorption using different adsorbent traps.38 The first and second sampling sequences target components with high (>20 kPa) and moderate (1–20 kPa) vapor pressure using carbon-based material at 25 °C. The third and final sequence uses a Tenax TA trap at 80 °C to target components with low vapor pressure (<1 kPa) and/or hydrophilic characteristics. The three traps are sequentially thermally desorbed, trapped, and concentrated in a programmed temperature vaporizing (PTV) inlet and analyzed in a single GC-MS run.
Batch Stripping
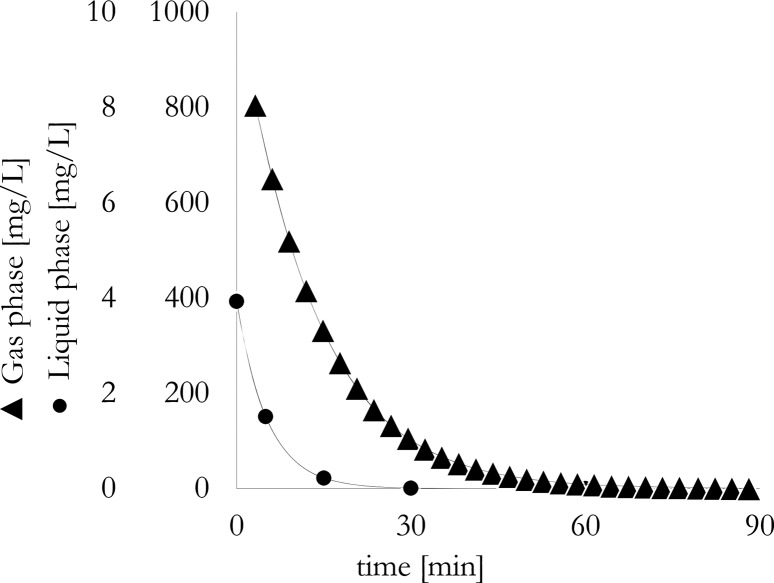
This method is used if direct gas-phase analysis is not possible. Because Henry’s coefficient is an important design parameter for stripping columns, this equipment can be used to derive its value from liquid samples taken as a function of time39 (see also Data Analysis section). On the basis of our experience (Figure 1), significant separation takes place early on; therefore, measurement time intervals need to be tuned accordingly.
Figure 1.
Concentration depletion in gas and liquid phase during stripping of 500 ppm isoamyl acetate solution in water with CO2.
2.1.3. Equilibrium Dialysis
This is one of the oldest methods;40 two cells of equal volume separated by a membrane are filled with, e.g., a buffer containing flavor, and a protein solution and allowed to equilibrate. The key points are to ensure true equilibrium, the absence of adsorption to the membrane,10 and the sample container (relevant at low solubility). For this, appropriate blank measurements can be used.41 Equilibrium analysis suffers from comparatively higher uncertainty: Beyeler and Solms42 investigated binding between 12 ligands and soy protein and bovine serum albumin and found similar binding constants, whereas Mills and Solms43 found notable differences using headspace analysis. Most likely, protein–membrane interactions are responsible for this.
2.1.4. High-performance liquid affinity chromatography (HPLAC)
Sostmann and Guichard27 introduced this method to investigate the interaction of β-lactoglobulin (BLG) with flavor compounds. They immobilized BLG on silica support, and by injecting flavor compounds, differences are observed related to protein–flavor interactions. One of the drawbacks is that the support materials are not inert to all flavors.
These are the most common methods used for food, but there are others applied in, e.g., biology, biophysics, or biochemistry. For example, the Hummel and Dreyer method is used by Pelletier et al.29 to determine the number of binding sites in BLG for selected flavors, but these methods are too specific for this Review and considered out of scope.
2.2. Data Analysis
In most methods mentioned earlier, gas chromatography is used to measure headspace composition. The GC peak areas are used in different ways; for instance, Weel et al.44 took peak area variation to report the effect of whey protein gel on diacetyl and ethyl butyrate release, whereas Nahon, Roozen, and de Graaf45 used the sum of the average peak area to investigate possible interactive effects between sweetness and aroma compounds. Mostly, a flavor in an aqueous food matrix is reported relative to a standard such as a flavor–water system.46−48 Nahon et al.49 reported partition coefficients for ethyl acetate, methyl butanoate, ethyl butanoate, hexanal, and octanal as a function of sucrose concentration, which is much more generally applicable.
Next, we describe various characterization parameters. Wang and Arntfield50 used “binding percentage” and expressed it as a function of their gas chromatograms peak area A as
| 9 |
Landy et al.51 replaced peak area values with the vapor–liquid partition coefficient expressed in molar fraction and called it “retention percentage”.
In various investigations,22,40,52,53 the “double reciprocal equation” is used to analyze equilibrium dialysis data using
| 10 |
with v moles of bound flavor per mole protein, Cf the free flavor concentration, n the number of binding sites in the protein, and κ the global binding constant. This method heavily depends on measurement accuracy at low concentration; nonlinear fitting is preferred (see Conclusions).
The amount of bound component54 can be determined using
| 11 |
where CGbb is the headspace concentration for the buffer blank, CG is the headspace concentration for protein-buffer solution, and C is the flavor concentration. Seuvre et al.55 used the Henry coefficient as a starting point and derived the “retention percentage” defined as
| 12 |
in which KGWxy and KGM are Henry’s volatility parameters of a volatile in water and solution, respectively. Landy et al.51 reported the vapor–liquid partition coefficient of aroma compounds in a solution containing nonvolatile constituents through
| 13 |
where t is the time, At0 and At are the peak area of volatiles at time t = 0 and t, respectively, T is the temperature (K), N is the number of moles of liquid phase, Q̇G is the carrier gas flow rate, P is the total pressure, and R is the gas constant.
Using activity coefficients, Fares et al.56 and Langourieux and Crouzet57 derived
| 14 |
where pi is vapor pressure of the pure solute and γi∞ is the activity coefficient at infinite dilution.
As mentioned, if there is a limitation in gas-phase sampling, the partition coefficient can be measured with a batch stripping column. For the flavor concentration in the liquid phase at known stripping gas flow rate,39 the following equation is used
| 15 |
where CL0 and CL are the initial and sequential liquid-phase concentrations, respectively, KH is Henry’s volatility coefficient (pc indicates dimension pressure over concentration in the phase), Q̇G is the gas flow rate, T is the temperature, Vcolumn is the column volume, R is the universal gas constant, and t is time. From a linear plot of ln C versus time, KHpc is determined. Please note, the system needs to be (i) isothermal, (ii) liquid phase well-mixed, (iii) vapor phase ideal, (iv) Henry’s law valid, (v) volume of liquid constant, (vi) partial pressure of the solute low compared to the total pressure, and (vii) exit vapor at equilibrium with the liquid. Gosset et al.58 mentioned that equilibrium in the outlet and well-mixed system is difficult to warrant, and we believe that the liquid volume is not that constant when taking multiple samples.
For high-performance liquid affinity chromatography, the flavor–protein interactions are reported as a “binding constant”27,29,59−61
| 16 |
where tR and t are retention times of the compound with protein and without protein present on the column, respectively, CP is the protein concentration, and t0 is the void time.
2.3. Predictive Approach
Various modeling approaches have been successfully applied; here, we focus on phase equilibria and mass transfer starting with the partition coefficient that underlies both.62 We give special attention to experimental work in the conclusions because it forms the basis for fully theoretical concepts such as UNIFAC,63 NRTL,64 or the interaction-parameter-based Wilson method.65
2.3.1. Phase Equilibrium
Buttery et al.66 estimated partition coefficients (eq 17) for aliphatic aldehydes in water–oil mixtures starting from binary air-to-water partition coefficients, air-to-oil partition coefficients, and the oil and water fraction in the product
| 17 |
where CG and CM are the concentrations of flavor in gas and liquid mixtures, respectively. The overall concentration of the flavor in the liquid mixture can be expressed through a component mass balance with FW and FO the fractions of water and oil, respectively.
| 18 |
Thus
| 19 |
Doyen et al.46 used the same expression to investigate volatile release from emulsified lipids based on concentrations; Roberts et al.48 investigated the effect of lipids on flavor retention in milk-based liquids using oil-to-water partitioning.
| 20 |
| 21 |
For systems containing proteins instead of lipids, Andriot et al.23 reformulated the partition coefficient based on available unbound flavors by introducing an effective partition coefficient.
| 22 |
where Cb and Cf are the concentrations
of bound and free flavors in the liquid phase, respectively. At equilibrium,
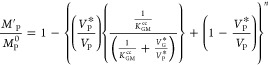
the concentration of bound flavor is a function of binder concentration CB, the concentration of free flavor in the liquid
phase Cfeq, and global equilibrium binding constant 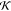 b.
b.
| 23 |
These
authors assume that protein reduces available flavor for
transport to the gas phase by a factor (1 + 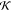 bCbeq), or in other
words, they assumed irreversible binding to the protein and
bCbeq), or in other
words, they assumed irreversible binding to the protein and
| 24 |
The equations mentioned above form a good basis. Obviously, if the flavor compounds interact, this will complicate the situation,67 and there are factors that need to be treated with caution, such as temperature, acid–base equilibria, sorption to suspended particles, and other phase transitions such as crystallization,62 as described next.
Temperature, Pressure, and Phase Composition
We discuss these factors together because they are linked: equilibrium is established when the chemical potential of a component in the two phases is equal. For gas and liquid
| 25 |
and by definition
| 26 |
where μ is chemical potential, p is vapor pressure, and R and T are the universal gas constant and temperature, respectively. Superscript 0 and “sat” are indicators of values in standard and saturated conditions, respectively. The chemical potential is not only affected by temperature and pressure but is also a function of the activity of the flavors in the liquid phase.
If the temperature, pressure, and composition were the only factors to consider, modeling should not be complicated because commercial software such as Aspenplus can predict gas-product equilibria. However, some components affect the activity of volatiles without binding, such as ethanol,68,69 salt,18 and sugar,45,70 and this is not yet covered in this software. These components can also influence the equilibrium indirectly, e.g., by changing the binding constant of proteins71 or through denaturation.40 Furthermore, phase transitions, such as crystallization,11 can reduce the phase volume for flavors to interact with,72 which consequently leads to higher gas-to-mixture partitioning. To be complete, for sugars, comparative effects were reported: sugar hydration reduces free water and increases flavor concentration (some observations are in the next section).
Flavor Binding and Entrapment
Flavor binding to a food matrix is “sorption” in its broadest sense, so adsorption, absorption, and physicochemical and chemical binding.1 Bound, free, and total concentration can be distinguished, and only the freely dissolved components contribute to the gas-phase concentration. Exchange of flavor compounds between bound and free state is often faster in liquid foods than flavor transfer to the gas phase;73 transport across the water–gas interface is thus the rate-limiting step. Flavors can also be entrapped in small regions, e.g., created by carbohydrates,74 and suspended solid particles can have binding properties albeit the rate of equilibration is often slow because of diffusion limitation;62 therefore, we only mention this to be complete.
2.3.2. Interfacial Mass Transfer
Transfer of flavors from the aqueous phase to either air or another liquid phase such as saliva can be described using theoretical concepts from chemical engineering.3 As early as 1855, Fick expressed the mass transfer rate as a linear function of a molar concentration gradient.75 By introducing hM and hG as mass transfer coefficients for mixture and gas phase, mass flux J for either side is given by
| 27 |
| 28 |
where CM, CMi, CG, and CG are concentrations of flavor in mixture bulk, and interface and gas bulk, and interface, respectively. (CGi – CG) is the driving force for mass transfer on the gas side, which is often smaller than that on the liquid side (CM – CM).73,76,77 The concentration CMi at the interface determines the concentration in the bulk air phase CM = CG/KGMcc (see also Figure 2); thus, eq 27 can be rewritten to
| 29 |
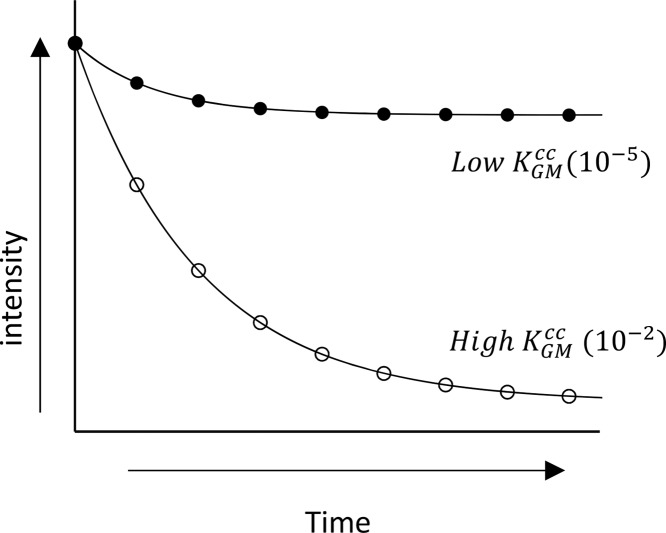
Figure 2.
Schematic representation of headspace depletion for compounds with different partition coefficients.80
The value of mass transfer coefficient hM depends on how we describe mass transfer phenomena in the interface (see Coulson et al.78 for a review). Two classic theories, the two-film theory77 and the penetration theory,79 are still extensively used in prediction of flavor release, and later, we introduce them briefly.
Mass transfer is a process resulting from either the random movement of molecules (molecular diffusion) or convective eddies present in turbulent fluids (eddy diffusion), and both are relevant for food research. Eddy diffusion is much faster than molecular diffusion and independent of flavor type.62 Doyen et al.46 showed that eddy diffusion can be used to predict ethyl hexanoate release from an emulsion with low-fat content and molecular diffusion is suited to predict ethyl octanoate release, whereas the headspace behavior of ethyl butyrate can be described by eddy diffusion. In general, eddy diffusion gives better predictions for systems with high partition coefficients and molecular diffusion for systems with a low partition coefficient (see Figure 2(80) for corresponding headspace concentration profiles).
The Two-Film Theory
In this theory, it is assumed that turbulence creates concentration uniformity in gas and product while bringing molecules close to the interface where eddies die out and form a laminar stagnant region where the resistance to transfer is located. In these regions, diffusion is molecular, and the mass transport coefficient hM is given by
| 30 |
where DM and δM are molecular diffusivity of flavor and film thickness on the mixture side, respectively.
Penetration Theory
This theory suggests that eddies in the bulk bring an element to the interface for a finite time, exposing it to the second phase after which it returns to the bulk. In this way, the bulk is exposed to the second phase, and equilibrium is established immediately through molecular diffusion.62 The short exposure time does not restrict components to reach the surface layer,78 and the mass transfer coefficient is given by
| 31 |
where D is the average diffusion coefficient and te is the exposure time to the second phase. Van Elk et al.81 introduced a modified theory for finite liquid bulk to which we refer the interested reader.
In both the film and penetration theory, the resistance in the liquid phase controls mass transfer, which is valid if there is no concentration gradient in the gas phase. For stagnant water phase and a turbulent gas, this holds for high partition coefficients KGMcc > 10–3.62 If mass transfer resistances in both phases exist, this can be taken into account using the relationship82
| 32 |
Mass flux across the interface is related to the bulk concentrations of the flavor in both phases that can be derived from an overall mass transfer coefficient hO defined as
| 33 |
Because no concentrations build up in the boundary layers at the interface, this gives
| 34 |
Nonequilibrium Partition Model
De Roos and Wolswinkel83 described partitioning of volatile compounds in matrices for eddy diffusion in highly agitated systems to allow exchange between product volume element VP* and gas volume element VG. Furthermore, the whole liquid boundary layer is considered at equilibrium with the gas boundary layer.3Eq 35 shows the amount of released volatile M′p after MP0 extraction steps relative to MP, the initial concentration.
 |
35 |
The term VG*/VP is indicative of mass transfer resistance; for high product resistance, the product element in equilibrium with a fixed volume element of gas is smaller, leading to higher VG*/VP. De Roos and Wolswinkel83 showed that their approach also holds for some less agitated systems.
Mathematical Models for Predicting Flavor Equilibration in the Headspace above Aqueous Mixtures
In 1997, Harrison and colleagues76 presented a mathematical model for diacetyl release from a water–sunflower oil emulsion using equilibrium partitioning66 and the penetration theory
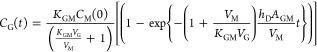 |
36 |
where AGM is the gas–liquid mixture surface area. This correlation actually dates back to McNulty’s Ph.D. thesis84 that focused on flavor transport from emulsions to saliva.85−87 For a long exposure time t → ∞, the headspace concentration reaches equilibrium; thus
| 37 |
Andriot et al.23 state that CG(∞) must be the same as that used for the effective partition coefficient in eq 24. For a short exposure time t → 0, eq 36 results in
| 38 |
In the early stages of flavor transfer, the partition coefficient KGM has no effect on the release (see eq 38). Harrison et al.76 conclude, based on eq 36, that the effect of emulsion composition and microstructure on initial flavor release is through an effect on the interfacial mass transfer coefficient hD that they used as a fitting parameter. They also found that surfactant or protein had no effect on flavor diffusion between the phases and could successfully describe the release of 2-heptanone from 60:40 oil-in-water emulsion. Despite these interesting findings, we believe that a case-by-case evaluation is needed using the thermodynamic kinetic models as a reference. Seuvre et al.55 showed that increasing lipid (miglyol) concentration from 0.5 to 1% completely masks flavor binding to BLG, and McNulty and Karel86 saw a drop in overall mass transfer coefficient for long-chain alcohols as the surfactant is added to the oil/water emulation. A similar observation by Guichard and Langourieux61 shows that, even if the addition of fat induced a greater change in favor retention than the addition of protein, BLG at the oil/water interface does limit the transfer of hydrophobic compounds from oil to water. In the next section, the effects that have been attributed to the various food components are discussed based on their category.
3. Flavor–Matrix Interaction in Beverages
Except for water, the majority of drinks are complex mixtures of water, carbohydrates, lipids, proteins, and other organic compounds, and all of them can interact with and/or bind flavors.88 The duality of health and acceptability of a beverage usually has contradicting aspects; e.g., separation of fat from milk or ethanol from beer leads to loss of desirable flavors, whereas the product as such could be more healthy. In this section, we will look at flavor–matrix interactions in liquid food products. Because of the massive amount of research,7,89,90 it is not feasible to address all flavor–matrix interactions; therefore, we present relevant categories below.
3.1. Lipids
In beverages, oils and fats may be dispersed as droplets91 that are thermodynamically unstable; therefore, surfactants (emulsifiers) such as proteins are added to protect oil drops from coalescence.92,93 Lipids can accommodate hydrophobic components1,46,94,95 and, consequently, have high flavor retention (depending on the logP value of the component) compared to other food ingredients (see later sections).94 The physical state of the lipid also affects aroma retention.96 McNulty and Karel86 using stirred diffusion cells showed that hydrogenated vegetable oils decreased flavor release rates by 1 order of magnitude when going from oil to a solid fat index of 66, thereby influencing the overall partitioning coefficient as discussed before. Roberts et al.48 used headspace sampling with a solid-phase microextraction fiber and investigated this further using a milk-based emulsion with 1.36% lipid content consisting of hydrogenated palm fat and milk fat. Investigations at various temperatures showed lower flavor release at higher solid fat content for practically all systems with the exception of 2-pentylfuran and limonene in milk fat, which may be due to crystal exclusion effects.
3.2. Proteins
Protein-containing beverages cover a broad spectrum, including dairy, soft drinks, sports drinks, and fermented beverages, and may contain proteins from animal and plant origin. Even though proteins do not contribute to the flavor of products directly,97 they can interact with flavors either reversibly22,27 or irreversibly98,99 (if hydrolyzed, proteins are known to form peptides that can be extremely bitter depending on their size and hydrophobicity100). Covalent chemical linkage such as amide and ester formation and condensation of aldehydes with sulfhydryl (SH) groups101 are irreversible, and noncovalent hydrophobic and electrostatic interactions, hydrogen bonds, and van der Waals interactions are reversible.9 For example, β-lactoglobulin is known to have reversible interactions with flavors,27 and aldehyde flavors can bind reversibly and irreversibly to proteins.97,102
Proteins may also convey undesirable off-flavors to foods, soy protein in particular is known for this.103,104 Furthermore, proteins can change food structure, which reduces flavor perception due to inhibited mass transfer.105−107 Flavor–protein interactions are more diverse than those with lipids or carbohydrates due to the variability of the chemical structure, including varying amino acid side chains, terminal ends, and more hydrophobic regions.89
Whey Protein
Whey protein has long been considered a byproduct of cheese formation but is now highly valued and contains, among others, α-lactalbumin, bovine serum albumin (BSA), immunoglobulins, and most abundantly β-lactoglobulin.108
β-Lactoglobulin
BLG, of which the characteristics and structure are well-known,9,109 is also known to bind various flavors such as alkanones,22 esters,29 methyl ketones,110 alcohols,61 and lactones9 reversibly through hydrophobic interactions.27 The binding capacity increases going from alcohols to ketones and aldehydes,9 and within a chemical class, the affinity constant increases with increasing hydrophobic chain length61 except for terpenic compounds,60 acids, and pyrazines.29 O’Neill and Kinsella,22,111 using an equilibrium dialysis method, observed a reduction in binding capacity as a consequence of structure loss due to urea treatment (disulfide bonds or ethylation). Further, it was reported that β-lactoglobulin at the oil/water interface limits transfer of hydrophobic compounds and reduces flavor release.61 Furthermore, by using headspace analysis and exponential dilution technique, Seuvre et al.55 showed that the relative volatility of 2-nonanone in a mixture of water with 3% β-lactoglobulin and 0.5% miglyol is higher when using an emulsion, whereas isoamyl acetate was not affected, which could hint at cooperative effects.
α-Lactalbumin
α-Lactalbumin has a lower binding capacity compared to other whey proteins even though it can bind ketones and aldehydes.112,113 Charles et al.114 used static headspace analysis to compare flavor release of ethyl hexanoate and allyl isothiocyanate from emulsions with β-lactoglobulin and α-lactalbumin; the flavor retention of the latter emulsion was significantly less.
Bovine Serum Albumin (BSA)
BSA binds a variety of compounds: retinol,115 long-chain fatty acids,116−119 alkanes,120−122 aldehydes, and ketones.40,41,112 By using a liquid–liquid partition equilibrium method, Damodaran and Kinsella123 found that the binding constant for ketones depends on chain length, functional group, and protein structure. In general, binding constants for BSA decrease in the order aldehydes > ketones > alcohols.124 Compared to casein, BSA binds larger amounts, using as many as 5 or 6 out of 21 primary carbonyl binding sites.90,113
Caseins
Caseins are less ordered and more flexible than the globular whey proteins that have secondary and tertiary structure.125 In aqueous solutions, caseins show retention of several flavor compounds: limonene, linalool, terpinyl acetate, β-ionone, and 2-octanone.126 Caseins are used in a wide variety of food emulsions,127 and the effects are diverse: aqueous-phase mass transfer resistance increases for ethyl acetate, whereas interface resistance is higher for ethyl butanoate and ethyl hexanoate.128
Flavor retention depends on aroma compounds and the protein content, as was the case for the other proteins. For a homologous series of ethyl esters (ethyl acetate, butanoate, and hexanoate), Landy et al.51 using either headspace analysis or exponential dilution method showed that retention increased with carbon chain length from 0 to 38% and 0 to 61% for caseinate contents of 5 and 50 g/L, respectively. For diacetyl, the corresponding increase is 0 to 23%, which is in line with data for ethyl acetate.129
Fares et al.56 employed exponential dilution and equilibrium dialysis and compared activity coefficients of aroma compounds in casein solution (25 and 75 g/L) and found no retention for acetone, ethyl acetate, or 2-propanol but did find that diacetyl and benzaldehyde interacted through strong and weak bonds. The binding behavior of diacetyl is in agreement with findings of Landy et al.;51 activity coefficients of selected aroma compounds in an aqueous casein solution (25 g/L) gave no significant change for acetone and ethyl acetate, but for diacetyl, benzaldehyde, and 2-propanol, the activity coefficient increased 360, 150, and 130%, respectively, which we interpret as an increase in volatility. At higher casein concentration (75 g/L), significant binding for benzaldehyde, acetone, and ethyl acetate is found, and volatility of diacetyl and 2-propanol increased. In another study performed by Le Thanh et al.,130 this increase in activity coefficient was observed for acetone and ethyl acetate using headspace analysis and sorption
Soy Protein
Soy protein consists of four protein fractions, 2S, 7S, 11S, and 15S, according to their Svedberg units. The main protein fractions are the globulins 7S and β-conglycinin (37–39% of total protein) and 11S and glycinin (31–44% of total protein).131 Damodaran and Kinsella132 studied binding of 2-nonanone using the equilibrium dialysis method and found that 11S has a very weak affinity compared to that of 7S, which acted similarly to whole soy protein, suggesting preferential interaction with the 7S fraction.
Gremli133 and co-workers used the headspace sampling method along with what they named high vacuum transfer method and investigated flavor interactions with soy protein; unsaturated aldehydes strongly interact (a percentage is permanently bound due to irreversible bonds) compared to that of saturated ones. Furthermore, at 100 mg/L, no evidence of flavor–flavor interactions of aldehyde and ketones were found in 5% protein solution. From the maximum amount of volatiles bound to the protein, they concluded that, at conventional dosage levels, ∼70% of added heptanal and 60% of 2-nonanone might be lost in soy protein-containing beverages.
By using the micropartitioning method, Li et al.134 studied interactions of vanillin with soy, casein, and whey proteins. At 12 °C, they found that the enthalpy and entropy of binding for casein and whey protein are negative and thus enthalpy-driven, whereas for soy protein, this is highly positive and entropy-driven. Protein-flavor binding is strongest in the following order: soy > gelatin > ovalbumin > casein > corn.89 Beyeler and Solms42 also found that soy protein, β-lactoglobulin, and bovine serum albumin showed increased binding with chain length, which points to hydrophobic interactions.22,28,40
3.3. Sugars
In food products such as ice cream, beverages, jellies, and sauces, carbohydrates are used as sweeteners, thickeners, stabilizers, and gelling agents. The impact of carbohydrates on aroma compounds is quite diverse and difficult to predict because they are able to induce both retention and release effects depending on the conditions used and on the actual flavor molecules.135 In beverages, small sugar molecules (mono- and disaccharides) affect flavor partitioning via binding with water molecules, leaving flavors to be concentrated in the remaining available water45,136,137 as reflected in increased activity coefficients of acetone, ethyl acetate, and octanol in solution in the presence of glucose130 and sucrose.36 Cyclic oligosaccharides, such as cyclodextrin, and polysaccharides (starch, gum, and pectin) are known for their ability to form inclusion complexes with aromatic compounds, making them good flavor carriers and encapsulation materials.138−140 These interactions have been investigated141−143 and reviewed144 but are considered outside the scope of the current paper.
In expresso coffee beverages, the addition of sucrose, fructose, or lactose was shown to lead to a significant release of some furan compounds and a lower release of pyrazines,145 whereas in ready-to-drink coffee, the presence of sugars induced either no change or a retention effect depending on the sugar type. Even though salting-out should not be excluded, Paravisini and Guichard believe that retention can be a result of interactions between other nonvolatile compounds and aroma compounds. For example, the nonvolatile matrix of coffee contains up to 30% of brown polymers called melanoidins that are known to interact with aroma compounds.135
Using the headspace analysis technique, Kieckbusch and Judson King146 showed that, for esters, the partition coefficient increases in maltodextrin solutions with increasing carbon number as Nawar147 observed later on for sucrose/water solutions and ketones using the same method. Nawar also reports a radical increase in headspace concentration when the flavor was added to water/sugar solution compared to adding sugar to water/flavor solution. Bredie et al.148 showed that, in 20% glucose/water solution, volatility of compounds with low water solubilities, such as menthol and limonene, increased, whereas isoamyl acetate and diacetyl, which have some solubility, were not affected. The activity of flavors with low solubility is much more affected by the addition of sugars.
3.4. Ethanol
Ethanol odor is described as sweet,14 and the concentration ranges from 2.5 to 70% in commercial beverages. Ethanol is polar and fully miscible with water, which increases the solubility of hydrophobic flavors and thus enhances flavor retention,149−151 which can be traced back to a book written by Young152 that shows that partitioning of esters and higher alcohols is reduced with increasing volume percentage of ethanol. Bakker et al.153 showed this also for 10 mg/L of isoamyl acetate, and Conner et al.154 using headspace analysis showed that activity coefficients of esters decreased for ethanol concentration >17% (v/v) depending on their acid chain length. At concentrations below 17% (v/v), the activity is not affected because of limited solubility of these hydrophobic compounds.
Indirectly, ethanol is involved in structural changes of certain proteins;155,156 in 13%(v/v), ethanol denaturation of β-lactoglobulin was observed, which influences flavor interactions157 through reduction of accessible binding sites.158 Andriot et al.157 used two complementary static headspace and HPLC techniques to find that heat treatment did not affect the retention of benzaldehyde in β-lactoglobulin solution, whereas in the presence of NaCl or ethanol retention of benzaldehyde decreased, which was attributed to aggregation of the protein.
3.5. Salts
Salts are known to influence flavor compounds in aqueous systems,159−161 which is often referred to as salting in and salting out effects, and in emulsions they are known to influence the partition coefficient because of this. Although most foods do not contain large amounts of salt, it can still also be relevant as some salts have much greater effects than others (e.g., CaCl2). We mention here a number of effects that were reported to be complete. Saturation of paraffin oil/water emulsion with sodium sulfate increases the partial pressure of volatiles 12–20 times.18 Salts can also alter protein conformation, possibly exposing hydrophobic binding sites, leading to changes in their binding capacity162,163 and possibly even aggregation. Damodaran and Kinsella53 using equilibrium dialysis investigated NaCl, Na2So4, NaSCN, and CL3CCOONa in relation to binding of 2-nonanone, 2-octanone, and 2-heptanone to BSA. They found that NaCl and Na2So4 increased the activity coefficient of 2-nonanone, leading to its removal from the protein phase to the salt solution. Wang and Arntfield50 using headspace analysis investigated pea protein isolate and rated the effect of different salts on flavor binding strength as Na2SO4 ≫ NaCl > NaCH3 = no salt > NaSCN. By using solid-phase microextraction (SPME) and GC/MS analysis for porcine protein, Pérez-Juan et al.164 observed that KCl and NaCl increased branched aldehydes, hexanal, and methional concentration in the headspace by 5–10 times, whereas no effect was found for octanal and 2-pentanone and MgCl2 and CaCl2 showed no effect for all flavors with the exception of branched aldehydes that were completely released in the presence of 1.0 M MgCl2. Last but not least, Bortnowska165 used static headspace analysis and studied the effect of salt in oil/water emulsions with dried egg yolk (DEY) or starch sodium octenylsuccinate (SOE) as emulsifiers and observed a decrease in diacetyl retention with increasing salt concentration regardless of emulsifier type.
From the above it is clear that salts can have various effects starting from a direct effect on the activity of flavor components present in the water phase (depending on their solubility) to indirect effects, mostly on proteins. Salt can influence charges of binding sites for flavors, lead to exposure of more hydrophobic patches, and even lead to aggregation of proteins. All these effects can influence the release and retention of flavors, and what we see in the literature is that often the more complex explanations are preferred while overlooking the direct thermodynamic effects on activity, which is a true omission.
3.6. Environmental Conditions
Viscosity is an internal friction of a fluid and acts on molecular diffusion as suggested by the Stokes–Einstein and Wilke–Chang equations. De Roos62 reports that adding 1% carboxymethylcellulose (CMC) to an aqueous flavor solution results in lower release rates from the viscous CMC solution than from water with the differences being highest for the most volatile compounds. By using headspace SPME, Rabe et al.166 studied the effect of viscosity using sucrose solutions on the release of 13 flavors and found very diverse behaviors. In general, highly volatile flavors are most affected by viscosity compared to that of less volatile ones,70 which is logical because highly volatile compounds hardly experience resistance from the gas phase, and the movement across the liquid phase is rate-limiting for mass transfer. Marin et al.167 found little effect on the mass transfer coefficient of flavors in water, but the temperature and viscosity play a critical role. Hansson et al.136 suggested that binding with viscosity enhancers also need to be considered.
Starting from the penetration theory, it is clear that the diffusion coefficient is influenced by viscosity,7 and this is the case for both molecular and eddy diffusion, which is a complex matter because diffusion of small molecules does not obey the Stokes–Einstein relation. The macroscopic viscosity of the system dramatically differs from the microscopic viscosity as “sensed” by the diffusing molecules.168 McClements169 offers an interesting example from Basaran et al.170 who used an ultrasonic imaging technique and showed that sugar molecules move through xanthan solutions at almost the same rate as they move through pure water. The macroscopic viscosity of the xanthan solutions (measured at low shear rates) is much higher than that of water, but the sugar molecules can pass through the pores rather unhindered.
Temperature affects retention of aroma compounds either directly or indirectly. At higher temperature, more flavor will be found in the headspace, which is a direct effect. Fares et al.56 used exponential dilution and equilibrium dialysis and showed that temperature increases the activity coefficient of flavors and that this can be further influenced by the presence of small molecules as discussed earlier. Indirectly, temperature influences binding sites; for example, soy protein binding of hexane to glycinin occurs at 5 °C but not at higher temperatures,171 whereas carbonyls interacted independent of temperature at temperatures above 25 °C but binding increased drastically at 5 °C.40 These findings were attributed to changes in the tertiary and quaternary structures; more examples can be found in the protein section. In general, binding is favorable at low temperature for β-lactoglobulin,111 casein and whey protein,134 and bovine serum albumin and model wine solution.172 As mentioned in the fat section, crystallization of lipids127 can also influence the distribution of flavors.
pH is one of the main reasons for protein denaturation, and through that also influences flavor binding. Emulsions stabilized by proteins are particularly sensitive;173 β-lactoglobulin undergoes several conformational changes between pH 2 and 9,174 and these changes affect the affinity for flavors as well as the emulsification strength of the protein. Jouenne and Crouzet28,175 show that binding of β-ionone, limonene, and ketones systematically increased going from pH 3 to 9 with the sharpest increase from pH 6 to 9 but diminishes at pH 11, where denaturation takes place. Binding of hydrocarbons by proteins was investigated by Mohammadzadeh et al.121 using lysozyme, ovalbumin, ovotransferrin, α-chymotrypsin, and α-chymotrypsinogen. For all proteins, there was a noticeable increase in binding of heptane at lower pH values except for α-chymotrypsinogen.
4. Concluding Remarks
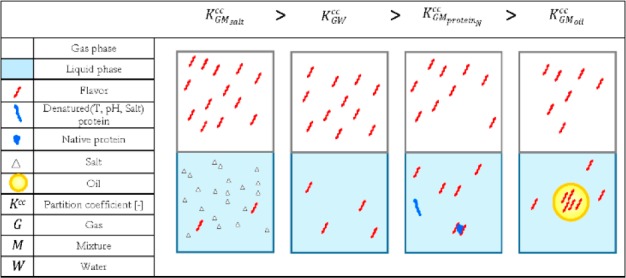
From the previous sections it is clear that retention and release of flavor components are a complex matter, but at the same time, there is a theoretical background that can help interpretation of experimental data using thermodynamics as a starting point and as schematically illustrated in Figure 3. A flavor will partition between liquid and gas (2nd panel), and this partitioning can be influenced by the presence of small components (1st panel) that affect the chemical activity of the flavor. When introducing a binder such as protein, depending on its state (native or denatured), the flavor will bind more or less to it while obeying sorption relations (3rd panel), whereas introduction of an additional phase (4th panel) such as oil will lead to redistribution of the flavor over all available phases depending on the partitioning coefficients.
Figure 3.
Schematic representation of the effect of different beverage ingredients on the partition coefficient of flavors.
Although many investigations have been done on flavors, application of models in food design is still a step to take. We have shown that there are ample methods, thermodynamic insights, and kinetic models. We think that the theoretical background is not used that often due to the peculiarities of the components: they are mostly present at a very low concentration, and the models are validated for conditions in which the ratio of components is not that extreme.
This is also linked to the analysis threshold that can induce a relatively large measurement error at the low flavor concentrations in foods. To that needs to be added that, even the smallest loss of flavor to, e.g., adsorption to a wall, can influence the measured concentration greatly, and through that also, for example, the partition coefficient. We want to stress that the methods that are standardly used to derive parameter values (as described in that section), either through linearization in a log plot or taking reciprocal values, are very prone to small differences in concentration. For example, in a reciprocal plot, those measurements that are done at low concentration give a lot of weight to the parameter values that are derived from, for example, the slope because they would be positioned at the high end of the x-axis. Because these concentrations are also prone to the highest experimental error (often in the range as the measured values), this can very rapidly lead to misinterpretation. Because of these aspects, we recommend the use of fitting procedures that are nonlinear and are directly applied to measured data. This is a well-established fact in, for example, enzyme kinetics research in which linearization was traditionally used in the Lineweaver–Burke approach, which leads to over- and underestimation of parameters, whereas this does not occur using a nonlinear approach.
We already mentioned the analysis threshold, and we think that this is an undervalued aspect of flavor research, especially in combination with interaction analysis that makes the situation as described above even more complex. The concentrations that are to be measured will in most cases be lower than in a system that contains liquids and flavor, which puts even more relevance to the measurement method. It cannot be ignored that this may also have led to misinterpretation of parameter values and consequently models that are unable to capture the observed release behavior. We thoroughly believe that more attention needs to be paid to how concentrations are measured and their influence on parameter values and model predictions.
The authors declare no competing financial interest.
References
- van Ruth S. M.; Roozen J. P.. Delivery of flavours from food matrices. In Food Flavour Technology; Taylor A. J., Ed.; Sheffield Academic Press Ltd., 2002; p 167. [Google Scholar]
- Taylor A. J.; Adamson A. W. Physical chemistry of flavour. Int. J. Food Sci. Technol. 1998, 33, 53–62. 10.1046/j.1365-2621.1998.00157.x. [DOI] [Google Scholar]
- Taylor A. J. Release and transport of flavors in vivo: Physicochemical, physiological, and perceptual considerations. Compr. Rev. Food Sci. Food Saf. 2002, 1, 45–57. 10.1111/j.1541-4337.2002.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Taylor A. J.; Roozen J. P. Volatile flavor release from foods during eating. Crit. Rev. Food Sci. Nutr. 1996, 36, 765–784. 10.1080/10408399609527749. [DOI] [PubMed] [Google Scholar]
- Berger R. G.; Girault G. Macromolecule-ligand binding studied by the Hummel and Dreyer method: Current state of the methodology. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2003, 797, 51–61. 10.1016/S1570-0232(03)00482-3. [DOI] [PubMed] [Google Scholar]
- Delwiche J. The impact of perceptual interactions on perceived flavor. Food Qual. Prefer. 2004, 15, 137–146. 10.1016/S0950-3293(03)00041-7. [DOI] [Google Scholar]
- Guichard E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev. Int. 2002, 18, 49–70. 10.1081/FRI-120003417. [DOI] [Google Scholar]
- Kinsella J. E.; Morr C. V. Milk proteins: Physicochemical and functional properties. CRC Crit. Rev. Food Sci. Nutr. 1984, 21, 197–262. 10.1080/10408398409527401. [DOI] [PubMed] [Google Scholar]
- Kühn J.; Considine T.; Singh H. Interactions of milk proteins and volatile flavor compounds: Implications in the development of protein foods. J. Food Sci. 2006, 71, R72. 10.1111/j.1750-3841.2006.00051.x. [DOI] [Google Scholar]
- Overbosch P.; Afterof W. G. M.; Haring P. G. M. Flavor release in the mouth. Food Rev. Int. 1991, 7, 137–184. 10.1080/87559129109540906. [DOI] [Google Scholar]
- de Roos K. B. Effect of texture and microstructure on flavour retention and release. Int. Dairy J. 2003, 13, 593–605. 10.1016/S0958-6946(03)00108-0. [DOI] [Google Scholar]
- de Roos K. B. Understanding and controlling the behaviour of aroma compounds in thermally processed foods. Trends Food Sci. Technol. 2006, 17, 236–243. 10.1016/j.tifs.2005.11.008. [DOI] [Google Scholar]
- Salles C.; et al. In-mouth mechanisms leading to flavor release and perception. Crit. Rev. Food Sci. Nutr. 2010, 51, 67–90. 10.1080/10408390903044693. [DOI] [PubMed] [Google Scholar]
- Villamor R. R.; Ross C. F. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. 10.1146/annurev-food-030212-182707. [DOI] [PubMed] [Google Scholar]
- Wang K.; Arntfield S. D. Effect of protein-flavour binding on flavour delivery and protein functional properties: A special emphasis on plant-based proteins. Flavour Fragrance J. 2017, 32, 92–101. 10.1002/ffj.3365. [DOI] [Google Scholar]
- Xu L.; Basheer C.; Lee H. K. Developments in single-drop microextraction. J. Chromatogr. A 2007, 1152, 184–192. 10.1016/j.chroma.2006.10.073. [DOI] [PubMed] [Google Scholar]
- Kinsella J. E.; Damodaran S.. Flavor problems in soy proteins: Origin nature, control and binding phenomena. In The analysis and control of less desirable flavors in foods and beverages; Charalambous G., Ed.; Academic Press: New York, 1980; pp 95–109. [Google Scholar]
- Nelson P. E.; Hoff J. E. Food volatiles: Gas chromatographic determination of partition coefficients in water-lipid Systems. J. Food Sci. 1968, 33, 479–482. 10.1111/j.1365-2621.1968.tb03659.x. [DOI] [Google Scholar]
- Mana Kialengila D.; Wolfs K.; Bugalama J.; Van Schepdael A.; Adams E. Full evaporation headspace gas chromatography for sensitive determination of high boiling point volatile organic compounds in low boiling matrices. J. Chromatogr. A 2013, 1315, 167–175. 10.1016/j.chroma.2013.09.058. [DOI] [PubMed] [Google Scholar]
- Sander R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. 10.5194/acp-15-4399-2015. [DOI] [Google Scholar]
- Land D. G. Perspectives on the effects of interactions on flavor perception: An overview. Flavor-Food Interact. 1994, 633, 2–11. 10.1021/bk-1996-0633.ch001. [DOI] [Google Scholar]
- O’Neill T. E.; Kinsella J. E. Binding of alkanone flavors to β-Lactoglobulin: Effects of conformational and chemical modification. J. Agric. Food Chem. 1987, 35, 770–774. 10.1021/jf00077a030. [DOI] [Google Scholar]
- Andriot I.; Harrison M.; Fournier N.; Guichard E. Interactions between methyl ketones and β-Lactoglobulin: Sensory analysis, headspace analysis, and mathematical modeling. J. Agric. Food Chem. 2000, 48, 4246–4251. 10.1021/jf991261z. [DOI] [PubMed] [Google Scholar]
- Relkin P.; Molle D.; Marin I. Non-covalent binding of benzaldehyde to β-lactoglobulin: Characterisation by spectrofluorimetry and electrospray ionisation mass-spectrometry. J. Dairy Res. 2001, 68, 151–155. 10.1017/S0022029900004684. [DOI] [PubMed] [Google Scholar]
- Rogacheva S.; Espinosa Díaz M. A.; Voilley A. Transfer of aroma compounds in water-lipid systems: Binding tendency of β-lactoglobulin. J. Agric. Food Chem. 1999, 47, 259–263. 10.1021/jf9808372. [DOI] [PubMed] [Google Scholar]
- Mitropoulou A.; Hatzidimitriou E.; Paraskevopoulou A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva. Food Res. Int. 2011, 44, 1561–1570. 10.1016/j.foodres.2011.04.023. [DOI] [Google Scholar]
- Sostmann K.; Guichard E. Immobilized β-lactoglobulin on a HPLC-column: A rapid way to determine protein-flavour interactions. Food Chem. 1998, 62, 509–513. 10.1016/S0308-8146(97)00182-9. [DOI] [Google Scholar]
- Jouenne E.; Crouzet J. Determination of apparent binding constants for aroma compounds with β-lactoglobulin by dynamic coupled column liquid chromatography. J. Agric. Food Chem. 2000, 48, 5396–5400. 10.1021/jf000423k. [DOI] [PubMed] [Google Scholar]
- Pelletier E.; Sostmann K.; Guichard E. Measurement of interactions between β-Lactoglobulin and flavor compounds (esters, acids, and pyrazines) by affinity and exclusion size chromatography. J. Agric. Food Chem. 1998, 46, 1506–1509. 10.1021/jf970725v. [DOI] [Google Scholar]
- Souza E. S.; et al. Estimating the octanol/water partition coefficient for aliphatic organic compounds using semi-empirical electrotopological index. Int. J. Mol. Sci. 2011, 12, 7250–7264. 10.3390/ijms12107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi I.; Hirono S.; Liu Q.; Nakagome I.; Matsushita Y. Simple Method of Calculating Octanol/Water Partition Coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. 10.1248/cpb.40.127. [DOI] [Google Scholar]
- Arthur C. L.; Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. 10.1021/ac00218a019. [DOI] [Google Scholar]
- Jeannot M. A.; Przyjazny A.; Kokosa J. M. Single drop microextraction-Development, applications and future trends. J. Chromatogr. A 2010, 1217, 2326–2336. 10.1016/j.chroma.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Kolb B.; Ettre L. S.. Static Headspace-Gas Chromatography: Theory and Practice; John Wiley and Sons, Inc., 2006. [Google Scholar]
- Soria A. C.; García-Sarrió M. J.; Ruiz-Matute A. I.; Sanz M. L. Headspace techniques for volatile sampling. Compr. Anal. Chem. 2017, 76, 255–278. 10.1016/bs.coac.2017.02.001. [DOI] [Google Scholar]
- Voilley A.; Simatos D.; Loncin M. Gas-phase concentration of volatiles in equilibrium with a liquid aqueous phase. Leb. Technol. 1977, 10, 45–49. [Google Scholar]
- Kolb B.; Ettre L. S.. Static Headspace–Gas Chromatography; John Wiley and Sons, Inc., 2013; Vol. 1. [Google Scholar]
- Ochiai N.; Tsunokawa J.; Sasamoto K.; Hoffmann A. Multi-volatile method for aroma analysis using sequential dynamic headspace sampling with an application to brewed coffee. J. Chromatogr. A 2014, 1371, 65–73. 10.1016/j.chroma.2014.10.074. [DOI] [PubMed] [Google Scholar]
- Mackay D.; Shiu W. Y.; Sutherland R. P. Determination of air-water Henry’s law constants for hydrophobic pollutants. Environ. Sci. Technol. 1979, 13, 333–337. 10.1021/es60151a012. [DOI] [Google Scholar]
- Damodaran S.; Kinsella J. E. Interaction of carbonyls with soy protein: Thermodynamic effects. J. Agric. Food Chem. 1981, 29, 1249–1253. 10.1021/jf00108a037. [DOI] [Google Scholar]
- Jung D.-M.; Ebeler S. E. Headspace solid-phase microextraction method for the study of the volatility of selected flavor compounds. J. Agric. Food Chem. 2003, 51, 200–5. 10.1021/jf020651+. [DOI] [PubMed] [Google Scholar]
- Beyeler M.; Solms J. Interaction of flavor model compounds with soy protein and bovine serumalbumin. Leb. Wissensch Technol. 1974, 7, 217–219. [Google Scholar]
- Mills O. E.; Solms J. Interaction of selected flavour compounds with whey proteins. Leb. Technol. 1984, 17, 331–335. [Google Scholar]
- Weel K. G. C.; et al. Flavor release and perception of flavored whey protein gels: Perception is determined by texture rather than by release. J. Agric. Food Chem. 2002, 50, 5149–5155. 10.1021/jf0202786. [DOI] [PubMed] [Google Scholar]
- Nahon D. F.; Roozen J. P.; de Graaf C. Flavor release from mixtures of sodium cyclamate, sucrose, and an orange aroma. J. Agric. Food Chem. 1998, 46, 3426–3430. 10.1021/jf980097x. [DOI] [Google Scholar]
- Doyen K.; Carey M.; Linforth R. S. T.; Marin M.; Taylor A. J. Volatile release from an emulsion: Headspace and in-mouth studies. J. Agric. Food Chem. 2001, 49, 804–810. 10.1021/jf000853a. [DOI] [PubMed] [Google Scholar]
- Robinson A. L.; et al. Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning. J. Agric. Food Chem. 2009, 57, 10313–10322. 10.1021/jf902586n. [DOI] [PubMed] [Google Scholar]
- Roberts D. D.; Pollien P.; Watzke B. Experimental and modeling studies showing the effect of lipid type and level on flavor release from milk-based liquid emulsions. J. Agric. Food Chem. 2003, 51, 189–195. 10.1021/jf025646k. [DOI] [PubMed] [Google Scholar]
- Nahon D. F.; Harrison M.; Roozen J. P. Modeling flavor release from aqueous sucrose solutions, using mass transfer and partition coefficients. J. Agric. Food Chem. 2000, 48, 1278–1284. 10.1021/jf990464k. [DOI] [PubMed] [Google Scholar]
- Wang K.; Arntfield S. D. Effect of salts and pH on selected ketone flavours binding to salt-extracted pea proteins: The role of non-covalent forces. Food Res. Int. 2015, 77, 1–9. 10.1016/j.foodres.2015.03.017. [DOI] [Google Scholar]
- Landy P.; Druaux C.; Voilley A. Retention of aroma compounds by proteins in aqueous solution. Food Chem. 1995, 54, 387–392. 10.1016/0308-8146(95)00069-U. [DOI] [Google Scholar]
- O’Neill T. E.; Kinsella J. E. Flavor protein interactions: Characteristics of 2-Nonanone binding to isolated soy protein fractions. J. Food Sci. 1987, 52, 98–101. 10.1111/j.1365-2621.1987.tb13980.x. [DOI] [Google Scholar]
- Damodaran S.; Kinsella J. E. The effects of neutral salts on the stability of macromolecules. A new approach using a protein-ligand binding system. J. Biol. Chem. 1981, 256, 3394–3398. [PubMed] [Google Scholar]
- O’keefe S. F.; Wilson L. A.; Murphy P. A.; Resurreccion A. P. Determination of the binding of hexanal to soy glycinin and O-conglycinin in an aqueous model system using a headspace technique. J. Agric. Food Chem. 1991, 39, 1022–1028. 10.1021/jf00006a003. [DOI] [Google Scholar]
- Seuvre A. M.; Espinosa Díaz M. A.; Voilley A. Influence of the food matrix structure on the retention of aroma compounds. J. Agric. Food Chem. 2000, 48, 4296–4300. 10.1021/jf990825w. [DOI] [PubMed] [Google Scholar]
- Fares K.; Landy P.; Guilard R.; Voilley A. Physicochemical interactions between aroma compounds and milk proteins: Effect of water and protein modification. J. Dairy Sci. 1998, 81, 82–91. 10.3168/jds.S0022-0302(98)75554-7. [DOI] [Google Scholar]
- Langourieux S.; Crouzet J. Study of aroma compounds-polysaccharides interactions by dynamic exponential dilution. LWT - Food Sci. Technol. 1994, 27, 544–549. 10.1006/fstl.1994.1107. [DOI] [Google Scholar]
- Gosset J.; Cameron C.; Eckstrom B.; Goodman C.; Lincoff A.. Mass transfer coefficients and Henry’s constants for packed-tower air stripping of volatile organics: Measurements and correlation, 1985.
- Nilsson K.; Larsson P.-O. High-performance liquid affinity chromatography on silica-bound alcohol dehydrogenase. Anal. Biochem. 1983, 134, 60–72. 10.1016/0003-2697(83)90264-6. [DOI] [PubMed] [Google Scholar]
- Reiners J.; Nicklaus S.; Guichard E. Interactions between β-lactoglobulin and flavour compounds of different chemical classes. Impact of the protein on the odour perception of vanillin and eugenol. Lait 2000, 80, 347–360. 10.1051/lait:2000130. [DOI] [Google Scholar]
- Guichard E.; Langourieux S. Interactions between β-lactoglobulin and flavour compounds. Food Chem. 2000, 71, 301–308. 10.1016/S0308-8146(00)00181-3. [DOI] [Google Scholar]
- de Roos K. B.Physiochemical models of flavor release from foods. In Flavor Release; Roberts D. D., Taylor A. J., Eds.; American Chemical Society, 2000; pp 126–141. [Google Scholar]
- Cao W.; Knudsen K.; Fredenslund A.; Rasmussen P. Group-contribution viscosity predictions of liquid mixtures using UNIFAC-VLE parameters. Ind. Eng. Chem. Res. 1993, 32, 2088–2092. 10.1021/ie00021a034. [DOI] [Google Scholar]
- Novak L. T.; Chen C.; Song Y. Segment-Based Eyring - NRTL Viscosity Model for Mixtures Containing Polymers. Ind. Eng. Chem. Res. 2004, 43, 6231–6237. 10.1021/ie0401152. [DOI] [Google Scholar]
- Sadeghi R. Segment-based Eyring–Wilson viscosity model for polymer solutions. J. Chem. Thermodyn. 2005, 37, 445–448. 10.1016/j.jct.2004.10.009. [DOI] [Google Scholar]
- Buttery R. G.; Guadagni D. G.; Ling L. C. Flavor compounds: Volatilities in vegetable oil and oil-water mixtures. Estimation of odor thresholds. J. Agric. Food Chem. 1973, 21, 198–201. 10.1021/jf60186a029. [DOI] [Google Scholar]
- de Roos K. B.; Sarelse J. A.. Volatile acids and nitrogen compounds in prawn powder. In Flavour Science: Recent Developments; Taylor A. J., Mottram D. S., Eds.; The Royal Society of Chemistry, 1996; pp 13–18. [Google Scholar]
- Escalona-Buendia H.; Piggott J. R.; Conner J. M.; Paterson A. Effect of ethanol strength on the release of higher alcohols and aldehydes in model solutions. Dev. Food Sci. 1998, 40, 615–620. 10.1016/S0167-4501(98)80081-2. [DOI] [Google Scholar]
- Williams A. A.; Rosser P. R. Aroma enhancing effects of ethanol. Chem. Senses 1981, 6, 149–153. 10.1093/chemse/6.2.149. [DOI] [Google Scholar]
- Roberts D. D.; Elmore J. S.; Langley K. R.; Bakker J. Effects of sucrose, guar gum, and carboxymethylcellulose on the release of flavor compounds under dynamic conditions. J. Agric. Food Chem. 1996, 44, 1321–1326. 10.1021/jf950567c. [DOI] [Google Scholar]
- Arakawa T.; Timasheff S. N. Mechanism of protein salting in and salting out by divalent cation salts: Balance between hydration and salt binding. Biochemistry 1984, 23, 5912–5923. 10.1021/bi00320a004. [DOI] [PubMed] [Google Scholar]
- McNulty P. B.Food Structure and Behaviour. In Food Structure and Behaviour; Blanshard J. M. V., Lillford P., Eds.; Academic Press: London, 1987, p 245. [Google Scholar]
- Harrison M.; Hills B. P. Mathematical model of flavor release from liquids containing aroma-binding macromolecules. J. Agric. Food Chem. 1997, 45, 1883–1890. 10.1021/jf9607876. [DOI] [Google Scholar]
- Kinsella J. E. Flavor perception and binding to food components. Flavor Chemistry of Lipid Foods; 1989, 376–403. [Google Scholar]
- Fick A.Ueber Difusion, 1851; pp 59–86. [Google Scholar]
- Harrison M.; Hills B. P.; Bakker J.; Clothier T. Mathematical models of flavor release from liquid emulsions. J. Food Sci. 1997, 62, 653–664. 10.1111/j.1365-2621.1997.tb15429.x. [DOI] [Google Scholar]
- Whitman W. G. The two film theory of gas absorption. Chem. Metall. Eng. 1923, 29, 146–148. [Google Scholar]
- Coulson J. M.; Richardson J. F.; Backhurst J. R.; Harker J. H.; Barlow W.. Chemical engineering: fluid flow, heat transfer and mass transfer; Butterworth-Heinemann, 1999; Vol. 1. [Google Scholar]
- Higbie R. The rate of absorption of a pure gas into a still liquid during short periods of exposure. Trans. Am. Inst. Chem. Eng. 1935, 35, 36–60. [Google Scholar]
- Linforth R. S. T. Modelling Flavour Release. Food Flavour Technology 2010, 207–228. 10.1002/9781444317770.ch8. [DOI] [Google Scholar]
- van Elk E. P.; Knaap M. C.; Versteeg G. F. Application of the penetration theory for gas - liquid mass transfer without liquid bulk: differences with system with a bulk. Chem. Eng. Res. Des. 2007, 85, 516–524. 10.1205/cherd06066. [DOI] [Google Scholar]
- Marin M.; Baek I.; Taylor A. J.. Flavor release as a unit operation: A mass transfer approach based on a dynamic headspace dilution method. In Flavor Release; Roberts D. D., Taylor A. J., Eds.; American Chemical Society, 2000; Vol. 763, pp 153–165. [Google Scholar]
- De Roos K. B.; Wolswinkel K.. Non-equilibrium partition model for predicting flavor release in the mouth. In Trends in Flavor Research; Maarse H., van der Heij D. G., Eds.; Elsevier, 1994; pp 15–32. [Google Scholar]
- McNulty P. B.Factors affecting flavour release and uptake in O/W emulsions; Massachusetts Inst. Technol., 1972. [Google Scholar]
- McNulty P. B.; Karel M. Factors affecting flavour release and uptake in O/W emulsions I. Release and uptake models. Int. J. Food Sci. Technol. 1973, 8, 309–318. 10.1111/j.1365-2621.1973.tb01719.x. [DOI] [Google Scholar]
- McNulty P. B.; Karel M. Factors affecting flavour release and uptake in oil-water emulsions II. Stirred cell studies. Int. J. Food Sci. Technol. 1973, 8, 319–331. 10.1111/j.1365-2621.1973.tb01720.x. [DOI] [Google Scholar]
- McNulty P. B.; Karel M. Factors affecting flavour release and uptake in oil-water emulsions. III. Scale-up model and emulsion studies. Int. J. Food Sci. Technol. 1973, 8, 415–427. 10.1111/j.1365-2621.1973.tb01728.x. [DOI] [Google Scholar]
- Nahon D. F.; Roozen J. P.; de Graaf C. Sweetness flavour interactions in soft drinks. Food Chem. 1996, 56, 283–289. 10.1016/0308-8146(96)00025-8. [DOI] [Google Scholar]
- Reineccius G.Flavor chemistry and technology; CRC Press, 2006. [Google Scholar]
- Tromelin A.; Andriot I.; Guichard E. Protein-flavour interactions. Flavour Food 2006, 172–207. 10.1533/9781845691400.2.172. [DOI] [Google Scholar]
- Ziegler H.Flavourings: Production, composition, applications, regulations; WILEY-VCH Verlag, 2007. [Google Scholar]
- Coupland J. N.; McClements D. J. Lipid oxidation in food emulsions. Trends Food Sci. Technol. 1996, 7, 83–91. 10.1016/0924-2244(96)81302-1. [DOI] [Google Scholar]
- McClements D. J.Lipid-based emulsions and emulsifiers. In Food Lipids: Chemistry, Nutrition, and Biotechnology; Akoh C. C., Min D. B., Eds.; CRC Press, 2008; pp 63–97. [Google Scholar]
- Hills B. P.; Harrison M. Two-film theory of flavour release from solids. Int. J. Food Sci. Technol. 1995, 30, 425–436. 10.1111/j.1365-2621.1995.tb01390.x. [DOI] [Google Scholar]
- Giroux H. J.; Perreault V.; Britten M. Characterization of hydrophobic flavor release profile in oil-in-water emulsions. J. Food Sci. 2007, 72, S125. 10.1111/j.1750-3841.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- Relkin P.; Fabre M.; Guichard E. Effect of fat nature and aroma compound hydrophobicity on flavor release from complex food emulsions. J. Agric. Food Chem. 2004, 52, 6257–6263. 10.1021/jf049477a. [DOI] [PubMed] [Google Scholar]
- Heng L.; et al. Protein-flavour interactions in relation to development of novel protein foods. Trends Food Sci. Technol. 2004, 15, 217–224. 10.1016/j.tifs.2003.09.018. [DOI] [Google Scholar]
- Hansen a P.; Heinis J. J. Benzaldehyde, citral, and d-limonene flavor perception in the presence of casein and whey proteins. J. Dairy Sci. 1992, 75, 1211–1215. 10.3168/jds.S0022-0302(92)77869-2. [DOI] [PubMed] [Google Scholar]
- Hansen A. P.; Heinis J. J. Decrease of vanillin flavour perception in the presence of casein and whey proteins. J. Dairy Sci. 1991, 74, 2936–2940. 10.3168/jds.S0022-0302(91)78477-4. [DOI] [PubMed] [Google Scholar]
- Maehashi K.; Huang L. Bitter peptides and bitter taste receptors. Cell. Mol. Life Sci. 2009, 66, 1661–1671. 10.1007/s00018-009-8755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram D. S.; Szauman-Szumski C.; Dodson A. Interaction of Thiol and Disulfide Flavor Compounds with Food Components. J. Agric. Food Chem. 1996, 44, 2349–2351. 10.1021/jf960170o. [DOI] [Google Scholar]
- Wang K.; Arntfield S. D. Binding of carbonyl flavours to canola, pea and wheat proteins using GC/MS approach. Food Chem. 2014, 157, 364–372. 10.1016/j.foodchem.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Mills F. D.; Solms J. Interaction of selected flavour compounds with whey proteins. Lebensm.-Wiss.u.-Technol. 1984, 17, 331–335. [Google Scholar]
- Semenova M. G.; Antipova A. S.; Misharina T. A.; Golovnya R. V. Binding of aroma compounds with legumin. I. Binding of hexyl acetate with 11S globulin depending on the protein molecular state in aqueous medium. Food Hydrocolloids 2002, 16, 557–564. 10.1016/S0268-005X(02)00017-6. [DOI] [Google Scholar]
- Carr J.; et al. The effect of gelling agent type and concentration on flavor release in model systems. Flavor-Food Interactions 1996, 633, 98–108. 10.1021/bk-1996-0633.ch009. [DOI] [Google Scholar]
- Jaime I.; Mela D. J.; Bratchell N. A study of texture-flavor interactions using free-choice profiling. J. Sens. Stud. 1993, 8, 177–188. 10.1111/j.1745-459X.1993.tb00212.x. [DOI] [Google Scholar]
- Wilson C. E.; Brown W. E. Influence of food matrix structure and oral breakdown during mastication on temporal perception of flavor. J. Sens. Stud. 1997, 12, 69–86. 10.1111/j.1745-459X.1997.tb00054.x. [DOI] [Google Scholar]
- Kinsella J. E.; Whitehead D. M.. Proteins in Whey: Chemical, Physical, and Functional Properties. In Advances in Food and Nutrition Research; Kinsella J. E., Ed.; Academic Press, 1989; Vol. 33, pp 343–438. [DOI] [PubMed] [Google Scholar]
- Batt C. A.; Brady J.; Sawyer L. Design improvements of β-lactoglobulin. Trends Food Sci. Technol. 1994, 5, 261–265. 10.1016/0924-2244(94)90019-1. [DOI] [Google Scholar]
- Andriot I.; Harrison M.; Fournier N.; Guichard E. Interactions between methyl ketones and β-lactoglobulin: Sensory analysis, headspace analysis, and mathematical modeling. J. Agric. Food Chem. 2000, 48, 4246–4251. 10.1021/jf991261z. [DOI] [PubMed] [Google Scholar]
- O’Neill T. E.; Kinsella J. E. Effect of heat treatment and modification on conformation and flavor binding by β-Lactoglobulin. J. Food Sci. 1988, 53, 906–909. 10.1111/j.1365-2621.1988.tb08982.x. [DOI] [Google Scholar]
- Franzen K. L.; Kinsella J. E. Parameters affecting the binding of volatile flavor compounds in model food systems. I. Proteins. J. Agric. Food Chem. 1974, 22, 675–678. 10.1021/jf60194a040. [DOI] [Google Scholar]
- Jasinski E.; Kilara A.. Flavor binding by whey proteins. Milchwissenschaft; 1985; Vol. 596. [Google Scholar]
- Charles M.; Lambert S.; Brondeur P.; Courthaudon J.-L.; Guichard E.. Influence of formulation and structure of an oil-in-water emulsion on the flavour release. In Flavor Release; Roberts D. D., Taylor A. J., Eds.; American Chemical Society, 2000; pp 342–354. [Google Scholar]
- Futterman S.; Heller J. The enhancement of fluorescence and the decreased susceptibility to enzymatic oxidation of retinol complexed with bovine serum albumin, beta-lactoglobulin, and the retinol-binding protein of human plasma. J. Biol. Chem. 1972, 247, 5168–5172. [PubMed] [Google Scholar]
- Burova T. V.; et al. Flavour release in model bovine serum albumin/pectin/ 2-octanone systems. Food Hydrocolloids 1999, 13, 7–14. 10.1016/S0268-005X(98)00059-9. [DOI] [Google Scholar]
- Morrisett J. D.; Pownall H. J.; Gotto JR A. M. Bovine serum albumin: study of the fatty acid and steroid binding sites using spin-labeled lipids. J. Biol. Chem. 1974, 260, 2487–2494. [PubMed] [Google Scholar]
- Pérez M. D.; et al. Interaction of fatty acids with beta-lactoglobulin and albumin from ruminant milk. J. Biochem. 1989, 106, 1094–7. 10.1093/oxfordjournals.jbchem.a122971. [DOI] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [PubMed] [Google Scholar]
- Mohammadzadeh-K A.; Smith L. M.; Feeney R. E. Hydrophobic binding of hydrocarbons by proteins. I. Relationship of hydrocarbon structure. Biochim. Biophys. Acta, Protein Struct. 1969, 194, 256–264. 10.1016/0005-2795(69)90201-3. [DOI] [PubMed] [Google Scholar]
- Mohammadzadeh-K A.; Smith L. M.; Feeney R. E. Hydrophobic binding of hydrocarbons by proteins. II. Relationship of protein structure. Biochim. Biophys. Acta, Protein Struct. 1969, 194, 256–264. 10.1016/0005-2795(69)90201-3. [DOI] [PubMed] [Google Scholar]
- Mohammadzadeh-K A.; Feeney R. E.; Samuels R. B.; Smith L. M. Solubility of alkanes in protein solutions. Biochim. Biophys. Acta, Protein Struct. 1967, 147, 583–589. 10.1016/0005-2795(67)90018-9. [DOI] [PubMed] [Google Scholar]
- Damodaran S.; Kinsella J. E. Flavor protein interactions. Binding of carbonyls to bovine serum albumin: Thermodynamic and conformational effects. J. Agric. Food Chem. 1980, 28, 567–571. 10.1021/jf60229a019. [DOI] [PubMed] [Google Scholar]
- Burova T. V.; et al. Flavour release in model bovine serum albumin/pectin/2-octanone systems. Food Hydrocolloids 1999, 13, 7–14. 10.1016/S0268-005X(98)00059-9. [DOI] [Google Scholar]
- Swaisgood H. E. Review and update of casein chemistry. J. Dairy Sci. 1993, 76, 3054–3061. 10.3168/jds.S0022-0302(93)77645-6. [DOI] [PubMed] [Google Scholar]
- Jouenne E.; Crouzet J.. Aroma compounds: proteins interactions using headspace techniques. In Headspace Analysis of Foods and Flavors: Theory and Practice; Rouseff R. L., Cadwallader K. R., Eds.; Springer, 2001; Vol. 488, pp 33–41. [PubMed] [Google Scholar]
- McClements D. J.Food Emulsion Principle,Practices, and Techniques; CRC Press, 2004. [Google Scholar]
- Landy P.; Rogacheva S.; Lorient D.; Voilley A. Thermodynamic and kinetic aspects of the transport of small molecules in dispersed systems. Colloids Surf., B 1998, 12, 57–65. 10.1016/S0927-7765(98)00057-5. [DOI] [Google Scholar]
- Mannhold R.; Rekker R. F. The hydrophobic fragmental constant approach for calculating log P in octanol/water and aliphatic hydrocarbon/water systems. Perspect. Drug Discovery Des. 2000, 18, 1–18. 10.1023/A:1008782809845. [DOI] [Google Scholar]
- Le Thanh M.; Thibeaudeau P.; Thibaut M. A.; Voilley A. Interactions between volatile and non-volatile compounds in the presence of water. Food Chem. 1992, 43, 129–135. 10.1016/0308-8146(92)90226-R. [DOI] [Google Scholar]
- Pourcelly G.; Bazinet L.; Pabby A. K.; Rizvi S. S. H.; Sastre A. M. Developments of bipolar membrane technology in food and bio-Industries. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications 2008, 581–633. 10.1201/9781420009484.ch21. [DOI] [Google Scholar]
- Damodaran S.; Kinsella J. E. Interaction of carbonyls with soy protein: Conformational effects. J. Agric. Food Chem. 1981, 29, 1253–1257. 10.1021/jf00108a038. [DOI] [PubMed] [Google Scholar]
- Gremli H. A. Interaction of flavor compounds with soy protein. J. Am. Oil Chem. Soc. 1974, 51, 95–97. 10.1007/BF02542100. [DOI] [Google Scholar]
- Li Z.; Grun I. U.; Fernando L. N. Interaction of vanillin with soy and dairy proteins in aqueous model systems: A thermodynamic study. J. Food Sci. 2000, 65, 997–1001. 10.1111/j.1365-2621.2000.tb09406.x. [DOI] [Google Scholar]
- Paravisini L.; Guichard E. Interactions between aroma compounds and food matrix: From Food to Perception. Flavour: From Food to Perception 2016, 208–234. 10.1002/9781118929384.ch9. [DOI] [Google Scholar]
- Hansson A.; Andersson J.; Leufven A. The effects of sugars end pectin on flaovur release from a soft drink-related model system. Food Chem. 2001, 72, 363–368. 10.1016/S0308-8146(00)00243-0. [DOI] [Google Scholar]
- King B. M.; et al. Sweetener/sweetness-induced changes in flavor perception and flavor release of Fruity and Green character in beverages. J. Agric. Food Chem. 2006, 54, 2671–2677. 10.1021/jf060195f. [DOI] [PubMed] [Google Scholar]
- Conde-Petit B.; Escher F.; Nuessli J. Structural features of starch-flavor complexation in food model systems. Trends Food Sci. Technol. 2006, 17, 227–235. 10.1016/j.tifs.2005.11.007. [DOI] [Google Scholar]
- Ordoñez M.; Herrera A. Morphologic and stability cassava starch matrices for encapsulating limonene by spray drying. Powder Technol. 2014, 253, 89–97. 10.1016/j.powtec.2013.11.005. [DOI] [Google Scholar]
- Reineccius T. A.; Reineccius G. A.; Peppard T. L. Potential for beta-cyclodextrin as partial fat replacer in low-fat foods. J. Food Sci. 2004, 69, 334–341. 10.1111/j.1365-2621.2004.tb06336.x. [DOI] [Google Scholar]
- Lubbers S.; Guichard E. The effects of sugars and pectin on flavour release from a fruit pastille model system. Food Chem. 2003, 81, 269–273. 10.1016/S0308-8146(02)00422-3. [DOI] [Google Scholar]
- Menting L. G.; Hoogstad B.; Thijssen H. A. G. Diffusion coefficients of water and organic volatiles in carbohydrate-water systems. Int. J. Food Sci. Technol. 1970, 5, 111–126. 10.1111/j.1365-2621.1970.tb01549.x. [DOI] [Google Scholar]
- Gunning Y. M.; et al. Factors affecting the release of flavor encapsulated in carbohydrate matrixes. J. Agric. Food Chem. 1999, 47, 5198–5205. 10.1021/jf990039r. [DOI] [PubMed] [Google Scholar]
- Goubet I.; Le Quere J. L.; Voilley A. J. Retention of aroma compounds by carbohydrates: Influence of their physicochemical characteristics and of their physical state. A review. J. Agric. Food Chem. 1998, 46, 1981–1990. 10.1021/jf970709y. [DOI] [Google Scholar]
- Piccone P.; Lonzarich V.; Navarini L.; Fusella G.; Pittia P. Effect of sugars on liquid-vapour partition of volatile compounds in ready-to-drink coffee beverages. J. Mass Spectrom. 2012, 47, 1120–1131. 10.1002/jms.3073. [DOI] [PubMed] [Google Scholar]
- Kieckbusch T. G.; King C. J. Partition coefficients for acetates in food systems. J. Agric. Food Chem. 1979, 27, 504–507. 10.1021/jf60223a033. [DOI] [Google Scholar]
- Nawar W. W. Some variables affecting composition of headspace aroma. J. Agric. Food Chem. 1971, 19, 1057–1059. 10.1021/jf60178a003. [DOI] [Google Scholar]
- Bredie W. L. P.; Mottram D. S.; Birch G. G.. Aroma binding in maltodextrin solutions. In Trends in Flavour Research; Maarse H., van der Heij D. G., Eds.; Elsevier, 1994; pp 139–143. [Google Scholar]
- Aznar M.; Tsachaki M.; Linforth R. S. T. T.; Ferreira V.; Taylor A. J. Headspace analysis of volatile organic compounds from ethanolic systems by direct APCI-MS. Int. J. Mass Spectrom. 2004, 239, 17–25. 10.1016/j.ijms.2004.09.001. [DOI] [Google Scholar]
- Câmara J. S.; Alves M. A.; Marques J. C. Development of headspace solid-phase microextraction-gas chromatography-mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal. Chim. Acta 2006, 555, 191–200. 10.1016/j.aca.2005.09.001. [DOI] [Google Scholar]
- Whiton R. S.; Zoecklein B. W. Optimization of headspace solid-phase microextraction for analysis of wine aroma compounds. Am. J. Enol. Vitic. 2000, 51, 379–382. [Google Scholar]
- Young S.Distillation principles and processes; Macmillan and Co., 1922. [Google Scholar]
- Bakker J.et al. Effect of the food matrix on flavour release and perception. In Flavour Science: Recent Developments; Taylor A. J., Mottram D. S., Eds.; The Royal Society of Chemistry, 1996; pp 369–374. [Google Scholar]
- Conner J. M.; Birkmyre L.; Paterson A.; Piggott J. R. Headspace concentrations of ethyl esters at different alcoholic strengths. J. Sci. Food Agric. 1998, 77, 121–126. . [DOI] [Google Scholar]
- Dib R.; Chobert J. M.; Dalgalarrondo M.; Haertle T. Secondary structure changes and peptic hydrolysis of β-lactoglobulin induced by diols. Biopolymers 1996, 39, 23–30. . [DOI] [PubMed] [Google Scholar]
- Yoshida K.; et al. Alcohol induced structural and dynamic changes in β-lactoglobulin in aqueous solution: A neutron scattering study. Biochim. Biophys. Acta, Proteins Proteomics 2012, 1824, 502–510. 10.1016/j.bbapap.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Andriot I.; Marin I.; Feron G.; Relkin P.; Guichard E. Binding of benzaldehyde by β-lactoglobulin, by static headspace and high performance liquid chromatography in different physico-chemical conditions. Lait 1999, 79, 577–586. 10.1051/lait:1999647. [DOI] [Google Scholar]
- Fischer N.; Widder S. How proteins influence food flavor. Food Technol. 1997, 51, 68–70. [Google Scholar]
- Florez Menendez J. C.; Fernandez Sanchez M. L.; Sanchez Uria J. E.; Fernandez Martıinez E.; Sanz-Medel A. Static headspace, solid-phase microextraction and headspace solid-phase microextraction for BTEX determination in aqueous samples by gas chromatography. Anal. Chim. Acta 2000, 415, 9–20. 10.1016/S0003-2670(00)00862-X. [DOI] [Google Scholar]
- Poll L.; Flink J. M. Aroma analysis of apple juice: Influence of salt addition on headspace volatile composition as measured by gas chromatography and corresponding sensory evaluations. Food Chem. 1984, 13, 193–207. 10.1016/0308-8146(84)90073-6. [DOI] [Google Scholar]
- Druaux C.; Voilley A. Effect of food composition and microstructure. Trends Food Sci. Technol. 1997, 8, 364–368. 10.1016/S0924-2244(97)01095-9. [DOI] [Google Scholar]
- Lubbers S.; Landy P.; Voilley A. Retention and release of aroma compounds in food containing proteins. Food Technol. 1998, 52, 68–74. [Google Scholar]
- Arntfield S. D.; Murray E. D.; Ismond M. A. H. Influence of salts on the microstructural and rheological properties of heat-induced protein networks from ovalbumin and vicilin. J. Agric. Food Chem. 1990, 38, 1335–1343. 10.1021/jf00096a008. [DOI] [Google Scholar]
- Pérez-Juan M.; Flores M.; Toldrá F. Effect of ionic strength of different salts on the binding of volatile compounds to porcine soluble protein extracts in model systems. Food Res. Int. 2007, 40, 687–693. 10.1016/j.foodres.2006.11.013. [DOI] [Google Scholar]
- Bortnowska G. Effects of pH and ionic strength of NaCl on the stability of diacetyl and (−)-α-pinene in oil-in-water emulsions formed with food-grade emulsifiers. Food Chem. 2012, 135, 2021–2028. 10.1016/j.foodchem.2012.06.082. [DOI] [PubMed] [Google Scholar]
- Rabe S.; Krings U.; Berger R. G. Dynamic flavor release from sucrose solutions. J. Agric. Food Chem. 2003, 51, 5058–5066. 10.1021/jf0302411. [DOI] [PubMed] [Google Scholar]
- Marin M.; Baek I.; Taylor A. J. Volatile release from aqueous solutions under dynamic headspace dilution conditions. J. Agric. Food Chem. 1999, 47, 4750–4755. 10.1021/jf990470g. [DOI] [PubMed] [Google Scholar]
- Walstra P. Physical chemistry of foods 2003, 1. 10.1016/j.ejpb.2003.10.013. [DOI] [Google Scholar]
- McClements D. J.Food emulsions: principles, practices, and techniques; CRC Press, 2004. [Google Scholar]
- Basaran T. K.; Demetriades K.; McClements D. J. Ultrasonic imaging of gravitational separation in emulsions. Colloids Surf., A 1998, 136, 169–181. 10.1016/S0927-7757(97)00338-5. [DOI] [Google Scholar]
- O’keefe S. F.; Resurreccion A. P.; Wilson L. A.; Murphy P. A. Temperature effect on binding of volatile flavor compounds to soy protein in aqueous model systems. J. Food Sci. 1991, 56, 3–7. 10.1111/j.1365-2621.1991.tb05386.x. [DOI] [Google Scholar]
- Druaux C.; Lubbers S.; Charpentier C.; Voilley A. Effects of physico-chemical parameters of a model wine on the binding of γ-decalactone on bovine serum albumin. Food Chem. 1995, 53, 203–207. 10.1016/0308-8146(95)90789-A. [DOI] [Google Scholar]
- Akoh C. C.; Min D. B.. Food lipids: chemistry, nutrition, and biotechnology; CRC Press, 2008. [Google Scholar]
- Sawyer L.; McSweeney P. L.; Fox P. F. β-Lactoglobulin. Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects 2013, 1, 211–259. 10.1007/978-1-4614-4714-6_7. [DOI] [Google Scholar]
- Jouenne E.; Crouzet J.; Taylor A. J. Interaction of aroma compounds with β-lactoglobulin. Flavour Science: Recent Developments 1996, 425–429. 10.1533/9781845698232.7.425. [DOI] [Google Scholar]